Abstract
Bone remodeling depends on the spatial and temporal coupling of bone formation by osteoblasts and bone resorption by osteoclasts; however, the molecular basis of these inductive interactions is unknown. We have previously shown that osteoblastic overexpression of TGF-β2 in transgenic mice deregulates bone remodeling and leads to an age-dependent loss of bone mass that resembles high-turnover osteoporosis in humans. This phenotype implicates TGF-β2 as a physiological regulator of bone remodeling and raises the question of how this single secreted factor regulates the functions of osteoblasts and osteoclasts and coordinates their opposing activities in vivo. To gain insight into the physiological role of TGF-β in bone remodeling, we have now characterized the responses of osteoblasts to TGF-β in these transgenic mice. We took advantage of the ability of alendronate to specifically inhibit bone resorption, the lack of osteoclast activity in c-fos−/− mice, and a new transgenic mouse line that expresses a dominant-negative form of the type II TGF-β receptor in osteoblasts. Our results show that TGF-β directly increases the steady-state rate of osteoblastic differentiation from osteoprogenitor cell to terminally differentiated osteocyte and thereby increases the final density of osteocytes embedded within bone matrix. Mice overexpressing TGF-β2 also have increased rates of bone matrix formation; however, this activity does not result from a direct effect of TGF-β on osteoblasts, but is more likely a homeostatic response to the increase in bone resorption caused by TGF-β. Lastly, we find that osteoclastic activity contributes to the TGF-β–induced increase in osteoblast differentiation at sites of bone resorption. These results suggest that TGF-β is a physiological regulator of osteoblast differentiation and acts as a central component of the coupling of bone formation to resorption during bone remodeling.
INTRODUCTION
During development and adult life, bone undergoes continuous remodeling through the coordinated processes of bone formation and bone resorption. Bone is formed by osteoblasts, which are of mesenchymal origin, and is resorbed by osteoclasts, which are derived from the hematopoietic system. In the adult skeleton, constant bone mass is maintained through the close microanatomical coupling of osteoblastic and osteoclastic activities (for review, see Parfitt, 1994). Deregulation of this coupling underlies the pathological loss of bone mass seen in osteoporosis and other metabolic bone diseases. Since new bone formation requires the continuous generation of new osteoblasts, osteoclastic resorption is not only coupled to the activity of osteoblasts, but also to the differentiation of osteoblasts from osteoprogenitor cells. In spite of their importance for our understanding of normal bone metabolism and the pathogenesis of metabolic bone diseases, the molecular mechanisms that govern the coordination of these processes are largely unknown.
One secreted factor that modulates the differentiation of osteoblasts and the proliferation of osteoprogenitor cells is transforming growth factor-β (TGF-β) (for reviews see Bonewald and Dallas, 1994; Centrella, et al., 1994). Both osteoblasts and osteoclasts secrete TGF-β, and all TGF-β isoforms (TGF-β1, -β2, and -β3) are present in their latent form within bone matrix (Seyedin et al., 1985; Robey et al., 1987; Sandberg et al., 1988; Pelton et al., 1991). Since bone explants release TGF-β during bone resorption (Pfeilschifter and Mundy, 1987) and osteoclasts have the ability to activate latent TGF-β (Oreffo, et al., 1989; Oursler, 1994), it has been suggested that TGF-β plays a role in the coupling of bone formation to bone resorption. Thus, TGF-β deposited in bone matrix by osteoblasts may be released and activated at sites of resorption by osteoclasts, which in turn leads to the induction of nearby osteoblastic differentiation. However, this model requires experimental verification in vivo, which so far has been difficult. For example, it is not clear whether TGF-β directly induces osteoblastic differentiation in the adult skeleton, and there have been no good in vivo experimental systems to detect the release of TGF-β from bone matrix during bone resorption or to assess the physiological relevance of bone matrix-derived TGF-β for osteoblastic differentiation.
We have previously generated transgenic mice that overexpress TGF-β2 from the osteocalcin promoter, which is osteoblast-specific (Erlebacher and Derynck, 1996). Transgenic mice showed a dramatic, age-dependent loss of bone mass similar to that seen in osteoporosis and hyperparathyroidism, yet showed relatively few defects in skeletal development or growth. In the transgenic line with the highest level of TGF-β2 expression, i.e., the D4 line, the phenotype was associated with three major histological alterations consistent with increased rates of bone remodeling: an increase in the density of bone matrix-embedded osteocytes, an increase in the rate of bone formation by osteoblasts, and an increase in the rate of bone resorption by osteoclasts.
These transgenic mice provided us with a unique model with which to characterize the regulation of osteoblast and osteoclast function by TGF-β during bone remodeling. We focused on the two endpoint osteoblastic responses, i.e., the increase in osteocyte density and the increase in bone formation. Through a combination of anatomical, genetic, and pharmacological approaches, we found that the increase in bone formation, contrary to expectation, was a secondary consequence of increased bone resorption. In contrast, the increase in osteocyte density resulted from a direct stimulation of osteoblastic differentiation by TGF-β2. This effect was greatly enhanced by osteoclastic activity, suggesting that TGF-β activity is functionally increased at sites of bone resorption in vivo. Our results suggest that TGF-β is a physiological regulator of osteoblast differentiation and a key mediator of the coupling of osteoblast differentiation to osteoclastic bone resorption required for skeletal homeostasis.
MATERIALS AND METHODS
Transgenic Mice
The generation of D4 mice that overexpress TGF-β2 from the osteocalcin promoter has been described (Erlebacher and Derynck, 1996). The generation and characterization of E1 mice, which express a cytoplasmically truncated type II TGF-β receptor from the osteocalcin promoter, will be described elsewhere (Filvaroff et al., unpublished data). Both lines were generated and maintained on a (DBA/2 × C57BL/6J) F1 background (Jackson Laboratories, Bar Harbor, ME). D4 mice were identified by the distinct appearance of their calvariae (Erlebacher and Derynck, unpublished observations) or by PCR of tail DNA using the primers 5′-GTGCTGGTTGTTGTGCTGCTC-3′ within the β-globin sequences of the transgene, and 5′-CCTTGGCGTAGTACTCTTCGTC-3′ within the TGF-β2 cDNA (Erlebacher and Derynck, 1996). E1 mice were genotyped by PCR of tail DNA as described (Filvaroff et al., unpublished data). D4/E1 mice were generated by crossing D4 hemizygotes with E1 hemizygotes or D4/E1 hemizygotes, and offspring were genotyped by PCR of tail DNA. TGF-β2 expression levels were measured in bone powder extracts prepared from mice at day 35 as described (Erlebacher and Derynck, 1996) using an ELISA that is TGF-β2 specific and does not recognize TGF-β1 or -β3 (RD Systems, Minneapolis, MN). Mice homozygous for the D4 transgene were embryonic lethal (Erlebacher and Derynck, 1996) and were therefore not present in these analyses.
Mice heterozygous for an inactivated allele of c-fos (Johnson et al., 1992) were generously provided by Randall Johnson and were on a (129/SvJ × C57BL/6J) F1 background. The inactivated c-fos allele was tracked by PCR of tail DNA as described (Johnson et al., 1992). To generate c-fos−/− and c-fos−/−/D4 mice, c-fos+/− mice were crossed with D4 mice, and the resultant F1 c-fos+/−/D4 mice and c-fos+/− mice were then intercrossed. All day 16 measurements of osteocyte density, mineral apposition rate, serum calcium and phosphorus levels, and growth rate were performed on the litter mates of this cross. Mice homozygous for the targeted c-fos allele were identified by their failure to undergo tooth eruption (Johnson et al., 1992; Wang et al., 1992), and the D4 transgene was detected by PCR of tail DNA. c-fos−/− mice and control litter mates maintained past weaning were fed a liquid diet of dissolved powdered milk and rice cereal. Serum calcium and phosphorus levels were determined from retro-orbital bleeds using colorimetric assays (Sigma Chemical, St. Louis, MO).
Scanning Electron Microscopy
Femurs from 16- and 35-d mice were deorganified in 5.25% sodium hypochlorite (Chlorox), coated with gold, and viewed at 10 kV with a Jeol JSM-840A scanning electron microscope (Jeol, Tokyo, Japan). At least three D4 and three wild-type mice were analyzed at each time point.
Osteocyte Density Measurements
Osteocyte density was determined using hematoxylin and eosin-stained 5-μm sections of bones that were fixed in 2% paraformaldehyde/PBS, decalcified, and embedded in paraffin as described previously (Erlebacher and Derynck, 1996). Osteocyte numbers in cortical bone for each mouse were determined in the dorsal, ventral and medial aspects of the femoral diaphysis from two cross-sections spaced 200 μm apart at the level of the third trochanter. Osteocyte numbers in epiphyseal cancellous bone were measured in two longitudinal sections separated by 200 μm. Sectioned bone surfaces were scanned into a computer using Adobe Photoshop (Mountain View, CA), and their surface areas were measured using NIH Image (Wayne Rasband, National Institutes of Health). Osteocyte densities (osteocytes per mm2) for each mouse were converted into three-dimensional densities as described (Sissons and O’Connor, 1977), assuming a 15-μm diameter for an osteocyte.
Mineral Apposition Rate Measurements
The mineral apposition rate was determined from 4.5-μm sections of undecalcified bone fixed in 70% ethanol, stained en bloc in Villanueva bone stain (osteochrome stain, Polysciences, Niles, IL), and embedded in methylmethacrylate. For analyses at day 16, mice were injected with 10 mg/kg calcein (Sigma) on day 12 (or on day 10 for some c-fos−/− and c-fos−/−/D4 mice), and on day 15 with 25 mg/kg tetracycline (Sigma). For day 35 analyses, mice were injected on day 30 with calcein and at day 34 with tetracycline.
The mineral apposition rate was measured from photomicrographs of sections of bone viewed under UV light. The periosteal mineral apposition rate of each mouse was measured along the dorsal and medial aspects of the femur from at least two cross-sections spaced 200 μm apart at the level of the third trochanter. The epiphyseal mineral apposition rate in the femur of each mouse was determined in at least two longitudinal sections spaced 200 μm apart. Individual measurements (50–100 per mouse) were taken along double-labeled surfaces at a spacing of about 40 μm. The mineral apposition rate for each mouse was calculated as the average of the distances between the fluorochrome labels divided by the time between their injection. The SEM of these measurements per mouse was always lower than 10% their average value. Mineralization lag time was calculated as the average of individual measurements of the osteoid seam width divided by the mineral apposition rate measured at the same location.
Alendronate Treatment and Parathyroidectomy
Alendronate was generously provided by Gideon Rodan (Merck Research Laboratories, West Point, PA) or prepared as the soluble component of Fosamax (Alendronate Sodium Tablets, Merck & Co., West Point, PA), dissolved in PBS. Both sources gave identical results. Mice were intraperitoneally injected with 0.3 mg/kg alendronate or PBS every other day from days 15 to 35. Alendronate treatment during this period of rapid growth caused a mild osteopetrosis, slightly reduced serum phosphorus levels, but no effect on serum calcium levels, and thus may have resulted in a mild hyperparathyroidism due to an increased demand by the growing bones for calcium.
Parathyroidectomy or sham operations were performed on day 21, after weaning. Mice were anesthetized with intramuscular injections of 100 mg/kg ketamine (Sigma), 5 mg/kg xylazine (Sigma), and 1.25 mg/kg acetopromazine (Sigma), and the parathyroid glands were removed by blunt dissection as described previously (Meyer et al., 1989). Incision sites were sutured shut and sealed with collodion (Mallinckrodt Baker, Paris, KY). Sham operation mimicked the entire operation without the actual removal of the parathyroid glands. Untreated mice were fed a normal diet containing 0.7–0.8% calcium and 0.6% phosphorus; sham-operated and parathyroidectomized mice were fed a high-calcium diet containing 1.46% calcium and 0.99% phosphorus (Purina Test Diets, Purina Mills, Richmond, IN) after surgery to minimize the risk of hypocalcemia. Mice were intraperitoneally injected on day 30 with calcein and on day 34 with tetracycline for analysis of the mineral apposition rate. Mice were fasted overnight on day 34 and retro-orbital bleeds were taken immediately before mice were killed on day 35. Bones were dissected and fixed and stored in 70% ethanol at 4°C. For analysis of the osteocyte density, bones were refixed in 2% paraformaldehyde/PBS before further processing (see above).
Day 35 serum calcium and phosphorus levels, determined by colorimetric assay (Sigma), were used to score for successful parathyroidectomies. The prior overnight fast was included to minimize the dietary absorption of calcium. Sham-operated, fasted mice had a serum calcium of 9.5 ± 0.4 mg/dl (n = 21) and a serum phosphorus of 7.6 ± 1.0 mg/dl (n = 21); we defined a successful parathyroidectomy as one resulting in a serum calcium level 2 SDs below the mean, and a serum phosphorus level 1 SD above the mean. Thus, only mice with a serum calcium less than 8.7 mg/dl and a serum phosphorus more than 8.6 mg/dl were included for further analysis. These mice formed a clearly defined group relative to sham-operated mice and mice in which the surgery was unsuccessful.
Analysis of Osteoblast Differentiation with Bromodeoxyuridine (BrdU)
Mice were given two intraperitoneal injections of BrdU (Boehringer Mannheim, Indianapolis, IN) spaced 8 h apart on day 12 or day 16. Mice injected on day 12 were killed 96 h after the second injection, and mice injected on day 16 were killed 4 h after the second injection. Bones were fixed overnight at 4°C in 4% paraformaldehyde/PBS, decalcified for 5 d in 10% EDTA, 0.1 M Tris, pH 7.0, at 4°C, and embedded in paraffin. Cross-sections (5 μm) were taken at the level of the third trochanter and stained for labeled nuclei using the BrdU staining kit (Zymed Laboratories, South San Francisco, CA). Periosteal osteoblasts were identified as cuboidal cells abutting the bone surface. For each mouse, labeled periosteal osteoblast and subperiosteal osteocyte nuclei were counted from six sections spaced at least 50 μm apart, covering the dorsal, ventral, and medial aspects of the diaphysis. Three hundred to 800 osteoblasts were scored per mouse.
Effects of Alendronate on Plasma TGF-β Levels
Before the plasma level of TGF-β2 was measured at day 35, mice were injected intraperitoneally with 0.3 mg/kg alendronate or PBS vehicle every other day for a total of three injections before retro-orbital blood collection at day 35. Heparinized tubes were used to collect plasma, and samples were acid treated as described (Erlebacher and Derynck, 1996) before TGF-β2 measurement by a TGF-β2-specific ELISA (RD Systems). To measure the plasma level of TGF-β2 at 3 mo, mice were injected for 3 consecutive days with 3 mg/kg alendronate or PBS vehicle before the collection of plasma and TGF-β2 ELISA.
Statistical Analysis and Derivation of the Osteocyte Formation Rate
The statistical significance of all comparisons of wild-type, D4, E1, and D4/E1 mice, or measurements of individual and combined effects of alendronate and parathyroidectomy, was determined by analysis of variance followed by the Bonferroni t test for multiple comparisons. p < 0.0083 was used as the criterion for significance for each comparison, giving a final significance level of p < 0.05 for the six comparisons per set of four experimental groups. All other comparisons were pairwise using Student’s t test at a significance level of p < 0.05. The osteocyte formation rate was calculated as the mathematical product of the mean epiphyseal osteocyte density with the mean epiphyseal mineral apposition rate, with errors propagated as described (Taylor, 1997).
RESULTS
Increased Osteocyte Density in Mice with Osteoblastic Overexpression of TGF-β2 Does Not Require Osteoclastic Bone Resorption
Because of the close functional relationships between osteoblastic differentiation, bone formation, and osteoclastic bone resorption, we first assessed whether the increased osteocyte density in D4 mice was a direct effect of overexpressed TGF-β2 on osteoblasts or whether it depended on bone resorption by osteoclasts. This evaluation was pursued using anatomical and genetic approaches.
As an anatomical approach, we assessed the osteocyte density of bone at a location that is naturally devoid of osteoclastic activity, i.e., under the periosteum of the diaphysis of a long bone. More specifically, we measured the osteocyte density of subperiosteal cortical bone in the diaphysis of the femur of 16-d-old mice in the region opposite to the third trochanter (Figure 1C). The bone surface at this location undergoes intramembranous ossification in the absence of osteoclastic bone resorption, which is easily verified by scanning electron microscopy (Boyde, 1972). Thus, the diaphyseal surfaces of both wild-type and D4 mice clearly lacked characteristic resorption pits (lacunae), in contrast to the continuous resorptive surface of the distal metaphysis (Figure 1, A, B, and D).
Figure 1.
Scanning electron microscopy of bone surfaces. Periosteal surfaces of wild-type (A) and D4 transgenic (B) femurs corresponding to the area of the diaphysis bracketed in panel C. The third trochanter is located at the top of both micrographs. Osteoclastic resorption lacunae are absent from the diaphyseal surfaces of both bones and are only visible distal to the third trochanter in the transition to the distal metaphysis (asterisk in panel A). The transition zone between diaphysis and metaphysis in the D4 bone (boxed in panel B) is shown at higher magnification in panel D. The resorptive surface of the metaphysis (r) is clearly distinct from the diaphysis, which has the characteristic appearance of forming and mineralizing bone (f). These micrographs also revealed a dramatic increase in the size of the vasculature in D4 bone compared with wild-type. One vascular pore is indicated with an arrow in panel D. The mineralizing surface in D4 bone appears somewhat disorganized compared with the wild-type surface, consistent with the irregular cross-sectional appearance of fluorochrome labels in D4 bone (Erlebacher and Derynck, 1996). Osteocyte lacunae are indicated with arrowheads in panel D. Scale bars, 200 μm in A and B; 100 μm in D.
Scanning electron micrographs also showed that the diaphyseal osteocyte density, as assessed by the density of surface osteocyte lacunae, was clearly higher in D4 bones than in normal bones at 16 d of age (Figure 1, A and B). This increase was also histologically apparent in cross-sections of diaphyseal bone (Figure 2, A and B). In these sections, we quantitated the osteocyte density in the subperiosteal 25 μm of cortical bone (bracketed in Figure 2A). From our analysis of the mineral apposition rate (see below), we know that the outer 25 μm of cortical bone corresponds to ∼5.8 d of radial growth up to day 16. As shown in Figure 2E, the subperiosteal osteocyte density in D4 mice was about 1.6-fold higher than in wild-type controls. A similar increase in osteocyte density was also observed at day 35 (see below).
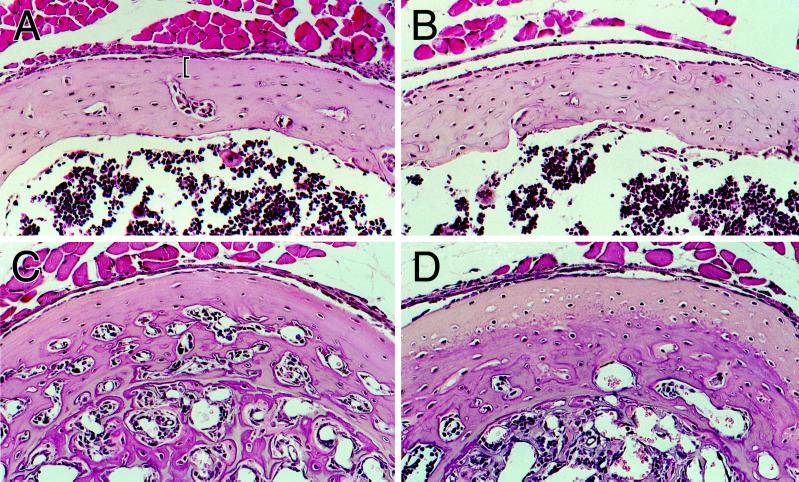
Figure 2.
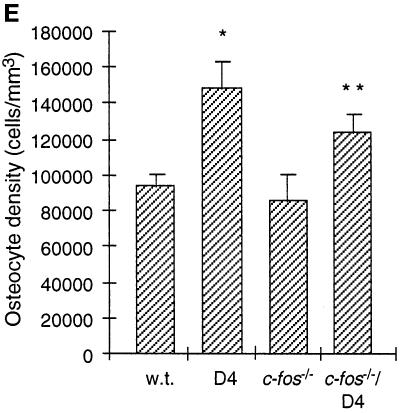
Osteocyte density in the absence of bone resorption. (A and B) Hematoxylin and eosin-stained sections of cortical bone from the femoral diaphyses of wild-type (A) and D4 transgenic (B) mice at day 16. The sections are at the level of the third trochanter, where bone resorption is absent on periosteal surfaces. The brackets in panel A demarcate the 25 μm of subperiosteal cortex in which we quantitated the osteocyte density in wild-type and D4 mice. Osteoblasts are visible as the monolayer of cuboidal cells directly abutting the periosteal surface; osteoprogenitor cells are located more peripherally. (C and D) Similar sections of cortical bone from femurs of c-fos−/− (C) and c-fos−/−/D4 mice at day 16 (D). The dense, slightly ossified calcified cartilage that fills the bone marrow spaces of c-fos−/− mice can be seen toward the bottom of both micrographs. (E) Osteocyte densities in the subperiosteal 25 μm of cortical bone of femurs from 16-d-old wild-type (n = 5) and D4 (n = 4) mice, and in the entire cortex of femurs from c-fos−/− (n = 3) and c-fos−/−/D4 (n = 3) mice. Values represent the mean ± SD. ∗, Significantly different from wild-type (p < 0.0005); ∗∗, Significantly different from c-fos−/− (p = 0.02).
To genetically evaluate the role of osteoclastic bone resorption in the increase of osteocyte density caused by TGF-β2 overexpression, we crossed D4 mice with c-fos−/− mice, which have an osteopetrotic phenotype due to a complete block in osteoclastic differentiation (Grigoriadis et al., 1994). Despite the absence of osteoclasts in c-fos−/−/D4 mice, their osteocyte density in cortical bone was still increased when compared with c-fos−/− and wild-type controls (Figure 2, C–E). These results are consistent with our anatomical and histological analyses. Taken together, our findings strongly suggest that the increase in osteocyte density in D4 transgenic mice does not require osteoclastic bone resorption.
Increased Osteocyte Density in D4 Bones Requires Osteoblastic Responsiveness to TGF-β
To conversely test whether the increase in osteocyte density depended on osteoblastic responsiveness to TGF-β, we used a newly generated line of transgenic mice that overexpress a cytoplasmically truncated version of the type II TGF-β receptor from the osteocalcin promoter (the E1 line, Filvaroff et al., unpublished data). Overexpression of this truncated receptor in cell culture has been shown to interfere with endogenous TGF-β signaling in a dominant negative manner (Chen et al., 1993), and since the osteocalcin promoter is osteoblast-specific (Baker et al., 1992), we expected this transgenic line to have impaired TGF-β signaling in osteoblasts.
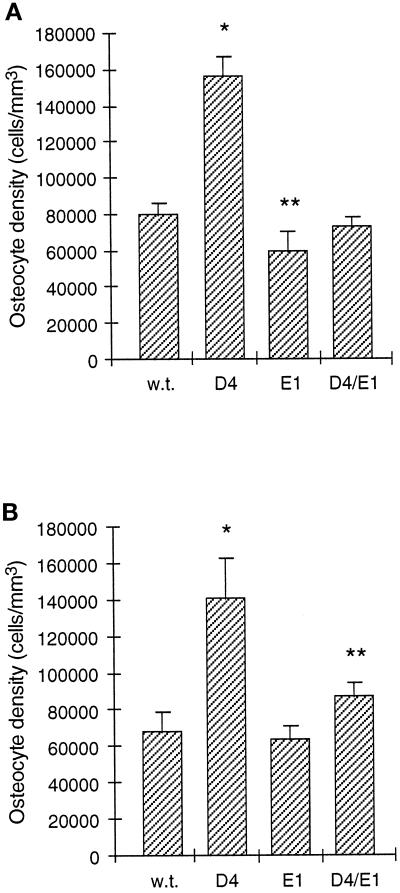
We crossed our D4 mice with the E1 transgenic mice to generate double transgenic D4/E1 mice that overexpress both the TGF-β2 and the truncated type II TGF-β receptor transgenes. Expression of the truncated receptor transgene in D4/E1 mice did not inhibit expression of the TGF-β2 transgene, because the high level of TGF-β2 in the bone matrix of D4 mice was not reduced in D4/E1 mice (data not shown). However, expression of the truncated receptor in D4/E1 mice dramatically reduced the high osteocyte density seen in D4 mice to almost wild-type levels. This effect was seen in both cortical bone where resorption is absent (Figure 3A), as well as in the femoral epiphyses where resorption is present (Figure 3B). Furthermore, the osteocyte density of E1 mice was mildly (25%) reduced below the wild-type level in the femoral epiphysis, yet was not significantly different from wild-type mice in cortical bone (Figure 3, A and B). In contrast to the decrease in osteocyte density in D4/E1 mice, expression of the truncated receptor did not significantly affect the increased bone formation rate (see below) or the overall loss of cancellous bone mass caused by TGF-β2 overexpression. Furthermore, the hypoplastic clavicles and patent anterior fontanels noted in D4 mice (Erlebacher and Derynck, 1996; our unpublished observations) were also still present in D4/E1 double transgenic mice. Thus, these results strongly suggest that osteoblastic responsiveness to TGF-β is required for both the increase in osteocyte density caused by osteoblastic overexpression of TGF-β, as well as for the generation of wild-type osteocyte density at some anatomical sites.
Figure 3.
Effect of osteoblastic expression of a dominant-negative type II TGF-β receptor on osteocyte density in wild-type and D4 mice at day 35. Transgenic E1 mice overexpressing a truncated TGF-β receptor in osteoblasts were crossed to D4 mice overexpressing osteoblastic TGF-β2 to generate double transgenic D4/E1 mice. Values represent the mean ± SD. (A) Osteocyte density in the distal epiphysis of the femur, where bone resorption is present. The osteocyte density in D4 mice (n = 5; ∗) was significantly elevated compared with wild-type (n = 6; p < 0.0001), and the osteocyte density in E1 mice (n = 5; ∗∗) was significantly reduced compared with wild-type (p < 0.005). The osteocyte density in D4/E1 mice (n = 6) was not significantly different from wild-type. (B) Osteocyte density in cortical bone from the femoral diaphysis at the level of the third trochanter, where periosteal bone resorption is absent. The osteocyte density in D4 mice (n = 5; ∗) was significantly elevated compared with wild-type (n = 6; p < 0.0001), whereas the osteocyte density in E1 mice (n = 5) was not significantly reduced compared with wild-type. The osteocyte density in D4/E1 mice (n = 6; ∗∗) was significantly elevated compared with wild-type (p < 0.005) and significantly reduced compared with D4 (p < 0.0005).
Alendronate Reduces Epiphyseal Osteocyte Density and Plasma TGF-β2 Levels
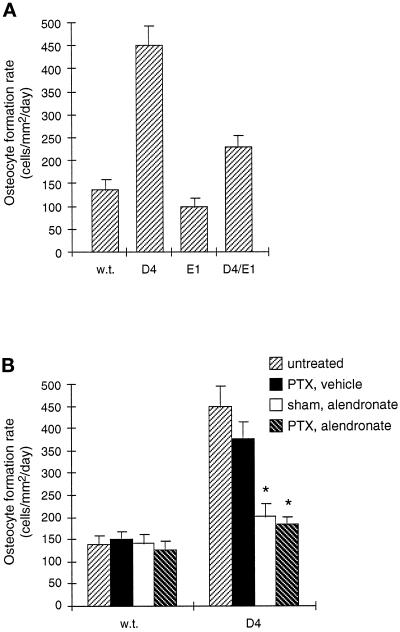
From the experiments described above, we conclude that the increase in osteocyte density caused by TGF-β2 overexpression was a direct effect of TGF-β on osteoblasts that did not require osteoclastic bone resorption. However, these analyses do not rule out the possibility that osteoclastic bone resorption might contribute to the increased osteocyte density at sites where active resorption occurs. We therefore treated mice with alendronate, a bisphosphonate that potently inhibits osteoclastic bone resorption in vivo (for a review see Rodan and Fleisch, 1996), for 3 wk before animals were killed at day 35. We then measured the osteocyte density in the femoral epiphysis, a site of ongoing resorption where osteocyte density was 1.9-fold higher in D4 mice than in wild-type mice. Since this treatment produced a mild osteopetrosis and changes in calcium and phosphorus serum levels consistent with secondary hyperparathyroidism (data not shown), we included parallel experimental groups of mice that had undergone parathyroidectomy at day 21. As shown in Figure 4A, alendronate treatment resulted in a moderate decrease in epiphyseal osteocyte density in D4 mice, but not in wild-type mice. Parathyroidectomy did not significantly affect osteocyte density. In contrast to its effects in the femoral epiphysis, and consistent with our results on the diaphyseal osteocyte density, alendronate did not affect the subperiosteal osteocyte density (Figure 4B). Thus, alendronate decreased the osteocyte density in D4 mice only at sites of osteoclastic bone resorption.
Figure 4.
Effect of alendronate and parathyroidectomy on osteocyte density in wild-type and D4 mice at day 35. Mice were treated with alendronate or vehicle starting at day 15 and underwent parathyroidectomy or sham operation at day 21. Untreated mice were the same as those analyzed in Figure 3. Values represent the mean ± SD. (A) Osteocyte density in the distal epiphysis of the femur, where bone resorption is present. Osteocyte densities in D4 mice treated with alendronate and sham operation (n = 3; ∗), or alendronate and parathyroidectomy (n = 6; ∗) were significantly reduced compared with untreated D4 mice (n = 5; p < 0.005). This reduction is more striking after calculation of the osteocyte formation rate (Figure 10). The osteocyte density in D4 mice treated with vehicle and parathyroidectomy (n = 4) was not significantly different from untreated D4 mice. Osteocyte densities in wild-type mice treated with vehicle and parathyroidectomy (n = 2), alendronate and sham operation (n = 3), or alendronate and parathyroidectomy (n = 6) were not significantly different from untreated wild-type mice (n = 6). (B) Osteocyte density in the diaphyseal cortex of the femur at the level of the third trochanter, where periosteal bone resorption is absent. Osteocyte densities were measured in only the subperiosteal 36 μm of cortical bone, which corresponds to periosteal bone made since day 21. The number of mice analyzed was as in panel A. Osteocyte densities in wild-type and D4 mice treated with alendronate and parathyroidectomy did not significantly differ from respective untreated wild-type and D4 controls.
These results suggested that bone resorption somehow locally enhanced the increase in osteocyte density caused by TGF-β2 overexpression. One possible mechanism, as previously suggested (Pfeilschifter and Mundy, 1987), is that osteoclastic bone resorption causes the release and activation of bone matrix-bound TGF-β and thereby increases its local concentration on nearby bone surfaces. Since the level of TGF-β2 in bone matrix of D4 mice is considerably higher than the TGF-β2 level in wild-type bone (Erlebacher and Derynck, 1996), its release from the matrix might lead to dramatic increases in the local TGF-β concentration at bone surfaces. To assess this possibility, we measured the effect of alendronate on the plasma level of TGF-β2 in D4 mice. This level was not significantly reduced by alendronate treatment for 1 wk prior to day 35 and at the same dose as in our experiments above (data not shown). However, three daily injections of a higher dose of alendronate in 3-mo-old mice resulted in reduced plasma TGF-β2 levels (Figure 5). At this age, the direct contribution of the TGF-β2 secreted by osteoblasts to the plasma level of TGF-β2 is likely to be less than at day 35, when bone growth and modeling are much more active. These results suggest that osteoclastic bone resorption can lead to the release of TGF-β from bone matrix and contribute to the elevation of plasma TGF-β levels.
Figure 5.
The effect of alendronate on plasma TGF-β2 levels in wild-type and D4 mice. Three-month-old mice were injected daily for 3 d with 3 mg/kg alendronate or PBS vehicle before blood drawing. Total plasma TGF-β2 levels were measured using a TGF-β2–specific ELISA. Values represent the mean ± SD of wild-type mice treated with vehicle (n = 7), wild-type mice treated with alendronate (n = 6), D4 mice treated with vehicle (n = 6), and D4 mice treated with alendronate (n = 10). The high level of plasma TGF-β2 in PBS-treated D4 mice was significantly inhibited by alendronate treatment (∗, p < 0.0005).
Kinetics of Osteoblast Differentiation
To gain further insight into the cause of the osteocyte density increase in D4 mice, we analyzed the kinetics of osteoblast differentiation using in vivo BrdU incorporation. Twelve-day-old mice were injected twice with BrdU, which, due to its short half-life in vivo (Packard, et al., 1973), resulted in two short periods of mitotic cell labeling. We then determined at day 16 the labeling index of mature periosteal osteoblasts on the femoral diaphysis, where resorption is absent. Mature osteoblasts, which are generally postmitotic in vivo (Young, 1962), were identified as the monolayer of cuboidal cells abutting the bone surface (Young, 1962, Kimmel and Jee, 1980; see Figure 2, A and B). These cells are actively engaged in bone matrix synthesis, with the same rate in wild-type and D4 mice (see below). Fibroblastic osteoprogenitor cells, which are premitotic, were located more peripherally.
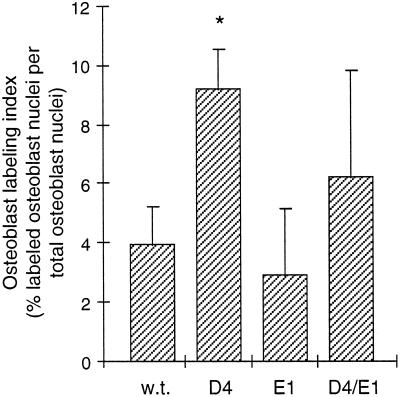
As shown in Figure 6, the percentage of BrdU-labeled periosteal osteoblasts after the 4-d chase period was about 2.4-fold increased in D4 mice compared with wild-type mice. Furthermore, the number of labeled subperiosteal osteocytes per section was increased eightfold from 0.5 (±0.6) in wild-type mice (n = 4) to 4.1 (±1.0) in D4 mice (n = 4), which is proportionally much greater than would have been expected from the 1.6-fold increase in subperiosteal osteocyte density. Labeled osteoblasts and osteocytes could conceivably have been derived from osteoblasts dividing on day 12 that had either remained on the bone surface or subsequently matured into osteocytes. Alternatively, they could have been derived from osteoprogenitor cells dividing on day 12 that had subsequently differentiated. To distinguish between these possibilities, mice were given two pulses of BrdU and killed the same day. With this schedule, D4 mice showed a low percentage of osteoblast labeling (1.6 ± 1.3 [n = 4]) that was not significantly different from wild-type mice (0.45 ± 0.03 [n = 2]), and sections showed no labeled osteocytes. This result is consistent with previous observations that mature osteoblasts rarely divide in vivo (Young, 1962). Furthermore, D4 and wild-type mice killed 2 d after injection also showed low levels of osteoblast labeling (data not shown), indicating that a longer time period was required to generate appreciable numbers of labeled mature osteoblasts. Thus, the majority of BrdU-labeled osteoblasts and osteocytes after 4 d represent cells that had newly differentiated from osteoprogenitor cells that were dividing on day 12. Subtracting the same-day labeling index from the 4-d labeling index, we estimate that the percentage of osteoblast labeling attributable to new cell differentiation over 4 d was increased about 2.2-fold, i.e., from 3.5 (±1.4) in wild-type bone to 7.6 (±1.9) in D4 bone.
Figure 6.
Kinetics of osteoblast differentiation in the femoral periosteum. Mice were injected with BrdU on day 12 and killed on day 16. The labeling index was calculated as the percentage of labeled periosteal osteoblast nuclei per total periosteal osteoblast nuclei counted in immunostained sections of the femoral diaphysis at the level of the third trochanter. Values represent the mean ± SD of four mice per group. Overexpression of TGF-β2 in D4 mice increased the osteoblast labeling index significantly over wild-type (∗, p < 0.002); the labeling index in E1 mice was not significantly different from wild-type.
In parallel to the experiments described above, we also analyzed the kinetics of osteoblast differentiation in our transgenic mice that overexpress the truncated type II TGF-β receptor in osteoblasts. After BrdU labeling at day 12 and analysis at day 16, the osteoblastic labeling index (Figure 6) and the number of labeled subperiosteal osteocytes per section (data not shown) were similar in E1 mice and wild-type mice. In D4/E1 double transgenic animals, these values were intermediate between wild-type and D4 mice but showed variability between individual animals. Significantly, both results are in agreement with the effect of truncated receptor overexpression on the subperiosteal osteocyte density in wild-type and D4 mice (Figure 3). Taken together, these kinetic studies are consistent with the notion that increased TGF-β2 expression in osteoblasts stimulates the rate of osteoblastic differentiation, and that this stimulation underlies the increased osteocyte density in D4 mice.
Increased Mineral Apposition Rate in D4 Transgenic Mice Does Not Require Osteoblastic TGF-β Receptor Function, Yet Is Inhibited by Alendronate
The rate of bone deposition is often measured by the mineral apposition rate. This rate is determined through sequential injection of two fluorochromes that incorporate into bone matrix at sites of ongoing mineralization. Injection of these fluorochromes with an interval of several days results in the histological visualization of two parallel lines at sites of bone formation. The mineral apposition rate is then measured as the distance between the fluorochrome labels divided by the time between their injection (Parfitt et al., 1987).
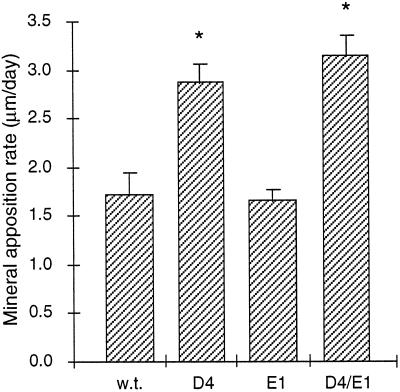
We have previously shown that the mineral apposition rate at endosteal surfaces in the tibia was increased ∼70% in D4 mice compared with wild-type mice (Erlebacher and Derynck, 1996). Consistent with this observation, the mineral apposition rate at endosteal surfaces in the femoral epiphysis at day 35 was increased 80% in D4 mice (Figure 7). To evaluate whether this increase required TGF-β signaling in osteoblasts, we again used D4/E1 double transgenic mice that overexpress a cytoplasmically truncated type II TGF-β receptor in osteoblasts. In contrast to the dramatic inhibitory effect of this truncated receptor on the osteocyte density increase, D4/E1 mice showed the same 80% increase in mineral apposition rate as D4 mice, and E1 mice showed no difference in their mineral apposition rate compared with wild-type (Figure 7). Thus, dominant negative inhibition of TGF-β receptor signaling in osteoblasts did not affect the mineral apposition rate, which suggests that its increase in D4 mice does not require osteoblastic responsiveness to TGF-β.
Figure 7.
The effect of osteoblastic expression of a dominant-negative type II TGF-β receptor on the mineral apposition rate in the femoral epiphyses of wild-type and D4 mice at day 35. Transgenic E1 mice were crossed to D4 mice to generate doubly transgenic D4/E1 mice. Values represent the mean ± SD of wild-type (n = 5), D4 (n = 4), E1 (n = 5), and D4/E1 (n = 4) mice. Mineral apposition rates in D4 and D4/E1 mice were significantly elevated compared with wild-type (∗, p < 0.0001), yet were not significantly different from one another. The mineral apposition rate in E1 mice was not significantly different from wild-type.
To assess whether the increased mineral apposition rate in D4 mice depended on osteoclastic bone resorption, we again tested the effect of alendronate administered over a 3-wk period before animals were killed on day 35. In parallel, we included experimental groups that underwent parathyroidectomy at day 21. Consistent with its inhibitory effect on bone resorption and, as a consequence, on overall bone formation (Rodan and Fleisch, 1996), alendronate decreased the percentage of fluorochrome-labeled bone surfaces from ∼60% in the femoral epiphyses of both wild-type and D4 mice to ∼30%. In addition, as shown in Figure 8, treatment with alendronate dramatically reduced the epiphyseal mineral apposition rate in D4 bones to a level similar to wild-type, yet did not affect the mineral apposition rate in wild-type mice, which is consistent with previous results (Rodan and Fleisch, 1996). The decrease in mineral apposition rate in D4 mice was not due to impaired bone mineralization, since alendronate did not increase the lag time between osteoid deposition and its mineralization in wild-type or D4 mice (data not shown). Parathyroidectomy did not affect the mineral apposition rate in D4 or wild-type mice with or without alendronate treatment. Taken together, our observations suggest that the increase in mineral apposition rate in D4 mice does not depend on the responsiveness of osteoblasts to TGF-β, yet requires osteoclastic bone resorption.
Figure 8.
The effect of alendronate and parathyroidectomy on the epiphyseal mineral apposition rate in wild-type and D4 mice at day 35. Mice were treated with alendronate or vehicle starting at day 15, and underwent parathyroidectomy or sham operation at day 21. The mineral apposition rate was determined in the distal epiphysis of the femur, a site of bone resorption. Untreated mice were the same as those analyzed in Figure 7. Values represent the mean ± SD. The mineral apposition rates in D4 mice treated with alendronate and sham operation (n = 3; ∗) or alendronate and parathyroidectomy (n = 5; ∗) were significantly reduced compared with untreated D4 mice (n = 4; p < 0.001) and not significantly different from untreated wild-type mice (n = 5). The mineral apposition rate in D4 mice treated with vehicle and parathyroidectomy (n = 5) was not significantly different from untreated D4 mice. Mineral apposition rates in wild-type mice treated with vehicle and parathyroidectomy (n = 4), alendronate and sham operation (n = 3), or alendronate and parathyroidectomy (n = 5) were not significantly different from untreated wild-type mice.
The Mineral Apposition Rate in D4 Mice Is Not Increased on Periosteal Surfaces Lacking Bone Resorption
The conclusion that the increased mineral apposition rate in D4 mice depends on osteoclastic activity predicts that bone surfaces that lack osteoclastic resorption would not have an increased mineral apposition rate. We therefore measured the mineral apposition rate on the periosteal surface of the femoral diaphysis, a site devoid of osteoclastic activity. As shown in Figure 9, this rate was the same in D4 as in wild-type at both days 16 and 35. The higher periosteal mineral apposition rate at day 16, compared with day 35, is consistent with the faster growth rate of younger mice. Fluorochromes injected at this time formed continuous concentric rings parallel to the periosteum, showing that radial growth was continuous (data not shown). Consistent with the absence of osteoclastic activity on the femoral diaphyseal periosteum, alendronate treatment and parathyroidectomy did not affect the mineral apposition rate in wild-type or D4 mice on periosteal surfaces (Figure 9A).
Figure 9.
Mineral apposition rates in the absence of bone resorption. Mineral apposition rates were determined on the periosteal surfaces of the femoral diaphysis at the level of the third trochanter, where bone resorption is absent. (A) Periosteal mineral apposition rates at day 35. The mineral apposition rate in untreated D4 mice (n = 4) was not significantly different from untreated wild-type mice (n = 4), and alendronate and parathyroidectomy treatment did not significantly affect periosteal mineral apposition rates in either wild-type (n = 3) or D4 mice (n = 3). (B) Periosteal mineral apposition rates at day 16. Like day 35, the mineral apposition rate in D4 mice (n = 7) was not significantly different from wild-type mice (n = 7). The mineral apposition rate in c-fos−/−/D4 mice (n = 8; ∗) was significantly elevated compared with c-fos−/− mice (n = 8; p < 0.005). The mineral apposition rate in c-fos−/− mice was significantly reduced compared with wild-type mice (p < 0.0001).
Surprisingly, increased TGF-β2 expression in c-fos−/−/D4 mice led to an increased periosteal mineral apposition rate compared with c-fos−/− controls (Figure 9B). c-fos−/−/D4 and c-fos−/− mice also showed lower serum calcium and phosphorus levels than wild-type mice (Table 1). However, the increase in periosteal mineral apposition rate in c-fos−/−/D4 mice over c-fos−/− mice could not be ascribed to a differential defect in bone mineralization, since the mineralization lag time, i.e., the lag time between osteoid deposition and its mineralization (Table 1), was not significantly different between the two types of mice, although it was dramatically increased over normal. Thus, increased osteoblastic expression of TGF-β2 resulted in increased periosteal bone deposition in a c-fos−/− background, in contrast to the unaltered mineral apposition rate in a wild-type background. We were unable to accurately assess the mineral apposition rate of endosteal surfaces in c-fos−/− and c-fos−/−/D4 mice because these surfaces lacked double fluorochrome labeling. The density of the slightly ossified calcified cartilage that fills the bone marrow cavities of c-fos−/− mice was unchanged in c-fos−/−/D4 mice (data not shown).
Table 1.
Growth parameters, serological values, and periosteal mineralization lag times in wild-type, D4, c-fos−/−, and c-fos−/−/D4 at day 16
| Mouse | Day 16 weight (g) | Day 12 to day 16 growth rate (g/day) | Serum calcium (mg/dl) | Serum phosphorus (mg/dl) | Mineralization lag time (days) |
|---|---|---|---|---|---|
| Wild-type | 8.3 ± 0.7 (17) | 0.31 ± 0.09 (17) | 10.6 ± 0.6 (12) | 11.5 ± 1.7 (12) | 1.3 ± 0.1 (7) |
| D4 | 7.7 ± 1.0 (23) | 0.29 ± 0.06 (23) | 10.7 ± 0.5 (12) | 10.6 ± 1.2 (12) | 1.5 ± 0.4 (7) |
| c-fos−/− | 7.3 ± 1.0 (13)a | 0.07 ± 0.05 (5)b | 8.8 ± 0.9 (5)b | 6.0 ± 0.9 (5)b | 7.8 ± 2.8 (8)b |
| c-fos−/−/D4 | 7.1 ± 1.5 (15)a | 0.13 ± 0.08 (7)b | 9.4 ± 0.6 (7)b | 6.6 ± 1.7 (7)b | 8.7 ± 3.5 (8)b |
Significantly different from wild-type; a p < 0.01; b p < 0.001. The number of mice analyzed is shown in parentheses.
Whereas TGF-β2 overexpression increased the periosteal mineral apposition rate in a c-fos−/− background, mineral apposition rates in c-fos−/− mice were generally reduced compared with wild-type mice (Figure 9B), consistent with their smaller size and dramatically reduced growth rates (Johnson et al., 1992; Table 1). TGF-β2 overexpression in osteoblasts itself did not affect the weights and growth rates of wild-type or c-fos−/− mice (Table 1). Lastly, c-fos−/−/D4 mice evaluated at day 35 had a dramatically increased incidence of long bone fractures. As previously observed, D4 mice occasionally showed spontaneous fractures (Erlebacher and Derynck, 1996); however, five of nine c-fos−/−/D4 mice showed tibial fractures (with bilateral fractures observed in four cases), whereas none of the six c-fos−/− mice examined showed hind limb fractures.
DISCUSSION
Various studies have shown that TGF-β affects the activity and differentiation of osteoblasts and osteoclasts (Bonewald and Dallas, 1994; Centrella et al., 1994), but the complexity of these data does not allow us to infer the role of skeletal TGF-β in bone development and remodeling. In the present study, we used our transgenic mice that overexpress TGF-β2 in osteoblasts to characterize the responses of osteoblasts to TGF-β during bone remodeling. We focused on the two endpoint osteoblastic responses to increased TGF-β2 expression, i.e., the increases in osteocyte density and bone formation.
Increased Osteocyte Density Results from a Direct Effect of TGF-β on Osteoblasts
We have previously shown that increased TGF-β2 expression in osteoblasts results in increased osteocyte density (Erlebacher and Derynck, 1996). Our data now strongly suggest that this increase does not depend on osteoclastic activity. First, TGF-β overexpression increases the osteocyte density in the subperiosteal cortical bone of the diaphysis, a site devoid of osteoclasts. Second, the increase in osteocyte density persists in a c-fos−/− background, which lacks osteoclasts. Furthermore, the normal and increased osteocyte densities both require osteoblastic responsiveness to TGF-β, since dominant negative inhibition of TGF-β receptor function decreases the osteocyte density in both wild-type and D4 mice, respectively. The TGF-β–induced positive regulation of osteocyte density therefore results most likely from a direct, autocrine effect on osteoblasts and occurs even at endogenous levels of TGF-β expression.
Although this parameter can be conveniently assessed, the increased osteocyte density in response to TGF-β does not accurately reflect the rate of differentiation from osteoblast to osteocyte. For example, increased osteocyte density could result from decreased bone formation without a change in osteocyte differentiation. Therefore, the rate of osteocyte formation should take into account the final osteocyte density and the rate of bone deposition, i.e., by multiplying the osteocyte density (cells/mm3) with the mineral apposition rate (mm3/mm2/day). This index in cells/mm2/day may therefore be a better measure of the effects of TGF-β on osteocyte differentiation.
Using this index, the 1.6-fold increase in subperiosteal osteocyte density in D4 mice compared with wild-type translates into a 1.6-fold increase in osteocyte formation rate, since the mineral apposition rate was unchanged. In contrast, the 1.9-fold increase in osteocyte density in the femoral epiphysis of D4 mice corresponds to a 3.3-fold increase in osteocyte formation rate, because of the 1.7-fold increase in mineral apposition rate at that site (Figure 10A). In addition, while the truncated type II TGF-β receptor reduced the epiphyseal osteocyte density of D4 mice to the wild-type level (Figure 3), the osteocyte formation rate at that site in D4/E1 mice is still elevated 1.7-fold over normal (Figure 10A) because of the increased local mineral apposition rate (Figure 7). This partial inhibition is consistent with the competitive nature of dominant negative interference. Lastly, the modest inhibition of the epiphyseal osteocyte density in D4 mice by alendronate (Figure 4A) underestimates a dramatic inhibition of the osteocyte formation rate (Figure 10B), since alendronate strongly inhibited the mineral apposition rate increase in D4 epiphyses (Figure 8). Alendronate, however, did not affect the periosteal osteocyte formation rate, since it had no effect on the periosteal mineral apposition rate or the subperiosteal osteocyte density.
Figure 10.
Osteocyte formation rates in cells/mm2/day in the femoral epiphysis at day 35, calculated as the mathematical product of the epiphyseal osteocyte density (cells/mm3) and the epiphyseal mineral apposition rate (mm3/mm2/day). This index represents the production rate of new osteocytes per unit forming bone surface. Values are shown as mean ± SD. (A) Effects of TGF-β2 overexpression and osteoblastic expression of a truncated type II TGF-β receptor on the epiphyseal osteocyte formation rate. Transgenic E1 mice were crossed to D4 mice to generate double transgenic D4/E1 mice. Although the epiphyseal osteocyte density in D4/E1 mice is at the wild-type level (Figure 3A), the osteocyte formation rate in these mice is partially reduced compared with D4 mice since their epiphyseal mineral apposition rate is still elevated (Figure 7). (B) Effects of alendronate and parathyroidectomy on the epiphyseal osteocyte formation rate. Alendronate treatment with or without parathyroidectomy (∗) dramatically inhibits the increase in osteocyte formation rate in D4 mice because it inhibits both the osteocyte density increase (Figure 4A) and the mineral apposition rate increase (Figure 8) caused by TGF-β2 overexpression.
The Increased Mineral Apposition Rate Depends on Osteoclastic Activity and Not on the Direct Response of Osteoblasts to TGF-β
In addition to the increase in osteocyte density, TGF-β2 overexpression in osteoblasts also increased bone formation as measured by the mineral apposition rate (Erlebacher and Derynck, 1996). However, several observations suggest that this is a secondary consequence of increased bone resorption and not a direct effect of TGF-β2 on osteoblasts. First, osteoblastic overexpression of a truncated type II TGF-β receptor did not affect the mineral apposition rate in the femoral epiphyses of wild-type or D4 mice, even though it reduced their osteocyte density. Second, TGF-β2 overexpression increased the osteocyte density of subperiosteal cortical bone, which forms in the absence of bone resorption, but did not affect the periosteal mineral apposition rate. Lastly, inhibition of bone resorption by alendronate prevented the TGF-β2–induced increase in mineral apposition rate at sites of bone resorption, but not on nonresorbing surfaces. This effect was not due to inhibition of TGF-β signaling, since alendronate did not affect the increase in subperiosteal osteocyte density.
The dependence of the mineral apposition rate on osteoclast activity stands in contrast to the osteoblast-mediated effects of TGF-β2 on osteocyte density. Considering the differential inhibition of distinct TGF-β responses by dominant negative receptors (Chen et al., 1993; Derynck and Feng, 1997), the increases in mineral apposition rate and osteocyte density might result from distinct responses of osteoblasts to TGF-β2 occurring at different thresholds. The inhibition of the increase in epiphyseal osteocyte density, but not the increase in the mineral apposition rate at that site, would then imply that higher TGF-β concentrations are required for the former than for the latter response. This, however, is difficult to reconcile with the findings that TGF-β increases the subperiosteal osteocyte density without changing the periosteal mineral apposition rate, and that the epiphyses of alendronate-treated D4 mice have a normal mineral apposition rate but an elevated osteocyte density.
Instead, our results are consistent with the interpretation that increased TGF-β2 expression leads to an enhanced epiphyseal mineral apposition rate as a secondary response to its stimulation of bone resorption, independent of the direct effects of TGF-β2 on osteoblasts. Since dominant negative interference with TGF-β receptor signaling in osteoblasts did not affect the mineral apposition rate, and TGF-β2 overexpression did not increase the fraction of total bone surface undergoing mineralization, our results suggest that TGF-β does not directly regulate the rate of bone formation during normal bone remodeling. The previously observed increases in bone formation after subperiosteal injections of TGF-β (Noda and Camilliere, 1989; Joyce et al., 1990; Centrella et al., 1994) may reflect a microfracture repair process consistent with the role of TGF-β in wound healing.
The increased mineral apposition rate in D4 mice may reflect a homeostatic response that maintains bone integrity past a critical threshold of resorption. Similarly, the increase in mineral apposition rate by high doses of parathyroid hormone also results from stimulation of bone resorption (Hock and Gera, 1992; Uzawa et al., 1995). Such a response may be sensitive to impaired mechanical and structural properties of bone. Thus, mice with osteogenesis imperfecta show increased periosteal bone formation as a compensatory response to impaired skeletal integrity (Bonadio et al., 1993; Pereira et al., 1995). This mechanism may also explain the increase in periosteal mineral apposition rate in c-fos−/−/D4 mice when compared with c-fos−/− mice, even though D4 and wild-type mice have the same periosteal mineral apposition rate. TGF-β2 overexpression probably adds to the inherent structural defects of osteopetrotic c-fos−/− bones by decreasing their matrix quality, since c-fos−/−/D4 mice have a dramatic increase in fracture incidence over c-fos−/− mice, even though both mice have the same total bone mass.
TGF-β Increases the Rate of Osteoblastic Differentiation
By labeling differentiating osteoblasts using BrdU, we showed a 2.2-fold higher labeling index of periosteal osteoblasts in D4 mice than in wild-type mice. However, the surface density of mature osteoblasts in D4 bone remained at the wild-type level (Erlebacher and Derynck, 1996; Figure 2, A and B). These findings suggest that TGF-β increases the steady-state rate of osteoblastic differentiation. This may be due, in part, to an acceleration of the maturation rate of already committed osteoprogenitor cells; however, the normal to increased number of osteoprogenitors in D4 bone (Figure 2; Erlebacher and Derynck, 1996) strongly suggests that this effect is largely due to increased differentiation of osteoprogenitor cells coupled to increased osteoprogenitor cell proliferation. Thus, the increased birth rate of osteoblasts in D4 bone explains the 1.6-fold increase in periosteal osteocyte formation rate and the high number of labeled subperiosteal osteocytes. Overexpression of TGF-β2 may also affect the rate of apoptosis of osteoprogenitors and osteoblasts; however, we did not detect any differences in the very low level of apoptotic cells, as assessed by 4,6-diamidino-2-phenylindole staining of the femoral periosteum at day 16 (data not shown).
Consistent with the direct osteoblastic effect of TGF-β on osteocyte density, the increased labeling of osteoblasts and osteocytes induced by TGF-β2 overexpression occurred in the absence of bone resorption and was partially inhibited by dominant negative interference with TGF-β signaling in osteoblasts. Thus, the increased rate of osteoblastic differentiation also reflects a direct effect of TGF-β on osteoblasts. Since the osteocalcin promoter used to drive expression of the truncated TGF-β receptor is activated only after mature osteoblasts have stopped dividing (Bronckers et al., 1985; Groot et al., 1986), this effect may be to induce the secretion of a second signal that stimulates osteoprogenitor cell proliferation and differentiation in a paracrine manner. In addition, TGF-β may directly regulate other aspects of osteoprogenitor cell physiology before activation of the osteocalcin promoter.
We chose the femoral periosteum to analyze the kinetics of osteoblast differentiation to avoid concurrent effects of TGF-β2 on bone resorption and formation. However, TGF-β is likely to increase osteoblast differentiation at other sites as well, e.g., in the femoral epiphysis where the D4 osteocyte formation rate is 3.3-fold higher than wild-type. In addition, the decrease in epiphyseal osteocyte density in E1 mice with no change in mineral apposition rate suggests that endogenous TGF-β signaling is most likely required to maintain the normal rate of epiphyseal osteoblastic differentiation. In contrast, periosteal osteocyte differentiation and cortical osteocyte density were not significantly reduced by interfering with TGF-β signaling in wild-type osteoblasts. These results may reflect a lower level of endogenous TGF-β activity on periosteal surfaces compared with endosteal surfaces. Differences in distribution of TGF-β activity in bone may at least partially explain the higher rate of osteoblastic differentiation on endosteal surfaces compared with periosteal surfaces (Young, 1962). Accordingly, increases in TGF-β activity may be involved in the increased osteocyte density seen in several metabolic bone diseases such as osteoporosis (Mullender et al., 1996), hyperparathyroidism (Malluche and Faugere, 1990), and osteogenesis imperfecta (Bonadio et al., 1993; Whyte, 1996). Since osteocytes may mediate skeletal responses to mechano-sensation (Aarden et al., 1994), the regulation of their density by TGF-β may significantly affect bone metabolism.
The developmental phenotype of D4 mice (Erlebacher and Derynck, 1996) strikingly resembles the phenotype of mice heterozygous for an inactivated allele of Cbfa1, a transcription factor required for normal ossification and osteoblast differentiation (Ducy et al., 1997; Komori et al., 1997; Otto et al., 1997). Both mice show the hypoplastic clavicles, patent anterior fontanels, and a general delay in ossification, characteristic of cleidocranial dysplasia. This similar phenotype suggests that TGF-β may down-regulate the embryonic expression of Cbfa1. Since the cleidocranial phenotype in D4 mice does not require osteoblastic responsiveness to TGF-β, its underlying mechanism is likely to be distinct from the direct stimulatory effects of TGF-β2 on the rate of osteoblastic differentiation.
Osteoclasts Contribute to the TGF-β–induced Increase in Osteoblastic Differentiation
Although the increases in osteoblastic differentiation rate and osteocyte density are direct effects of TGF-β on osteoblasts and do not require bone resorption, alendronate dramatically reduced the osteocyte formation rate in D4 mice in the femoral epiphysis, a site of bone resorption (see above and Figure 10B). This effect was associated with decreased osteoclastic activity and was not due to inhibition of TGF-β signaling, since alendronate did not alter the increased periosteal osteocyte formation rate. Thus, at sites of bone resorption, osteoclastic activity augments the direct effects of TGF-β2 on the rate of osteocyte formation and osteoblastic differentiation. The similarly drastic inhibition of the osteocyte formation rate at these sites by overexpression of the truncated type II TGF-β receptor therefore suggests a substantial contribution of osteoclasts to the local level of TGF-β activity. Osteoclasts may activate latent TGF-β (Oreffo et al., 1989; Oursler, 1994) or lead to the release of bone matrix-bound TGF-β. This latter possibility is suggested by experiments in organ culture (Pfeilschifter and Mundy, 1987) and is consistent with the ability of alendronate to decrease the plasma level of TGF-β2 in D4 mice.
The coupling of osteoblastic differentiation to osteoclastic activity is one of the central tenets of bone remodeling, yet the molecular regulation of this process remains poorly understood. Since the direct stimulation of osteoblastic differentiation by TGF-β can be augmented by osteoclastic activity, TGF-β may be an important mediator of the coupling of osteoblastic differentiation to sites of bone resorption. Since overexpression of TGF-β in transgenic mice also leads to increased bone resorption (Erlebacher and Derynck, 1996), TGF-β may be involved in regulating and coordinating the activities of osteoblasts and osteoclasts during bone remodeling. Deregulation of skeletal TGF-β expression, activation, and responsiveness in humans may have important physiological consequences and contribute to pathological bone-remodeling states.
ACKNOWLEDGMENTS
We thank Ilse Sauerwald and Nilda Ubana for their assistance with the histological analyses and Margaret Mayes for her assistance with the scanning electron microscopy. We also thank Gideon Rodan for the alendronate and Randall Johnson for the c-fos−/− mice. We are grateful to Deborah Zimmerman, Alfred Kuo, Steve Gitelman, Bernard Halloran, Gideon Rodan, and Zena Werb for helpful discussions, and Jill Helms for use of the fluorescence microscope used in the kinetic analyses. This research was supported by National Institutes of Health grants DE-10306 and AR-41126 (to R.D.) and a postdoctoral fellowship from the American Heart Association (to E.H.F.). A.E. is a member of the Medical Scientist Training Program supported by grant NIGMS GM07618.
REFERENCES
- Aarden EM, Burger EH, Nijweide PJ. Function of osteocytes in bone. J Cell Biochem. 1994;55:287–299. doi: 10.1002/jcb.240550304. [DOI] [PubMed] [Google Scholar]
- Baker AR, Hollingshead PG, Pitts MS, Hansen S, Taylor R, Stewart TA. Osteoblast-specific expression of growth hormone stimulates bone growth in transgenic mice. Mol Cell Biol. 1992;12:5541–5547. doi: 10.1128/mcb.12.12.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonadio J, Jepsen KJ, Mansoura MK, Jaenisch R, Kuhn JL, Goldstein SA. A murine skeletal adaptation that significantly increases cortical bone mechanical properties. Implications for human skeletal fragility. J Clin Invest. 1993;92:1697–1705. doi: 10.1172/JCI116756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonewald LF, Dallas SL. Role of active and latent transforming growth factor β in bone formation. J Cell Biochem. 1994;55:350–357. doi: 10.1002/jcb.240550312. [DOI] [PubMed] [Google Scholar]
- Boyde A. Biochemistry and Physiology of Bone. Vol. 1. G. H. Bourne, New York: Academic Press; 1972. Scanning electron microscope studies of bone; pp. 259–310. [Google Scholar]
- Bronckers AL, Gay S, Dimuzio MT, Butler WT. Immunolocalization of γ-carboxyglutamic acid containing proteins in developing rat bones. Collagen Relat Res. 1985;5:273–281. doi: 10.1016/s0174-173x(85)80017-0. [DOI] [PubMed] [Google Scholar]
- Centrella M, Horowitz MC, Wozney JM, McCarthy TL. Transforming growth factor-β gene family members and bone. Endocr Rev. 1994;15:27–39. doi: 10.1210/edrv-15-1-27. [DOI] [PubMed] [Google Scholar]
- Chen RH, Ebner R, Derynck R. Inactivation of the type II receptor reveals two receptor pathways for the diverse TGF-β activities. Science. 1993;260:1335–1338. doi: 10.1126/science.8388126. [DOI] [PubMed] [Google Scholar]
- Derynck R, Feng X-H. TGF-β receptor signaling. Biochim Biophys Acta Rev Cancer. 1997;1333:F105–F150. doi: 10.1016/s0304-419x(97)00017-6. [DOI] [PubMed] [Google Scholar]
- Ducy P, Zhang R, Geoffry V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- Erlebacher A, Derynck R. Increased expression of TGF-β2 in osteoblasts results in an osteoporsis-like phenotype. J Cell Biol. 1996;132:195–210. doi: 10.1083/jcb.132.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriadis AE, Wang ZQ, Cecchini MG, Hofstetter W, Felix R, Fleisch HA, Wagner EF. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994;266:443–448. doi: 10.1126/science.7939685. [DOI] [PubMed] [Google Scholar]
- Groot CG, Danes JK, Blok J, Hoogendijk A, Hauschka PV. Light and electron microscopic demonstration of osteocalcin antigenicity in embryonic and adult rat bone. Bone. 1986;7:379–385. doi: 10.1016/8756-3282(86)90259-0. [DOI] [PubMed] [Google Scholar]
- Hock JM, Gera I. Effects of continuous and intermittent administration and inhibition of resorption on the anabolic response of bone to parathyroid hormone. J Bone Miner Res. 1992;7:65–72. doi: 10.1002/jbmr.5650070110. [DOI] [PubMed] [Google Scholar]
- Johnson RS, Spiegelman BM, Papaioannou V. Pleiotropic effects of a null mutation in the c-fos proto-oncogene. Cell. 1992;71:577–586. doi: 10.1016/0092-8674(92)90592-z. [DOI] [PubMed] [Google Scholar]
- Joyce ME, Roberts AB, Sporn MB, Bolander ME. Transforming growth factor-β and the initiation of chondrogenesis and osteogenesis in the rat femur. J Cell Biol. 1990;110:2195–2207. doi: 10.1083/jcb.110.6.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel DB, Jee WS. A quantitative histological analysis of the growing long bone metaphysis. Calcif Tissue Int. 1980;32:113–122. doi: 10.1007/BF02408530. [DOI] [PubMed] [Google Scholar]
- Komori T, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- Malluche HH, Faugere M. Bone biopsies: histology and histomorphometry of bone. In: Avioli LV, Krane SM, editors. Metabolic Bone Disease and Clinically Related Disorders. Philadelphia: W.B. Saunders; 1990. pp. 283–328. [Google Scholar]
- Meyer RA, Jr, Tenenhouse HS, Meyer MH, Klugerman AH. The renal phosphate transport defect in normal mice parabiosed to X-linked hypophosphatemic mice persists after parathyroidectomy. J Bone Miner Res. 1989;4:523–532. doi: 10.1002/jbmr.5650040411. [DOI] [PubMed] [Google Scholar]
- Mullender MG, van der Meer DD, Huiskes R, Lips P. Osteocyte density changes in aging and osteoporosis. Bone. 1996;18:109–113. doi: 10.1016/8756-3282(95)00444-0. [DOI] [PubMed] [Google Scholar]
- Noda M, Camilliere JJ. In vivo stimulation of bone formation by transforming growth factor-β. Endocrinology. 1989;124:2991–2994. doi: 10.1210/endo-124-6-2991. [DOI] [PubMed] [Google Scholar]
- Oreffo RO, Mundy GR, Seyedin SM, Bonewald LF. Activation of the bone-derived latent TGF-β complex by isolated osteoclasts. Biochem Biophys Res Commun. 1989;158:817–823. doi: 10.1016/0006-291x(89)92795-2. [DOI] [PubMed] [Google Scholar]
- Otto F, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- Oursler MJ. Osteoclast synthesis and secretion and activation of latent transforming growth factor β. J Bone Miner Res. 1994;9:443–452. doi: 10.1002/jbmr.5650090402. [DOI] [PubMed] [Google Scholar]
- Packard DS, Jr, Menzies RA, Skalko RG. Incorporation of thymidine and its analogue, bromodeoxyuridine, into embryos and maternal tissues of the mouse. Differentiation. 1973;1:397–404. doi: 10.1111/j.1432-0436.1973.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Parfitt AM. Osteonal and hemi-osteonal remodeling: the spatial and temporal framework for signal traffic in adult human bone. J Cell Biochem. 1994;55:273–286. doi: 10.1002/jcb.240550303. [DOI] [PubMed] [Google Scholar]
- Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- Pelton RW, Saxena B, Jones M, Moses HL, Gold LI. Immunohistochemical localization of TGF-β1, TGF-β2, and TGF-β3 in the mouse embryo: expression patterns suggest multiple roles during embryonic development. J Cell Biol. 1991;115:1091–1105. doi: 10.1083/jcb.115.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira RF, Hume EL, Halford KW, Prockop DJ. Bone fragility in transgenic mice expressing a mutated gene for type I procollagen (COL1A1) parallels the age-dependent phenotype of human osteogenesis imperfecta. J Bone Miner Res. 1995;10:1837–1843. doi: 10.1002/jbmr.5650101202. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J, Mundy GR. Modulation of type β transforming growth factor activity in bone cultures by osteotropic hormones. Proc Natl Acad Sci USA. 1987;84:2024–2028. doi: 10.1073/pnas.84.7.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey PG, Young MF, Flanders KC, Roche NS, Kondaiah P, Reddi AH, Termine JD, Sporn MB, Roberts AB. Osteoblasts synthesize and respond to transforming growth factor-type β (TGF-β) in vitro. J Cell Biol. 1987;105:457–463. doi: 10.1083/jcb.105.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan GA, Fleisch HA. Bisphosphonates: mechanisms of action. J Clin Invest. 1996;97:2692–2696. doi: 10.1172/JCI118722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg M, Vuorio T, Hirvonen H, Alitalo K, Vuorio E. Enhanced expression of TGF-β and c-fos mRNAs in the growth plates of developing human long bones. Development. 1988;102:461–470. doi: 10.1242/dev.102.3.461. [DOI] [PubMed] [Google Scholar]
- Seyedin SM, Thomas TC, Thompson AY, Rosen DM, Piez KA. Purification and characterization of two cartilage-inducing factors from bovine demineralized bone. Proc Natl Acad Sci USA. 1985;82:2267–2271. doi: 10.1073/pnas.82.8.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sissons HA, O’Connor P. Quantitative histology of osteocyte lacunae in normal human cortical bone. Calcif Tissue Res. 1977;22:530–533. doi: 10.1007/BF02064153. [DOI] [PubMed] [Google Scholar]
- Taylor JR. An Introduction to Error Analysis: The Study of Uncertainties in Physical Measurements. 2nd ed. Sausalito, CA: University Science Books; 1997. [Google Scholar]
- Uzawa T, Hori M, Ejiri S, Ozawa H. Comparison of the effects of intermittent and continuous administration of human parathyroid hormone(1–34) on rat bone. Bone. 1995;16:477–484. doi: 10.1016/8756-3282(95)90194-9. [DOI] [PubMed] [Google Scholar]
- Wang ZQ, Ovitt C, Grigoriadis AE, Mohle-Steinlein U, Ruther U, Wagner EF. Bone and haematopoietic defects in mice lacking c-fos. Nature. 1992;360:741–745. doi: 10.1038/360741a0. [DOI] [PubMed] [Google Scholar]
- Whyte MP. Osteogenesis imperfecta. In: Favus MJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Philadelphia: Lippincott-Raven; 1996. pp. 382–384. [Google Scholar]
- Young RW. Cell proliferation and specialization during endochondral osteogenesis in young rats. J Cell Biol. 1962;14:357–370. doi: 10.1083/jcb.14.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]