Abstract
In recent years a significant increase in the incidence of Serratia marcescens infections was noted at the Chang Gung Memorial Hospital, Taoyuan, Taiwan. A review of laboratory (1991 to 2002) and infection control (1995 to 2002) records showed the possibility of an extended epidemic of nosocomial urinary tract infections (UTIs) caused by S. marcescens. Therefore, in 1998 and 1999, 87 isolates were collected from patients with such infections and examined and another 51 isolates were collected in 2001 and 2002. The patients were mostly elderly or the infections were associated with the use of several invasive devices. S. marcescens was usually the only pathogen found in urine cultures in our study. Neither prior infections nor disseminated infections with the organism were observed in these patients. Resistance to most antibiotics except imipenem was noted. Two genotyping methods, pulsed-field gel electrophoresis and infrequent-restriction-site PCR, were used to examine the isolates. A total of 12 genotypes were identified, and 2 predominant genotypes were found in 72 (82.8%) of the 87 isolates derived from all over the hospital. However, 63.9% of the isolates of the two genotypes were from neurology wards. A subsequent intervention by infection control personnel reduced the infection rate greatly. The number and proportion of the two predominant genotypes were significantly reduced among the 51 isolates collected in 2001 and 2002. Thus, a chronic and long-lasting epidemic of nosocomial UTIs caused by S. marcescens was identified and a successful intervention was carried out. Both a cautious review of laboratory and infection control data and an efficient genotyping system are necessary to identify such a cryptic epidemic and further contribute to the quality of patient care.
Serratia marcescens, a gram-negative bacillus belonging to the family Enterobacteriaceae, was considered a nonpathogenic saprophytic water organism until late in the 20th century. Its pathogenicity for humans was first noted in 1913 (31); however, the prevalence of S. marcescens in human diseases had been underestimated for years before the first known outbreak of nosocomial S. marcescens infections in 1951 (30). Since 1960, infections with this organism have been reported with increasing frequency (1, 7, 24).
S. marcescens is now recognized as a prominent opportunistic pathogen causing significant outbreaks of nosocomial infections of various severities, such as urinary tract infections (UTIs) (5, 15, 16, 20, 28), respiratory tract infections (28), bacteremia (12, 16, 18, 28, 34), conjunctivitis (11), endocarditis (6), meningitis (34), and wound infections (18, 28). In recent years, outbreaks associated with extended-spectrum β-lactamase-producing (9, 17) or imipenem-resistant (26) S. marcescens strains have emerged as an important infection control problem.
Efficient typing methods that differentiate between strains are essential in epidemiological investigations. Genotyping methods, such as pulsed-field gel electrophoresis (PFGE), which analyzes the whole genomic DNA, are considered the most reliable systems for the fingerprinting of S. marcescens (9, 12, 18, 20). However, the expertise and specialized equipment required to perform PFGE seem to limit its popularity. A PCR-based typing method, infrequent-restriction-site PCR (IRS-PCR) (10), has the advantage of having few technical difficulties and requires no previous genetic knowledge of the target organism. The procedure has been used to fingerprint a number of bacterial species and produces results comparable to those obtained by PFGE (21-23). Thus, IRS-PCR has the potential to be used as an efficient tool in epidemiological investigations.
The incidence of S. marcescens infections at the Chang Gung Memorial Hospital (CGMH), Taoyuan, Taiwan, had been increasing significantly in the past few years. Therefore, records from the Clinical Microbiology Laboratory and infection control were retrospectively reviewed, and a prospective molecular epidemiological survey by the two genotyping methods, PFGE and IRS-PCR, was undertaken.
MATERIALS AND METHODS
Setting.
CGMH is a 4,000-bed university-affiliated medical center located in northern Taiwan. To serve patients with different clinical needs, the hospital consists of 13 departments, including the Neurology Department. A total of 407 beds are distributed among the seven general wards and three intensive care units in the Neurology Department.
Laboratory-based surveillance.
Records of clinical isolates have been continuously monitored in the Clinical Microbiology Laboratory, Department of Clinical Pathology, of CGMH since 1991. Each year, data on the number of isolates and their antimicrobial susceptibility patterns are provided to physicians in the hospital as a reference for antimicrobial therapy.
All clinical isolates of S. marcescens were cultured and identified by standard methods (2). No major changes in methods for the identification of S. marcescens were made during the study period. Antimicrobial susceptibility was tested by a standard disk diffusion method (13) for nonblood isolates and by the broth microdilution method (14) for blood isolates. The antimicrobial agents used were amikacin (30 μg), ampicillin (10 μg), cefamandole (30 μg), cephalothin (30 μg), cefotaxime (30 μg), ceftazidime (30 μg), ceftizoxime (30 μg), ceftriaxone (30 μg), cefuroxime (30 μg), chloramphenicol (30 μg), ciprofloxacin (5 μg), gentamicin (10 μg), imipenem (10 μg), nalidixic acid (30 μg), nitrofurantoin (300 μg), piperacillin (100 μg), sulfamethoxazole-trimethoprim (SXT) (1.25/23.75 μg), tetracycline (30 μg), and ticarcillin (75 μg). Nalidixic acid and nitrofurantoin were included only when urinary isolates were tested. Cefixime, ciprofloxacin, and ceftizoxime were not available until 1994, 1996, and 1997, respectively. Susceptible and resistant isolates were defined according to the criteria suggested by the National Committee for Clinical Laboratory Standards (13, 14). Isolates in the intermediate category were deemed resistant in this study.
Nosocomial infection surveillance and environmental culture.
Active hospital-wide surveillance for nosocomial infections has been ongoing since 1976 (8). Beginning in 1995, a case of nosocomial infection was defined according to the updated criteria of the Centers for Disease Control and Prevention (3). A nosocomial UTI was defined as the acquisition of a UTI 72 h after hospitalization with clinical symptoms, such as fever, dysuria, or suprapubic tenderness, and positive laboratory findings (including positive findings by bacterial culture, Gram stain smear, or the leukocyte esterase test or the detection of pyuria). The medical records of patients regarded as having nosocomial infections were reviewed, and data on the patients' demographic characteristics, underlying diseases, antimicrobial agents received, and invasive procedures received were extracted. When appropriate, environmental surveillance was performed, and specimens, such as swabs of solid surfaces, were subjected to bacterial culture as described above.
Microbiological examination.
The study was performed in two stages. Stage I was conducted between April 1998 and March 1999. A total of 203 nonrepetitive clinical isolates of S. marcescens were collected from urinary specimens, and 87 (42.9%) of them were subsequently identified as nosocomial and were submitted for genotype analysis. Stage II, which was performed as described above for stage I, was conducted between April 2001 and March 2002, and 51 nosocomial isolates of S. marcescens were obtained from 100 urinary specimens and were also submitted for genotyping analysis.
PFGE of genomic DNA digested with XbaI and IRS-PCR were used to fingerprint the isolates by previously described procedures (22), with the only modification being that in the IRS-PCR the overnight broth culture was washed once and heated at 94°C for 30 min to inactivate the DNase. The criteria suggested by Tenover et al. (25) were used to interpret the fragment patterns obtained by PFGE. For IRS-PCR, banding patterns with more than four band differences were arbitrarily categorized as different genotypes, according to our previous experience (21-23).
Statistical analysis.
The chi-square test and Student's two-tailed t test were used for statistical analysis. A difference was considered statistically significant when the P value by the two-tailed t test was less than 0.05. All statistical analyses were performed with the SPSS statistical software package for Windows. Results were expressed as means ± standard deviations unless stated otherwise.
RESULTS
Increasing number of S. marcescens infections.
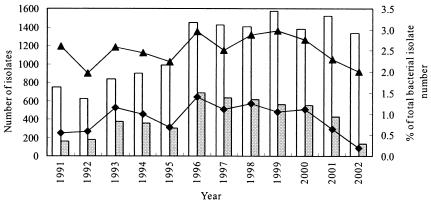
In the 12 years between 1991 and 2002, a total of 14,172 S. marcescens infections, including 4,972 UTIs, were identified. Laboratory records for clinical isolates were retrospectively studied, and so these may have included multiple isolates from the same patient. As the policy for the requisition of bacterial cultures has remained the same over this period, any change in the number of isolates was considered related to the frequency of infections. As shown in Fig. 1, the annual number of S. marcescens isolates increased steadily, from 749 in 1991 to 991 in 1995, followed by a rapid increase to 1,450 in 1996. The number then leveled off at about 1,400 between 1996 and 2002, with a maximum of 1,571 in 1999. Compared to the total number of bacteria isolated in the CGMH laboratory (173,715 from 1991 to 1995, 257,037 from 1996 to 2000, and 132,544 in 2001 and 2002), the proportion of S. marcescens organisms identified increased significantly, from 2.36% before 1995 to 2.81% from 1996 to 2000, and then decreased to 2.15% in 2001 and 2002 (Fig. 1) (average, 2.52%; maximum, 2.97% in 1999 [P < 0.05]). The number of urinary isolates of S. marcescens also increased, from 159 in 1991 to 299 in 1995, followed by a sudden upsurge to 688 in 1996, and then gradually decreased to 134 in 2002 (Fig. 1). A similar trend in the proportion of S. marcescens isolates from urinary origins compared to the total number of bacteria was also observed (Fig. 1). Among the S. marcescens population, the proportion of urinary isolates also increased significantly from 1996 (33.48% from 1991 to 1995 versus 42.05% from 1996 to 2000 [P < 0.05]) and decreased drastically and continuously to 28.21% in 2001 and 10.06% in 2002 (Fig. 1).
FIG. 1.
Trend in the annual number of S. marcescens isolates from all specimens (open bars) or urine cultures (gray bars) and the respective percentage compared with the total bacterial isolate number in CGMH from 1991 to 2002. ▴, proportion of S. marcescens isolates among the total number; ♦, proportion of urinary S. marcescens isolates among the total number.
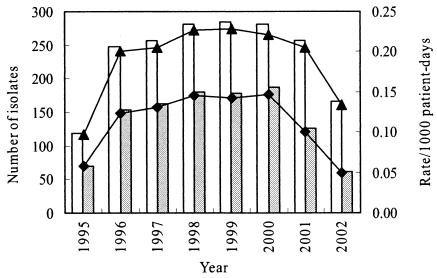
The most conspicuous change was noted in the rate of S. marcescens nosocomial infections per 1,000 patient-days after 1995 (Fig. 2): from 0.098‰ in 1995 to 0.217‰ from 1996 to 2000 (P < 0.001) and then gradually down to 0.205‰ in 2001 and 0.133‰ in 2002 (Fig. 2). Furthermore, a similar trend was observed in nosocomial S. marcescens UTIs (Fig. 2): an increase from 0.058‰ in 1995 to 0.138‰ from 1996 to 2000 (P < 0.001) and then to 0.100‰ in 2001 and 0.049‰ in 2002 (P < 0.001) (Fig. 2).
FIG. 2.
Trend in the annual number of nosocomial S. marcescens isolates from all sources (open bars) or urinary tract infection (gray bars) and the respective rates of infection per 1,000 patient-days in CGMH from 1995 to 2002. ▴, total S. marcescens infection rate; ♦, urinary S. marcescens infection rate.
Antimicrobial resistance of isolates.
Nearly 100% resistance to ampicillin, cefamandole, cefuroxime, cephalothin, chloramphenicol, nitrofurantoin, and tetracycline was shown for recent isolates. Rates of resistance to amikacin, gentamicin, nalidixic acid, piperacillin, SXT, and ticarcillin have remained relatively steady at 50 to 70% during the past decade. Among the extended-spectrum cephalosporins and other newer drugs, the rates of resistance only to ceftazidime, ceftizoxime, and ciprofloxacin were lower than 50%. Imipenem, to which only less than 1% of the population was resistant, remained the most effective antimicrobial agent. The nosocomial population generally showed higher rates of resistance to all of these antibiotics than the nonnosocomial population.
Clinical characteristics of patients with nosocomial UTIs.
Since there was a burst of S. marcescens infections, including UTIs, beginning in about 1996 and the levels stayed high (Fig. 1), particularly those of nosocomial infections (Fig. 2), it was important to determine the source of the infection. During stage I of the study, between April 1998 and March 1999, 87 patients were identified as having S. marcescens nosocomial UTIs. The clinical characteristics of these patients were examined, and some salient findings are described below. For comparison, the patients were divided into two groups: neurology patients and others (Table 1).
TABLE 1.
Clinical characteristics of the 87 patients with nosocomial UTIs caused by S. marcescens studied in stage I of the study
| Factors | Mean ± SD (range) or no. (%) of patients
|
P valuea | Odds ratio (95% CIb) | ||
|---|---|---|---|---|---|
| All patients (n = 87) | Neurology wards (n = 49) | Other wards (n = 38) | |||
| Sex | |||||
| Male | 54 (62.1) | 33 (67.3) | 21 (55.3) | NS | 1.67 (0.64-4.40) |
| Female | 33 (37.9) | 16 (32.7) | 17 (44.7) | ||
| Age (yr) | 58.8 ± 19.9 (2-86) | 58.1 ± 20.6 (6-86) | 59.8 ± 19.2 (2-86) | NS | |
| Duration (days) of hospital admission at onset of UTI | 21.4 ± 21.2 (4-138) | 18.7 ± 20.8 (4-138) | 24.8 ± 21.6 (6-105) | NS | |
| Diagnosis of admission | |||||
| Brain dysfunction or head injury | 33 (37.9) | 28 (57.1) | 5 (13.2) | <0.0001 | |
| Infections at other sites | 18 (20.7) | 4 (8.2) | 14 (36.8) | <0.005 | |
| Malignancy | 15 (17.2) | 5 (10.2) | 10 (26.3) | <0.05 | |
| Cardiovascular disease | 12 (13.8) | 8 (16.3) | 4 (10.5) | NS | 1.66 (0.40-7.26) |
| Diabetes mellitus | 8 (9.2) | 5 (10.2) | 3 (7.9) | NS | 1.33 (0.25-7.61) |
| Invasive devices at onset of UTIs | |||||
| Intravenous catheter | 74 (85.1) | 44 (89.8) | 30 (78.9) | NS | 2.35 (0.61-9.29) |
| Nasogastric tube | 69 (79.3) | 39 (79.6) | 30 (78.9) | NS | 1.04 (0.32-3.32) |
| Urinary catheter | 66 (75.9) | 38 (77.6) | 28 (73.7) | NS | 1.23 (0.41-3.69) |
| Tracheotomy | 37 (42.5) | 27 (55.1) | 10 (26.3) | <0.01 | |
| Central venous pressure tip | 34 (39.1) | 17 (34.7) | 17 (44.7) | NS | 0.66 (0.25-1.71) |
| Mechanical ventilation | 32 (36.8) | 24 (49.0) | 8 (21.1) | <0.01 | |
| Surgical drains | 11 (12.6) | 8 (16.3) | 3 (7.9) | NS | 2.28 (0.49-11.84) |
| Total parenteral nutrition infusion | 6 (6.9) | 0 (0) | 6 (15.8) | <0.01 | |
| Duration (days) of urinary catheter before onset of UTIs | 13.3 ± 10.5 (1-56) | 13.3 ± 9.5 (4-55) | 13.3 ± 11.9 (1-56) | NS | |
| Management after onset of UTIs | |||||
| Withdrawal of urinary catheters | 24 (36.4) | 16 (42.1) | 8 (28.6) | NS | 1.82 (0.57-5.89) |
| Antimicrobial treatment | 84 (96.6) | 46 (93.9) | 38 (100) | NS | 0.00 (0.00-2.90) |
| Symptoms and signs at onset of UTIs | |||||
| Fever | 58 (66.7) | 32 (65.3) | 26 (68.4) | NS | 0.87 (0.32-2.35) |
| Pyuria | 7 (8.0) | 5 (10.2) | 2 (5.3) | NS | 2.05 (0.32-16.30) |
| Other complaints | 2 (2.3) | 0 (0) | 2 (5.3) | NS | 0.00 (0.00-3.17) |
| Asymptomatic | 20 (23.0) | 12 (24.5) | 8 (21.1) | NS | 1.22 (0.40-3.79) |
| Antimicrobial resistance of the isolates, multiresistant to all antibiotics tested except imipenem | 42 (48.3) | 26 (53.1) | 16 (42.1) | NS | 1.55 (0.61-4.00) |
| Imipenem + ciprofloxacin | 10 (11.5) | 4 (8.2) | 6 (15.8) | NS | 0.47 (0.10-2.12) |
| Imipenem + ceftizoxime | 3 (3.4) | 1 (2.0) | 2 (5.3) | NS | 0.38 (0.01-5.59) |
| Imipenem + ciprofloxacin + ceftizoxime | 14 (16.1) | 10 (20.4) | 4 (10.5) | NS | 2.18 (0.56-9.18) |
The χ2 test and two-tailed Student's t test were used for statistical analysis of the difference between blood and urinary isolates. NS, not statistically significant (P > 0.05).
CI, confidence interval.
The ratio of males to females was about 5:3, and the ages ranged from 2 to 86 years (average, 58.8 ± 19.9 years), with three of the patients being younger than 6 years of age and seven of the patients being older than 80 years of age. Forty-seven (54.0%) patients were 60 to 79 years of age. At admission, most of the patients had brain dysfunction or head injury (37.9%) or infection with various pathogens at sites other than the urinary tract (20.7%). Other underlying diseases, such as malignancy (17.2%), cardiovascular diseases (13.8%), and diabetes mellitus (9.2%), were also frequently found. Significant differences were observed between the patients in neurology wards and the patients in other wards, in that brain dysfunction or head injury was the most frequent finding in the neurology group (57.1%; P < 0.0001), while infections at other sites (36.8%; P < 0.005) and malignancy (26.3%; P < 0.05) were more prevalent in the patients in other wards. Nosocomial UTIs occurred a mean of 21.4 ± 21.2 days (range, 4 to 138 days) posthospitalization. All patients had received implants with two to eight different invasive devices, including intravenous catheters (85.1%) and nasogastric tubes (79.3%).
At the onset of the UTIs, 66 (75.9%) patients had had indwelling urinary catheters for a mean of 13.3 ± 10.5 days (range, 1 to 56 days). Fifteen (17%) of the remaining 21 patients had infections occurring in a mean 5.3 ± 4.1 days (range, 1 to 14 days) after the removal of the urinary catheters. Therefore, urinary catheters were the most common invasive device used in these patients (93.1%). Six (6.9%) patients never received a urinary catheter. When a UTI was diagnosed, 84 (96.6%) patients were treated with antimicrobial agents, and the urinary catheter was removed from 24 (36.4%) of the patients. For the remaining three patients who did not receive antimicrobial treatment, the urinary catheter was not withdrawn but was simply replaced with a new catheter.
S. marcescens was usually the only predominant pathogen isolated from the urine cultures. Other pathogens were concomitantly isolated from only five (5.7%) patients: Acinetobacter baumannii (n = 2), Candida albicans (n = 1), Enterococcus faecalis (n = 1), and Pseudomonas aeruginosa (n = 1). Neither prior S. marcescens infections at other sites nor S. marcescens infections that disseminated to other sites were observed in these patients.
The S. marcescens isolates causing nosocomial UTIs exhibited higher levels of resistance than the other S. marcescens isolates to ampicillin (100%), cephalothin (100%), cefuroxime (100%), gentamicin (98.9%), piperacillin (97.7%), nitrofurantoin (97.7%), SXT (96.6%), amikacin (94.3%), nalidixic acid (88.5%), ceftizoxime (72.4%), and ciprofloxacin (57.5%). None of the isolates, however, was resistant to imipenem. The organisms isolated from 42 (48.3%) patients were resistant to all of the antibiotics mentioned above except imipenem.
Environmental surveillance of neurology wards.
To elucidate whether there was a clustering of S. marcescens nosocomial infections in any particular ward, the frequency of such infections in each ward was determined. The survey generally focused on UTIs, since it was becoming apparent during the investigation that the proportion of S. marcescens UTIs was quite high (∼60%) and that the trend in the annual number of S. marcescens UTI cases was similar to the total number of cases of S. marcescens nosocomial infections (Fig. 2). The proportion of S. marcescens isolates causing UTIs was significantly higher in the neurology wards than in the other wards in 1998 (102 of 433 versus 78 of 504, respectively [P < 0.002]).
The environment in the neurology wards was then checked for factors that might have predisposed patients to S. marcescens UTIs. Serious problems were found in the procedures for the handling of urine bottles. The urine was collected in a urine bag attached to the catheter. At a designated time, the urine was drained from the urine bag into the urine bottle for measurement of the urine volume and other tests. During the procedure, although gloves were used, they were left at the bedside and were reused for the same procedure the next time. The same medical assistant collected the urine bottles from different patients in the same ward, and the urine was poured off once per day. The urine bottles were then reused after only a rough rinse with tap water, the thinking being that the bottles did not directly contact the patient, the catheter, or the urine bag. Infection control personnel, however, detected S. marcescens on the bottles. Surveillance cultures were performed continuously for several weeks, and S. marcescens isolates of the same genotypes were obtained from the urine bottles as well as from the urine of new patients residing in the same beds, suggesting that the S. marcescens strains were being transmitted to the next patient who used the contaminated urine bottles (data not shown).
Infection control personnel therefore recommended the following new measures. Two new bottles were prepared and used alternately for each new patient. Before each bottle was reused, it was washed thoroughly, treated with 0.6% sodium hypochlorite, and air dried at least overnight. After measurement of the urine volume, the nurse caring for the patient poured off the urine directly from the urine bottle. A new pair of gloves was used each time that urine was drained from the bag. Contact of the bag with the bottle was absolutely prohibited. The new intervention measures were initiated in late 1999, but it took quite a long time to completely convince the medical personnel in the wards of the need for the new measures and to implement the new measures. The effect was significant, as seen in Fig. 2: the rate of S. marcescens nosocomial infections per 1,000 patient-days dropped dramatically (66%), from 0.146‰ in 2000 to 0.049‰ in 2002 (P < 0.001).
Molecular fingerprinting.
At the beginning of the present investigation, to elucidate whether the significant increase in the number of S. marcescens isolates was due to the transmission of an epidemic strain(s) among the neurology patients, the genotypes of the 87 isolates obtained between April 1998 and March 1999 (stage I) were determined by PFGE and IRS-PCR. Again, at the end of the study, to confirm that the epidemic was under control, another 51 isolates obtained between April 2001 and March 2002 (stage II) were also genotyped by IRS-PCR.
A total of 12 genotypes were found among the 87 isolates recovered during stage I (Table 2), including two dominant genotypes, genotypes 1 (40 isolates, 46.0%) and 2 (32 isolates, 36.8%), among the isolates from 6 intensive care units and 24 general wards. The other 10 genotypes were found among the remaining 15 (17.2%) isolates derived from 4 intensive care units and 11 general wards. Table 2 shows the differences in the distributions of the various genotypes in the neurology wards compared with those in the other wards. Concordant results were obtained by IRS-PCR and PFGE, except that by PFGE two isolates that had the genotype 1 pattern by IRS-PCR had genotypes different from those of the other genotype 1 isolates (data not shown).
TABLE 2.
Number of S. marcescens isolates with the respective genotypes obtained from patients with nosocomial UTIs in stages I and II
| Genotypea | No. of S. marcescens isolates
|
|||||
|---|---|---|---|---|---|---|
| Stage I
|
Stage II
|
|||||
| Total (n = 87) | Neurology wards (n = 49) | Other wards (n = 38) | Total (n = 51) | Neurology wards (n = 11) | Other wards (n = 40) | |
| 1 | 40 | 26 | 14 | 8 | 0 | 8 |
| 2 | 32 | 20 | 12 | 16 | 5 | 11 |
| 3 | 6 | 2 | 4 | 0 | 0 | 0 |
| 4 | 1 | 1 | 0 | 0 | 0 | 0 |
| 5-11 | 7b | 0 | 7b | 0 | 0 | 0 |
| 12 | 1 | 0 | 1 | 1 | 1 | 0 |
| 13 | 0 | 0 | 0 | 1 | 1 | 0 |
| 14 | 0 | 0 | 0 | 1 | 1 | 0 |
| 15 | 0 | 0 | 0 | 6 | 1 | 5 |
| 16 | 0 | 0 | 0 | 2 | 1 | 1 |
| 17 | 0 | 0 | 0 | 2 | 1 | 1 |
| 18 | 0 | 0 | 0 | 4 | 0 | 4 |
| 19 | 0 | 0 | 0 | 2 | 0 | 2 |
| 20-27 | 0 | 0 | 0 | 8b | 0 | 8b |
Genotypes were obtained by IRS-PCR.
One isolate of each genotype.
In stage II, many new genotypes that were different from those obtained in stage I were identified by IRS-PCR of the 51 isolates (Table 2). However, genotypes 1 (8 isolates; 15.7%) and 2 (16 isolates; 31.4%) remained the most prevalent, although a substantial decrease in the numbers of isolates, particularly in the neurology wards, compared to the numbers in stage I was noted (Table 2). It was also noted that isolates with different genotypes could exhibit the same antibiogram, while isolates with the same genotype could have different resistance patterns.
DISCUSSION
Retrospective review of laboratory records and recent surveys revealed that since 1991 the total number of S. marcescens isolates as well as the total numbers of S. marcescens isolates and S. marcescens UTI isolates as a proportion of the total number of bacteria detected in CGMH showed a gradual but steady increase (Fig. 1). In recent years, however, a surge in nosocomial S. marcescens infections as well as S. marcescens UTIs was noted (Fig. 2). The increase was statistically significant and therefore could be regarded as an epidemic (29). The epidemic, particularly that of nosocomial S. marcescens UTIs, had not been noted earlier, despite the implementation since 1976 of active hospital-wide surveillance of nosocomial infections in this hospital. This finding underlines the importance of regular analysis and review of the data accumulated in a clinical microbiology laboratory, in addition to the routine isolation and identification of microorganisms, for the identification of potential outbreaks of nosocomial infections.
The rates of nosocomial S. marcescens UTIs were particularly high in the neurology wards compared to the rates in the other wards (Table 2). Molecular typing indicated that two genotypes were dominant among the S. marcescens isolates collected from patients with nosocomial UTIs, with only three isolates collected from the neurology wards showing molecular fingerprints (genotypes 3 and 4) other than genotypes 1 and 2 (Table 2). In contrast, among the isolates collected from nonneurology wards, although genotypes 1 and 2 were still the most prevalent (26 of 38 isolates), 9 different genotypes were found among the other 12 isolates. The results indicate that genotypes 1 and 2 might have been responsible for an extended epidemic of nosocomial UTIs, particularly in the neurology wards.
Various reservoirs, such as contaminated antiseptics (28), indwelling urinary catheters (5), urinals (20), and urine-measuring containers and urinometers (19), have been associated with outbreaks of S. marcescens UTIs. Indwelling urinary catheters were frequently used in our neurology wards (Table 1). The urine was collected in a measuring bottle, which was reused without proper disinfection. This procedure apparently predisposed patients to nosocomial UTIs, as seen by the significant decline in the rate of nosocomial UTIs, from 3.71% in 1999 to 0.92% in 2002, following the implementation of intervention measures in late 1999. Consistent with this decline was the fact that the number of isolates of the two dominant genotypes in neurology wards in 1998 to 1999 was reduced significantly in 2001 and 2002, and concurrently, the numbers of isolates of other genotypes that had not been seen before increased, although the overall rate of infection decreased (Table 2). This observation implies that the major source of the epidemic strains may have been the neurology wards, with subsequent transmission to the whole hospital. Further investigation is required to elucidate whether similar problems were present in other, nonneurology wards. Furthermore, for early detection of the recurrence, if any, of such an extended epidemic in the future, urinary isolates of S. marcescens are still collected continuously in the hospital's microbiology laboratory. When there is any suspicion of an increase in the rate of nosocomial infections associated with this organism, the isolates can be promptly genotyped to clarify whether there is an outbreak.
S. marcescens characteristically causes low-level but long-lasting outbreaks of cross infections (4, 9, 15, 19). In the present study, although epidemic strains were finally identified, the development of the epidemic was so chronic that infection control personnel were barely aware of its existence. It was not recognized until the number of nosocomial isolates affected the incidence of S. marcescens infections in the whole hospital and the epidemic-causing strains were identified by molecular typing methods. Similar examples can be found in previous reports describing prolonged outbreaks that lasted for more than 1 year (4, 5, 9, 15, 19). This suggests that no increase in the incidence of nosocomial infections associated with S. marcescens should be ignored.
Another characteristic of S. marcescens outbreaks is that they usually involve more than one epidemic strain (4, 9, 12, 15, 27). In the present study, two strains prevalent within the whole hospital were clearly demonstrated by the two molecular typing methods. Genotyping is also very important when epidemic strains are resistant to many antimicrobial agents. Although antibiotic susceptibility patterns are simple to determine and are frequently used by infection control personnel, they may not accurately predict the molecular relationships among the isolates (12, 27). Genetically distinct pathogens may share similar antibiograms, and conversely, different antimicrobial resistance patterns can be acquired from genetically related bacteria during the course of an outbreak (32). Both situations existed in the present study. This may also partly explain why the epidemic was not identified for quite some time.
Thus, as shown above, genotyping is particularly useful in the analysis of an outbreak. The data help to focus efforts either on the detection of a single source or on general improvement of infection control measures when multiple strains are identified. PFGE is regarded as the most accurate method, although some limitations do exist. The 87 strains obtained during 1998 and 1999 were tested by PFGE and IRS-PCR, and the latter yielded a result comparable to that obtained by PFGE. According to our experience, IRS-PCR is much simpler and less time-consuming and therefore was used to type the isolates collected in 2001 and 2002. IRS-PCR has been used with small numbers of S. marcescens isolates (33). However, our use of the technique, as described here, is the first large-scale application of IRS-PCR with a substantial number of S. marcescens isolates.
Reports have indicated several risk factors for S. marcescens infections (27). In the present study, the use of intravenous catheters, nasogastric tubes, indwelling urinary catheters, and urine bottles appeared to be closely related to the development of the S. marcescens UTIs (Table 1). Indwelling devices disrupt integumental integrity or connect normally sterile body sites to the environment. Intensive contact during the manual handling of these devices further increased the risk of nosocomial infections. It may not be avoidable for patients who rely on such procedures for survival. However, a more stringent infection control policy that is executed strenuously should be helpful in reducing such risks.
In conclusion, a hidden extended epidemic of nosocomial UTIs associated with S. marcescens was identified. Genotyping provided firm evidence that two genetically different strains of S. marcescens were responsible for the prolonged epidemic. Both laboratory and infection control personnel should monitor the incidence of infections with care. When a slight but steady increase in the number of pathogens possessing characteristics similar to those of the S. marcescens isolates described here is noted, further molecular typing analysis, such as by IRS-PCR, is warranted to identify the potential epidemic.
Acknowledgments
This work was supported in part by grant CMRP798 (to T.-L. Wu) and grant CMRP697 (to J. T. Ou) from CGMH and grant NSC91-2314-B-182A-069 (to L.-H. Su) from the National Science Council, Executive Yuan, Taipei, Taiwan.
Both L.-H. Su and J. T. Ou are senior authors who contributed equally to this paper.
REFERENCES
- 1.Dodson, W. H. 1968. Serratia marcescens septicaemia. Arch. Intern. Med. 121:145-150. [PubMed] [Google Scholar]
- 2.Farmer, J. J., III. 1995. Enterobacteriaceae: introduction and identification, p. 438-449. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 6th ed. American Society for Microbiology, Washington, D.C.
- 3.Garner, J. S., W. R. Jarvis, T. G. Emori, T. C. Horan, and J. M. Hughes. 1988. CDC definitions for nosocomial infections, 1988. Am. J. Infect. Control 16:128-140. [DOI] [PubMed] [Google Scholar]
- 4.Hejazi, A., H. M. Aucken, and F. R. Falkiner. 2000. Epidemiology and susceptibility of Serratia marcescens in a large general hospital over an 8-year period. J. Hosp. Infect. 45:42-46. [DOI] [PubMed] [Google Scholar]
- 5.John, J. F., Jr., and W. F. McNeill. 1981. Characteristics of Serratia marcescens containing a plasmid coding for gentamicin resistance in nosocomial infections. J. Infect. Dis. 143:810-817. [DOI] [PubMed] [Google Scholar]
- 6.Körner, R. J., A. Nicol, D. S. Reeves, A. P. MacGowan, and J. Hows. 1994. Ciprofloxacin resistant Serratia marcescens endocarditis as a complication of non-Hodgkin's lymphoma. J. Infect. 29:73-76. [DOI] [PubMed] [Google Scholar]
- 7.Lancaster, L. J. 1962. Role of Serratia species in urinary tract infections. Arch. Intern. Med. 109:536. [DOI] [PubMed] [Google Scholar]
- 8.Leu, H. S. 1995. The impact of US-style infection control programs in an Asian country. Infect. Control Hosp. Epidemiol. 16:359-364. [DOI] [PubMed] [Google Scholar]
- 9.Luzzaro, F., R. Perilli, R. Migliavacca, G. Lombardi, P. Micheletti, A. Agodi, S. Stefani, G. Amicosante, and L. Pagani. 1998. Repeated epidemics caused by extended-spectrum beta-lactamase-producing Serratia marcescens strains. Eur. J. Clin. Microbiol. Infect. Dis. 17:629-636. [DOI] [PubMed] [Google Scholar]
- 10.Mazurek, G. H., V. Reddy, B. J. Marston, W. H. Haas, and J. T. Crawford. 1996. DNA fingerprinting by infrequent-restriction-site amplification. J. Clin. Microbiol. 34:2386-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNaughton, M., N. Mazinke, and E. Thomas. 1995. Newborn conjunctivitis associated with triclosan 0.5% antiseptic intrinsically contaminated with Serratia marcescens. Can. J. Infect. Control 10:7-8. [PubMed] [Google Scholar]
- 12.Miranda, G., C. Kelly, F. Solorzano, B. Leanos, R. Coria, and J. E. Patterson. 1996. Use of pulsed-field gel electrophoresis typing to study an outbreak of infection due to Serratia marcescens in a neonatal intensive care unit. J. Clin. Microbiol. 34:3138-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NCCLS. 2000. Performance standards for antimicrobial disk susceptibility tests; approved standard M2-A7, 7th ed. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 14.NCCLS. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard M7-A5, 5th ed. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 15.Okuda, T., N. Endo, Y. Osada, and H. Zen-Yoji. 1984. Outbreak of nosocomial urinary tract infections caused by Serratia marcescens. J. Clin. Microbiol. 20:691-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ostrowsky, B. E., C. Whitener, H. K. Bredenberg, L. A. Carson, S. Holt, L. Hutwagner, M. J. Arduino, and W. R. Jarvis. 2002. Serratia marcescens bacteremia traced to an infused narcotic. N. Engl. J. Med. 346:1529-1537. [DOI] [PubMed] [Google Scholar]
- 17.Pagani, L., F. Luzzaro, P. Ronza, A. Rossi, P. Micheletti, F. Porta, and E. Romero. 1994. Outbreak of extended-spectrum beta-lactamase producing Serratia marcescens in an intensive care unit. FEMS Immunol. Med. Microbiol. 10:39-46. [DOI] [PubMed] [Google Scholar]
- 18.Passaro, D. J., L. Waring, R. Armstrong, F. Bolding, B. Bouvier, J. Rosenberg, A. W. Reingold, M. McQuitty, S. M. Philpott, W. R. Jarvis, S. B. Werner, L. S. Tompkins, and D. J. Vugia. 1997. Postoperative Serratia marcescens wound infections traced to an out-of-hospital source. J. Infect. Dis. 175:992-995. [DOI] [PubMed] [Google Scholar]
- 19.Rutala, W. A., V. A. Kennedy, H. B. Loflin, and F. A. Sarubbi, Jr. 1981. Serratia marcescens nosocomial infections of the urinary tract associated with urine measuring containers and urinometers. Am. J. Med. 70:659-663. [DOI] [PubMed] [Google Scholar]
- 20.Shi, Z.-Y., P. Y.-F. Liu, Y.-J. Lau, Y.-H. Lin, and B.-S. Hu. 1997. Use of pulsed-field gel electrophoresis to investigate an outbreak of Serratia marcescens. J. Clin. Microbiol. 35:325-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su, L. H., C. H. Chiu, T. L. Wu, C. Chu, J. H. Chia, A. J. Kuo, C. C. Lee, C. F. Sun, and J. T. Ou. 2002. Molecular epidemiology of Salmonella enterica serovar Enteritidis isolated in Taiwan. Microbiol. Immunol. 46:833-840. [DOI] [PubMed] [Google Scholar]
- 22.Su, L. H., H. S. Leu, Y. P. Chiu, J. H. Chia, A. J. Kuo, C. F. Sun, T. Y. Lin, and T. L. Wu. 2000. Molecular investigation of two clusters of nosocomial bacteraemia caused by multiresistant Klebsiella pneumoniae using pulsed-field gel electrophoresis and infrequent-restriction-site PCR. J. Hosp. Infect. 46:110-117. [DOI] [PubMed] [Google Scholar]
- 23.Su, L. H., J. H. Chia, H. S. Leu, S. W. Cheng, A. J. Kuo, C. F. Sun, and T. L. Wu. 2000. DNA polymorphism of Mycobacterium abscessus analyzed by infrequent-restriction-site polymerase chain reaction. Changgeng Yi Xue Za Zhi 23:467-475. [PubMed] [Google Scholar]
- 24.Taylor, G., and P. M. Keane. 1962. Cross infection with Serratia marcescens. J. Clin. Pathol. 15:145-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Troillet, N., Y. Carmeli, L. Venkataramant, P. DeGirolami, and M. H. Samore. 1999. Epidemiological analysis of imipenem-resistant Serratia marcescens in hospitalized patients. J. Hosp. Infect. 42:37-43. [DOI] [PubMed] [Google Scholar]
- 27.Van der Sar-van der Brugge, S., S. M. Arend, A. T. Bernards, G. A. M. Berbee, R. G. J. Westendorp, J. D. M. Feuth, and P. J. van den Broek. 1999. Risk factors for acquisition of Serratia marcescens in a surgical intensive care unit. J. Hosp. Infect. 41:291-299. [DOI] [PubMed] [Google Scholar]
- 28.Vigeant, P., V. G. Loo, C. Bertrand, C. Dixon, R. Hollis, M. A. Pfaller, A. P. McLean, D. J. Briedis, T. M. Perl, and H. G. Robson. 1998. An outbreak of Serratia marcescens infections related to contaminated chlorhexidine. Infect. Control Hosp. Epidemiol. 19:791-794. [DOI] [PubMed] [Google Scholar]
- 29.Wendt, C., and L. A. Herwaldt. 1997. Epidemics: identification and management, p. 175-213. In R. P. Wenzel (ed.), Prevention and control of nosocomial infections, 3rd ed. The Williams & Wilkins Co., Baltimore, Md.
- 30.Wheat, R. P., A. Zuckerman, and L. A. Rantz. 1951. Infection due to Chromobacteria: report of eleven cases. Arch. Intern. Med. 88:461-466. [DOI] [PubMed] [Google Scholar]
- 31.Woodward, H. M. M., and K. B. Clarke. 1913. A case of infection in man by the bacterium Prodigiosum. Lancet i:314-315. [Google Scholar]
- 32.Wu, T. L., L. H. Su, H. S. Leu, C. H. Chiu, Y. P. Chiu, J. H. Chia, A. J. Kuo, C. F. Sun, and the Infection Control Group. 2002. Molecular epidemiology of nosocomial infections associated with multiresistant Acinetobacter baumannii by infrequent-restriction-site PCR. J. Hosp. Infect. 51:27-32. [DOI] [PubMed] [Google Scholar]
- 33.Yoo, J. H., J. H. Choi, W. S. Shin, D. H. Huh, Y. K. Cho, K. M. Kim, M. Y. Kim, and M. W. Kang. 1999. Application of infrequent-restriction-site PCR to clinical isolates of Acinetobacter baumannii and Serratia marcescens. J. Clin. Microbiol. 37:3108-3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaidei, M., J. Sifuentes, M. Bobadilla, D. Moncada, and S. P. de León. 1989. Epidemic of Serratia marcescens bacteremia and meningitis in a neonatal unit in Mexico City. Infect. Control Hosp. Epidemiol. 10:14-20. [DOI] [PubMed] [Google Scholar]