Abstract
To investigate the species distribution of Ehrlichia present in Missouri dogs, we tested 78 dogs suspected of having acute ehrlichiosis and 10 healthy dogs. Blood from each dog was screened with a broad-range 16S rRNA gene PCR assay that detects known pathogenic species of Ehrlichia and Anaplasma. The species was determined by using species-specific PCR assays and nucleotide sequencing. Ehrlichia antibody testing was performed by using an indirect immunofluorescence assay with Ehrlichia chaffeensis as the antigenic substrate. The broad-range assay detected Ehrlichia or Anaplasma DNA in 20 (26%) of the symptomatic dogs and 2 (20%) of the asymptomatic dogs. E. ewingii accounted for 20 (91%), and E. chaffeensis accounted for 1 (5%) of the positives. Anaplasma phagocytophilum DNA was detected in one dog, and the sequences of regions of the 16S rRNA gene and the groESL operon amplified from the blood of this dog matched the published sequences of this organism. Antibodies reactive with E. chaffeensis were detected in 14 (67%) of the 21 PCR-positive dogs and in 12 (19%) of the 64 PCR-negative dogs. Combining the results of PCR and serology indicated that 33 (39%) of 85 evaluable dogs had evidence of past or current Ehrlichia infection. We conclude that E. ewingii is the predominant etiologic agent of canine ehrlichiosis in the areas of Missouri included in this survey. E. canis, a widely recognized agent of canine ehrlichiosis, was not detected in any animal. The finding of E. ewingii in asymptomatic dogs suggests that dogs could be a reservoir for this Ehrlichia species.
Ehrlichiosis is an important emerging infection of dogs and humans. The first species recognized, Ehrlichia canis, causes monocytic ehrlichiosis in dogs. A closely related species, E. chaffeensis, was subsequently identified as the cause of human monocytic ehrlichiosis (1). E. chaffeensis has also been detected in dogs (12), coyotes (21), goats (13), and deer (3, 10). Another closely related species, E. ewingii, was initially recognized as the cause of granulocytic ehrlichiosis in dogs (15) and was recently found to cause some cases of granulocytic ehrlichiosis in humans (7). Most cases of human granulocytic ehrlichiosis are caused by a species referred to as the agent of human granulocytic ehrlichiosis (4). This bacterium has also been detected in dogs (19), deer (5), horses (20), and rodents (31). The name Anaplasma phagocytophilum has recently been proposed to include this bacterium, in addition to the species previously known as E. phagocytophilum and E. equi (14), and this proposed name is used in the present study.
Most studies of the prevalence of infection with Ehrlichia spp. in dogs have been based on serologic methods assays that often used antigens derived from E. canis. Because of serologic cross-reactions between E. canis and other Ehrlichia species, including E. chaffeensis and E. ewingii (25, 29), these studies do not provide identification of the species that elicits production of anti-Ehrlichia antibodies in the host animal. Four studies have used molecular techniques and/or cell culture methods to identify the Ehrlichia species infecting dogs. In these studies, carried out in North Carolina (6, 22), Virginia (11), and Oklahoma (25), 24 dogs were infected with E. chaffeensis, 21 were infected with E. canis, 19 were infected with E. ewingii, 10 were infected with E. platys, and 1 was infected with A. phagocytophilum. A recent study described 15 dogs with E. ewingii infection proven by PCR (18).
In our laboratory at Washington University Medical Center in St. Louis, Mo., we have detected nearly 200 cases of human ehrlichiosis in recent years by using PCR; 89% of these cases were caused by E. chaffeensis and 11% were caused by E. ewingii. To learn more about possible relationships between human and canine ehrlichiosis, we studied the occurrence and species distribution of Ehrlichia in pet dogs in Missouri. The focus of the study was on ill dogs with clinical manifestations suggestive of ehrlichiosis, but we also studied a smaller number of asymptomatic dogs.
MATERIALS AND METHODS
Canine subjects and blood samples.
Participating Missouri veterinarians were recruited by the staff at the University of Missouri College of Veterinary Medicine. Participating veterinarians were asked to submit blood samples from dogs that they suspected of having ehrlichiosis on the basis of a distributed list of clinical manifestations of granulocytic or monocytic ehrlichiosis; these clinical manifestations included fever, evidence of musculoskeletal disease, hepatomegaly, splenomegaly, uveitis, seizures, hemorrhage, cytopenias, hyperglobulinemia, presence of morulae in a preripheral blood smear, and presence of ticks on the dog. EDTA-anticoagulated whole blood and serum specimens were collected from each dog for laboratory testing. For each dog with suspected ehrlichiosis included in the study, veterinarians were also asked to submit whole-blood and serum specimens from another dog under their care at the same time that was not ill (e.g., dogs being seen for routine immunizations or dogs being boarded under the supervision of the veterinarian). Thirty-five veterinarians submitted samples from 88 dogs from March 2000 through January 2001; the samples were mailed to the Virology Laboratory at St. Louis Children's Hospital. The veterinarians also provided clinical and epidemiologic data for each dog by using a standardized data collection form. The first day of observed illness was known for 23 dogs. For these 23, samples were obtained after a median interval of 4 days (range, 0 to 31).
PCR testing.
Leukocyte lysates were prepared from whole-blood specimens as described previously (7). Broad range Ehrlichia PCR was performed with primers (ECA and HE3) that bind to segments of the 16S rRNA gene that are conserved among all pathogenic Ehrlichia and A. phagocytophilum. The Ehrlichia species was determined by additional reactions with sets of primers specific for E. chaffeensis (HE1 and HE3) (2), E. ewingii (EW1 and HE3) (33), and E. canis (11). Samples positive with the broad-range primers were also tested with primers EHR 521 and EHR 747 that amplify A. phagocytophilum, as well as other Anaplasma spp. (27). Samples positive with EHR 521 and EHR 747 were also tested with primers GE9F and GE2 that amplify a portion of the 16S rRNA gene of A. phagocytophilum, as well as the closely related white-tailed deer agent (23), and also with a nested assay that specifically amplifies a 1,256-bp segment of the A. phagocytophilum groESL operon (28).
Serology.
Canine serum specimens were tested for immunoglobulin G (IgG) antibodies reactive with E. chaffeensis by using an indirect immunofluorescent-antibody assay (IFA), as described previously (9). Fluorescein isothiocyanate-labeled goat anti-dog IgG (γ-specific) conjugate was used at a dilution of 1/150. Serum samples were screened at a 1/32 dilution; specimens reactive at this dilution were titrated to the end point. Antibody titers were expressed as the greatest reciprocal dilution for which specific reactivity was observed. Dogs were considered seropositive if the IFA titer was ≥64.
Sequencing.
Amplified products from the Ehrlichia broad-range assay performed on DNA extracted from canine whole blood were sequenced at Washington University School of Medicine. The sequencing reaction contained 125 ng of purified amplicon, 3.2 pmol of primer, BigDye terminators (Applied Biosystems, Inc., Foster City, Calif.), and AmpliTaq FS DNA polymerase. Extension products were analyzed in an automated DNA sequncer (model 377; Applied Biosystems). The primers used for sequencing of the broad-range PCR product were HE3 (2) and PER-1R (17).
Nucleotide sequence accession number.
The GenBank accession number of the 1,256-bp groESL sequence amplified from a Missouri dog is AY219849.
Statistical methods.
Categorical data were compared by using the chi-square test or the Fisher exact test. A P value of <0.05 was considered statistically significant. Statistical analyses were carried out by using Epi Info 2000 (Centers for Disease Control and Prevention).
RESULTS
A total of 88 pet dogs were included in the study, including 78 (89%) that were ill and 10 (11%) that were asymptomatic. The dogs included a wide variety of breeds, of which the most common were Labradors and Golden Retrievers (n = 22, including mixes). Fifty-six percent were female, and the mean age was 4.6 years (range, 1 to 13 years). Fever and musculoskeletal signs (i.e., lameness, reluctance to rise or move, walking with a stiff or stilted gait, or painful or swollen joints) were the most frequent clinical findings. Other reported findings included current or recent hemorrhage, organomegaly, uveitis, and neurologic signs.
Routine laboratory test results were available for only a minority of the dogs and indicated that 19 dogs had thrombocytopenia (platelet count, <200,000/μl), 20 had anemia, 10 had leukopenia, and 4 had hyperglobulinemia. Granulocytic morulae were observed on peripheral blood smears from two dogs that were later found to be PCR positive for E. ewingii.
The results of PCR testing of the 88 dogs are shown in Table 1. Ehrlichia or Anaplasma DNA was detected in the blood of 22 (25%) of the 88 dogs, including 20 (26%) of the ill dogs and 2 (20%) of the asymptomatic dogs. Species-specific PCR testing revealed 19 infections with E. ewingii, 1 with E. chaffeensis, and 1 with A. phagocytophilum. One additional dog was determined to be positive by the broad-range assay but negative by the species-specific assays. The species identity of this dog's infection was determined to be E. ewingii by nucleotide sequencing of a portion of the 16S rRNA gene. The failure of the species-specific assay to yield the species identity was probably related to the fact that the species-specific assays are less sensitive than the broad-range assay for the detection of Ehrlichia DNA (unpublished data). E. canis was not detected in any dog. Two dogs positive for E. ewingii were also positive in the screening assay for A. phagocytophilum (primers EHR 521 and EHR 747) but negative with the confirmatory assays that amplify segments of the 16S ribosomal DNA gene and the groESL operon of A. phagocytophilum and were thus considered to be positive only for E. ewingii. Thus, infection with multiple Ehrlichia species was not detected in any dogs in the present study.
TABLE 1.
Results of Ehrlichia PCR testing of Missouri dogs
| Status | Total no. of dogs | No. (%) of dogs PCR positive for:
|
|||
|---|---|---|---|---|---|
| E. ewingii | E. chaffeensis | E. canis | A. phagocyto- philum | ||
| Ill | 78 | 18 (23) | 1 (1) | 0 | 1 (1) |
| Asymptomatic | 10 | 2 (20) | 0 | 0 | 0 |
| Combined | 88 | 20 (20) | 1 (1) | 0 | 1 (1) |
Because human cases of ehrlichiosis caused by A. phagocytophilum have been rare in Missouri (24), we carried out nucleotide sequencing of portions of the 16S rRNA gene and the groESL heat shock operon amplified from the blood of the dog that was positive for A. phagocytophilum. Sequencing of the 16S ribosomal gene segment was performed by using the sequencing primer PER-1R, which provides the sequence of a 126-bp segment that spans the highly variable region. The sequence determined matched the published sequence of A. phagocytophilum (GenBank accession no. U02521) (8). The nucleotide sequence of the segment of the groESL operon amplified by PCR was very similar to or identical to sequences previously determined for A. phagocytophilum.
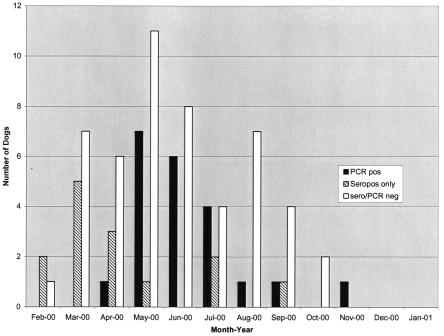
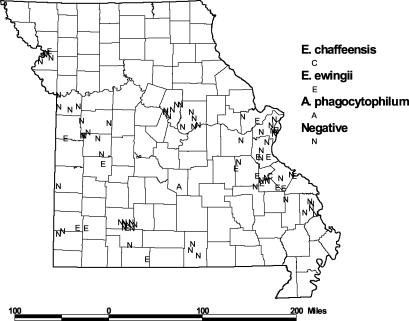
There were no significant differences between PCR-positive and PCR-negative dogs in gender, proportion fertile, mean age, or the presence of fever or musculoskeletal symptoms, thrombocytopenia, or anemia (Table 2). Definite tick exposure (tick currently embedded or recently removed) was reported in 75% of PCR-positive compared to 50% of PCR-negative dogs (P = 0.05 [chi square]). As shown in Fig. 1, most (81%) of the PCR-positive cases occurred during May through July. Figure 2 shows the distribution of PCR-positive and PCR-negative samples within the state of Missouri. Most specimens were submitted from the southern portion of the state. Positive dogs were located throughout this region, with a cluster of positives in four counties (Jefferson, Washington, St. Francois, and St. Genevieve) located south of St. Louis and a smaller cluster in the southwest portion of the state.
TABLE 2.
Clinical characteristics of Missouri dogs tested for Ehrlichia
| Characteristic or finding | No. of animals with data available | No. (%) of animals that werea:
|
|||
|---|---|---|---|---|---|
| Ill (n = 78)
|
Asymptomatic (n = 10)
|
||||
| PCR positive (n = 20) | PCR negative (n = 58) | PCR positive (n = 2) | PCR negative (n = 8) | ||
| Male | 85 | 6 (32) | 26 (46) | 1 (100) | 4 (50) |
| Fertile | 81 | 5 (29) | 19 (35) | 0 | 1 (13) |
| Mean age (yr) | 85 | 5.1 | 4.7 | 4.8 | 3.1 |
| Febrile | 78 | 9 (45) | 26 (45) | NA | NA |
| Musculoskeletal findingsb | 78 | 15 (75) | 37 (64) | NA | NA |
| Tick exposure | 78 | 15 (75)* | 29 (50)* | NA | NA |
| Thrombocytopenia | 22 | 6 (86) | 14 (93) | NA | NA |
| Anemia | 22 | 3 (60) | 17 (100) | NA | NA |
Except where indicated (i.e., mean age), entries in the table indicate the number of dogs and the percentage of those tested that had the indicated characteristic or finding. *, P = 0.05 (chi-square analysis). NA, not available.
Defined as lameness, reluctance to move, walking with a stiff or stilted gait, or painful or swollen joints.
FIG. 1.
Seasonal occurrence of ehrlichiosis in Missouri dogs.
FIG. 2.
Geographical distribution of dogs included in the present study by county of residence. The results of Ehrlichia PCR assays are shown as designated in the key.
Sera from 85 dogs, including 76 ill and 9 asymptomatic animals, were tested by IFA for antibodies reactive with E. chaffeensis. Table 3 shows the results compared to results of PCR testing. Of the 85 dogs, 26 (31%) had IgG antibodies reactive with E. chaffeensis at a titer of ≥64, including 14 (67%) of 21 that were PCR positive and 12 (19%) of 64 that were PCR negative (P < 0.001 [chi square]). IFA was performed on samples from 19 dogs that were PCR positive for E. ewingii by PCR. Of these, 13 (68%) had titers of ≥64 (range, <32 to 2,048; geometric mean titer, 142). The single dog that was positive for E. chaffeensis by PCR had a reciprocal titer of 64, and the single dog that was positive for A. phagocytophilum by PCR was negative for antibodies reactive with E. chaffeensis. Of the 76 ill dogs tested, 24 (32%) were IFA positive compared to 2 (22%) of the asymptomatic dogs (P = 0.7 [Fisher exact test]). In all, 33 (39%) of the 85 dogs tested by PCR and IFA had evidence of either past or current Ehrlichia exposure based on either a positive PCR or positive serology.
TABLE 3.
Results of Ehrlichia serologic testing of Missouri dogs compared to results of Ehrlichia PCR
| Status | No. of dogs evaluated | No. (%) of dogs with resulta
|
|||
|---|---|---|---|---|---|
| PCR+/ IFA+ | PCR+/ IFA− | PCR−/ IFA+ | PCR−/ IFA− | ||
| Ill | 76 | 14 (18)* | 6 (8) | 10 (13) | 46 (61) |
| Asymptomatic | 9 | 0 | 1 (11) | 2 (22) | 6 (66) |
| Combined | 85 | 14 (16) | 7 (8) | 12 (14) | 52 (61) |
Test results are indicated as PCR+/IFA+, PCR positive and IFA positive, etc.
Although the most likely explanation for the finding of positive serology with a negative PCR in 12 dogs is that they had past infection, another possible explanation is the effect of antibiotic therapy given for the acute illness. Antibiotic prescribing information was available for 60 dogs at the time of sample collection. Nineteen had received antibiotics for at least 1 day before testing (range, 1 day to 7 months prior to sample collection); seven of these animals had received an antibiotic with significant anti-Ehrlichia activity (doxycycline or chloramphenicol). One of the seven was PCR positive and IFA negative after 6 days of chloramphenicol treatment, one was PCR negative but IFA positive after receiving 4 weeks of doxycycline, and the remaining five were PCR negative and IFA negative.
One possible explanation for the finding of seronegativity in seven PCR-positive dogs (six ill and one asymptomatic) could have been that blood samples were obtained early in the illness before a serologic response had occurred. Information on the day of onset of illness was available for three of the six ill dogs with this finding; in these dogs, the samples were obtained on days 3, 3, and 30 after onset of symptoms.
DISCUSSION
This study of the Ehrlichia species present in dogs in Missouri revealed several notable results. The first was the finding that more than 90% of dogs with molecular evidence of current Ehrlichia infection were infected with E. ewingii. Although E. ewingii had previously been demonstrated as a cause of ehrlichiosis in Missouri dogs (30), no study had yet documented its presence by molecular methods. The distribution of Ehrlichia species in Missouri dogs differs dramatically from that in humans with ehrlichiosis acquired in the state. In our laboratory, which receives human specimens from a geographic region similar to the region from which dog samples were provided for the present study, E. chaffeensis has accounted for 89% of the cases, with E. ewingii accounting for the remaining 11%. One explanation for this discrepancy may be differences in host pathogenicity; namely, E. chaffeenis may be more pathogenic for humans, and E. ewingii may be more pathogenic for dogs. Additional molecular studies of the prevalence of Ehrlichia in asymptomatic dogs would help clarify these results.
The absence of E. canis in the present study is also noteworthy. One other molecular study of canine ehrlichiosis, performed in Virginia, found only E. chaffeensis and E. ewingii, without any cases of E. canis infection (11). We do not think the absence of E. canis in the present study is the result of the failure of the PCR assay used to detect E. canis, since the PCR primers in the broad-range assay used for initial screening can amplify the DNA of E. canis. Previous studies of the causes of canine ehrlichiosis that were based on serology may have failed to make definitive species identification because of serologic cross-reactions among members of the Ehrlichia, including E. chaffeensis and E. ewingii. Specifically, it is possible that some cases of E. ewingii infection were mistakenly attributed to E. canis infection. An alternative explanation for the preponderance of infections with E. ewingii is that participating veterinarians selected dogs for inclusion in the present study who had symptoms such as arthritis that are associated with E. ewingii infection.
The detection of a dog infected with A. phagocytophilum or a closely related species was surprising. We have not detected A. phagocytophilum in our extensive experience with human ehrlichiosis in Missouri. It is possible that the organism does exist at low levels in Missouri and simply escapes detection as a human pathogen. It is also possible that the agent detected was a species related to but not identical to A. phagocytophilum, although we think this is unlikely because of the very close similarity of the groESL sequence determined in the present study to many different A. phagocytophilum sequences determined in the laboratory of one of the authors (J.W.S.). Because complete travel histories were not available, we cannot exclude the possibility that this dog was infected out of the state.
Serologic testing for antibodies reactive with E. chaffeensis revealed that 31% of dogs had serologic evidence of past or present infection with Ehrlichia. Combining the results of molecular and serologic testing, 39% of all dogs tested had evidence of past or present infection with Ehrlichia, indicating frequent exposure of Missouri dogs residing in the survey regions of Missouri to this group of bacteria. Discrepancies between the results of serologic and molecular tests observed for some animals were not unexpected. The 12 dogs that were seropositive but PCR negative probably had past Ehrlichia infection. The fact that these cases were evenly distributed throughout the year supports this explanation. It is also possible that some of these dogs had recent infection but were PCR negative because of antibiotic treatment. However, only seven dogs in the study were known to have received antibiotics with activity against Ehrlichia, and only one was PCR positive and IFA negative, which indicates that antibiotic therapy was not the explanation for this finding. Finally, the sensitivity of PCR as a method for detecting acute canine ehrlichiosis has not been determined, and it is possible that PCR was falsely negative in some of these dogs.
Several explanations are possible for the seven dogs that were PCR positive but seronegative. Some of these dogs may have been sampled very early in the course of their infection before an antibody response had occurred. Unfortunately, the interval between the day of onset of symptoms and the day when the blood sample was obtained was not available for all dogs. Another possible explanation may have been failure to make an antibody responses to acute Ehrlichia infection in some of these dogs. Convalescent-phase samples were not available to test this hypothesis. It is also possible that in sera from some dogs, the E. chaffeensis antigen used in the IFA may have failed to detect antibodies produced in response to infection with E. ewingii. This possibility is supported by the observation of inconsistent seroreactivity with E. canis antigen in serum from dogs found to be positive for E. ewingii DNA by PCR (16, 18).
There were no differences among the dogs with or without confirmed ehrlichiosis by sex, age, breed, or fertility status. The larger overall representation of retrievers in the study sample may be explained by the popularity of these breeds as pets, but data on breed prevalence for the state were not available. Expected early summer peaks in both total suspected tick-borne illnesses and in actual PCR-positive cases of ehrlichiosis were noted. Prior studies have noted higher incidence, mortality rate, and chronicity among German shepherd dogs in South Africa with E. canis infection (32). However, no particular breed stood out in our study as having increased incidence.
We highlight here the potential relationships between human and canine ehrlichiosis. The finding that two of ten asymptomatic dogs were PCR positive for E. ewingii suggests that dogs might serve as a reservoir for E. ewingii. Goodman et al. (18) also recently showed evidence of asymptomatic dogs that were PCR positive for E. ewingii. The two asymptomatic PCR-positive dogs in the present study were sampled in March and April, months which are earlier in the year than those in which most cases of human ehrlichiosis occur in Missouri. This finding raises the possibility that chronic canine Ehrlichia infection could be a source for subsequent infections with Ehrlichia in humans residing in the same areas. It is probably more likely that dogs and humans share similar exposures to infecting ticks, suggesting that cases of canine ehrlichiosis may serve as sentinels for human cases, as described for other tick-borne infections, including Rocky Mountain spotted fever (26). Most cases of suspected canine ehrlichiosis do not currently undergo testing to reveal the etiologic agent. If confirmatory testing becomes more widely adopted, results could assist human public health officials in identifying environments where the risk of acquiring human ehrlichiosis is high.
Acknowledgments
We are indebted to the veterinarians of Missouri who contributed samples for this study. We are grateful to Jim Struthers and Mario Schootman from the Epidemiology and Statistics Section of the Division of Health Behavior Research, Departments of Pediatrics and Medicine, Washington University School of Medicine, for assistance in preparing the figure showing the location of study dogs throughout the state of Missouri and to Barbara Hartman for assistance with preparation of the manuscript.
REFERENCES
- 1.Anderson, B. E., J. E. Dawson, D. C. Jones, and K. H. Wilson. 1991. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J. Clin. Microbiol. 29:2838-2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, B. E., J. W. Sumner, J. E. Dawson, T. Tzianabos, C. R. Greene, J. G. Olson, D. B. Fishbein, M. Olsen-Rasmussen, B. P. Holloway, E. H. George, and A. F. Azad. 1992. Detection of the etiologic agent of human ehrlichiosis by polymerase chain reaction. J. Clin. Microbiol. 30:775-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arens, M. Q., A. M. Liddell, G. Buening, M. Gaudreault-Keener, J. W. Sumner, J. A. Comer, R. S. Buller, and G. A. Storch. 2003. Detection of Ehrlichia spp. in the blood of wild white-tailed deer in Missouri by PCR assay and serologic analysis. J. Clin. Microbiol. 41:1263-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakken, J. S., J. S. Dumler, S. M. Chen, M. R. Eckman, L. L. Van Etta, and D. H. Walker. 1994. Hum. granulocytic ehrlichiosis in the upper Midwest United States: a new species emerging? JAMA 272:212-218. [PubMed] [Google Scholar]
- 5.Belongia, E. A., K. D. Reed, P. D. Mitchell, C. P. Kolbert, D. H. Persing, J. S. Gill, and J. J. Kazmierczak. 1997. Prevalence of granulocytic Ehrlichia infection among white-tailed deer in Wisconsin. J. Clin. Microbiol. 35:1465-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breitschwerdt, E. B., B. C. Hegarty, and S. I. Hancock. 1998. Sequential evaluation of dogs naturally infected with Ehrlichia canis, Ehrlichia chaffeensis, Ehrlichia equi, Ehrlichia ewingii, or Bartonella vinsonii. J. Clin. Microbiol. 36:2645-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buller, R. S., M. Arens, S. P. Hmiel, C. D. Paddock, J. W. Sumner, Y. Rikhisa, A. Unver, M. Gaudreault-Keener, F. A. Manian, A. M. Liddell, N. Schmulewitz, and G. A. Storch. 1999. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N. Engl. J. Med. 341:148-155. [DOI] [PubMed] [Google Scholar]
- 8.Chen, S. M., J. S. Dumler, J. S. Bakken, and D. H. Walker. 1994. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J. Clin. Microbiol. 32:589-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Comer, J. A., W. L. Nicholson, C. D. Paddock, J. W. Sumner, and J. E. Childs. 2000. Detection of antibodies reactive with Ehrlichia chaffeensis in the raccoon. J. Wildl. Dis. 36:705-712. [DOI] [PubMed] [Google Scholar]
- 10.Davidson, W. R., J. M. Lockhart, D. E. Stallknecht, E. W. Howerth, J. E. Dawson, and Y. Rechav. 2001. Persistent Ehrlichia chaffeensis infection in white-tailed deer. J. Wildl. Dis. 37:538-546. [DOI] [PubMed] [Google Scholar]
- 11.Dawson, J. E., K. L. Biggie, C. K. Warner, K. Cookson, S. Jenkins, J. F. Levine, and J. G. Olson. 1996. Polymerase chain reaction evidence of Ehrlichia chaffeensis, an etiologic agent of human ehrlichiosis, in dogs from southeast Virginia. Am. J. Vet. Res. 57:1175-1179. [PubMed] [Google Scholar]
- 12.Dawson, J. E., and S. A. Ewing. 1992. Susceptibility of dogs to infection with Ehrlichia chaffeensis, causative agent of human ehrlichiosis. Am. J. Vet. Res. 53:1322-1327. [PubMed] [Google Scholar]
- 13.Dugan, V. G., S. E. Little, D. E. Stallknecht, and A. D. Beall. 2000. Natural infection of domestic goats with Ehrlichia chaffeensis. J. Clin. Microbiol. 38:448-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumler, J. S., A. F. Barbet, C. P. Bekker, G. A. Dasch, G. H. Palmer, S. C. Ray, Y. Rikihisa, and F. R. Rurangirwa. 2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and “HGE agent” as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 51:2145-2165. [DOI] [PubMed] [Google Scholar]
- 15.Ewing, S. A., W. R. Roberson, R. G. Buckner, and C. S. Hayat. 1971. A new strain of Ehrlichia canis. J. Am. Vet. Med. Assoc. 159:1771-1774. [PubMed] [Google Scholar]
- 16.Goldman, E. E., E. B. Breitschwerdt, C. B. Grindem, B. C. Hegarty, J. J. Walls, and J. S. Dumler. 1998. Granulocytic ehrlichiosis in dogs from North Carolina and Virginia. J. Vet. Intern. Med. 12:61-70. [DOI] [PubMed] [Google Scholar]
- 17.Goodman, J. L., C. Nelson, B. Vitale, J. E. Madigan, J. S. Dumler, T. J. Kurtti, and U. G. Munderloh. 1996. Direct cultivation of the causative agent of human granulocytic ehrlichiosis. N. Engl. J. Med. 334:209-215. [DOI] [PubMed] [Google Scholar]
- 18.Goodman, R. A., E. C. Hawkins, N. J. Olby, C. B. Grindem, B. Hegarty, and E. B. Breitschwerdt. 2003. Molecular identification of Ehrlichia ewingii infection in dogs: 15 cases (1997-2001). JAVMA 222:1102-1107. [DOI] [PubMed] [Google Scholar]
- 19.Greig, B., K. M. Asanovich, P. J. Armstrong, and J. S. Dumler. 1996. Geographic, clinical, serologic, and molecular evidence of granulocytic ehrlichiosis, a likely zoonotic disease, in Minnesota and Wisconsin dogs. J. Clin. Microbiol. 34:44-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansson, K. E., B. Pettersson, M. Uhlen, A. Gunnarsson, M. Malmqvist, and E. Olsson. 1995. Identification of the causative agent of granulocytic ehrlichiosis in Swedish dogs and horses by direct solid phase sequencing of PCR products from the 16S rRNA gene. Res. Vet. Sci. 58:109-112. [DOI] [PubMed] [Google Scholar]
- 21.Kocan, A. A., G. C. Levesque, L. C. Whitworth, G. L. Murphy, S. A. Ewing, and R. W. Barker. 2000. Naturally occurring Ehrlichia chaffeensis infection in coyotes from Oklahoma. Emerg. Infect. Dis. 6:477-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kordick, S. K., E. B. Breitschwerdt, B. C. Hegarty, K. L. Southwick, C. M. Colitz, S. I. Hancock, J. M. Bradley, R. Rumbough, J. T. McPherson, and J. N. MacCormack. 1999. Coinfection with multiple tick-borne pathogens in a Walker Hound kennel in North Carolina. J. Clin. Microbiol. 37:2631-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massung, R. F., K. Slater, J. H. Owens, W. L. Nicholson, T. N. Mather, V. B. Solberg, and J. G. Olson. 1998. Nested PCR assay for detection of granulocytic ehrlichiae. J. Clin. Microbiol. 36:1090-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McQuiston, J. H., C. D. Paddock, R. C. Holman, and J. E. Childs. 1999. The human ehrlichioses in the United States. Emerg. Infect. Dis. 5:635-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy, G. L., S. A. Ewing, L. C. Whitworth, J. C. Fox, and A. A. Kocan. 1998. A molecular and serologic survey of Ehrlichia canis, E. chaffeensis, and E. ewingii in dogs and ticks from Oklahoma. Vet. Parasitol. 79:325-339. [DOI] [PubMed] [Google Scholar]
- 26.Paddock, C. D., O. Brenner, C. Vaid, D. B. Boyd, J. M. Berg, R. J. Joseph, S. R. Zaki, and J. E. Childs. 2002. Short report: concurrent Rocky Mountain spotted fever in a dog and its owner. Am. J. Trop. Med. Hyg. 66:197-199. [DOI] [PubMed] [Google Scholar]
- 27.Pancholi, P., C. P. Kolbert, P. D. Mitchell, K. D. Reed, Jr., J. S. Dumler, J. S. Bakken, S. R. Telford III, and D. H. Persing. 1995. Ixodes dammini as a potential vector of human granulocytic ehrlichiosis. J. Infect. Dis. 172:1007-1012. [DOI] [PubMed] [Google Scholar]
- 28.Petrovec, M., J. W. Sumner, W. L. Nicholson, J. E. Childs, F. Strle, J. Barlic, S. Lotric-Furlan, and T. Avsic Zupanc. 1999. Identity of ehrlichial DNA sequences derived from Ixodes ricinus ticks with those obtained from patients with human granulocytic ehrlichiosis in Slovenia. J. Clin. Microbiol. 37:209-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rikihisa, Y., S. A. Ewing, and J. C. Fox. 1994. Western immunoblot analysis of Ehrlichia chaffeensis, E. canis, or E. ewingii infections in dogs and humans. J. Clin. Microbiol. 32:2107-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stockham, S. L., D. A. Schmidt, K. S. Curtis, B. G. Schauf, J. W. Tyler, and S. T. Simpson. 1992. Evaluation of granulocytic ehrlichiosis in dogs of Missouri, including serologic status to Ehrlichia canis, Ehrlichia equi, and Borrelia burgdorferi. Am. J. Vet. Res. 53:63-68. [PubMed] [Google Scholar]
- 31.Telford, S. R., III, J. E. Dawson, P. Katavolos, C. K. Warner, C. P. Kolbert, and D. H. Persing. 1996. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc. Natl. Acad. Sci. USA 93:6209-6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Heerden, J. 1982. A retrospective study on 120 natural cases of canine ehrlichiosis. J. S. Afr. Vet. Assoc. 53:17-22. [PubMed] [Google Scholar]
- 33.Warner, C., and J. Dawson. 1996. Genus- and species-level identification of Ehrlichia species by PCR and sequencing, p. 100-105. In D. H. Persing (ed.), PCR protocols for emerging infectious diseases: a supplement to diagnostic molecular microbiology: principles and applications. ASM Press, Washington, D.C.