Abstract
Enteroviral meningoencephalitis was diagnosed in a patient with an immunodeficiency syndrome acquired after treatment with rituximab for a relapsed primary B-cell lymphoma. A second meningoencephalitic episode was diagnosed 6 months later and was successfully treated with a combination of immunoglobulins and pleconaril. The infection was persistent since the enterovirus genome was detected in five sequential specimens of cerebrospinal fluid collected over 9 months. An echovirus 13 isolate was isolated in the first three samples. The viral sequence encoding the VP1 capsid protein of the three isolates was determined and was compared with that of four control viruses. The virus isolates recovered from the patient shared >99% nucleotide sequence similarity with one another. In a phylogenetic tree, they were directly related to a control virus obtained from a patient hospitalized in 2000 during an outbreak of enterovirus meningitis. The epidemiological origin of a chronic echovirus infection in a patient with immune deficiency suggests that the echovirus had been continuously circulating in the general population after the outbreak that had revealed its emergence.
Enteroviral meningoencephalitis is a life-threatening infection in patients with severe antibody deficiencies such as X-linked agammaglobulinemia (9, 15, 22, 27, 28). Treatment with intravenous and intrathecal immunoglobulin has resulted in clinical and virological improvements in some patients, but reverse transcription (RT)-PCR has shown evidence of viral persistence even after therapy (11, 15). An efficient antienterovirus drug, pleconaril (VP63843; ViroPharma, Inc., Exton, Pa.), was successfully used to treat immunocompromised patients with life-threatening infections (16, 24).
Published reports have shown that the enteroviruses most commonly recovered from patients with meningoencephalitis syndrome are, in decreasing order, echovirus types 11 (more than 12 cases), 30, 3, 5, 9, 25, 2, 7, 17, 19, 24, 29, and 33 (14, 15). In rare cases coxsackievirus types B3, B4, and A15 have also been isolated (14, 17). Echovirus 13 is an enterovirus that has rarely been detected in Europe or the United States, and so the spectrum of the diseases associated with this virus is not fully known (2, 6, 8). Only one case of echovirus 13 meningoencephalitis has been described in the literature (27). The sequential isolation of virus from patients with enterovirus infections provides an opportunity to study the genomic changes that enterovirus strains undergo during prolonged replication in a human host. Genome variation over time during chronic enterovirus infection in immunodeficient patients has been described (3, 12, 17) but has never been reported in patients with chronic meningoencephalitis.
We report on a protracted course of enterovirus meningoencephalitis in an adult with evidence of immunodeficiency after chemotherapy for relapsed lymphoma (21). The genomic sequence encoding the VP1 capsid protein of the three echovirus 13 isolates collected from cerebrospinal fluid (CSF) specimens over a period of 3 months was determined. A phylogenetic analysis based on the VP1 sequence was performed to investigate the epidemiological origin of the echovirus 13 identified in the patient.
CASE REPORT
The case described here has been described in detail elsewhere (21). Briefly, a 53-year-old man was diagnosed with follicular lymphoma in August 1998 at the University Hospital of Clermont-Ferrand (Clermont-Ferrand, France) and was treated with 12 courses of low-dose chemotherapy plus interferon. He made a complete recovery. In December 2000, a first relapse of his lymphoma was treated with four infusions of the chimeric anti-CD20 monoclonal antibody rituximab (375 mg · m−2 · week−1), which induced a second complete remission. In June 2001, he presented with signs of meningoencephalitis. Clinical manifestations included fever, headaches, diffuse paresthesia, concentration difficulties, sensorimotor deafness, diplopia, a pyramidal syndrome, and ataxia. Magnetic resonance imaging (MRI) revealed thoracic myelitis and signal enhancement of meninges after gadolinium injection. Cytological and immunophenotyping revealed only 2% malignant B cells. Serum immunoglobulin levels were low (immunoglobulin G [IgG], 5.5 g/liter; IgA, 0.69 g/liter; IgM, 0.15 g/liter). An echovirus 13 isolate was isolated from three CSF samples at 4-week intervals. Concomitantly, histological examination of a duodenal biopsy specimen revealed a second relapse of the patient's lymphoma. Thus, before the diagnosis of enterovirus meningoencephalitis was considered, the patient was treated for 5 consecutive months with high-dose corticosteroids, salvage systemic polychemotherapy, and repeated intrathecal corticosteroid and chemotherapy infusions, which induced a third complete remission. In November 2001 he received high-dose consolidation chemotherapy, followed by autologous hematopoietic stem cell transplantation (HSCT). While the patient was receiving systemic and intrathecal corticosteroids and chemotherapy, MRI showed complete regression of the thoracic myelitis, and his neurological symptoms improved partially, albeit with persistence of mild deafness and paresthesia. Nevertheless, 2 months after HSCT, in January 2002, the patient experienced a recurrence of mild fever, complete sensorimotor deafness, and neurological symptoms identical to those previously described in June 2001. The MRI findings at this time were normal. An RT-PCR for enterovirus detection in CSF was positive. Marked hypogammaglobulinemia was also observed (serum IgG level, 3.7 g/liter). An antienteroviral treatment was therefore started in January 2002 and comprised repeated intravenous immunoglobulin injections at a dose of 0.7 g per kg of body weight over a period of 12 months in order to achieve residual serological gamma globulin levels higher than 15 g per liter. In February 2002 pleconaril was administered orally at a dose of 1,200 mg per day for 10 consecutive days. A few weeks later, the patient was well and alert, the dyesthesia and ataxia had improved, and only mild pyramidal syndrome and deafness persisted.
MATERIALS AND METHODS
Clinical specimens and virus identification.
Eight CSF samples were sequentially recovered from the patient for virus isolation, enterovirus RT-PCR, and cytological and biochemical analyses. For virus isolation, CSF samples and, when available, throat swab and stool specimens were inoculated onto human lung embryonic (MRC5) and human oral epidermoid carcinoma (KB) cell lines (BioMerieux, Marcy l'Etoile, France) by a previously described method (7). Lym-Benyesh-Melnick antiserum pools were used for identification of the enterovirus isolates, as described elsewhere (13).
Seven virus isolates were analyzed in the present study. Isolates S1, S2, and S3 were recovered from three sequential CSF specimens obtained from the patient in June, July, and August 2001, respectively. Four control isolates were also included: echovirus 13 prototype strain Del Carmen (E-13), isolated in 1953 from a rectal swab from a healthy infant, and three echovirus 13 isolates recovered from patients hospitalized in Clermont-Ferrand in 1991 (isolate CF1083-91), 1995 (isolate CF957-95), and 2000 (isolate CF1393-00). Isolates CF1083-91 and CF957-95 were isolated from the stools of two infants, and isolate CF1393-00 was isolated from the CSF of a 7-year-old child hospitalized during an outbreak of meningitis in 2000.
Diagnostic RT-PCR assays.
Isolation of viral RNAs was performed with the QIAamp viral RNA mini kit (Qiagen, Courtaboeuf, France) and 140 μl of CSF, according to the recommendations of the manufacturer. Nucleic acids were eluted in 40 μl of the buffer provided in the kit. In each assay, one negative control of sterile distilled water and one positive control consisting of an echovirus 25 culture supernatant with a known virus titer were included.
Synthesis of the cDNAs was performed with the ThermoScript RT-PCR system (Invitrogen, Cergy Pontoise Cedex, France) with random hexanucleotides as primers, according to the instructions of the manufacturer. Each reaction was carried out in a final volume of 20 μl that included 9 μl of viral RNA, 4 μl of 5× reverse transcriptase buffer, 5 μM dithiothreitol, 40 U of RNaseOut, the four deoxynucleotides at a concentration of 1 mM each, and 15 U of ThermoScript reverse transcriptase. The amplification reactions with the partial 5′ noncoding sequence were performed as follows. Briefly, the reaction mixture contained 7 μl of cDNA, the two primers described elsewhere (7) at a concentration of 20 μM each, the four deoxynucleotides at a concentration of 2 mM each, and 0.4 μl of Taq polymerase. Thermal cycling comprised 37 cycles, as follows: 1 cycle of denaturation for 2 min at 94°C, followed by 35 cycles of denaturation for 15 s at 94°C, annealing for 20 s at 50°C, and elongation for 50 s at 72°C, with a final cycle of 10 min at 72°C. The RT-PCR products were detected by DNA enzyme immunoassay (GEN-ETI-K DEIA; DiaSorin, Antony, France) as described previously (7).
Amplification of the VP1-encoding gene sequences of virus isolates.
The complete VP1-encoding sequences were amplified from the genomes of the virus isolates with two subgeneric degenerate oligonucleotides, ecox1 and ecox2, by the RT-PCR method described elsewhere (4). Oligonucleotides ecox1 (5′-GC GGA TCC GCG GCC GCG AGC TCI GCR TGC AAY GAY TTY TCW G-3′) and ecox2 (5′-GCT GCA GGG CGC GCC TCT AGA RTC YCT RTT RTA RTC YTC CCA-3′) had a composite sequence: a degenerate sequence (underlined) at the 3′ end and a heterologous sequence at the 5′ end. The amplification reactions were performed with 4 μl of cDNA in a reaction mixture containing the two primers at a concentration of 200 nM each, the four deoxynucleotides at a concentration of 200 μM each, and 0.4 μl of the enzyme mixture provided with the Expand High Fidelity PCR system (Roche Molecular Biochemicals, Meylan, France). Thermal cycling comprised 37 cycles, as follows: 1 cycle of denaturation for 2 min at 94°C, followed by 35 cycles of denaturation for 15 s at 94°C, annealing for 20 s at 55°C, and elongation for 50 s at 72°C, with a final cycle of 10 min at 72°C.
Nucleotide sequencing of PCR products and comparative sequence analysis.
After purification of the amplified products with the Qiaquick PCR purification kit (Qiagen), the nucleotide sequences of both strands of the PCR products were determined at Euro Sequence Gene Service (Evry, France). Forward and reverse sequencing reactions were performed with oligonucleotides BNS (5′-GC GGA TCC GCG GCC GCG AGC-3′) and XAP (5′-GCT GCA GGG CGC GCC TCT AGA-3′), which correspond to the heterologous sequences in oligonucleotides ecox1 and ecox2, respectively.
The sequences determined in the present study were compared with one another and with the echovirus 13 sequences available in international databases. A multiple-sequence alignment was constructed, stored, and edited with the computer program Genedoc (version 2.6; K. B. Nicholas and H. B. Nicholas, Jr. [available at http://www.psc.edu/biomed/genedoc/]). Comparative analysis of the sequences was performed with the computer program MEGA (version 2; S. Kumar, K. Tamura, I. Jakobsen, and M. Nei [available at http://www.megasoftware.net]). The dendrograms reconstructed from the VP1-encoding sequences were obtained by the neighbor-joining method (25), as implemented in the MEGA computer program and edited with TreeExplorer (version 2.12; K. Tamura [available at http://evolgen.biol.metro-u.ac.jp]). The reliability of the phylogenetic topologies (branching patterns) was determined by Felsenstein's boostrap test (10).
Nucleotide sequence accession numbers.
The nucleotide sequences reported in the present study were deposited in the EMBL database under accession numbers AJ241427 (echovirus 13 prototype strain) and AJ537604 to AJ537609 (echovirus 13 isolates recovered from patients hospitalized in Clermont-Ferrand, France).
RESULTS
Laboratory data.
Eight CSF specimens were analyzed over 12 months (Table 1). CSF examination showed raised protein levels, which ranged from 0.67 to 1.91 g/liter (normal values, 0.1 to 0.55 g/liter); normal or mildly low levels of glucose, which ranged from 2 to 4.4 mmol/liter (normal values, 2.5 to 4.5 mmol/liter); and pleocytosis, with white blood cell counts ranging from 11 to 286 cells per mm3 and with a predominance of lymphocytes-monocytes (median, 97% of white blood cell counts) in all but the third sample. Bacteriological investigations of all specimens were negative, as was a test for the detection of the herpes simplex virus genome in CSF ordered on the first admission.
TABLE 1.
Laboratory data for CSF specimens from the patient with meningoencephalitis
| CSF specimen | Cytological and biochemical analysis of CSF
|
||||
|---|---|---|---|---|---|
| No. | Date (mo.day.yr) | WCC/mm3a | % Lymphocytesb monocytes | Protein concn (g/liter) | Glucose concn (nmol/liter) |
| 1 | 06.15.01 | 249 | 95 | 1.53 | 3.9 |
| 2 | 07.18.01 | 286 | 97 | 1.91 | 4.4 |
| 3 | 08.14.01 | 50 | 5 | 0.67 | 2.2 |
| 4 | 01.24.02 | 18 | 100 | 0.74 | 2.5 |
| 5 | 02.05.02 | 11 | 96 | 0.71 | 2 |
| 6 | 02.18.02 | 18 | 98 | 0.75 | 2.4 |
| 7 | 04.02.02 | 18 | 99 | 0.81 | 2.6 |
| 8 | 06.03.02 | 49 | 92 | 0.89 | 2.6 |
WCC, white cell count in CSF.
Relative percentage of lymphocytes-monocytes compared to the number of neutrophils.
The RT-PCR for enterovirus detection gave positive results for the first five CSF samples (Table 2). The results were negative for the other three CSF samples obtained after antienteroviral treatment with immunoglobulins and pleconaril.
TABLE 2.
Results of enterovirus RT-PCR tests and cell cultures for CSF samples from the patient
| CSF specimen | Virus detection
|
Virus isolates
|
||
|---|---|---|---|---|
| No. | Date (mo.day.yr) | In-house RT-PCR resulta | Cell culture resultb | Strain name, designation (accession no.) |
| 1 | 06.15.01 | + | + | CF1504-00, S1 (AJ537607) |
| 2 | 07.18.01 | + | + | CF1737-00, S2 (AJ537608) |
| 3 | 08.14.01 | + | + | CF1925-00, S3 (AJ537609) |
| 4 | 01.24.02 | + | − | |
| 5 | 02.05.02c | + | − | |
| 6 | 02.18.02d | − | − | |
| 7 | 04.02.02 | − | − | |
| 8 | 06.03.02 | − | − | |
The nucleic extraction was performed with the QIAamp viral RNA mini kit (Qiagen) for RT and PCR amplification in two steps.
MCR5 and KB cells were used (see Materials and Methods).
CSF recovered before pleconaril treatment.
CSF recovered after pleconaril treatment.
The CSF specimens were inoculated onto standard cultures of MRC5 and KB cells, and three echovirus 13 isolates were identified from the specimens obtained in three consecutive months (June to August 2001), the period during which the patient first exhibited manifestations of meningoencephalitis (Table 2). No virus was isolated from throat swab or stool specimens over the 12-month period of investigation.
A good correlation between viral isolation and detection of the enterovirus genome was observed for the first three CSF samples. The RT-PCR method detected the enterovirus genome in the fourth and fifth CSF samples, but the cultures were negative.
Genomic analysis of the echovirus 13 isolates recovered from the patient.
The complete VP1-encoding sequences (861 nucleotides) of the echovirus 13 isolates recovered from the patient's first three CSF specimens (virus isolates S1, S2, and S3) were determined. Pairwise comparisons of the three sequences showed a maximum of 5 nucleotide differences between isolates S1 and S3 collected 3 months apart (data not shown). Overall, the three echovirus 13 isolates shared a high degree of similarity in the VP1-encoding sequence (99.4 to 99.6% nucleotide similarity, 99.7 to 100% amino acid similarity). Of the nucleotide differences observed between the isolates, only 1 (nucleotide position 258) led to an amino acid change, at residue 168 in S2 and S3 (Fig. 1). The sequences of isolates S1, S2, and S3 were also compared with those of four control viruses: echovirus 13 prototype strain Del Carmen (E-13) and three echovirus 13 isolates recovered from patients hospitalized in Clermont-Ferrand (isolates CF1083-91, CF957-95, and CF1393-00). Isolates S1, S2, and S3 showed the greatest similarity with isolate CF1393-00 (97.9 to 98.4% nucleotide similarity, 98.9 to 99.3% amino acid similarity), a virus isolated from a patient hospitalized during a widespread outbreak of enterovirus meningitis in 2000 (see below). Of the 18 nucleotide differences observed between isolates CF1393-00 and S3, 3 resulted in amino acid changes, at residues 86 (Ala→Thr), 168 (Thr→Ile), and 271 (Pro→Ser). The sequences of isolates S1, S2, and S3 were significantly different from that of the prototype strain (E-13) isolated in 1953 (78.3 to 78.5% nucleotide similarity, 91.6 to 92% amino acid similarity). For instance, 187 nucleotide differences were observed between the prototype strain and isolate S3, resulting in 24 amino acid changes (Fig. 1). The majority of these amino acid changes clustered in the N terminus (6 changes) and C terminus (10 changes) of the VP1 sequence.
FIG. 1.
Multiple-sequence alignment of the VP1 capsid proteins of the echovirus 13 Del Carmen prototype strain (E-13), the CF1393-00 control isolate, and the three sequential echovirus 13 isolates (isolates S1, S2, S3) recovered from the CSF specimens of the patient. Dots indicate amino acid similarity with the VP1 sequence of the prototype strain.
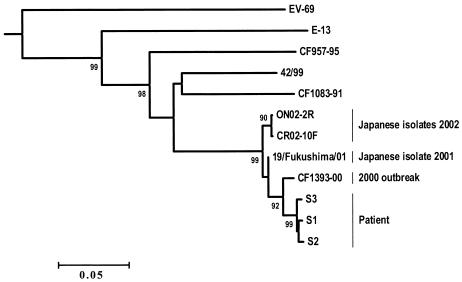
An unrooted dendrogram was constructed with the seven sequences determined (Fig. 2). To make the results more reliable, the sequences of four echovirus 13 isolates (isolates 42/99, 19/Fukushima/01, ON02-2R, and CR02-10F) from international databases were also included in the phylogenetic analysis. All the echovirus 13 isolates clustered together with a high bootstrap confidence value (PB) of 99 (99% of 1,000 resampled data gave the observed cluster). The sequences of isolates S1, S2, and S3 grouped together in a specific lineage (PB = 92) which also comprised isolate CF1393-00, collected during the meningitis outbreak in 2000. During the outbreak, echovirus 13 was identified in patients hospitalized from June to November and accounted for 23% of the enteroviruses detected in all cases of meningitis (35 of 151 enterovirus isolates). Isolates S1, S2, S3, and CF1393-00 were closely related to the viruses isolated from Japanese patients in 2001 and 2002 (PB = 99). In contrast, they were only distantly related to the control isolates recovered from patients hospitalized in Clermont-Ferrand in 1991 (isolate CF1083-91) and 1995 (isolate CF957-95). These isolates were the only two echovirus 13 isolates identified from 1991 to 1999 in Clermont-Ferrand. Finally, the most recent isolates, including those detected in the case patient, clustered in a specific genotype, showing widespread circulation of the virus.
FIG. 2.
Phylogenetic tree based on the VP1-encoding sequence illustrating the relationships of echovirus 13 clinical isolates. The reliability of internal branches was estimated by the resampling method (bootstrap test, 1,000 replicates) and is indicated as a percentage. Values <90% are not given. The sequences of four echovirus 13 isolates, 42/99 (GenBank accession number AF295467), 19/Fukushima/01 (GenBank accession number AB086858), ON02-2R (GenBank accession number AB092984), and CR02-10F (GenBank accession number AB092985), were retrieved from international databases and were included in the analysis to increase the reliability of the results of the phylogenetic inference. Strain ON02-2R was isolated from the CSF of a child with aseptic meningitis, and strains CR02-10F and the 19/Fukushima/01 were isolated from stools of children with exanthema and aseptic meningitis, respectively. No isolation source was given for strain 42/99. The nucleotide sequence of the echovirus 13 Del Carmen prototype strain (E-13) was determined in our laboratory (EMBL database accession number AJ241427). The sequence of enterovirus 69 (EV-69; GenBank accession number AF081349) was used as the outgroup to root the tree.
DISCUSSION
This is the first report to describe a case of persistent echovirus 13 meningoencephalitis and to provide a detailed genetic analysis of the three sequential echovirus 13 isolates recovered from a patient with relapsed lymphoma. The diagnosis of chronic enteroviral meningoencephalitis was established by detection of an enterovirus genome by RT-PCR and isolation of echovirus 13 isolates from CSF specimens.
The patient, who had been suffering from lymphoma since 1998, was admitted to hospital 6 months after a course of rituximab treatment in June 2001 for neurological symptoms. At that time a second relapse of the lymphoma was diagnosed and an echovirus 13 was isolated from three consecutive CSF samples. Before the diagnosis of enterovirus meningoencephalitis was considered, the patient was treated with systemic and local (intrathecal) corticosteroids and chemotherapy, including high-dose therapy and HSCT, but no antiviral treatment was given. His neurological symptoms improved, including normalization of the findings by MRI. Hence, the first neurological episode was probably related to the lymphoma; nevertheless, the possibility that the enterovirus infection was responsible is not excluded.
In January 2002, 8 months after isolation of the echovirus 13 isolate and 3 months after high-dose therapy and HSCT, similar neurological symptoms reappeared. Analysis of the patient's CSF by RT-PCR showed the persistence of the enteroviral genome. The patient's clinical symptoms improved after antiviral treatment, indicating that the second neurological episode resulted from the enterovirus infection. These findings confirmed the persistent nature of the enterovirus infection over the 8 months.
Enterovirus infections, particularly meningoencephalitis, are well documented in patients with X-linked agammaglobulinemia (19, 23, 26, 28). Quartier et al. (19) repeatedly isolated an enterovirus from different CSF specimens from a 14-year-old child with agammaglobulinemia over 8 months, and the enterovirus genome was detected over a prolonged period of 11 months. From our observations, the failure to obtain the virus from CSF samples during the second episode of meningoencephalitis may have resulted from the presence of neutralizing antibodies acquired through intravenous immunoglobulin therapy given in January and February 2002. Alternatively, the enterovirus may have persisted in the patient's nervous system tissue with or without active replication, which would explain the intermittent periods of culture negativity for the virus, despite a positive RT-PCR result. The second possibility was seen in a patient with end-stage cardiac disease involving an enterovirus (1).
Both the occurrence and the persistence of the echovirus 13 infection in our patient were probably due to severe humoral deficiency. Prior to the onset of viral meningoencephalitis 1 month after the first relapse of his lymphoma in January 2001, the patient had received the chimeric anti-CD20 monoclonal antibody rituximab. This drug is known to induce rapid and long-lasting depletion of the peripheral B-cell pool, which leads to hypogammaglobulinemia (20). The involvement of rituximab in this type of humoral deficiency was suspected by Quartier et al. (21), who recommended close medical monitoring of patients after rituximab therapy, particularly patients treated with a combination of rituximab and immunosuppressive or cytotoxic drugs. The administration of prophylactic intravenous immunoglobulins to some of these patients should be considered. Enteroviruses, unlike other viruses, which are largely contained by the cellular immune response, are cleared from the host by antibody-mediated mechanisms (22). Hence, rituximab was probably responsible to a large extent for the occurrence of the meningoencephalitis.
Within 2 weeks of the onset of the second meningoencephalitis episode, the patient was treated with intravenous immunoglobulin and 1 month later was treated with a combination of intravenous immunoglobulin and pleconaril. The drug was well tolerated, and except for persisting deafness, significant clinical improvements were observed during the following 4 months. Examination at 3, 57, and 85 days after completion of pleconaril therapy in combination with intravenous immunoglobulin revealed negative enterovirus PCR results and negative culture results for CSF specimens 6, 7, and 8, respectively. Only the protein level and cell counts remained moderately increased in the CSF specimen(s). In our experience and that of others, combination therapy with antiviral agents and intravenous immunoglobulin is a promising approach for immunocompromised patients with severe enterovirus infections (19, 24, 26).
Echovirus 13 was identified in the CSF samples by conventional seroneutralization tests and a molecular typing assay designed in the laboratory (4). To our knowledge, only one other case of meningoencephalitis due to echovirus 13 has been reported (27). The patient was a child with X-linked hypogammaglobulinemia, and the virus was isolated from a CSF sample obtained 1 month after admission. No other enterovirus was recovered over the following 12 months of the disease, which was fatal (27). The persistence of isolates of the same enterovirus serotype has been reported in agammaglobulinemic patients (9, 15). Misbah et al. (15) described the isolation of echovirus 11 over a 5-month period, and Dunn et al. (9) reported the persistence of echovirus 6 over a 7-year period. However, molecular analysis of the virus isolates was not performed in either study. In our study, comparative analysis of the genomic sequence encoding the VP1 capsid protein of the three virus isolates was performed (isolates S1, S2, and S3) and showed little variation between the three viruses. The nucleotide sequence similarities of the three viruses demonstrated the close relationship between the isolates and the slow evolution of the virus in the patient during the 3-month period of infection. This observation is in agreement with the genomic evolution of echovirus 30 observed during a chronic infection in a child with a severe immunodeficiency syndrome (3). The phylogenetic analysis of the VP1 sequences showed that isolates S1, S2 and S3 clustered in a group with all the echovirus 13 control isolates examined: prototype strain Del Carmen (E-13) and three isolates recovered from patients hospitalized in Clermont-Ferrand in 1991, 1995, and 2000, respectively. This analysis also showed that all the viruses isolated from 2000 to 2002 were closely related to one another and clustered in the same group, whatever their geographical origin. The three isolates from the patient were directly related to control isolate CF1393-00, which was recovered from a patient hospitalized during an outbreak of enterovirus meningitis that occurred from June to November 2000. Phylogenetic analysis showed that the patient was infected with an echovirus 13 isolate that had been circulating in the general population during and after the outbreak. From 1991 to 1999 the echovirus 13 isolate had been isolated only twice from patients hospitalized in Clermont-Ferrand. However, during the outbreak of meningitis in 2000, this serotype was the second most frequently isolated enterovirus, after echovirus 30 (5, 7, 18). During the same period, there were also notifications of echovirus 13 isolations in different regions of France by the National Reference Center for Enteroviruses, Lyon, France (personal communication). In addition, echovirus 13 isolations associated with meningitis were reported in the United States, the United Kingdom, and Germany (2, 6, 8).
The present study establishes an epidemiological connection between a sporadic echovirus 13 infection in a patient with meningoencephalitis and the unusual epidemic circulation of the virus. In this patient the virus persisted over a period of 8 months. The study also shows that the enteroviruses circulating in a period following an epidemic constitute a risk for patients receiving immunosuppressive treatment. Particular attention should be paid to adult patients, in whom enterovirus infection is less readily sought for than in children. Diagnosis of neurological infection, which can be swiftly and easily arrived at by detecting the genome in CSF, now has therapeutic implications for immunodepressed subjects.
Acknowledgments
We are grateful to Jean-Jacques Chomel of the World Health Organization Collaborating Center, National Reference Center for Enteroviruses, for providing us with the prototype strains. We thank Jeffrey Watts for revision of the English manuscript.
This work was supported in part by a grant (grant EA2148) from the Ministère de l'Education Nationale, de la Recherche et de la Technologie.
REFERENCES
- 1.Andréoletti, L., T. Bourlet, D. Moukassa, L. Rey, D. Hot, Y. Li, V. Lambert, B. Gosselin, J. F. Mosnier, C. Stankoviak, and P. Wattré. 2000. Enteroviruses can persist with or without active viral replication in cardiac tissue of patients with end-stage ischemic or dilated cardiomyopathy. J. Infect. Dis. 182:1222-1227. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. 2000. Viral meningitis associated with increase in echovirus type 13. Commun. Dis. Rep. Wkly. 10:277-280. [PubMed]
- 3.Bailly, J. L., M. Chambon, C. Henquell, J. Icart, and H. Peigue-Lafeuille. 2000. Genomic variations in echovirus 30 persistent isolates recovered from a chronically infected immunodeficient child and comparison with the reference strain. J. Clin. Microbiol. 38:552-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailly, J. L., A. Béguet, M. Chambon, C. Henquell, and H. Peigue-Lafeuille. 2000. Nosocomial transmission of echovirus 30: molecular evidence by phylogenetic analysis of the VP1 encoding sequence. J. Clin. Microbiol. 38:2889-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailly, J. L., D. Brosson, C. Archimbaud, M. Chambon, C. Henquell, and H. Peigue-Lafeuille. 2002. Genetic diversity of echovirus 30 during a meningitis outbreak, demonstrated by direct molecular typing from cerebrospinal fluid. J. Med. Virol. 68:558-567. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 2001. Echovirus type 13—United States, 2001. Morb. Mortal. Wkly. Rep. 50:777-780. [PubMed] [Google Scholar]
- 7.Chambon, M., C. Archimbaud, J. L. Bailly, C. Henquell, C. Regagnon, F. Charbonné, and H. Peigue-Lafeuille. 2001. Circulation of enteroviruses and persistence of meningitis cases in the winter of 1999-2000. J. Med. Virol. 65:340-347. [DOI] [PubMed] [Google Scholar]
- 8.Diedrich, S., and E. Schreier. 2001. Aseptic meningitis in Germany associated with echovirus type 13. BMC Infect. Dis. 1:14 [Online.] http://www.biomedcentral.com/1471-2334/1/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn, J. J., J. R. Romero, R. Wasserman, and H. A. Rotbart. 2000. Stable enterovirus 5′ nontranslated region over a 7-year period in a patient with agammaglobulinemia and chronic infection. J. Infect. Dis. 182:298-301. [DOI] [PubMed] [Google Scholar]
- 10.Felsenstein, J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783-791. [DOI] [PubMed] [Google Scholar]
- 11.Geller, T. J., and D. Condie. 1995. A case of protracted coxsackie virus meningoencephalitis in a marginally immunodeficient child treated successfully with intravenous immunoglobulin. J. Neurol. Sci. 129:131-133. [DOI] [PubMed] [Google Scholar]
- 12.Kew, O. M., R. W. Sutter, B. K. Nottay, M. J. McDonough, D. R. Prevots, L. Quick, and M. A. Pallansch. 1998. Prolonged replication of a type 1 vaccine-derived poliovirus in an immunodeficient patient. J. Clin. Microbiol. 36:2893-2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim, K. A., and M. Benyesh-Melnick. 1960. Typing of viruses by combination of antiserum pools. Application to typing of enteroviruses (coxsackie and ECHO). J. Immunol. 84:309-317. [PubMed] [Google Scholar]
- 14.McKinney, R. E., S. L. Katz, and C. M. Wilfert. 1987. Chronic enteroviral meningoencephalitis in agammaglobulinemic patients. Rev. Infect. Dis. 9:334-356. [DOI] [PubMed] [Google Scholar]
- 15.Misbah, S. A., G. P. Spickett, P. C. J. Ryba, J. M. Hockaday, J. S. Kroll, C. Sherwood, J. B. Kurtz, E. R. Moxon, and H. M. Chapel. 1992. Chronic enteroviral meningoencephalitis in agammaglobulinemia: case report and literature review. J. Clin. Immunol. 12:266-270. [DOI] [PubMed] [Google Scholar]
- 16.Nowak-Wegrzyn. A., W. Phipatanakul, J. A. Winkelstein, M. S. Forman, and H. M. Lederman. 2001. Successful treatment of enterovirus infection with the use of pleconaril in 2 infants with severe combined immunodeficiency. Clin. Infect. Dis. 32:e13-e14. [DOI] [PubMed] [Google Scholar]
- 17.O'Neil, K. M., M. A. Pallansch, J. A. Winkelstein, T. M. Lock, and J. F. Modlin. 1988. Chronic group A coxsackievirus infection in agammaglobulinemia: demonstration of genomic variation of serotypically identical isolates persistently excreted by the same patient. J. Infect. Dis. 157:183-186. [DOI] [PubMed] [Google Scholar]
- 18.Peigue-Lafeuille, H., N. Croquez, H. Laurichesse, P. Clavelou, O. Aumaître, J. Schmidt, M. Maillet-Vioud, C. Henquell, C. Archimbaud, J. L. Bailly, and M. Chambon. 2002. Enterovirus meningitis in adults in 1999-2000 and evaluation of clinical management. J. Med. Virol. 67:47-53. [DOI] [PubMed] [Google Scholar]
- 19.Quartier, P., S. Foray, J. L. Casanova, I. Hau-Rainsard, S. Blanche, and A. Fischer. 2000. Enteroviral meningoencephalitis in X-linked agammaglobulinemia: intensive immunoglobulin therapy and sequential viral detection in cerebrospinal fluid by polymerase chain reaction. Pediatr. Infect. Dis. J. 19:1106-1108. [DOI] [PubMed] [Google Scholar]
- 20.Quartier, P., B. Brethon, P. Philippet, J. Landman-Parker, F. Le Deist, and A. Fischer. 2001. Treatment of childhood autoimmune haemolytic anaemia with rituximab. Lancet 358:1511-1513. [DOI] [PubMed] [Google Scholar]
- 21.Quartier, P., O. Tournilhac, C. Archimbaud, L. Lazaro, C. Chaleteix, P. Millet, H. Peigue-Lafeuille, S. Blanche, A. Fischer, J. L. Casanova, P. Travade, and M. Tardieu. 2003. Enteroviral meningoencephalitis after anti-CD20 (rituximab) treatment. Clin. Infect. Dis. 36:e47-e49. [DOI] [PubMed] [Google Scholar]
- 22.Rotbart, H. A. 1995. Enteroviral infections of the central nervous system. Clin. Infect. Dis. 20:971-981. [DOI] [PubMed] [Google Scholar]
- 23.Rotbart, H. A., J. P. Kinsella, and R. L. Wasserman. 1990. Persistent enterovirus infection in culture-negative meningoencephalitis: demonstration by enzymatic RNA amplification. J. Infect. Dis. 161:787-791. [DOI] [PubMed] [Google Scholar]
- 24.Rotbart, H. A., and A. D. Webster for the Pleconaril Treatment Registry Group. 2001. Treatment of potentially life-threatening enterovirus infections with pleconaril. Clin. Infect. Dis. 32:228-235. [DOI] [PubMed] [Google Scholar]
- 25.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 26.Schmugge, M., R. Lauener, W. Bossart, R. A. Seger, and T. Güngör. 1999. Chronic enteroviral meningo-encephalitis in X-linked agammaglobulinemia: favourable response to anti-enteroviral treatment. Eur. J. Pediatr. 158:1010-1011. [DOI] [PubMed] [Google Scholar]
- 27.Van Maldergem, L., F. Mascart, D. Ureel, E. Jauniaux, W. Broeckx, and M. Vainsel. 1989. Echovirus meningoencephalitis in X-linked hypogammaglobulinemia. Acta Paediatr. Scand. 78:325-326. [DOI] [PubMed] [Google Scholar]
- 28.Webster, A. D. B., H. A. Rotbart, T. Warner, P. Rudge, and N. Hyman. 1993. Diagnosis of enterovirus brain disease in hypogammaglobulinemic patients by polymerase chain reaction. Clin. Infect. Dis. 17:657-661. [DOI] [PubMed] [Google Scholar]