Abstract
A disseminated Fusarium oxysporum infection with skin localization was diagnosed in a woman with a relapse of B-acute leukemia during induction chemotherapy. The infection was refractory to amphotericin B-lipid complex alone but responded successfully when voriconazole was added.
CASE REPORT
A 32-year-old female with relapsing acute lymphoblastic leukemia was hospitalized for induction chemotherapy on 14 June 2001. At the time of hospitalization, the patient did not show clinical alteration. Blood examination showed hyperleukocytosis (27,180 leukocytes/mm3) with 90% blastic forms and 500 polymorphonuclear leukocytes (PMN)/mm3.
She received broad-spectrum antibiotherapy (amino-penicillin, amikacin, and fluoroquinolone) and granulocyte-colony stimulating factor (Neupogen 30; Roche). On July 1 (day 0), she was aplastic. The patient left the hospital against medical advice on day 3, but she returned on day 6 with fever and neutropenia (≤500 PMN/mm3) that lasted for 16 days. Clinical and radiographic examination showed no abnormalities. However, as the patient presented again with fever, antibiotherapy was changed empirically to a combination of piperacillin-tazobactam (later changed to cefepime) and vancomycin and the central venous catheter was removed and cultured. Both the catheter and blood cultures remained negative. Four days after the onset of fever (day 10), vesicular and necrotic lesions appeared on both calves. The next day (day 11), the skin lesions were extended to all the surfaces of the legs (Fig. 1). The diagnosis of opportunistic mycosis was considered, and skin biopsy and blood cultures were performed. Consistently, the histopathological analysis of the skin biopsy showed fungal hyphae deeply localized in the skin tissue. Amphotericin B-lipid complex (ABLC) (5 mg/kg of body weight/day) was added on day 12 and controlled until the patient was released on day 30. Biopsy and blood sample cultures yielded Fusarium oxysporum, confirming a systemic fungal infection with skin invasion. Evidence of infection was not observed by either cerebral-, thoracic-, and abdominal-computed tomography or by cardiac echography. Despite therapy, fever persisted and the skin lesions extended to the whole body, confirming the disseminated character of the infection (Fig. 1). In this context, voriconazole (VCZ; Pfizer, Inc., New York, N.Y.) (loading dose, 6 mg/kg/day, followed by 4 mg/kg/day administered intravenously every 12 h) was added at day 18, 8 days after onset of the rash. Further blood cultures were negative. After 9 days of VCZ-ABLC treatment, the skin lesions improved remarkably and the fever disappeared, while the patient still had a severe neutropenia (<100 PMN/mm3). On day 28, the patient recovered from neutropenia (>1,000 PMN/mm3) and, 2 days later, refused to remain hospitalized and was released with a continued prescription of VCZ (400 mg per day taken orally). Antibiotherapy and ABLC (cumulative dose, 3.6 g) were stopped. Fusariosis did not relapse during a follow-up of nine months.
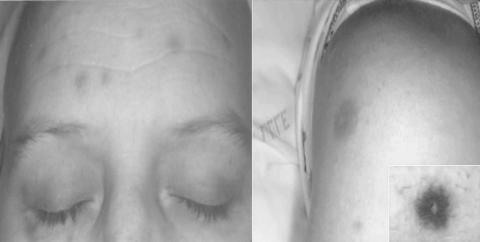
FIG. 1.
Disseminated fusariosis infection. The patient presented multiple skin varicelliform lesions on the face (left) and on the leg (right). A close-up view of a necrotic lesion (inset) shows more detail.
Fungi belonging to the genus Fusarium are ubiquitously present in soil, air, and water and are parasites of numerous plants. In humans, these microorganisms usually cause superficial or subcutaneous infections such as keratitis or onychomycosis, but they may cause severe disseminated infections in immunocompromised patients (1, 8, 13). Invasive or disseminated fusariosis is a rare but severe complication in hematological diseases (7, 9, 13, 14). To date, limited success with diverse antifungal drugs has been obtained in patients with persistent immune deficiency (13). In the present report, we describe the case of a patient with leukemia who developed disseminated fusariosis that was successfully treated with VCZ and ABLC.
Laboratory diagnosis of mycological infection.
F. oxysporum was recovered from 1 of 7 blood culture vials (Mycosis IC/F, Bactec 9050 system; Becton Dickinson). The sole positive blood culture was sampled on day 13. The time to detectable growth was 2 days. F. oxysporum developed in subcultures from both blood culture and skin biopsy (sampled on day 14) after a 2-to 3-day incubation at 37°C on 50% Sabouraud's glucose agar medium (supplemented with amikacin). Colonies with white to purple aerial mycelium grew rapidly. Microscopy showed short phialides bearing fusiform, septate (3-5) macroconidia and the presence of terminal or intercalary chlamydospores. Rare one-celled microconidia on predominantly lateral phialides were also observed. Cultures from peripheral samples (mouth, nose, and throat) and other clinical samples were negative for bacteria, viruses, Fusarium, and other fungi. Enzyme-linked immunosorbent assays for detecting either Aspergillus galactomannan or Candida mannan (Platelia; Bio-Rad, Paris, France) were also negative for several serum samples. In our hospital, these tests are routinely used in this kind of patient as part of a protocol to prevent systemic fungal disease.
Antifungal susceptibility testing (for amphotericin B, itraconazole, and VCZ) of the Fusarium oxysporum strain was performed both in France and in the United States. Briefly, MICs for the isolate were obtained simultaneously by the NCCLS M38-A broth microdilution (12) and E-test methods by following the specific recommendations for each test.
Invasive or disseminated fungal infections are a frequent complication in immunocompromised patients. The most commonly encountered genera are Candida and Aspergillus. However, other molds, like Fusarium spp., are emerging as serious opportunistic pathogens. Fusarium infections are associated with high mortality, ranging from 52 to 70% in patients with blood disorders (3, 13) despite treatment with amphotericin B. Patients in remission are generally observed to survive the infection (13).
On the practical side, when fever in neutropenic patients continues despite broad-spectrum antimicrobials, an antifungal drug is usually added. The most commonly used drug is amphotericin B, in either conventional or lipid-based formulations. However, this compound has variable activity against Fusarium spp. The response to amphotericin B treatment seems to be closely related to neutrophil recovery (3, 11) or to the concomitant administration of granulocyte colony-stimulating factor (3, 7, 13).
VCZ is a new broad-spectrum triazole that has a good safety profile (5, 10). To our knowledge, only one case of fusariosis treated with VCZ has been reported previously (16), in a patient with invasive ocular infection who received the drug intravenously and intracamerally. The outcome was favorable, but the patient was not immunocompromised. In contrast, our patient had persistent severe neutropenia for more than 44 days. Interestingly, even in this context, the addition of VCZ ABLC therapy resulted in healing of the skin lesions within 3 weeks, marked clinical improvement, and negative blood cultures. The present report underscores the importance of early detection and identification of the infecting organism in the clinical specimen. Fusarial hyphae were detected during the microscopical examination of the skin samples, but they can be confused with Aspergillus hyphae. Therefore, even if hyphae are detected microscopically in a clinical sample, a definite laboratory diagnosis can be established only by culture. However, the presence of the diagnostic phialides and phialoconidia in the direct examination of blood cultures allowed a presumptive identification of the Fusarium genus (11).
Amphotericin B MICs for the strain of F. oxysporum recovered from our patient were ≤2 μg/ml by two methods (E-test at 24 h and NCCLS broth microdilution). VCZ MICs for F. oxysporum have ranged from 0.25 to 8 μg of VCZ/ml (2, 6) with the NCCLS 38-A method for filamentous fungi. In the present report, our F. oxysporum isolate had different susceptibilities to the two triazoles, while the VCZ MIC was 1 μg/ml and the itraconazole MIC was >8 μg/ml by both methods. Although in vitro testing for susceptibility to azoles has not always correlated with the in vivo response (4), the combination of VCZ with ABLC resulted in a successful outcome in the present case. Remarkably, no relapse of the fungal disease was observed in this patient for the more-than-9-month follow-up, even when she was submitted to new cytotoxic therapeutic protocols. This result suggested that the Fusarium infection was efficiently neutralized by the VCZ-ABLC combination.
In conclusion, although the risk for systemic fusariosis seems lower than that for invasive aspergillosis, the frequency of the former is highly significant in patients suffering from hematological malignancies. In this context, fusariosis usually evolves as a severe deep fungal disease, which is associated with a high mortality rate. The description of the present case underlines both the importance of early detection and identification of the infecting fungal agent and the potential role of VCZ in the management of systemic fusariosis in the immunocompromised host, as reported recently (15).
REFERENCES
- 1.Anaissie, E., H. Kantarjian, P. Jones, B. Barlogie, M. Luna, G. Lopez-Berestein, and G. P. Bodey. 1986. Fusarium. A newly recognized fungal pathogen in immunosuppressed patients. Cancer 57:2141-2145. [DOI] [PubMed] [Google Scholar]
- 2.Arikan, S., M. Lozano-Chiu, V. Paetznick, S. Nangia, and J. H. Rex. 1999. Microdilution susceptibility testing of amphotericin B, itraconazole, and voriconazole against clinical isolates of Aspergillus and Fusarium species. J. Clin. Microbiol. 37:3946-39451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boutati, E. I., and E. J. Anaissie. 1997. Fusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years' experience at a cancer center and implications for management. Blood 90:999-1008. [PubMed] [Google Scholar]
- 4.Dei-Cas, E., L. Dujardin, M. E. Ribeiro Pinto, F. Ajana, J. Fruit, D. Poulain, D. Camus, and A. Vernes. 1991. Kinetic study of antifungal activity of amphotericin B, 5-fluorocytosine and ketoconazole against clinical yeast isolates using liquid-phase turbidimetry. Mycoses 34:167-172. [DOI] [PubMed] [Google Scholar]
- 5.Denning, D. W., P. Ribaud, N. Milpied, D. Caillot, R. Herbrecht, E. Thiel, A. Haas, M. Ruhnke, and H. Lode. 2002. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin. Infect. Dis. 34:563-571. [DOI] [PubMed] [Google Scholar]
- 6.Espinel-Ingroff, A., K. Boyle, and D. J. Sheehan. 2001. In vitro antifungal activities of voriconazole and reference agents as determined by NCCLS methods: review of the literature. Mycopathologia 150:101-115. [DOI] [PubMed] [Google Scholar]
- 7.Girmenia, C., A. P. Iori, F. Boecklin, A. Torosantucci, P. Chiani, P. De Fabritiis, F. Taglietti, A. Cassone, and P. Martino. 1999. Fusarium infections in patients with severe aplastic anemia: review and implications for management. Haematologica 84:114-118. [PubMed] [Google Scholar]
- 8.Guarro, J., and J. Gene. 1995. Opportunistic fusarial infections in humans. Eur. J. Clin. Microbiol. Infect. Dis. 14:741-754. [DOI] [PubMed] [Google Scholar]
- 9.Hennequin, C., V. Lavarde, J. L. Poirot, M. Rabodonirina, A. Datry, S. Aractingi, J. Dupouy-Camet, D. Caillot, F. Grange, L. Kures, O. Morin, B. Lebeau, S. Bretagne, C. Guigen, D. Basset, and R. Grillot. 1997. Invasive Fusarium infections: a retrospective survey of 31 cases. The French ‘Groupe d'Etudes des Mycoses Opportunistes' GEMO. J. Med. Vet. Mycol. 35:107-114. [PubMed] [Google Scholar]
- 10.Hoffman, H. L., and R. C. Rathbun. 2002. Review of the safety and efficacy of voriconazole. Expert Opin. Investig. Drugs 11:409-429. [DOI] [PubMed] [Google Scholar]
- 11.Letscher-Bru, V., F. Campos, J. Waller, R. Randriamahazaka, E. Candolfi, and R. Herbrecht. 2002. Successful outcome of treatment of a disseminated infection due to Fusarium dimerum in a leukemia patient. J. Clin. Microbiol. 40:1100-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NCCLS. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi, vol. 22. Approved standard M-38A. NCCLS, Wayne, Pa.
- 13.Okada, H., S. Hamatani, M. Kondo, T. Imai, S. Itoh, K. Isobe, and S. Onishi. 2000. Successful treatment of disseminated Fusarium infection in an infant with leukemia. Int. J. Hematol. 72:494-498. [PubMed] [Google Scholar]
- 14.Pagano, L., C. Girmenia, L. Mele, P. Ricci, M. E. Tosti, A. Nosari, M. Buelli, M. Picardi, B. Allione, L. Corvatta, D. D'Antonio, M. Montillo, L. Melillo, A. Chierichini, A. Cenacchi, A. Tonso, L. Cudillo, A. Candoni, C. Savignano, A. Bonini, P. Martino, and A. Del Favero. 2001. Infections caused by filamentous fungi in patients with hematologic malignancies. A report of 391 cases by GIMEMA Infection Program. Haematologica 86:862-870. [PubMed] [Google Scholar]
- 15.Perfect, J. R., K. A. Marr, T. J. Walsh, R. N. Greenberg, B. DuPont, J. de la Torre-Cisneros, G. Just-Nubling, H. T. Schlamm, I. Lutsar, A. Espinel-Ingroff, and E. Johnson. 2003. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin. Infect. Dis. 36:1122-1131. [DOI] [PubMed] [Google Scholar]
- 16.Reis, A., R. Sundmacher, K. Tintelnot, H. Agostini, H. E. Jensen, and C. Althaus. 2000. Successful treatment of ocular invasive mould infection (fusariosis) with the new antifungal agent voriconazole. Br. J. Ophthalmol. 84:932-933. [DOI] [PMC free article] [PubMed] [Google Scholar]