Abstract
Staphylococcus aureus is one of the most significant pathogens causing nosocomial and community-acquired infections. Among the secreted staphylococcal virulence factors, there is a growing list of enterotoxins which can induce gastroenteric syndrome and toxic shock syndrome. Here, we developed a real-time fluorescence PCR assay (TaqMan PCR) for the detection of genes encoding staphylococcal enterotoxins A, B, C1, and D (SEA, SEB, SEC1, and SED) of S. aureus as well as the mecA gene encoding methicillin resistance and the femB gene as a specific genomic marker for S. aureus. SEA to SED were selected because they are the four classically described enterotoxins of S. aureus and because they were detected by latex agglutination. In order to evaluate the reliability of TaqMan PCR, we investigated 93 isolates of S. aureus derived from patients at our hospital over 5 months and compared the results with data obtained by a commerciall available reversed passive latex agglutination assay (SET-RPLA) for these isolates. Thirteen enterotoxin genes were detected by TaqMan PCR; however, no proteins expressed by these genes were detected by SET-RPLA. As a result, more isolates of S. aureus (n = 44) were found positive by TaqMan PCR for one or more enterotoxin genes than by SET-RPLA for the respective proteins expressed by these genes (n = 40). We conclude that TaqMan PCR is more sensitive because it offers the possibility for determining enterotoxins on a genotypic basis. Additionally, the assay allows the parallel detection of genes for SEA to SED and methicillin resistance in S. aureus. Furthermore, real-time PCR is well suited for screening large numbers of samples at the same time, allowing rapid, reliable, efficient, and cost-saving routine laboratory diagnosis.
Staphylococcus aureus is a major human pathogen that produces a wide variety of exoproteins that cause various types of disease symptoms. Some S. aureus strains produce pyrogenic exotoxins, such as staphylococcal enterotoxins (SEs) and toxic shock syndrome toxin 1 (TSST-1) (13). Today, up to 15 SEs are known; the last one discovered was the recently identified SE U (SEU) (8). However, various studies have shown that not all of them play a role in food poisoning (8). Beside the classically described SEs (SEA to SEE), only SEH (19) and SEG and SEI (10, 14) have been proven to induce gastroenteric syndrome. The five major serological groups of enterotoxins fall into two subgroups: SEB, SEC1, SEC2, and SEC3, which have 66 to 98% amino acid sequence identity, and SEA, SED, and SEE, which have 53 to 81% amino acid sequence identity. The gene coding for TSST-1 has little sequence homology with SE or streptococcal pyrogenic exotoxin genes (9), although the toxins are structurally and functionally very similar.
The SEs are heat-stable exoproteins which induce gastroenteric syndrome in humans through ingestion. As superantigens, they may also cause toxic shock syndrome (9) by initiating the activation and proliferation of T cells with certain Vβ regions on their T-cell receptors (11). The biological effects of superantigens include pyrogenicity, enhancement of lethal endotoxin shock, and induction of inflammatory cytokines, such as tumor necrosis factor and interleukin 1 (5). Thus, SEs function as potent gastrointestinal toxins as well as superantigens. Although these are two separate functions localized on separate domains of the proteins, there is a high correlation between these activities (1). It has been suggested that S. aureus enterotoxins cause enterocolitis and that, when predominant in stool samples, S. aureus should be considered a possible etiologic agent for some cases of antibiotic-associated diarrhea (7) and toxic shock.
The most common toxin implicated in staphylococcal food poisoning is SEA (1). Like the gene for SEE (entE), the gene for SEA (entA) is carried by a temperate bacteriophage (4, 6, 22). The SEB gene (entB) is located on a chromosome (13). The genes for SEC (entC) and TSST-1 are also chromosomal (22). The genes for SED (entD) and SEJ (entJ) are plasmid carried (21).
To evaluate the reliability of a real-time PCR assay for the detection of SE genes, we focused on the four classically described enterotoxins of S. aureus (SEA to SED) because they were detected by a latex agglutination assay (15). Various methods for detecting enterotoxin production (17) have been developed so far. Among these methods, a commercially available reversed passive latex agglutination assay (SET-RPLA, an SE test kit for the detection of SEA to SED; Oxoid, Basingstoke, United Kingdom) is the most commonly used assay. Therefore, we used this assay as a reference method, although it limited the selection of enterotoxins to SEA to SED. In this assay, enterotoxins are identified by polyclonal antibodies (rabbit antiserum) specific for each of the enterotoxins.
Additionally, over the last few years, multiple PCR assays and multiplex PCR assays which detect specific gene sequences for SEs and TSST-1 by DNA amplification have been developed (2, 10, 16, 17). Conventional PCR techniques use agarose gel electrophoresis and/or probe detection formats to detect amplification products, but they are not well suited for the rapid screening of large numbers of samples, since they are rather time-consuming. For this reason, we developed a real-time fluorescence PCR assay (TaqMan PCR) that detects not only the genes for SEA to SED of S. aureus but also the mecA gene for methicillin resistance and the femB gene as a specific genomic marker for S. aureus. The system uses both PCR primers and a dye-labeled probe to detect the target sequence and provides an instantaneous reading of the PCR result. In order to evaluate the reliability of TaqMan PCR, 93 isolates of S. aureus derived from patients at our hospital over 5 months were analyzed. We compared phenotypic testing by a commercial agglutination assay with detection of toxin genes by the real-time PCR. Furthermore, we assessed the efficiency of TaqMan PCR compared to those of conventional methods, including classical PCR.
MATERIALS AND METHODS
Bacterial strains and culture media.
A total of 93 S. aureus strains were used in this study. All of these strains were derived from patients at a university hospital over 5 months. The following strains were used as positive controls in this study: S. aureus National Collection of Type Cultures (NCTC) 10652 (SEA positive), S. aureus NCTC 10654 (SEB positive), S. aureus NCTC 10655 (SEC positive), S. aureus NCTC 10656 (SED and SEA positive), S. aureus ATCC 29293 (SEA positive), and S. aureus MCCM 01974 (mecA positive and SEA positive; from the Medical Culture Collection Marburg).
All staphylococcal strains were cultivated on 5% sheep blood agar, incubated at 35°C for 24 h, and identified as S. aureus by standard microbiological methods. Methicillin resistance was determined by growth on Mueller-Hinton agar after incubation with 6 μg of oxacillin/ml and 2% NaCl at 35°C for 24 or 48 h.
Immunological detection of enterotoxins.
For enterotoxin detection, isolated S. aureus strains were inoculated in tryptone soy broth and incubated at 37°C for 18 to 24 h with shaking. After growth, the culture was centrifuged at 900 × g for 20 min, and the supernatant was used for enterotoxin detection. Enterotoxins were detected by the commercially available SET-RPLA according to the manufacturer's instructions. In brief, latex reagents sensitized with antisera to SEA to SED are mixed with supernatant dilutions and incubated overnight. The results of this test are available 2.5 days after isolation of the S. aureus strain.
Isolation of genomic DNA.
Total genomic DNA was isolated by using a QIAamp DNA mini kit (Qiagen, Hilden, Germany) for DNA purification. An inoculation loop of overnight-grown staphylococcal culture was suspended in 180 μl of buffer ATL (supplied with the QIAamp DNA mini kit), and 20 μl of proteinase K was added. Then, the mixture was vortexed and incubated at 56°C for 3 h with shaking. DNA isolation was then completed within 30 min according to the manufacturer's instructions for the kit. Two nanograms of this purified DNA was used as a template in PCR amplifications.
Oligonucleotide primers and TaqMan probes.
The oligonucleotide primers and fluorescence-labeled hybridization probes for the S. aureus enterotoxin genes (SEA, SEB, SEC1, and SED), the mecA gene for methicillin resistance, and the S. aureus-specific genomic marker femB were selected by using Primer Express software (Applied Biosystems). All oligonucleotides were synthesized by Applied Biosystems (OligoFactory, Weiterstadt, Germany). The sequences and the corresponding sequence locations of the oligonucleotide primers and TaqMan probes are shown in Table 1.
TABLE 1.
Oligonucleotide primers and TaqMan probes used in the PCR assaya
| Gene | Primer or probe | Sequence | Location of gene | Product size (bp) |
|---|---|---|---|---|
| SEA | SEA-fw | 5′-AAAATACAGTACCTTTGGAAACGGTT | 476-502 | 92 |
| SEA-rv | 5′-TTTCCTGTAAATAACGTCTTGCTTGA | 568-542 | ||
| Probe | FAM-AACGAATAAGAAAAATGTAACTGTTCAGGAGTTGGATC | 504-541 | ||
| SEB | SEB-fw | 5′-ACACCCAACGTTTTAGCAGAGAG | 307-330 | 81 |
| SEB-rv | 5′-CCATCAAACCAGTGAATTTACTCG | 388-364 | ||
| Probe | FAM-CAACCAGATCCTAAACCAGATGAGTTGCACA | 331-362 | ||
| SEC1 | SEC-fw | 5′-AATAAAACGGTTGATTCTAAAAGTGTGAA | 856-885 | 80 |
| SEC-rv | 5′-ATCAAAATCGGATTAACATTATCCATTC | 908-936 | ||
| Probe | FAM-TAGAAGTCCACCTTACAACAA | 887-907 | ||
| SED | SED-fw | 5′-TGATTCTTCTGATGGGTCTAAAGTCTC | 924-951 | 115 |
| SED-rv | 5′-GAAGGTGCTCTGTGGATAATGTTTT | 1039-1014 | ||
| Probe | FAM-TATGATTTATTTGATGTTAAGGGTGATTTTCCCGAA | 952-988 | ||
| MecA | mecA-fw | 5′-GCTTTGGTCTTTCTGCATTCCT | 127-149 | 91 |
| mecA-rv | 5′-ACGTTCAATTTAATTTTGTTAAAGAAGATG | 218-188 | ||
| Probe | FAM-AATGACGCTATGATCCCAATCTAACTTCCACAT | 154-187 | ||
| FemB | femB-fw | 5′-AATTAACGAAATGGGCAGAAACA | 277-299 | 93 |
| femB-rv | 5′-TGCGCAACACCCTGAACTT | 370-351 | ||
| Probe | FAM-AGAAATTAACTGGATGGTACGCGCGAAGA | 301-330 |
All oligonucleotides were selected by using Primer Express software and synthesized by Applied Biosystems. FAM, fluorescein.
TaqMan PCR.
Amplification mixtures contained, in a final volume of 25 μl, PCR buffer (10×) (Perkin-Elmer); 6 mM MgCl2; 10 mM each dATP, dCTP, and dGTP; 20 mM dUTP; 50 pmol of each primer; 150 nM TaqMan probe; 0.25 μl of AmpErase; 2.5 U of AmpliTaq; and 10 μl of template DNA. Amplification was performed with an ABI PRISM 7700 sequence detector programmed to hold at 50°C for 2 min, to hold at 95°C for 10 min, and to complete 45 cycles of 95°C for 15 s and 60°C for 1 min (total time, about 2 h). The data were analyzed in the real-time mode. PCR results were given as the increase in the fluorescence signal of the reporter dye detected by the ABI PRISM 7700 sequence detector. Test results are available 6 h after isolation of the S. aureus strain: DNA extraction requires 3.5 h; master mixture preparation, depending on the number of samples, requires about 30 min; and the TaqMan assay requires 2 h.
Determination of assay sensitivity.
To determine the sensitivity of the assay, a dilution series was performed with a representative strain (NCTC 10652) for the SEA gene and the S. aureus-specific genomic marker femB. Serial dilutions (1:4) of the cultured S. aureus strain were made beginning with 1.5 × 106 bacteria/ml and extending to 2.5 × 101 bacteria/ml. The amount of DNA purified from 1.5 × 106 bacteria was approximately 0.5 ng. The PCR was carried out in triplicate for SEA and FemB, and the cycle threshold (Ct) (mean and standard deviation) of the reactions was calculated.
RESULTS
A total of 93 S. aureus strains originating from stool samples derived from patients at our hospital over 5 months were tested for enterotoxin production by the commercially available agglutination assay (SET-RPLA) and real-time TaqMan PCR.
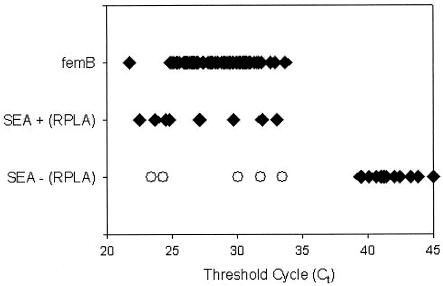
Figure 1 shows the results of TaqMan PCR for a representative panel of strains exemplary for SEA and the S. aureus-specific genomic marker femB. The TaqMan assay provided the advantage of instant detection of a PCR product by laser-activated fluorescence. In the real-time mode, the ABI PRISM 7700 instrument reads each well every few seconds and computes a mean baseline reading for early PCR cycles. Real-time results are reported as Ct values, representing the cycle at which fluorescence readings exceed the mean baseline. The Ct value depends on the amount of template DNA being used: the higher the amount of template DNA, the lower the Ct value.
FIG. 1.
Results of TaqMan PCR for representative panel of strains exemplary for SEA and the S. aureus-specific genomic marker femB. Open circles indicate five SEA strains which were detected by TaqMan PCR but not by SET-RPLA.
Our results show that there is a large area of detection of the cycle at which a statistically significant increase in ΔRn (Delta value of the normalized reporter signal minus the baseline signal) is first noted for positive gene detection (Ct values of 21.8 to 33.7 for femB and 22.5 to 33.5 for SEA; mean Ct values of 28.5 for femB and 27.9 for SEA). Ct values of 39.4 to 45 indicate no amplification or nonspecific amplification. There are no signals at Ct values of 33.5 to 39.4. Thus, the method allows clear discrimination between positive gene detection and no amplification. Simultaneous data were obtained for SEB, SEC1, and SED.
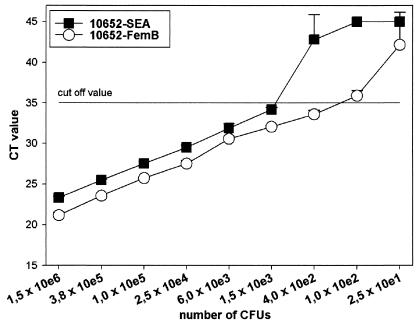
Determination of the analytical sensitivity of the PCR assay for the SEA gene and the S. aureus-specific marker femB with serial dilutions of cultured strain NCTC 10652 revealed detection limits of approximately 250 CFU (range, 100 to 400; genome equivalents) for the femB gene and approximately 1,000 CFU (range, 400 to 1,500; genome equivalents) for the SEA gene at a Ct cutoff of 35 (Fig. 2); this cutoff is 4.4 cycles below (or approximately 20-fold lower than) nonspecific or no amplification.
FIG. 2.
Sensitivity of real-time TaqMan PCR for SEA (closed symbols) and the specific genomic marker femB (open circles) of S. aureus reference strain NCTC 10652. A cutoff of 35 cycles was used; this cutoff is 4.4 cycles below (or 20-fold lower than) the lowest nonspecific detection seen in Fig. 1. Values represent means and standard deviations of triplicates. Two independent experiments gave identical results. e, exponent.
By TaqMan PCR, 44 of the 93 S. aureus strains were found positive for enterotoxins. Thirty-seven of these strains showed the production of only one enterotoxin. One strain was positive for SEA and SEB, one was positive for SEA and SEC1, one was positive for SEB and SEC1, and one was positive for SEC1 and SED. Two strains were positive for SEA, SEC1, and SED, and one was positive for SEB, SEC1, and SED (Table 2). With SET-RPLA, 40 of the 93 strains were found positive for enterotoxin production. Except for one strain which expressed SEB and SEC, all strains were positive for only one enterotoxin (Table 2).
TABLE 2.
Number of SE-positive strains detected by SET-RPLA or TaqMan PCRa
| No. of SEs detected | No. of strains found positive by:
|
|
|---|---|---|
| SET-RPLA | TaqMan PCR | |
| One | 39 | 37 |
| Two | 1 | 4 |
| Three | 3 | |
| Total | 40 | 44 |
A total of 93 S. aureus strains were tested.
Table 3 compares the results obtained for the individual enterotoxins by a conventional agglutination assay and real-time TaqMan PCR. By TaqMan PCR, 12 samples were found positive for SEA, 9 were positive for SEB, 20 were positive for SEC1, and 13 were positive for SED. By SET-RPLA, 7 samples were found positive for SEA, 8 were positive for SEB, 19 were positive for SEC, and 7 were positive for SED. In summary, SEA, SEB, SEC1, and SED were detected more often by TaqMan PCR than by SET-RPLA. As all strains found positive for SEC by SET-RPLA (n = 19) were detected by our SEC1 primer set, there seems to be no need to expand the PCR analysis to the rare SEC2 and SEC3 genes. A total of 41 samples which were SET-RPLA positive were also PCR positive. Additionally, 13 enterotoxin genes were detected by PCR but were not found by SET-RPLA. None of the SET-RPLA-positive strains were PCR negative.
TABLE 3.
SEs detected by SET-RPLA or TaqMan PCRa
| SE | No. of strains found positive by:
|
|
|---|---|---|
| SET-RPLA | TaqMan PCR | |
| SEA | 7 | 12 |
| SEB | 8 | 9 |
| SEC1 | 19 | 20 |
| SED | 7 | 13 |
| Total | 41 | 54 |
A total of 93 S. aureus strains were tested.
All 93 S. aureus strains were PCR positive for the S. aureus-specific genomic marker femB. Six strains possessed the mecA gene for methicillin resistance. This finding was confirmed by the results of conventional identification and susceptibility testing.
DISCUSSION
S. aureus is a major human pathogen and causes a variety of nosocomial and community-acquired infections. Among the wide range of staphylococcal virulence factors, there is a growing list of secreted superantigen toxins, including enterotoxins A to O and U (8). For the determination of SEs, the SET-RPLA is the most commonly used method. It detects a protein only if it is expressed in vitro and depends on sufficient amounts of toxin being produced. Recently, PCR assays have been developed to identify specific gene sequences for SEs (2, 10, 16, 17). However, these conventional PCR techniques use agarose gel electrophoresis to detect amplification products. Thus, they are not well suited for the rapid screening of large numbers of samples, since the number of samples that can be analyzed per gel is limited and loading, running, and staining of gels are rather time-consuming.
In contrast to conventional PCR, real-time PCR appears to be a much more suitable method, since it allows the analysis of a large number of samples at the same time. Therefore, we developed a real-time fluorescence PCR assay (TaqMan PCR) for the detection of the SEA to SED genes, the femB gene as a specific genomic marker for S. aureus, and the mecA gene for methicillin resistance. In order to assess the efficiency of TaqMan PCR, we investigated 93 strains of S. aureus and compared the results with data obtained by SET-RPLA for these strains. In contrast to SET-RPLA, TaqMan PCR allows the analysis of specific gene sequences with amplification and detection of the PCR product simultaneously in one reaction well. Thus, TaqMan PCR proved to be more time-saving than conventional PCR for examining a large number of samples. Lysis of isolates requires about 3.5 h, the TaqMan assay requires 2.5 h, the conventional PCR assay requires about 8 h, and the latex agglutination assay requires 2.5 days.
With TaqMan PCR, we found 44 of 93 strains positive for enterotoxins and 6 of 93 strains positive for the mecA gene. All strains were positive for the S. aureus-specific marker gene femB. More samples were found positive for enterotoxins by TaqMan PCR (n = 13) than by SET-RPLA. Furthermore, there were no false-negative results with the PCR. Our results also show that there is a large area of detection of the cycle at which a statistically significant increase in ΔRn is first noted for positive gene detection (Ct values of 22 to 34). However, there is no overlap between positive gene detection and no amplification (Ct values, 33.5 to 39.4), allowing clear discrimination between positive and negative signals. Since the TaqMan assay uses 45 amplification cycles, it is a very sensitive method. PCR experiments performed with NCTC 10652 revealed detection limits of approximately 250 CFU for the S. aureus-specific marker gene femB and approximately 1,000 CFU for the SEA gene. Therefore, we conclude that TaqMan PCR is more efficient for the detection of enterotoxins than the agglutination assay, possibly because SET-RPLA depends on various factors. The expression of a toxin requires a long incubation period (about 18 to 24 h) and is influenced by culture conditions. Furthermore, the production of a sufficient amount of toxin is essential for successful detection. Otherwise, this assay leads to false-negative results.
With TaqMan PCR, we found 12 strains positive for SEA (7 positive with SET-RPLA), 9 strains positive for SEB (8 positive with SET-RPLA), 20 strains positive for SEC1 (19 positive with SET-RPLA), and 13 strains positive for SED (7 positive with SET-RPLA). It is known that SEs are similar in structural and biological properties (9) but differ in amounts produced and in the mechanisms of gene regulation. SEB and SEC are expressed in greater quantities than the other enterotoxins. As shown by Bergdoll (3), SEA and SED are secreted in smaller amounts than SEB and SEC. We found that the levels of detection of SEB and SEC were genotypically and phenotypically almost identical. Furthermore, SEA is produced throughout the log phase of growth, while SEB, SEC, and SED are produced in greater quantities during the transition from the exponential to the stationary phase of growth (22). However, in accordance with the instructions of the manufacturer, SET-RPLA is performed with overnight liquid cultures, that is, cultures that are in the stationary phase. This factor might explain why more strains are found positive for SEA by genotype testing. The locations of the enterotoxin genes are also different. SEA is carried on a prophage (4), and SED is carried by a plasmid (21). The gene for SEC is localized on pathogenicity islands (22). The SEB gene (entB) is also located on a chromosome (13). It is also known that the transcription of SEB, SEC, and SED is subject to regulation by the accessory gene regulator (agr) two-component regulatory system, which controls most of the staphylococcal exoprotein virulence factors (12), and that the transcription of SEA is not regulated by the agr system (22). These data might also explain the sensitivity of TaqMan PCR, since SET-RPLA is based on the agr system.
Furthermore, with SET-RPLA it takes 2.5 days to get results, whereas with real-time PCR results are available within 6 h after isolation of the S. aureus strain. Lysis requires about 3.5 h, and the TaqMan assay requires about 2.5 h. The costs per strain are about $12 for SET-RPLA and about $8 for real-time PCR. Therefore, the PCR technique not only is faster but also is less expensive; in addition, TaqMan analysis is less labor-intensive.
In summary, we conclude that TaqMan PCR is a time-saving and efficient method, which also offers, unlike SET-RPLA, the possibility of detecting enterotoxins on a genotypic basis. Thus, this method is much more sensitive than SET-RPLA. Additionally, the assay allows the detection of SEA to SED and methicillin resistance of S. aureus at the same time. Furthermore, real-time PCR is well suited for screening a large number of samples at the same time, allowing rapid, reliable, efficient, and less costly routine laboratory diagnosis. Furthermore, in routine laboratory diagnosis, different assays can be performed with the ABI PRISM 7700 instrument in the same run; these could include the detection of enterohemorrhagic Escherichia coli (18), Chlamydia pneumoniae, Legionella pneumophila, or Mycoplasma pneumoniae (20). However, in smaller laboratories, where fewer tests would be performed, it would also be possible to use other real-time PCR platforms, such as LightCycler or SmartCycler systems.
Additionally, for further studies it would be conceivable to extend the detection of SE genes from the classical to the new and rarely described types.
Acknowledgments
We thank Sonja Kock and Alexander Dalpke for assistance in the preparation of the manuscript.
REFERENCES
- 1.Balaban, N., and A. Rasooly. 2000. Review. Staphylococcal enterotoxins. Int. J. Food. Microbiol. 61:1-10. [DOI] [PubMed] [Google Scholar]
- 2.Becker, K., R. Roth, and G. Peters. 1998. Rapid and specific detection of toxigenic Staphylococcus aureus: use of two multiplex PCR enzyme immunoassays for amplification and hybridization of staphylococcal enterotoxin genes, exfoliative toxin genes, and toxic shock syndrome toxin 1 gene. J. Clin. Microbiol. 36:2548-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergdoll, M. S. 1979. Staphylococcal intoxications, p. 443-493. In H. Riemann and F. L. Bryan (ed.), Foodborne infections and intoxications. Academic Press, Inc., New York, N.Y.
- 4.Betley, M. J., and J. J. Mekalanos. 1985. Staphylococcal enterotoxin A is encoded by phage. Science 229:185-187. [DOI] [PubMed] [Google Scholar]
- 5.Bohach, G. A., D. J. Fast, R. D. Nelson, and P. M. Schlievert. 1990. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit. Rev. Microbiol. 17:251-272. [DOI] [PubMed] [Google Scholar]
- 6.Borst, D. W., and M. J. Betley. 1994. Phage-associated differences in staphylococcal enterotoxin A gene (sea) expression correlate with sea allele class. Infect. Immun. 62:113-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gravet, A., M. Rondeau, C. Harf-Monteil, F. Grunenberger, H. Monteil, J. M. Scheftel, and G. Prevost. 1999. Predominant Staphylococcus aureus isolated from antibiotic-associated diarrhea is clinically relevant and produces enterotoxin A and the bicomponent toxin LukE-LukD. J. Clin. Microbiol. 37:4012-4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Letertre, C., S. Perelle, F. Dilasser, and P. Fach. 2003. Identification of a new putative enterotoxin SEU encoded by the egc cluster of Staphylococcus aureus. J. Appl. Microbiol. 95:38-43. [DOI] [PubMed] [Google Scholar]
- 9.Marrack, P., and J. Kappler. 1990. The staphylococcal enterotoxins and their relatives. Science 248:705-711. [DOI] [PubMed] [Google Scholar]
- 10.McLauchlin, J., G. L. Narayanan, V. Mithani, and G. O'Neill. 2000. The detection of enterotoxins and toxic shock syndrome toxin genes in Staphylococcus aureus by polymerase chain reaction. J. Food Prot. 63:479-488. [DOI] [PubMed] [Google Scholar]
- 11.Miethke, T., C. Wahl, K. Heeg, B. Echtenacher, P. H. Krammer, and H. Wagner. 1992. T cell-mediated lethal shock triggered in mice by the superantigen staphylococcal enterotoxin B: critical role of tumor necrosis factor. J. Exp. Med. 175:91-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Novick, R. P., H. F. Ross, S. J. Projan, J. Kornblum, B. Kreiswirth, and S. L. Moghazeh. 1993. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 12:3967-3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Novick, R. P., P. Schlievert, and A. Ruzin. 2001. Pathogenicity and resistance islands of staphylococci. Microbes Infect. 3:585-594. [DOI] [PubMed] [Google Scholar]
- 14.Omoe, K., M. Ishikawa, Y. Shimoda, D. L. Hu, S. Ueda, and K. Shinagawa. 2002. Detection of seg, seh, and sei genes in Staphylococcus aureus isolates and determination of the enterotoxin productivities of S. aureus isolates harboring seg, she, or sei genes. J. Clin. Microbiol. 40:857-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosec, J. P., and O. Gigaud. 2002. Staphylococcal enterotoxin genes of classical and new types detected by PCR in France. Int. J. Food Microbiol. 77:61-70. [DOI] [PubMed] [Google Scholar]
- 16.Schmitz, F. J., M. Steiert, B. Hofmann, J. Verhoef, U. Hadding, H. P. Heinz, and K. Kohrer. 1998. Development of a multiplex-PCR for direct detection of the genes for enterotoxin B and C, and toxic shock syndrome toxin-1 in Staphylococcus aureus isolates. J. Med. Microbiol. 47:335-340. [DOI] [PubMed] [Google Scholar]
- 17.Sharma, N. K., C. E. Rees, and C. E. Dodd. 2000. Development of a single-reaction multiplex PCR toxin typing assay for Staphylococcus aureus strains. Appl. Environ. Microbiol. 66:1347-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma, V. K., and E. A. Dean-Nystrom. 2003. Detection of enterohemorrhagic Escherichia coli O157:H7 by using a multiplex real-time PCR assay for genes encodin intimin and Shiga toxins. Vet. Microbiol. 93:247-260. [DOI] [PubMed] [Google Scholar]
- 19.Su, Y. C., and A. C. Lee Wong. 1995. Identification and purification of a new staphylococcal enterotoxin, H. Appl. Environ. Microbiol. 61:1438-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welti, M., K. Jaton, M. Altwegg, R. Sahli, A. Wenger, and J. Bille. 2003. Development of a multiplex real-time quantitative PCR assay to detect Chlamydia pneumoniae, Legionella pneumophila and Mycoplasma pneumoniae in respiratory tract secretions. Diagn Microbiol. Infect. Dis. 45:85-95. [DOI] [PubMed] [Google Scholar]
- 21.Zhang, S., J. J. Iandolo, and G. C. Stewart. 1998. The enterotoxin D plasmid of Staphylococcus aureus encodes a second enterotoxin determinant (sej). FEMS Microbiol. Lett. 168:227-233. [DOI] [PubMed] [Google Scholar]
- 22.Zhang, S., and G. C. Stewart. 2000. Characterization of the promoter elements for the staphylococcal enterotoxin D gene. J. Bacteriol. 182:2321-2325. [DOI] [PMC free article] [PubMed] [Google Scholar]