Abstract
Late endosomes and the Golgi complex maintain their cellular localizations by virtue of interactions with the microtubule-based cytoskeleton. We study the transport of mannose 6-phosphate receptors from late endosomes to the trans-Golgi network in vitro. We show here that this process is facilitated by microtubules and the microtubule-based motor cytoplasmic dynein; transport is inhibited by excess recombinant dynamitin or purified microtubule-associated proteins. Mapmodulin, a protein that interacts with the microtubule-associated proteins MAP2, MAP4, and tau, stimulates the microtubule- and dynein-dependent localization of Golgi complexes in semi-intact Chinese hamster ovary cells. The present study shows that mapmodulin also stimulates the initial rate with which mannose 6-phosphate receptors are transported from late endosomes to the trans-Golgi network in vitro. These findings represent the first indication that mapmodulin can stimulate a vesicle transport process, and they support a model in which the microtubule-based cytoskeleton enhances the efficiency of vesicle transport between membrane-bound compartments in mammalian cells.
INTRODUCTION
Cytoplasmic dyneins are part of a small family of minus end–directed, microtubule-based motors that have been implicated in a variety of organelle transport processes (Allan, 1996; Goodson et al., 1997). Cytoplasmic dynein is present on late endosomes and lysosomes (Lin and Collins, 1992) and also on the trans-Golgi network (TGN) (Fath et al., 1994) and likely participates in the centrosomal localization of these organelles. Indeed, movement of isolated endosomes and Golgi complexes along microtubules in vitro is supported by cytoplasmic dynein (Bomsel et al., 1990; Corthésy-Theulaz et al., 1992; Aniento et al., 1993; Blocker et al., 1997). A role for cytoplasmic dynein in Golgi and endosome localization was confirmed most dramatically by Harada et al. (1998), who found that disruption of the murine dynein heavy chain gene led to alteration in the distributions of endosomes and lysosomes and conversion of the Golgi complex into ministacks that were dispersed throughout the cytoplasm.
The ability of dynein to move membrane vesicles in vitro is enhanced by an auxiliary component called dynactin (Gill et al., 1991; Schroer and Sheetz, 1991). Dynactin is a large multiprotein complex (Allan, 1996; Schroer, 1996; Vallee and Sheetz, 1996). Overexpression in mammalian cells of the dynactin subunit dynamitin disrupts the dynactin complex and results in the dissociation of cytoplasmic dynein from prometaphase kinetochores and subsequent perturbation of mitosis (Echeverri et al., 1996). Moreover, dynamitin overexpression leads to a dramatic disruption of the Golgi complex into scattered structures, redistribution of endosomes and lysosomes to the cell periphery (Burkhardt et al., 1997), and inhibition of endoplasmic reticulum-to-Golgi transport (Presley et al., 1997). These data strongly support the conclusion that dynein and dynactin function in concert to drive organelle motility in living cells.
We have previously established an assay to monitor the microtubule- and cytoplasmic dynein-dependent localization of Golgi complexes in semi-intact Chinese hamster ovary (CHO) cells (Corthésy-Theulaz et al., 1992). In this assay, purified rat liver Golgi complexes are added to semi-intact CHO cells in the presence of desalted cytosol and an ATP-regenerating system. “Captured” Golgi complexes are quantified by centrifugation of the recipient, semi-intact cells through a sucrose cushion. We have recently purified a 31-kDa protein on the basis of its ability to stimulate this in vitro assay of Golgi localization (Ulitzur et al., 1997a). We named this protein mapmodulin because it binds the conserved microtubule-binding domains of MAP2, MAP4, and tau (Ulitzur et al., 1997a,b). Given that mapmodulin stimulates Golgi localization, we postulated that mapmodulin acts by trapping released MAPs that are in equilibrium with the microtubule surface; this process could lead to the overall displacement of MAPs from the path of organelles translocating along microtubules.
Although motors and cytoskeletal proteins are important for organelle distribution, they do not seem to be absolutely required for vesicular transport. Nevertheless, the relative positioning of organelles in relation to the cytoskeleton may enhance the probability of vesicle–target interactions, as seen for certain endosome fusion reactions (Bomsel et al., 1990; Aniento et al., 1993). Thus we were interested to explore the role of cytoplasmic dynein and related motility factors in the transport of proteins from endosomes to the TGN. In this paper, we show a role for dynein, dynactin, and mapmodulin in the transport of mannose 6-phosphate receptors (MPRs) from endosomes to the TGN. Each of these components enhances the initial rate but not the overall extent of vesicle trafficking.
MATERIALS AND METHODS
In Vitro Endosome–TGN Transport Assay
The assay was carried out as described (Itin et al., 1997). Briefly, transport is measured by monitoring the tyrosine sulfation of 46-kDa MPR (MPR46) that occurs upon its arrival at the TGN. Cells expressing MPR46 containing a consensus site for tyrosine sulfation and six N-terminal histidines for subsequent purification are cultured in sulfate-free α-minimum essential medium and sodium chlorate to accumulate unsulfated MPRs. Four hours before breaking the cells, cycloheximide is added so that newly synthesized MPRs exit the secretory pathway and reach their steady-state localization in late endosomes. Cells are then broken and preincubated with nonlabeled adenosine 3′-phosphate 5′-phosphosulfate (PAPS) to cold label any MPR46 molecules present in the TGN; membranes are collected on a sucrose cushion, frozen in liquid nitrogen, and stored at −80°C. Transport reactions were carried out in a final volume of 150 μl with 10 μl of 0.5 μCi/μl [35S]PAPS (∼20 μM final), 6 μl of membranes, cytosol, and an ATP-regenerating system. The extent of MPR46 sulfation (transport) was determined by direct scintillation counting of MPRs collected after detergent solubilization and Ni-NTA (Pharmacia) chromatography.
Protein Purification
Mapmodulin was purified from CHO cytosol exactly as described (Ulitzur et al., 1997b). Cytoplasmic dynein was purified from bovine brain cytosol essentially as described by Schroer and Sheetz (1991) and Gaglio et al. (1996) with the following modifications. High-speed supernatant was loaded on a Fastflow S-cation exchange column (Pharmacia, Piscataway, NJ) equilibrated with 25 mM 2-[N-morpholino]ethanesulfonic acid buffer, pH 6.0. Dynein-enriched fractions from a gradient of 0–500 mM NaCl were pooled, concentrated, and loaded on 11.5-ml 5–20% sucrose gradients in PMEE buffer (35 mM K-1,4-piperazinediethanesulfonic acid, pH 7.2, 5 mM MgSO4, 1 mM EGTA, and 0.5 mM EDTA). Dynein-enriched fractions were pooled and loaded on a MonoQ ion exchange column (Pharmacia fast protein liquid chromatography). A 100–400 mM KCl gradient (in PMEE buffer) was developed to separate dynein from dynactin. Aliquots were snap frozen after adding 1.25 M sucrose.
Recombinant dynamitin construct was a generous gift from Dr. Richard Vallee (University of Massachusetts, Worcester, MA) (Echeverri et al., 1996). The protein was purified essentially according to the method of Wittman and Hyman (1999). Briefly, cells were induced with isopropyl-1-thio-β-d-galactopyranoside for 4 h at 22°C, harvested, and sonicated in lysis buffer (PBS, 1 mM EGTA, 1 mM EDTA, 50 μg/ml lysozyme, 1 mM PMSF, and 0.1% β-mercaptoethanol). The supernatant was applied onto a Fastflow Q ion exchange column (Pharmacia) equilibrated in 50 mM Tris, pH 7.0, 100 mM KCl, 1 mM EGTA, 0.1% β-mercaptoethanol, and 20% glycerol. Dynamitin-enriched fractions were pooled from a 100–500 mM KCl gradient and further purified on a Sephacryl-100 gel filtration column equilibrated in 50 mM K-1,4-piperazinediethanesulfonic acid, pH 7.0, 100 mM KCl, 1 mM EGTA, and 0.1% β-mercaptoethanol.
Heat-stable MAPs were prepared as described by Herzog and Weber (1978). Briefly, twice-cycled microtubule pellets from bovine brain were stripped with 0.75 M NaCl. The microtubule protein mix was supplemented with 2% β-mercaptoethanol and 1 mM PMSF and boiled for 5 min. Denatured protein was removed by centrifugation at 4°C for 30 min at 10,000 × g. Aliquots (containing MAP2 and tau) were snap frozen.
RESULTS
Microtubules and Mapmodulin Stimulate the Initial Rate of Endosome-to-TGN Transport
We have established two cell-free systems that reconstitute the recycling of MPRs from endosomes to the TGN in vitro (Goda and Pfeffer, 1988; Itin et al., 1997). Our newer assay relies on the TGN localization of tyrosine sulfotransferase and monitors transport of MPRs engineered to contain a consensus sequence for modification by this enzyme. The engineered MPR is stably expressed in CHO cells; unsulfated MPRs are accumulated by preincubating cells with chlorate, a reversible inhibitor of sulfate precursor synthesis. Cells are gently broken and mixed with an ATP-regenerating system, cytosol, and [35S]PAPS at 30 or 37°C for various times. MPRs are then affinity purified, and the extent of transport is monitored by scintillation counting (Itin et al., 1997).
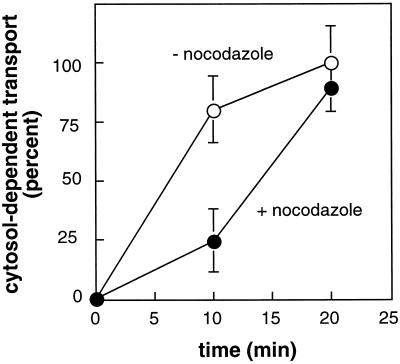
Endosome-to-TGN transport involves two organelles that are each localized adjacent to the centrosome. Thus, it was possible that the transport process was facilitated by microtubules, microtubule-based motor proteins, and factors such as mapmodulin that appear to stimulate dynein-mediated Golgi localization (Ulitzur et al., 1997a). We first tested whether microtubules were important for endosome-to-TGN transport using nocodazole to inhibit microtubule polymerization. As shown in Figure 1, nocodazole was a potent inhibitor of MPR transport at early times; at later times, it was without effect. These results demonstrate that early events in endosome-to-TGN transport are facilitated by microtubules.
Figure 1.
Nocodazole inhibits the initial rate of MPR transport from endosomes to the TGN in vitro. Reactions were carried out for the indicated times at 37°C in the presence (filled circles) or absence (open circles) of nocodazole (10 μg/ml) and 0.2 mg/ml cytosolic proteins and 100 ng/ml α-SNAP. Values shown represent the average of triplicate determinations; bars represent SD.
To test for a role for mapmodulin, we first established conditions in which this protein is limiting in our assay. We have previously shown that α-SNAP is a highly limiting component in our transport reactions (Itin et al., 1997). Thus we carried out reactions with limiting amounts of cytosol supplemented with α-SNAP to optimize our ability to detect the activity of other limiting constituents.
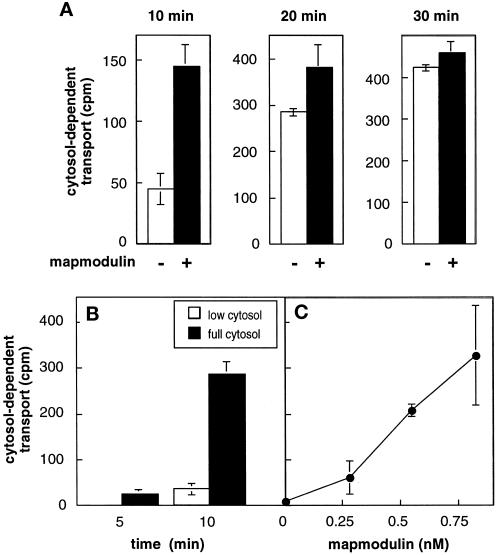
In the presence of low-cytosol (0.2 mg/ml) recombinant α-SNAP and Rab9–GDP dissociation inhibitor (GDI) complexes (Dirac-Svejstrup et al., 1994), 50 ng/ml (0.6 nM) mapmodulin stimulated the transport reaction (Figure 2A). Stimulation was limited to the initial phase of the reaction; at 30 min, mapmodulin no longer yielded levels of transport above those seen with low cytosol, indicating that other factors became more limiting at that point in the reaction.
Figure 2.
Mapmodulin stimulates the initial rate of MPR transport from endosomes to the TGN in vitro. (A) Reactions contained 0.2 mg/ml cytosol, 100 ng/ml α-SNAP, 50 ng/ml Rab9–GDI complex, and either no mapmodulin (−) or 50 ng/ml (0.6 nM) mapmodulin (+) and were incubated at 30°C for the indicated times. (B) Kinetics of endosome-to-TGN transport in low or full cytosol. Reactions contained 0.2 mg/ml cytosol, 100 ng/ml α-SNAP (low cytosol), or 1 mg/ml cytosol (full cytosol) and were incubated for 5 or 10 min at 30°C. (C) Purified mapmodulin was titrated in the presence of low cytosol as described in B; reactions were for 10 min. Cytosol-dependent transport was determined by subtracting transport measured in the absence of cytosol. Values shown represent the average of duplicate determinations; bars represent SD.
The ability of mapmodulin to stimulate at early times was consistent with this phase of the reaction being most dependent on the presence of intact microtubules (Figure 1). Moreover, the fact that other factors become limiting at later times is not surprising, because the transport of vesicles from endosomes to the TGN requires factors for vesicle budding, docking, and fusion (Pfeffer, 1996). Rab9–GDI complexes were found not to be limiting under these conditions (our unpublished results) and were therefore omitted from subsequent reactions.
Figure 2B compares the maximal extent of transport obtained in the presence of full cytosol (1 mg/ml, filled bars) versus low cytosol (0.2 mg/ml, white bars). After 5 min the reaction was still in the lag phase in the presence of either cytosol concentration; only low incorporation was seen. At 10 min, there was a substantial increase in transport in the presence of full cytosol; low cytosol reactions were still in the lag phase. At this time point in the transport reaction, mapmodulin (0.75 nM) added to reactions containing low cytosol stimulated MPR recycling to the same extent observed with full cytosol (Figure 2C), indicating that it is very limiting under these conditions. Mapmodulin stimulated the assay at concentrations comparable to those required for stimulation of in vitro Golgi localization (Ulitzur et al., 1997a).
Cytoplasmic Dynein Stimulates Transport
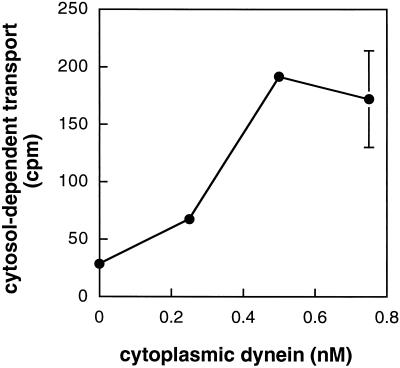
The ability of mapmodulin to stimulate MPR transport suggested a role for cytoplasmic dynein, consistent with mapmodulin’s proposed role in facilitating microtubule-based motor-dependent transport (Ulitzur et al., 1997a,b). Cytoplasmic dynein from bovine brain (Figure 3A) was added to transport reactions containing low cytosol (0.2 mg/ml cytosol and 100 ng/ml α-SNAP), and reactions were incubated for 10 min as before. As shown in Figure 4, low nanomolar concentrations of cytoplasmic dynein enhanced the ability of limiting cytosol concentrations to support MPR transport at early times.
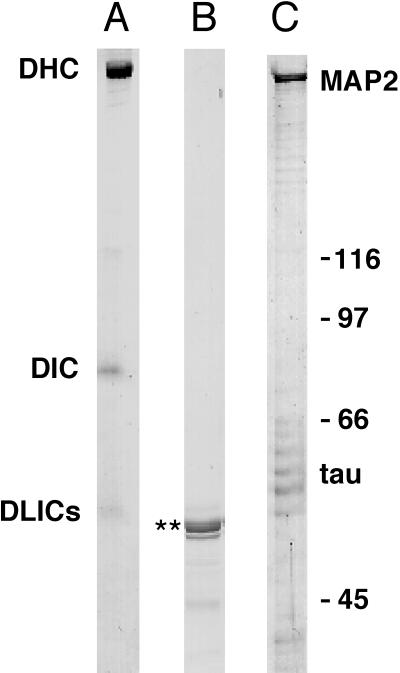
Figure 3.
Proteins used in this study. Coomassie blue–stained SDS-PAGE (7.5%) of (A) cytoplasmic dynein purified from bovine brain (A) (DHC, dynein heavy chain; DIC, dynein intermediate chain; DLICs, dynein light intermediate chains), recombinant dynamitin (B) (**), and heat-stable MAPs purified from bovine brain (C) (MAP2 and tau).
Figure 4.
Cytoplasmic dynein stimulates the initial rate of MPR transport from endosomes to the TGN. Reactions were carried out as described in Figure 1B with increasing concentrations of cytoplasmic dynein. Cytosol-dependent transport was obtained by subtracting transport measured in the absence of cytosol. Shown are the averages of duplicate determinations; bars represent SD.
To further assess the role of microtubules in the transport of MPRs from endosomes to the TGN, we stabilized the microtubules with Taxol before cell breakage and maintained Taxol throughout the assays. This led to increased amounts of microtubules and microtubule-bound membranes in the reactions. Under these conditions, higher concentrations of mapmodulin and dynein were required to stimulate transport (our unpublished results). This latter finding is not entirely surprising; these components would easily be titrated by the increase in available cognate binding sites.
Dynamitin Inhibits MPR Transport
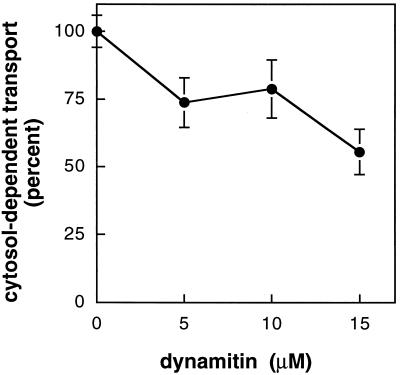
Overexpression or microinjection into cells of the dynactin complex subunit dynamitin was shown to block cytoplasmic dynein-dependent motility in vivo (Echeverri et al., 1996; Burkhardt et al., 1997; Presley et al., 1997; Ahmad et al., 1998). Given the stimulatory effect of cytoplasmic dynein, we tested whether recombinant dynamitin could inhibit MPR transport in vitro. Micromolar concentrations of recombinant dynamitin (Figure 3B) were tested; control reactions contained an equal amount of ultrapure BSA. Reactions were carried out in the presence of 1 mg/ml cytosol to obtain maximal transport and incubated for 15 min. As shown in Figure 5, 15 μM dynamitin could significantly inhibit transport by as much as 45%.
Figure 5.
Recombinant dynamitin inhibits MPR transport from endosomes to the TGN. Purified recombinant dynamitin was added to reactions containing 1 mg/ml cytosol. Transport is given relative to control reactions containing equal amounts of BSA. Cytosol-dependent transport was obtained by subtracting transport measured in the absence of cytosol. Shown are average and SDs of two independent experiments carried out in duplicate.
MAPs Inhibit MPR Transport
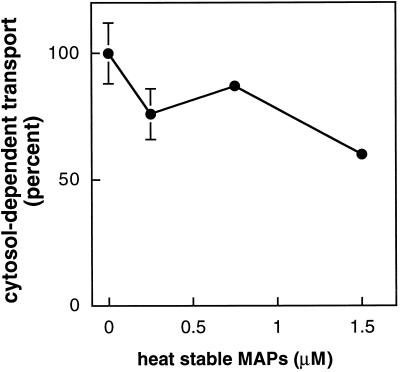
Several reports indicate that MAPs can inhibit cytoplasmic dynein- or kinesin-based motility. MAP2, tau, kinesin, and cytoplasmic dynein bind to overlapping or closely adjacent sites on the microtubule surface (Paschal et al., 1989; Rodionov et al., 1990; Hagiwara et al., 1994) and can inhibit dynein- or kinesin-mediated microtubule gliding in vitro (Von Massow et al., 1989; Lopez and Sheetz, 1993; Hagiwara et al., 1994). Recent studies have confirmed that overexpression of MAP4 inhibits organelle motility and trafficking in living cells (Bulinski et al., 1997). Similarly, MAP2C and tau overexpressed in COS cells caused a dramatic suppression of organelle movement (Sato-Harada et al., 1996). Consistent with these reports, heat-stable bovine MAPs (i.e., MAP2 and tau; Figure 3C) led to an inhibition of MPR recycling. As shown in Figure 6, when added at low micromolar concentrations, MAPs inhibited MPR transport by 40%.
Figure 6.
Heat-stable MAPs inhibit MPR transport from endosomes to the TGN. Purified heat-stable MAPs were added to reactions containing 1 mg/ml cytosol. Transport is given relative to control reactions containing equal amounts of BSA. Cytosol-dependent transport was obtained by subtracting transport measured in the absence of cytosol. Shown are averages of duplicates; bars represent SD.
DISCUSSION
We have shown here that the early phase of MPR transport from endosomes to the TGN is enhanced by the presence of polymerized microtubules, cytoplasmic dynein, along with the protein mapmodulin. The dynactin complex is probably also involved, because purified dynamitin could disrupt transport. Microtubules, dynein, and mapmodulin reduce the initial kinetic lag observed in our transport reactions but not the overall extent of transport. In addition, exogenous MAPs interfere with transport, as if they impede movement of membrane-bound organelles along microtubule tracks. A role for cytoplasmic dynein has been reported for other in vitro transport reactions (Bomsel et al., 1990; Aniento et al., 1993). However, presented here is the first demonstration that purified p50 dynamitin can inhibit an in vitro membrane transport reaction. Moreover, although mapmodulin was purified based on its ability to stimulate Golgi complex localization (Ulitzur et al., 1997a), its ability to enhance a vesicle trafficking reaction has not yet been examined.
The initial kinetic lag in our reactions will include all events that precede both membrane fusion and the enzymatic modification of MPRs used to monitor the transport process. This phase of the reaction will include organelle positioning, vesicle budding, and even possibly vesicle docking. It is quite reasonable to imagine a role for the microtubule-based cytoskeleton and the dynein–dynactin motor complex in any one (or all) of these individual steps.
Dynamitin overexpression experiments carried out by Burkhardt et al. (1997) have provided direct evidence for the involvement of dynactin and dynein in maintaining the intracellular distribution of the Golgi complex, intermediate compartment, early and late endosomes, and lysosomes. The inhibitory effect of dynamitin in our assay supports a role for the dynactin complex in endosome-to-TGN transport and lends further support for a dynein requirement in this process. Because the dynein–dynactin complex plays a key role in organelle positioning in vivo, it is possible that highly efficient transfer between membrane-bound compartments requires initial dynein–dynactin-mediated, microtubule-based organelle positioning.
Cytoplasmic dynein and mapmodulin stimulate transport at nanomolar concentrations, yet micromolar levels of dynamitin are needed to inhibit transport reactions. This is not entirely surprising, because excess dynamitin protein would be expected to be required to disrupt endogenous dynactin complexes. Indeed, dynein and dynactin are abundant proteins in the cytosol, and high-level overexpression was required by others to disrupt dynactin function in living cells (Echeverri et al., 1996; Burkhardt et al., 1997).
What roles do microtubules, dynein–dynactin and mapmodulin play in the early phases of MPR trafficking? As mentioned earlier, one possibility is that the initial lag phase represents a microtubule-based positioning of organelles. Microtubule-based motors and accessory proteins would optimize the organization of donor and acceptor compartments within the context of a broken cell extract. The transport assay uses gently broken cells. Nevertheless, in vitro reactions are significantly diluted relative to intact cells, and organelle redistribution may be important for maximal transport efficiency. Another possibility is the transport vesicles themselves move on microtubule-tracks en route to their targets. Although this is an attractive possibility, our data cannot discriminate between the two models. Nevertheless, our data demonstrate a role for the microtubule-based cytoskeleton in contributing to the efficiency of vesicle transport, regardless of the precise mechanism.
In reactions reconstituting the in vitro fusion of endosomal carrier vesicles with late endosomes, taxol-stabilized microtubules were shown to enhance both the initial rate and overall extent of the reaction (Aniento et al., 1993). The greatest effect was seen at early times, consistent with our findings. In contrast, no microtubule stimulation was detected in early–early or late–late endosome fusion reactions (Aniento et al., 1993). It is possible that homotypic fusion events rely more on linking proteins that can bridge organelles and facilitate their coalescence. In contrast, heterotypic fusion events may show a higher dependence on microtubule-based motors to facilitate docking reactions that are likely to require additional levels of target discrimination.
MAPs stabilize microtubule assemblies but inhibit organelle transport processes. How do cells maintain MAP-stabilized microtubule tracks in the face of large volumes of motor-mediated organelle transport? MAP–microtubule interactions are likely to be regulated by proteins such as kinases that modify MAPs (Drewes et al., 1997). Proteins such as mapmodulin may also regulate MAP–microtubule interactions. By binding to the microtubule-binding domain of MAPs, mapmodulin could retard the reassociation of MAPs that have been transiently released from the microtubule surface. In this manner, mapmodulin could modulate the equilibrium of MAPs with the microtubule lattice (Ulitzur et al., 1997a). Moreover, our recent organelle motility analysis in COS cells overexpressing mapmodulin revealed a significant increase in overall microtubule-based vesicle motility, suggesting that mapmodulin may serve a general role in motor-mediated transport (our unpublished results). In agreement with these findings, we have shown here that MPR transport between late endosomes and the TGN can also be stimulated by mapmodulin.
In conclusion, dynein and mapmodulin enhance early events in the transport of receptors between late endosomes and the TGN in vitro. Moreover, heat-stable MAPs interfere with this process. A future challenge will be to understand how these components link to endosomes and/or the TGN to localize them in the cell and to enhance intercompartmental transport.
ACKNOWLEDGMENTS
We are grateful to Drs. Richard Vallee and Christoph Echeverri for the dynamitin expression plasmid. This research was supported by National Institutes of Health grants GM-49843 and DK-37332. C.I. was supported by a European Molecular Biology Organization postdoctoral fellowship and a grant from the Roche Research Foundation.
REFERENCES
- Ahmad FJ, Echeverri CJ, Vallee RB, Baas PW. Cytoplasmic dynein and dynactin are required for the transport of microtubules into the axon. J Cell Biol. 1998;140:391–401. doi: 10.1083/jcb.140.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan V. Motor proteins: a dynamic duo. Curr Biol. 1996;6:630–633. doi: 10.1016/s0960-9822(09)00434-5. [DOI] [PubMed] [Google Scholar]
- Aniento F, Emans N, Griffiths G, Gruenberg J. Cytoplasmic dynein-dependent vesicular transport from early to late endosomes. J Cell Biol. 1993;123:1373–1387. doi: 10.1083/jcb.123.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blocker A, Severin FF, Burkhardt JK, Bingham JB, Yu H, Olivo JC, Schroer TA, Hyman AA, Griffiths G. Molecular requirements for bidirectional movement of phagosomes along microtubules. J Cell Biol. 1997;137:113–129. doi: 10.1083/jcb.137.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomsel M, Parton R, Kuznetsov SA, Schroer TA, Gruenberg J. Microtubule- and motor-dependent fusion in vitro between apical and basolateral endocytic vesicles from MDCK cells. Cell. 1990;62:719–731. doi: 10.1016/0092-8674(90)90117-w. [DOI] [PubMed] [Google Scholar]
- Bulinski JC, McGraw TE, Gruber D, Nguyen HL, Sheetz MP. Overexpression of MAP4 inhibits organelle motility and trafficking in vivo. J Cell Sci. 1997;110:3055–3064. doi: 10.1242/jcs.110.24.3055. [DOI] [PubMed] [Google Scholar]
- Burkhardt J K, Echeverri CJ, Nilsson T, Vallee RB. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J Cell Biol. 1997;139:469–484. doi: 10.1083/jcb.139.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthésy-Theulaz I, Pauloin A, Pfeffer SR. Cytoplasmic dynein participates in the centrosomal localization of the Golgi complex. J Cell Biol. 1992;118:1333–1345. doi: 10.1083/jcb.118.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirac-Svejstrup AB, Soldati T, Shapiro AD, Pfeffer SR. Rab-GDI presents functional rab9 to the intracellular transport machinery and contributes selectivity to rab9 membrane recruitment. J Biol Chem. 1994;269:15427–15430. [PubMed] [Google Scholar]
- Drewes G, Ebneth A, Preuss U, Mandelkow EM, Mandelkow E. MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell. 1997;89:297–308. doi: 10.1016/s0092-8674(00)80208-1. [DOI] [PubMed] [Google Scholar]
- Echeverri CJ, Paschal BM, Vaughan KT, Vallee RB. Molecular characterization of the 50-kDa subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J Cell Biol. 1996;132:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fath KR, Trimbur GM, Burgess DR. Molecular motors are differentially distributed on Golgi membranes from polarized epithelial cells. J Cell Biol. 1994;126:661–675. doi: 10.1083/jcb.126.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaglio T, Saredi A, Bingham JB, Hasbani MJ, Gill SR, Schroer TA, Compton DA. Opposing motor activities are required for the organization of the mammalian mitotic spindle pole. J Cell Biol. 1996;135:399–414. doi: 10.1083/jcb.135.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SRT, Schroer TA, Szilak I, Steuer ER, Sheetz MP, Cleveland DW. Dynactin, a conserved, ubiquitously expressed component of an activator of vesicle motility mediated by cytoplasmic dynein. J Cell Biol. 1991;115:1639–1650. doi: 10.1083/jcb.115.6.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda Y, Pfeffer SR. Selective recycling of the mannose 6-phosphate receptor to the trans Golgi network in vitro. Cell. 1988;55:309–320. doi: 10.1016/0092-8674(88)90054-2. [DOI] [PubMed] [Google Scholar]
- Goodson HV, Valetti C, Kreis TE. Motors and membrane traffic. Curr Opin Cell Biol. 1997;9:18–28. doi: 10.1016/s0955-0674(97)80147-0. [DOI] [PubMed] [Google Scholar]
- Hagiwara H, Yorifuji H, Sato-Yoshitake R, Hirokawa N. Competition between motor molecules (kinesin and cytoplasmic dynein) and fibrous microtubule-associated proteins in binding to microtubules. J Biol Chem. 1994;269:3581–3589. [PubMed] [Google Scholar]
- Harada A, Takei Y, Kanai Y, Tanaka Y, Nonaka S, Hirokawa N. Golgi vesiculation and lysosome dispersion in cells lacking cytoplasmic dynein. J Cell Biol. 1998;141:51–59. doi: 10.1083/jcb.141.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog W, Weber K. Fractionation of brain microtubule-associated proteins. Eur J Biochem. 1978;92:1–8. doi: 10.1111/j.1432-1033.1978.tb12716.x. [DOI] [PubMed] [Google Scholar]
- Itin C, Rancano C, Nakajima Y, Pfeffer SR. A novel assay reveals a role for soluble N-Ethylmalemide-sensitive fusion attachment protein in mannose 6-phosphate receptor transport from endosomes to the trans Golgi network. J Biol Chem. 1997;272:27737–27744. doi: 10.1074/jbc.272.44.27737. [DOI] [PubMed] [Google Scholar]
- Lin SX, Collins CA. Immunolocalization of cytoplasmic dynein to lysosomes in cultured cells. J Cell Sci. 1992;101:125–137. doi: 10.1242/jcs.101.1.125. [DOI] [PubMed] [Google Scholar]
- Lopez LA, Sheetz MP. Steric inhibition of cytoplasmic dynein and kinesin motility by MAP2. Cell Motil Cytoskeleton. 1993;24:1–16. doi: 10.1002/cm.970240102. [DOI] [PubMed] [Google Scholar]
- Paschal BM, Obar RA, Vallee RB. Interaction of brain cytoplasmic dynein and MAP2 with a common sequence at the C terminus of tubulin. Nature. 1989;342:569–572. doi: 10.1038/342569a0. [DOI] [PubMed] [Google Scholar]
- Pfeffer SR. Transport vesicle docking—SNAREs and associates. Annu Rev Cell Dev Biol. 1996;12:441–461. doi: 10.1146/annurev.cellbio.12.1.441. [DOI] [PubMed] [Google Scholar]
- Presley JF, Cole NB, Schroer TA, Hirschberg K, Zaal KJM, Lippincott-Schwartz J. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- Rodionov VI, Gyoeva FK, Kashina AS, Kuznetsov SA, Gelfand VI. Microtubule-associated proteins and microtubule-based translocators have different binding sites on tubulin molecule. J Biol Chem. 1990;265:5702–5707. [PubMed] [Google Scholar]
- Sato-Harada R, Okabe S, Umeyama T, Kanai Y, Hirokawa N. Microtubule-associated proteins regulate micortubule function as the track for intracellular membrane organelle transports. Cell Struct Funct. 1996;21:283–295. doi: 10.1247/csf.21.283. [DOI] [PubMed] [Google Scholar]
- Schroer TA. Structure and function of dynactin. Semin Cell Dev Biol. 1996;7:321–328. [Google Scholar]
- Schroer TA, Sheetz MP. Two activators of microtubule-based vesicle transport. J Cell Biol. 1991;115:1309–1318. doi: 10.1083/jcb.115.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitzur N, Humbert M, Pfeffer SR. Mapmodulin: a possible modulator of the interaction of microtubule-associated proteins with micortubules. Proc Natl Acad Sci USA. 1997a;94:5084–5089. doi: 10.1073/pnas.94.10.5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitzur N, Rancano C, Pfeffer SR. Biochemical characterization of mapmodulin, a protein that binds microtubule-associated proteins. J Biol Chem. 1997b;272:30577–30582. doi: 10.1074/jbc.272.48.30577. [DOI] [PubMed] [Google Scholar]
- Vallee RB, Sheetz MP. Targeting of motor proteins. Science. 1996;271:1539–1544. doi: 10.1126/science.271.5255.1539. [DOI] [PubMed] [Google Scholar]
- Von Massow A, Mandelkow EM, Mandelkow E. Interaction between kinesin, microtubules, and microtubule-associated protein 2. Cell Motil Cytoskeleton. 1989;14:562–571. doi: 10.1002/cm.970140413. [DOI] [PubMed] [Google Scholar]
- Wittman T, Hyman A. Methods in Cell Biology. San Diego: Academic Press; 1999. Production of active P50/dynamitin in bacteria. [Google Scholar]