Abstract
Luciferase reporter phages (LRPs) have proven to be efficient tools for drug susceptibility testing of Mycobacterium tuberculosis. Luminometric detection of LRP activity offers higher sensitivity and quantitative results, while a Polaroid film detection method offers a “low-tech” inexpensive alternative that is called the Bronx box. In this work we evaluated, improved, and compared the performance of the luminometer and the Bronx box formats for drug susceptibility testing with LRPs by using 51 clinical isolates of M. tuberculosis, with the agar proportion method (PM) serving as reference. The sensitivity in detecting resistance to isoniazid and rifampin, antibiotics that define multidrug resistance (MDR), was 100% for both methods. The turnaround time for results was reduced from 3 weeks for PM to 54 or 94 h for luminometry or the Bronx box, respectively. These results support the utility of LRPs as a screening test for the surveillance of MDR tuberculosis.
Early diagnosis of tuberculosis (TB) and drug-resistant TB allows the prescription of appropriate antibiotic regimens, leading to more efficient control of the disease (15). The increase in incidence of multidrug-resistant (MDR) TB (4) has clearly established the need to improve the drug susceptibility techniques available. Mycobacteriophages (phages) are promising tools for the early diagnosis of drug-resistant TB because they offer a phenotype-based result in a turnaround time similar to that of some molecular approaches at a low cost. Luciferase reporter phages (LRPs) are phages harboring the fflux reporter gene, which codes for the firefly luciferase, which in turn catalyzes a reaction that releases light in the presence of its substrate luciferin and ATP. LRPs are able to infect, replicate, and express their genome and the fflux gene only within viable mycobacterial cells. Luciferase activity can then be detected only if cellular ATP is present (8), allowing detection of M. tuberculosis in clinical samples (2, 13). If a decontaminated clinical specimen containing M. tuberculosis is pretreated with antibiotics and is then infected with LRPs, light emission will be proportional to mycobacterial viability; hence, LRPs are promising candidates for drug susceptibility testing (3, 12, 14). Detection of the luciferase activity is achieved by means of a luminometer or photographic film. The luminometer offers higher sensitivity and quantitative results (1, 2); the Polaroid film offers an inexpensive, “low-tech” alternative that is called the Bronx box (14), but its performance as a clinical tool requires further evaluation.
In this work we improve, evaluate, and compare the performance of the luminometer and the Bronx box formats for drug susceptibility with LRPs by using clinical isolates of M. tuberculosis obtained from a focus presenting high levels of resistance, with the agar proportion method (PM) (9) as the reference. Results indicate that both LRP assays show considerable agreement with the PM and that the turnaround time for results was reduced from 3 weeks for PM to 94 or 54 h by Bronx box or luminometry, respectively.
Clinical isolates and control strains.
Fifty-one clinical isolates of M. tuberculosis, obtained from a documented MDR focus in Colombia (11), were thawed from the strain collection of the International Center for Training and Medical Research in Cali, Colombia. Mycobacterium bovis BCG::pKB15 (constitutively expressing the fflux gene at high levels) was used as a reagent control (14). M. bovis BCG Pasteur and M. tuberculosis H37Rv were used as susceptible controls, and strains AWC (resistant to isoniazid [INH] and streptomycin [STR]) (16) and 14EBS (resistant to INH, rifampin [RIF], STR, and ethambutol [EMB]) (11) were used as resistant controls.
LRPs.
Stocks of phages phAE85 (3, 12) and phAE142 (1, 2) were amplified as previously indicated. Titers of phAE85 and phAE142 were 5 × 108 and 7.5 × 106 PFU/ml for the Bronx box and were 4.8 × 108 and 3.8 × 107 PFU/ml for luminometry, respectively.
Luciferin buffer.
A buffer composed of 12 mM MgSO4, 8 mM dithiothreitol, 50 mM sodium citrate, pH 5.3, and 0.2 mM luciferin (Molecular Probes, Eugene, Oreg.) was freshly prepared for each Bronx box assay (14). For luminometry, the buffer used (within a week of its preparation date) was 0.1 M sodium citrate, pH 4.5, and 0.33 mM luciferin.
Culture procedures.
M. tuberculosis isolates were thawed and cultured in Middlebrook 7H9 media supplemented with 10% albumin-dextrose-catalase (Sigma, St. Louis, Mo.) (MADC) and 0.025% Tween 80 (Sigma). Cultures were incubated at 37°C in a 5% CO2 atmosphere and were checked until turbidity was observed. A subculture was made in fresh medium to achieve logarithmic growth (5 to 14 days) with turbidity equivalent to a McFarland standard of 2 for Bronx box or a McFarland standard of 1 for luminometry.
Susceptibility test by Bronx box.
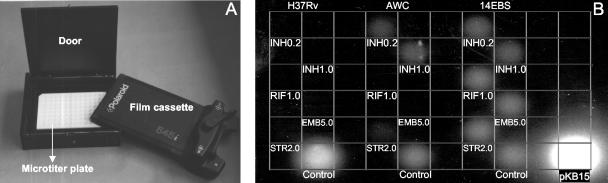
Mycobacterial cultures were washed twice with MADC without Tween and were readjusted to a turbidity equivalent of McFarland standard of 2. Aliquots were incubated at 37°C in 5% CO2 for 72 h in the presence and absence of antibiotics. The final antibiotic concentrations used (in micrograms per milliliter) were INH (0.2 and 1.0), RIF (1.0), STR (2.0), and EMB (5.0). In white 96-well microplates, aliquots of 100 μl of each culture were infected by adding 100 μl of the LRP solution followed by 8 h of incubation under the same conditions. Finally, 100 μl of the fresh luciferin buffer was added to each well, and the microplate was again covered with an acetate film and was loaded into the Bronx box. We improved the original Bronx box design (14) by converting the upper part of the box into a light-tight door (Fig. 1A). The Polaroid film (Polapan 57) was inserted on top of the microplate and was exposed overnight at 37°C in the dark. The film was developed, and spots of light were observed. The presence of light signal from the wells containing clinical samples incubated with antibiotic was considered resistance (Fig. 1B). When no signal was detected in the well without antibiotic, the result was not considered valid.
FIG. 1.
(A) Bronx box. The Polaroid film cassette is placed over the microtiter plate containing the LRP-infected mycobacterial cultures. The door is closed, and the overnight film exposure begins. (B) Drug susceptibility test result gained from the Bronx box. Strains H37RV, AWC, and 14EBS were incubated for 72 h in media supplemented with INH, RIF, STR, and EMB at the concentrations shown (in micrograms per milliliter). The cultures were infected with phAE142, incubated for 8 h at 37°C, when luciferin buffer was added, followed by overnight exposure to the Polaroid film. The lower row shows the controls without antibiotic. The well labeled pKB15 corresponds to M. bovis BCG::pKB15, which acts as a reagent control. The absence of signal indicates susceptibility to all antibiotics in H37Rv. Strain AWC shows resistance to INH and STR, while strain 14EBS resists all antibiotics.
Susceptibility test by luminometry.
Two milliliters of the mycobacterial culture was washed twice with MADC without Tween, and then the cell suspension was adjusted to a McFarland standard of 1. The isolate was incubated at 37°C in 5% CO2 in tubes with the antibiotics (same concentrations as with the Bronx box) for 48 h. Next, 100 μl of the isolate with the antibiotic was added to 50 μl of phage phAE142 in wells of a 96-well microplate. The microplate was sealed and incubated for 4 h under the same conditions. Afterwards, 100 μl of the luciferin buffer was added to each well, and light emission was registered immediately in the luminometer (Lucy 1; Anthos Biotech, Salzburg, Austria) as relative light units (RLU) (20-s integration and 1-s settle time). The positive growth control consisted of an isolate (not exposed to antibiotics) infected with phage, and the background was determined by measuring the number of RLU from 100 μl of the isolate (without phage) in the presence of luciferin buffer. To validate each sample, the number of RLU of growth control should be at least 10 times greater than that of the background. The inhibition index was calculated as previously indicated (12).
Susceptibility test by PM.
PM was performed as recommended by Kent and Kubica (9). Two independent observers and a third observer for discordant results evaluated the results of all susceptibility tests.
Statistical analysis.
The sensitivity and specificity of each method for each antibiotic were calculated by using PM as the “gold standard.” The agreement index of the results of each method with the gold standard was calculated, and statistical significance was tested by the McNemar χ2 test. Statistical significance was indicated (P < 0.05). All analyses were performed with Epi Info 2000 (http://www.cdc.gov/epiinfo/) and the SPSS software for Windows (release 7.5; SPSS Inc., Austin, Tex.).
LRP assay improvement.
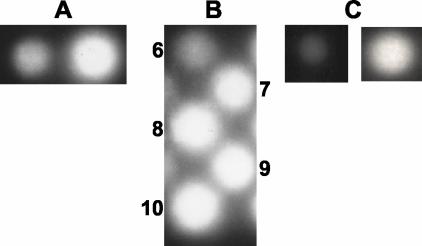
phAE142 was previously shown to be more efficient than phAE85 for the luminometer assay (2). We infected BCG Pasteur and M. tuberculosis H37Rv strains with phAE142 and phAE85 while using the same multiplicity of infection and compared the light signals with the Bronx box. phAE142 yielded stronger signals than phAE85 and was chosen (Fig. 2A).
FIG. 2.
Standardization of the Bronx box assay. (A) Light signals obtained after infection of BCG Pasteur with phAE85 (left) and phAE142 (right) with the same multiplicity of infection. (B) Standardization of the phAE142 infection time prior to luciferin addition. Time points (hours) are indicated next to the corresponding signal. Film was exposed overnight after substrate addition. (C) Shown are the light signals observed after 2 (left) and 14 (right) h of exposure to the Polaroid film (after the addition of luciferin to an 8-h phAE142-infected culture).
Light signals obtained were compared by using cell suspensions equivalent to McFarland standards of turbidity 1 to 4. For the Bronx box and the luminometer, McFarland standards of 2 and 1, respectively, were shown to be the lowest inocula that gave clear signals (data not shown).
By using the reference strains, we tested incubation times of 1, 6, 12, 24, 36, 48, 72, 96, and 120 h of the culture aliquots in the presence of antibiotics. Then cultures were infected with phAE142, and light signals were compared. The shortest periods presenting clear drug susceptibility pattern interpretation were chosen (results not shown). For the luminometer and Bronx box, the incubation times chosen were 48 and 72 h, respectively.
The infection time with the LRPs was tested every hour for 10 h. The shortest infection times allowing clear detection of luciferase activity were 4 and 8 h for the luminometer (data not shown) and Bronx box (Fig. 2B), respectively.
The exposure time of the Polaroid film to the infected culture after addition of the substrate was evaluated. Signals after 2 and 14 h were compared, and the overnight exposure was chosen (Fig. 2C). Because 8 h of LRP infection was required prior to film exposure, for practical reasons other time points were not tested.
Drug susceptibility results by PM.
From the 51 M. tuberculosis isolates tested, resistance to INH, RIF, STR, and EMB was detected in 24 (47.0%), 12 (23.5%), 20 (39.2%), and 5 (9.8%) isolates, respectively. Multidrug resistance was detected in 11 (21.6%) isolates, and 23 (45%) isolates were pansusceptible.
LRP assay results.
The 51 M. tuberculosis isolates tested were successfully infected by LRPs. Due to low signals in the antibiotic-free controls of some assays, a total of 44 isolates were evaluated in parallel by PM and Bronx box and 48 by PM and luminometry. The McNemar test used to evaluate the discordant pairs between Bronx box and luminometer with PM for each antibiotic was not statistically significant, which permits us to conclude that the agreement observed is significantly near 100%. The performance of both LRP techniques with PM as the gold standard is summarized in Table 1.
TABLE 1.
Comparison of antibiotic susceptibility determined by Bronx box and luminometry with PM as reference
| Antibiotic or resistance level patterna | Method | Agreementb | Sensitivityc | Specificityd |
|---|---|---|---|---|
| Antibiotics | ||||
| INH (0.2) | Luminometry | 97.9 (47/48) | 100 (23/23); 82.2-100 | 96.0 (24/25); 77.7-99.8 |
| Bronx box | 100 (44/44) | 100 (19/19); 79.1-100 | 100 (25/25); 83.4-100 | |
| INH (1.0) | Luminometry | 97.9 (47/48) | 100 (23/23); 82.2-100 | 96.0 (24/25); 77.7-99.8 |
| Bronx box | 90.9 (40/44) | 78.9 (15/19); 53.9-93.0 | 100 (25/25); 83.4-100 | |
| RIF (1.0) | Luminometry | 91.7 (44/48) | 100 (11/11); 67.9-100 | 89.2 (33/37); 73.6-96.5 |
| Bronx box | 95.5 (42/44) | 100 (10/10); 65.5-100 | 94.1 (32/34); 78.9-99.0 | |
| STR (2.0) | Luminometry | 87.5 (42/48) | 78.9 (15/19); 53.9-93.0 | 93.1 (27/29); 75.8-98.8 |
| Bronx box | 72.7 (32/44) | 80.0 (12/15); 51.4-94.7 | 69.0 (20/29); 49.0-84.0 | |
| EMB (5.0) | Luminometry | 89.6 (43/48) | 40.0 (2/5); 7.3-83.0 | 95.3 (41/43); 82.9-99.2 |
| Bronx box | 95.5 (42/44) | 33.3 (1/3); 1.8-87.5 | 100 (41/41); 89.3-100 | |
| Resistance patterns | ||||
| MDR (INH and RIF) | Luminometry | 89.6 (43/48) | 100 (10/10); 65.5-100 | 86.8 (33/38); 71.1-95.1 |
| Bronx box | 95.5 (42/44) | 100 (9/9); 62.9-100 | 94.3 (33/35); 75.9-99.0 | |
| Any resistance (INH, RIF, EMB, or STR) | Luminometry | 92.9 (223/240) | 91.4 (74/81); 82.5-96.2 | 93.7 (149/159); 88.4-96.8 |
| Bronx box | 90.9 (200/220) | 86.4 (57/66); 75.2-93.2 | 92.9 (143/154); 87.3-96.2 |
For antibiotics, values in parentheses are drug concentrations (in micrograms per milliliter). For each resistance patterns, the antibiotics to which resistance was demonstrated are listed in parentheses.
Values are percentages. The values in parentheses are numbers of concordant results/total numbers of results.
The value listed is a percentage indicating the ability of LRPs to detect resistance. For each entry, the value in parentheses is the number of isolates resistant by both methods/number of isolates resistant by PM and the 95% CI is given following the semicolon.
The value listed is a percentage indicating the ability of LRPs to detect susceptibility. For each entry, the value in parentheses is the number of isolates susceptible by both methods/number of isolates susceptible by PM and the 95% CI is given following the semicolon.
LRPs showed high agreement with the standard method, with high sensitivity and specificity for susceptibility testing. INH at 0.2 μg/ml was more accurate than at 1 μg/ml in the Bronx box. All cases of resistance to INH (at 0.2 μg/ml) and RIF, the antibiotics that define multidrug resistance, were detected by Bronx box and luminometry, with a 95% confidence index (CI) for sensitivity of 62.9 to 100.0% and 65.5 to 100.0% for the two methods, respectively. Due to some false-positive cases of MDR TB detected by LRP assay, the agreement with PM in detecting MDR TB was 89.6% for luminometry and 95.5% for the Bronx box. Larger samples should be tested to narrow these intervals and should confirm the potential of the LRPs as a tool for multidrug resistance screening.
Overall agreement between LRP and PM exceeded 89% for all antibiotics, except for STR, which had an agreement of 87.5% by luminometry and 72.7% by Bronx box, mainly due to false resistance in the latter. The sensitivity of LRP for detecting resistance to EMB in this group of isolates was only 40.0 and 33.3% for luminometry and Bronx box, respectively, although the small number of resistant cases (9.8% [5 of 51]) limited the stability of the sensitivity estimation (Table 1). Previous studies indicate that STR and EMB show difficulty of standardization with any method (6, 14, 17, 18). Banaiee et al. (1) showed higher agreement between the luminometer assay and BACTEC susceptibility in 197 out of 200 tests (98.5%), but in this study, the number of resistant isolates was lower and the cutoff values for RIF and STR were different (2.0 and 0.4 μg/ml, respectively). In our experience, it is not expected that these cutoffs would have improved our results. However, it may be that sensitivities determined by the liquid-phase BACTEC method more closely approximate the results seen with LRP assays for these drugs than does the PM. It may be possible to construct phages that are improved for assessment of STR and EMB resistance by incorporating a promoter to drive luciferase expression that directly senses the effects of these drugs. An improved understanding of STR and EMB action on mycobacteria could enhance this possibility. Nevertheless, since INH and RIF are the two most important susceptibilities in determining clinical outcome, the Bronx box test available could be useful in clinical settings in the developing world.
Falsely resistant cases could be due to a mixture of resistant and susceptible clones within the clinical isolate, selected by PM or LRPs. The thawing of frozen specimens could have influenced both growth of isolates as well as selecting for colonies with altered drug sensitivity. Other factors that could influence this result are the loss of an effective concentration of antibiotic over the time of the PM assay (7) or a higher inoculum in the LRP assays (12). The latter could also be the explanation for false resistance to STR by Bronx box but not by luminometry and will need to be evaluated further.
Although the phage infection capacity was verified prior to the evaluation of drug susceptibility, unsatisfactory mycobacterial infection could result in cases with false susceptibility to the antibiotics. First, a weak light signal could be obtained in the antibiotic-free control and a weaker signal indicative of resistance to drug would not be perceived. In the future, a 1:10 dilution control could be included that should be just barely visible for the assay to be considered valid. Second, false susceptibility, such as to EMB or STR, may relate to the effects of antibiotics in diminishing cellular ATP levels, which may occur without leading to actual killing of the mycobacterial cells.
The reliability of the results of any method is operationally defined in relation to a standard, in this case the PM. As new technologies increase the precision of detecting the survival and growth of microorganisms, standards will require reassessment. It is conceivable that LRP-based methods could detect survival and growth below the threshold of PM, because the results of the PM depend on the evaluation of bacterial growth, whereas LRP detects cell viability (10). In such cases, positive discordance could represent true rather than false positives. The availability of molecular markers of resistance is likely to provide a basis for evaluating discrepancies and in refining the reference standard. In the future, PM could be replaced by new technologies that have improved sensitivity and are able to provide faster and quantitative results, such as LRPs.
PM requires 3 weeks from the time of beginning the test until its final result; the Bronx box takes 94 h; luminometry requires 54 h. This short turnaround time represents an advantage if treatment needs to be modified. A recent study (1) comparing BACTEC with LRP and luminometry for the detection of resistance in isolates of M. tuberculosis from sputum provided excellent results for all of the antibiotics in only 2 days after detection, while BACTEC took 7 days (PM takes at least 3 weeks). LRP requires less labor and materials and fewer culture media, resulting in lower test costs. In addition, the time of exposure of laboratory personnel to mycobacterial cultures is minimal. The Bronx box does not require specialized equipment and is simple, but it is subjective and more laborious than luminometry. Photographic results could be further analyzed by densitometry, offering quantitative results, but this requires scanners and specialized software that complicates the assay while increasing the cost. On the other hand, the luminometer is easy to use and provides quick and quantitative results. For these reasons, the use of the luminometer for LRP methods would be recommended for reference laboratories having this resource.
Based on the results of this study and on the excellent sensitivity of LRP for detecting resistance to INH and RIF, we propose the application of this method as a screening test for the surveillance of MDR TB. LRPs could be a useful tool in public health programs for the control of drug resistance established in the DOTS-plus initiative (5). This method could also be applied in epidemiological studies for detection of foci of MDR M. tuberculosis. LRP can provide results faster and more cheaply than do other methods such as BACTEC and PM. The initiation of adequate treatment, as well as decision-making by the TB control program, could be accelerated by the use of the LRP technology. The validation and optimization of the LRP test, with some improvement of its specificity, could permit its use not only as a screening test but also in the routine diagnosis of resistance. A digital camera version of the Bronx box could combine the best elements of the box and the luminometer and is currently being evaluated. Studies aimed at further improving the system using LRPs that readily lysogenize M. tuberculosis are under way and could further enhance the sensitivity of the assay.
Acknowledgments
This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) (ID 991103 and AI26170 [W.R.J.]), COLCIENCIAS (contract 215-2000), the Fundación para la Promoción de la Investigación y la Tecnología, and the Colombian Ministry of Health.
We thank Stoyan Bardarov and Svetoslav Bardarov, Jr. for the training and technical advice, Nancy Saravia for her continuous scientific input and support, Mauricio Pérez for the statistical analysis, and Alfredo Ponce de León for the critical review of the manuscript.
REFERENCES
- 1.Banaiee, N., M. Bobadilla-del-Valle, S. Bardarov, Jr., P. F. Riska, P. M. Small, A. Ponce-de-Leon, W. R. Jacobs, Jr., G. F. Hatfull, and J. Sifuentes-Osornio. 2001. Luciferase reporter mycobacteriophages for detection, identification, and antibiotic susceptibility testing of Mycobacterium tuberculosis in Mexico. J. Clin. Microbiol. 39:3883-3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bardarov, S., Jr., H. Dou, K. Eisenach, N. Banaiee, S. Ya, J. Chan, W. R. Jacobs, Jr., and P. F. Riska. 2003. Detection and drug-susceptibility testing of M. tuberculosis from sputum samples using luciferase reporter phage: comparison with the Mycobacteria Growth Indicator Tube (MGIT) system. Diagn. Microbiol. Infect. Dis. 45:53-61. [DOI] [PubMed] [Google Scholar]
- 3.Carriere, C., P. F. Riska, O. Zimhony, J. Kriakov, S. Bardarov, J. Burns, J. Chan, and W. R. Jacobs, Jr. 1997. Conditionally replicating luciferase reporter phages: improved sensitivity for rapid detection and assessment of drug susceptibility of Mycobacterium tuberculosis. J. Clin. Microbiol. 35:3232-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Espinal, M. A., A. Laszlo, L. Simonsen, F. Boulahbal, S. J. Kim, A. Reniero, S. Hoffner, H. L. Rieder, N. Binkin, C. Dye, R. Williams, M. C. Raviglione, et al. 2001. Global trends in resistance to antituberculosis drugs. N. Engl. J. Med. 344:1294-1303. [DOI] [PubMed] [Google Scholar]
- 5.Farmer, P., and J. Y. Kim. 1998. Community based approaches to the control of multidrug resistant tuberculosis: introducing “DOTS-plus”. BMJ 317:671-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hazbón, M. H., M. del Socorro Orozco, L. A. Labrada, R. Tovar, K. A. Weigle, and A. Wanger. 2000. Evaluation of Etest for susceptibility testing of multidrug-resistant isolates of Mycobacterium tuberculosis. J. Clin. Microbiol. 38:4599-4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heifets, L. B., M. D. Iseman, and P. J. Lindholm-Levy. 1986. Ethambutol MICs and MBCs for Mycobacterium avium complex and Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 30:927-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs, W. R., Jr., R. G. Barletta, R. Udani, J. Chan, G. Kalkut, G. Sosne, T. Kieser, G. J. Sarkis, G. F. Hatfull, and B. R. Bloom. 1993. Rapid assessment of drug susceptibilities of Mycobacterium tuberculosis by means of luciferase reporter phages. Science 260:819-822. [DOI] [PubMed] [Google Scholar]
- 9.Kent, P. T., and G. P. Kubica. 1985. Public health mycobacteriology: a guide for the level III laboratory. Centers for Disease Control, Atlanta, Ga.
- 10.Kirk, S. M., R. F. Schell, A. V. Moore, S. M. Callister, and G. H. Mazurek. 1998. Flow cytometric testing of susceptibilities of Mycobacterium tuberculosis isolates to ethambutol, isoniazid, and rifampin in 24 hours. J. Clin. Microbiol. 36:1568-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laserson, K. F., L. Osorio, J. D. Sheppard, H. Hernandez, A. M. Benitez, S. Brim, C. L. Woodley, M. H. Hazbon, M. V. Villegas, M. C. Castano, N. Henriquez, E. Rodriguez, B. Metchock, and N. J. Binkin. 2000. Clinical and programmatic mismanagement rather than community outbreak as the cause of chronic, drug-resistant tuberculosis in Buenaventura, Colombia, 1998. Int. J. Tuberc. Lung Dis. 4:673-683. [PubMed] [Google Scholar]
- 12.Riska, P. F., and W. R. Jacobs, Jr. 1998. The use of luciferase-reporter phage for antibiotic-susceptibility testing of mycobacteria. Methods Mol. Biol. 101:431-455. [DOI] [PubMed] [Google Scholar]
- 13.Riska, P. F., W. R. Jacobs, Jr., B. R. Bloom, J. McKitrick, and J. Chan. 1997. Specific identification of Mycobacterium tuberculosis with the luciferase reporter mycobacteriophage: use of p-nitro-α-acetylamino-β-hydroxy propiophenone. J. Clin. Microbiol. 35:3225-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riska, P. F., Y. Su, S. Bardarov, L. Freundlich, G. Sarkis, G. Hatfull, C. Carrière, V. Kumar, J. Chan, and W. R. Jacobs, Jr. 1999. Rapid film-based determination of antibiotic susceptibilities of Mycobacterium tuberculosis strains by using a luciferase reporter phage and the Bronx Box. J. Clin. Microbiol. 37:1144-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turett, G. S., E. E. Telzak, L. V. Torian, S. Blum, D. Alland, I. Weisfuse, and B. A. Fazal. 1995. Improved outcomes for patients with multidrug-resistant tuberculosis. Clin. Infect. Dis. 21:1238-1244. [DOI] [PubMed] [Google Scholar]
- 16.Wanger, A., and K. Mills. 1996. Testing of Mycobacterium tuberculosis susceptibility to ethambutol, isoniazid, rifampin, and streptomycin by using Etest. J. Clin. Microbiol. 34:1672-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. 1997. 1997 Anti-tuberculosis drug resistance in the world: the W. H. O./IUTLD global project on anti-tuberculosis drug resistance surveillance. Publication W. H. O./TB/97.229. World Health Organization, Geneva, Switzerland.
- 18.World Health Organization. 2001. 2001 Anti-tuberculosis drug resistance in the world: the W. H. O./IUTLD global project on anti-tuberculosis drug resistance surveillance. Publication W. H. O./CDS/TB/2001.287. World Health Organization, Geneva, Switzerland.