Abstract
We evaluated the performance of three commercial measles immunoglobulin M enzyme immunoassays from Meddens, Denka Seiken, and Behring. The sensitivities were determined to be 96.7% for the Meddens and Denka Seiken assays and 87.9% for the Behring assay. The specificities of the assays were determined to be 94.6% for Meddens, 98.2% for Denka Seiken, and 98.7% for Behring.
The World Health Organization (WHO) estimates that there were >30 million measles cases, resulting in 777,000 deaths, in the year 2000 (20). Many countries have implemented measles vaccination programs and are working toward the elimination of the measles virus. The established role of the laboratory in measles surveillance is (i) to confirm measles cases by immunoglobulin M (IgM) serology and (ii) to genotype measles virus strains for molecular epidemiological purposes (16, 17, 18, 19).
Commercial enzyme immunoassay (EIA) kits are commonly used for measles IgM serology (17). It is important to fully understand the performance characteristics of measles IgM EIAs for measles laboratory surveillance, particularly in the elimination phase, when incidence is low, resulting in decreased positive predictive values (PPVs) for a test (4). In this study, we evaluated the Measles-IgM Comfort EIA μ-capture (Meddens Diagnostics BV, Vorden, The Netherlands), the Measles IgM (II) EIA “Seiken” (Denka Seiken, Tokyo, Japan), and the Enzygnost Anti-Measles Virus IgM (Dade Behring, Marburg, Germany) assays. Intra-assay variation was determined by calculating 95% confidence limits (see Table 2), and the statistical significance of interassay variation was done by using the z test to compare two proportions. The Bonferroni correction was used to correct for type I errors when comparing multiple tests. Corrected P values corresponding to <0.05, <0.01, or <0.001 were interpreted to indicate a statistically significant difference, while a P value of <0.10 was considered to indicate a trend toward statistical significance.
TABLE 2.
Relative overall sensitivities, specificities, and predictive values of measles virus IgM antibody testsa
| Assay | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Meddens | 96.7 (95.0, 98.4) | 94.6 (91.7, 97.6) | 97.4 (95.9, 98.9) | 94.6 (91.7, 97.6) |
| Denka Seiken | 96.7 (95.0, 98.4) | 98.2 (96.5, 99.9) | 99 (98.1, 100) | 94.4 (91.5, 97.4) |
| Behring | 87.9 (84.8, 91.0) | 98.7 (97.2, 100) | 99.7 (99.2, 100) | 88.8 (84.8, 92.7) |
Numbers in parentheses are 95% confidence intervals.
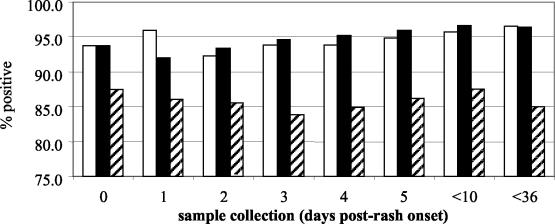
The measles panel consisted of paired sera from acute measles cases collected as part of the measles surveillance program in Iran (Table 1). Clinically diagnosed cases (17) were considered laboratory confirmed if consistent IgM-positive results were obtained using the three different measles IgM assays or by a >4-fold rise in titer between acute- and convalescent-phase sera by a plaque reduction neutralization test (12). A total of 423 sera from confirmed cases of measles were utilized to assess the sensitivities of the Meddens and Denka Seiken EIAs, and 421 sera were used for the Behring EIA. The Meddens and Denka Seiken EIAs showed identical sensitivities of 96.7%, whereas the Behring EIA showed a sensitivity of 87.9% (Table 2). The difference in sensitivity between the first two EIAs and the Behring EIA was statistically significant (P < 0.01). The dates of rash onset and serum collection were known, so the sensitivities of the EIAs could be assessed in relation to the timing of blood collection (Fig. 1). For the Meddens and Denka Seiken EIAs, sensitivity increased when the sample was collected >3 days after rash onset, as has been shown for the development of the IgM response to measles virus for both vaccinated and naturally infected individuals (7, 9, 13). However, the sensitivity of the Behring assay was essentially the same for samples collected before and after 3 days post-rash onset.
TABLE 1.
Distribution of results for measles IgM antibody testing using the measles and nonmeasles panels
| Assay | Measles panel no. of samples positive (n = 423) | Nonmeasles panel no. of samples positive or equivocal
|
|||
|---|---|---|---|---|---|
| Parvovirus (n = 4) | Rubella virus (n = 208) | HHV-6 (n = 12) | Total (n = 224) | ||
| Meddens | 409 | 1 | 8 | 3 | 12 |
| Denka Seiken | 409 | 0 | 2 | 2 | 4 |
| Behring | 370a | 0 | 2 | 1 | 3 |
n = 421.
FIG. 1.
Effect of timing of sample collection on measles IgM assay sensitivity. Open bars, Meddens assay; solid bars, Denka Seiken assay; hatched bars, Behring assay. Percent positive indicates the ratio of the cumulative number of positives as a percentage of the cumulative total of samples collected up to that time.
A number of different viruses, including measles and rubella viruses, parvovirus B19, enterovirus, and adenovirus, can give similar clinical presentations, and therefore laboratory confirmation is essential (5). It has been shown that false-positive measles IgM results can occur, particularly with parvovirus B19 and rubella virus, which have similar clinical presentations (3, 6, 8). In addition, it has been shown that reactivation of IgM responses to multiple viruses (including measles and rubella viruses and parvovirus B19) can occur in response to infection by one of the viruses (14). In this study, the nonmeasles panel (224 sera) consisted of sera from rubella virus, parvovirus B19, or human herpesvirus 6 (HHV-6) cases (Table 1). Rubella cases were confirmed when multiple rubella IgM kits (Meddens, Denka Seiken, and Behring) gave positive IgM results and/or if a >4-fold rise in IgG titer was detected between acute- and convalescent-phase sera by a hemagglutination inhibition test (2). Parvovirus B19 cases were defined by the presence of parvovirus B19-specific IgM antibodies (Biotrin International Ltd., Dublin, Ireland) and the absence of measles and rubella virus IgM antibodies. Roseola (HHV-6) cases were defined as follows: the age of the patient was <3 years and the sera tested positive for HHV-6-specific IgM antibodies, showed HHV-6-specific IgG seroconversion, and had low-avidity HHV-6-specific IgG antibodies (11, 15). In this study, the Denka Seiken EIA showed a specificity of 98.2%, the Behring EIA showed a specificity of 98.7%, and the Meddens EIA showed a specificity of 94.6% (Table 2). The difference between the specificities of the first two EIAs and that of the Meddens EIA was statistically significant (P < 0.05). The distribution of false-positive or equivocal measles virus IgM results for the three EIAs with respect to parvovirus B19, rubella virus, and HHV-6 cases is shown in Table 1.
The PPV of the Behring EIA (99.7%) was significantly better (P < 0.01) than that of the Meddens EIA (97.4%) but not that of the Denka Seiken EIA (99.0%) (Table 2). The Denka Seiken EIA showed a trend toward significance (P < 0.10) compared with the Meddens EIA. The negative predictive value (NPV) was 94.6% for the Meddens EIA, followed by the Denka Seiken EIA (94.4%) and the Behring EIA (88.8%). A trend toward statistical significance was shown between the NPVs of the Meddens and Denka Seiken EIAs and that of the Behring EIA (P < 0.10).
There are a few reports in the literature assessing the performances of both in-house measles IgM assays and commercial assays (1, 10, 13). A previous evaluation of four other measles IgM EIAs, along with the Behring EIA, found the last to have a sensitivity of 88.6%, a specificity of 96.7%, a PPV of 97.8%, and an NPV of 94.6% (13). The present study further confirms the performance of the Behring EIA through independent evaluation using different panels of sera. The Behring EIA has also been evaluated by others and found to perform well (91.8% sensitivity, 98.2% specificity, 98.2% PPV, and 92.0% NPV [1] and 97.2% sensitivity for outbreak cases as defined by increased complement fixation IgG titers [10]).
The decision to select a commercial measles IgM kit includes factors such as assay performance, cost, availability, ease of use, and turnaround time. The Meddens and Denka Seiken measles IgM capture EIA protocols are both easy to read and to follow. We find the Behring EIA procedure quite confusing and difficult to follow, at least for initial learning of the assay. The sample volumes required are 50 μl of serum for the Meddens assay, 10 μl for the Denka Seiken assay, and 20 μl for the Behring assay. In our experience, the Meddens assay requires ∼2.5 h, the Denka Seiken assay requires 4.5 h, and the Behring measles IgM EIA requires 4 h to complete. The incubations for the Meddens and Behring assays are done at 37°C, while the Denka Seiken assay incubations are done at room temperature (15 to 25°C).
We conclude from this study that the Meddens, Denka Seiken, and Behring IgM EIAs perform well and thus should be considered useful for measles laboratory surveillance.
Acknowledgments
We thank the Office of the Chief Scientist, Health Canada, and the World Health Organization for partial support of this study. We also thank Dan Chateau for assistance with the statistical analysis and David Safronetz, Jennifer Ball, and Elizabeth Oates for technical assistance.
REFERENCES
- 1.Arista, S., D. Ferraro, A. Cascio, E. Vizzi, and R. di Stefano. 1995. Detection of IgM antibodies specific for measles virus by capture and indirect enzyme immunoassays. Res. Virol. 46:225-232. [DOI] [PubMed] [Google Scholar]
- 2.Best, J. M., and J. E. Banatvala. 2000. Rubella, p. 387-415. In A. J. Zuckerman, J. E. Banatvala, and J. R. Pattison (ed.), Principles and practice of clinical virology, 4th ed. John Wiley and Sons, London, United Kingdom.
- 3.Cubel, R. C., M. M. Siqueira, E. O. Santos, M. F. Pires, C. M. Cruz, and J. P. Nascimento. 1996. Human parvovirus B19 infections among exanthematic diseases notified as measles. Rev. Soc. Bras. Med. Trop. 30:15-20. [DOI] [PubMed] [Google Scholar]
- 4.Cutts, F. T., and D. W. G. Brown. 1995. The contribution of field tests to measles surveillance and control: a review of available methods. Rev. Med. Virol. 5:35-40. [Google Scholar]
- 5.Davidkin, I., M. Valle, H. Peltola, T. Hovi, M. Paunio, M. Roivainen, K. Linnavuori, S. Jokinen, and P. Leinikki. 1998. Etiology of measles- and rubella-like illnesses in measles, mumps, and rubella-vaccinated children. J. Infect. Dis. 178:1567-1570. [DOI] [PubMed] [Google Scholar]
- 6.Donovon, S. M. 1997. False-positive results of an enzyme immunoassay for rubella in a case of measles. Clin. Infect. Dis. 24:271-272. [DOI] [PubMed] [Google Scholar]
- 7.Helfand, R. F., J. L. Heath, L. J. Anderson, E. F. Maes, D. Guris, and W. J. Bellini. 1997. Diagnosis of measles with an IgM capture EIA: the optimal timing of specimen collection after rash onset. J. Infect. Dis. 175:195-199. [DOI] [PubMed] [Google Scholar]
- 8.Jenkerson, S. A., M. Beller, J. P. Middaugh, and D. D. Erdman. 1995. False positive rubeola IgM tests. N. Engl. J. Med. 332:1103-1104. [DOI] [PubMed] [Google Scholar]
- 9.Nates, S., G. Rey, M. Giordano, S. Medeot, A. Depetris, J. Boshell, and C. D. de Wolff. 1997. Immunoglobulin M antibody response to measles virus following natural virus infection, primary vaccination, and reexposure to the virus. Viral Immunol. 10:165-173. [DOI] [PubMed] [Google Scholar]
- 10.Ozanne, G., and M. A. d'Halewyn. 1992. Performance and reliability of the Enzygnost measles enzyme-linked immunosorbent assay for detection of measles virus-specific immunoglobulin M antibody during a large measles epidemic. J. Clin. Microbiol. 30:564-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker, C. A., and J. M. Weber. 1993. An enzyme-linked immunosorbent assay for the detection of IgG and IgM antibodies to human herpesvirus type 6. J. Virol. Methods 41:265-276. [DOI] [PubMed] [Google Scholar]
- 12.Ratnam, S., V. Gadag, R. West, J. Burris, E. Oates, F. Stead, and N. Boullianne. 1995. Comparison of commercial enzyme immunoassay kits with plaque reduction neutralization test for detection of measles virus antibody. J. Clin. Microbiol. 33:811-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ratnam, S., G. A. Tipples, C. Head, M. Fauvel, M. Fearon, and B. J. Ward. 2000. Performance of indirect IgM serology tests and IgM capture assays for laboratory diagnosis of measles. J. Clin. Microbiol. 38:99-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas, H. I., E. Barrett, L. M. Hesketh, A. Wynne, and P. Morgan-Capner. 1999. Simultaneous IgM reactivity by EIA against more than one virus in measles, parvovirus B19 and rubella infection. J. Clin. Virol. 14:107-118. [DOI] [PubMed] [Google Scholar]
- 15.Ward, K. N., D. J. Turner, X. C. Parada, and A. D. Thiruchelvam. 2001. Use of immunoglobulin G antibody avidity for differentiation of primary human herpesvirus 6 and 7 infections. J. Clin. Microbiol. 39:959-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Working Group on Measles Elimination in Canada. 1999. Measles surveillance: guidelines for laboratory support. Can. Commun. Dis. Rep. 24:201-216. [PubMed] [Google Scholar]
- 17.World Health Organization. 2000. Manual for the laboratory diagnosis of measles virus infection. World Health Organization, Geneva, Switzerland. [Online.]
- 18.World Health Organization. 2001. Nomenclature for describing the genetic characteristics of wild-type measles viruses (update), part I. Wkly. Epidemiol. Rec. 76:242-247. [PubMed] [Google Scholar]
- 19.World Health Organization. 2001. Nomenclature for describing the genetic characteristics of wild-type measles viruses (update), part II. Wkly. Epidemiol. Rec. 76:249-251. [PubMed] [Google Scholar]
- 20.World Health Organization. 2002. W. H. O.-UNICEF joint statement on strategies to reduce measles mortality worldwide. Wkly. Epidemiol. Rec. 27:224-228. [PubMed] [Google Scholar]