Abstract
The immunoglobulin (Ig) molecule is composed of two identical heavy chains and two identical light chains (H2L2). Transport of this heteromeric complex is dependent on the correct assembly of the component parts, which is controlled, in part, by the association of incompletely assembled Ig heavy chains with the endoplasmic reticulum (ER) chaperone, BiP. Although other heavy chain-constant domains interact transiently with BiP, in the absence of light chain synthesis, BiP binds stably to the first constant domain (CH1) of the heavy chain, causing it to be retained in the ER. Using a simplified two-domain Ig heavy chain (VH-CH1), we have determined why BiP remains bound to free heavy chains and how light chains facilitate their transport. We found that in the absence of light chain expression, the CH1 domain neither folds nor forms its intradomain disulfide bond and therefore remains a substrate for BiP. In vivo, light chains are required to facilitate both the folding of the CH1 domain and the release of BiP. In contrast, the addition of ATP to isolated BiP–heavy chain complexes in vitro causes the release of BiP and allows the CH1 domain to fold in the absence of light chains. Therefore, light chains are not intrinsically essential for CH1 domain folding, but play a critical role in removing BiP from the CH1 domain, thereby allowing it to fold and Ig assembly to proceed. These data suggest that the assembly of multimeric protein complexes in the ER is not strictly dependent on the proper folding of individual subunits; rather, assembly can drive the complete folding of protein subunits.
INTRODUCTION
Immunoglobulin (Ig) molecules are the cornerstone of humoral immunity, playing essential roles as both membrane-bound receptor complexes in the development and activation of B lymphocytes and as secreted effector molecules. In addition, Ig molecules provide an excellent model for studies concerning the biosynthesis of multimeric protein complexes and the role of molecular chaperones in the folding and assembly of the individual protein subunits that form higher-order structures. In their simplest form, Igs are comprised of two identical heavy chains disulfide bonded to two identical light chains, forming an H2L2 molecule. Crystallographic analyses and in vitro folding studies indicate that each chain consists of a series of Ig domains that fold independently of each other (Goto and Hamaguchi, 1982; Lilie et al., 1995) into a compact structure composed of two twisted β sheets stabilized by a single disulfide bond (Amzel and Poljak, 1979). Developmentally, Ig heavy chains are synthesized before conventional light chain (LC) genes are rearranged and expressed. In pre-B cells, the heavy chains form homodimers (Kaloff and Haas, 1995); however, without LCs they are efficiently retained in the endoplasmic reticulum (ER) due to their association with the ER chaperone, BiP (Haas and Wabl, 1983; Bole et al., 1986). Initiation of LC protein expression allows the formation of H2L2 molecules. As is true of many other multimeric proteins, this assembly occurs in the ER, and incorrectly or incompletely assembled Ig molecules remain bound to BiP and are eventually targeted for degradation (Sitia et al., 1990; Gardner et al., 1993; Knittler et al., 1995).
BiP was first identified as a resident ER protein associated with incompletely assembled Ig heavy chains (Haas and Wabl, 1983; Bole et al., 1986). Subsequent studies have shown that BiP is expressed in all cell lineages (Bole et al., 1986) and associates transiently with numerous secretory pathway proteins and more stably with mutant counterparts of these proteins (Gething and Sambrook, 1992). This promiscuous binding suggests that BiP does not recognize a unique linear amino acid sequence. In vitro studies revealed that BiP preferentially binds peptides with the heptameric motif of HyXHyXHyXHy, where Hy is a bulky aromatic or hydrophobic residue and X is any amino acid (Blond-Elguindi et al., 1993). The alternating pattern of hydrophobic residues in the binding motif is compatible with BiP binding to proteins when they are in an extended conformation, in which the bulky aromatic/hydrophobic side chains lie on one side of the protein and presumably point into the protein-binding site of BiP. It is believed that these hydrophobic residues would be buried upon protein folding, thus providing a mechanism for the transient interaction of nascent proteins with BiP and the more stable association of mutant proteins that are defective in folding.
Although BiP binds transiently to the subunits of a wide variety of multimeric proteins (Gething and Sambrook, 1992), its associations with Ig heavy and light chains are among the best characterized. BiP binds transiently to the nascent Ig LC in vivo when the variable region is in an unfolded state (Hendershot et al., 1996; Skowronek et al., 1998; Hellman et al., 1999), which is in keeping with the predictions from the peptide-binding studies, and releases as the domain folds. Although BiP can bind transiently to multiple heavy-chain domains (Kaloff and Haas, 1995), the stable BiP binding site on unassembled heavy chains is the CH1 domain (Hendershot et al., 1987), which is involved in both hydrophobic and covalent interactions with the CL domain of the LC. Unlike wild-type (WT) heavy chains, mutants that lack the CH1 domain can be secreted as partially assembled molecules (Hendershot et al., 1987), demonstrating that BiP regulates the transport of assembling Ig molecules. The reason that BiP binds stably to the CH1 domain, but only transiently or not at all with other Ig domains, is not understood. It is possible that BiP can bind to both linear hydrophobic stretches on nascent chains before they fold (e.g., VL) and to hydrophobic faces on folded protein subunits before they assemble into multimeric complexes (e.g., CH1 of Ig heavy chain). Conversely, BiP may bind to unfolded regions of the proteins in both cases if the CH1 domain is unable to fold until it successfully pairs with a LC. Because transport of the Ig heavy chain appears to be central to the fidelity of B cell development and the integrity of the antibody response and because Ig molecules can serve as a paradigm for the mechanisms that control subunit assembly of multimeric proteins, we have defined, at the molecular level, the sequence of events that control Ig assembly and transport. We demonstrate that BiP and LC cooperate to ensure that only properly assembled Ig molecules are transported from the ER by controlling the final folding of the heavy chain.
MATERIALS AND METHODS
Construction of Ig Heavy-Chain Mutants
CH1 Deletion Mutant.
To produce the various heavy-chain constructs needed for these analyses, we started with a chimeric mouse–human γ heavy chain cDNA (generously provided by Dr. Randy Robinson, Xoma Corporation) that we have used previously for transient expression and BiP-binding experiments. The chimeric cDNA was constructed by joining the variable region from a mouse monoclonal antibody to the last five amino acids of a human J gene and the constant region of the human γ1 gene (Liu et al., 1987). We removed the CH1 domain from this construct using the deletional overlap-extension PCR method (Ho et al., 1989). Oligonucleotides were designed to produce in-frame joining of the final residue of the variable region, Ser113, with the first amino acid of the hinge region, Glu212. The PCR product was digested with XhoI and SacII and ligated into the chimeric γ cDNA in place of the WT sequence. The entire cDNA clone was sequenced before it was recloned into the pSVL eucaryotic expression vector (γΔCH1) for use in transfection studies. The deletion was based on a murine plasmacytoma in which the CH1 exon was removed by an alternative splice that occurred due to a mutation in the splice acceptor of the CH1 domain (Brandt et al., 1984). It was anticipated that this cDNA would encode a heavy chain mutant that would dimerize and be secreted in the absence of LC association (Morrison, 1978; Hendershot et al., 1987).
V-CH1 Truncation Mutant.
Using the chimeric heavy-chain cDNA, we produced a truncation mutant that was comprised of only the variable and CH1 domain. This was accomplished by PCR amplification of a region between the AspI site in the CH1 domain and the end of the CH1 domain-coding sequence. The 3′-oligo was designed to incorporate a stop codon immediately after the 3′-end of the domain (valine211) followed by a BamHI site. The product was digested and inserted into the heavy-chain cDNA in place of the original sequence. After sequencing, the truncated cDNA was inserted into the pSVL eucaryotic expression vector (γCH1). In addition to lacking the CH2 and CH3 domains, the truncation mutant no longer contained the hinge region that includes the cysteine residue required for covalent attachment of LC and the cysteine residues involved in heavy-chain dimerization. Two copies of a nine-amino acid epitope (YPYDVPDYA) from the influenza hemagglutinin protein (HA-tag) were added to its carboxy terminus. This was accomplished by first amplifying the variable and CH1 domains using T7 as the 5′-primer and GGAATTCAGCCCGTAGTCTGGGACGTC-GTATGGGTAT TGGCCAACTTTCTTGTCCACCTTGG as the 3′-primer. The MscI and EcoRI sites are underlined, the sequence encoding the tag is in boldface type, and the sequence complementary to the end of the CH1 domain is in lightface type. The PCR product was cut with XhoI and EcoRI and inserted into the pBEX vector, which places it immediately upstream and in frame with the HA-tag epitope. A second construct was made with a single epitope tag, by cutting the PCR product with XhoI and MscI and inserting into pBEX. This removed the tag from the PCR product and allowed in-frame insertion upstream of the HA-tag. Both were analyzed and provided similar experimental results; only data on the double-tagged truncation mutant are presented here because its size was more easily distinguished from the λ LC. The tagged construct was sequenced and recloned into pSVL (HA-γCH1) for transient expression experiments.
Transient Expression and Immunoprecipitations
The various γ heavy chain constructs were transiently expressed along with either WT or ATPase mutant hamster BiP cDNAs (Wei et al., 1995). Although BiP is already present in the COS cells, we cotransfected cells with a cDNA encoding hamster BiP in some experiments to ensure that BiP levels were not limiting. To examine the requirement for LCs in BiP release and heavy-chain folding, cDNAs encoding various LC constructs were cotransfected with the truncated heavy chains. These included either WT λI LC, mutant LCs that are unable to complete folding of either the variable domain (Vmut) or constant domain (Cmut) due to pair-wise substitution of serine residues for the cysteine residues involved in intradomain disulfide bond formation (C41 and C109 in the variable domain and C156 and C212 in the constant domain), or an ER-targeted constant domain (Hellman et al., 1999) construct. A cDNA encoding the NS-1 κ LC (Skowronek et al., 1998) was used as a control to monitor the effects of BiP release on domain folding and oxidation. COS-1 cells (2.5 × 106) were plated in 60-mm plastic dishes and transfected the following day by the DEAE-Dextran method as described previously (Hendershot et al., 1995). The constructs used in each experiment are indicated in the description of our results and the figure legends.
Cells were metabolically labeled with [35S] Translabel (ICN, Irvine, CA) 40 h after transfection, as indicated in RESULTS and figure legends. When labeled proteins were analyzed under reducing conditions, cells were washed twice with ice-cold PBS and lysed in our standard lysing buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 0.5% deoxycholic acid, and 0.5% NP40). When labeled proteins were analyzed under nonreducing conditions, cells were washed first with ice-cold PBS containing 20 mM N-ethylmaleimide (NEM) before lysing. NEM (20 mM) and apyrase (10 U/ml) were included in the lysing buffer. Variations in labeling and lysing conditions are detailed in RESULTS and figure legends. Labeled lysates were immunoprecipitated with either a polyclonal rabbit anti-rodent BiP antiserum (Hendershot et al., 1995), a monoclonal anti-BiP antibody (Bole et al., 1986), a polyclonal goat anti-mouse λ or goat anti-human γ antiserum (Southern Biotechnology Associates, Birmingham, AL), or a monoclonal antibody to the HA epitope (a kind gift of Dr. Al Reynolds, Vanderbilt University). Immune complexes were precipitated by binding to protein A-Sepharose beads (Sigma Chemical, St. Louis, MO). Proteins were resolved on SDS-polyacrylamide gels under either reducing or nonreducing conditions and visualized by fluorography using the Amplify Reagent (Amersham, Arlington Heights, IL).
BiP was released in vitro from the truncated heavy chain and the NS-1 κ LC by omitting NEM and apyrase from the lysing buffer and incubating the cell lysate with 2 mM Mg-ATP and 25 mM KCl at room temperature for 30 min. Heavy and light chains were immunoprecipitated with chain-specific antisera and analyzed on nonreducing gels to monitor oxidation of the CH1 and VL domains, respectively.
RESULTS
Is the Interaction of Mouse Heavy Chains with BiP in COS Fibroblast Cells Similar to That Described for Mouse Lymphoid Cells?
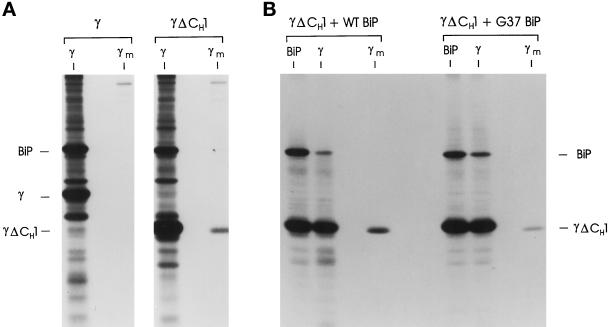
To examine the “stable” binding of BiP with the CH1 domain of unassembled Ig heavy chains, we wished to use the COS transient expression system, which is a much more tractable approach for examining numerous different constructs and combinations of them. First, we needed to ensure that monkey BiP would bind stably to the CH1 domain of the mouse Ig heavy chain and prevent its secretion as it does in mouse plasmacytomas (Hendershot et al., 1987). We produced an in-frame deletion mutant of the γ heavy chain that eliminated the CH1 domain (γΔCH1) but retained the hinge region that controls heavy chain dimerization. This construct was modeled on a mouse myeloma protein that no longer binds stably to BiP and can be secreted in the absence of LC pairing (Morrison, 1978; Brandt et al., 1984; Hendershot et al., 1987). cDNAs encoding the full-length γ heavy chain and the γΔCH1 mutant were transiently expressed in COS monkey fibroblast cells. The WT heavy chains associated with BiP and were not secreted during the 2-h labeling period (Figure 1A), which is consistent with heavy chain expression in mouse plasmacytoma lines (Bole et al., 1986; Hendershot et al., 1987). When cells expressing the γΔCH1 mutant were similarly analyzed, we found that although there was detectable association of the mutant heavy chain with BiP in the cell lysates, deletion of the CH1 domain allowed the heavy chain to be secreted without LC assembly (Figure 1A). Again, this is consistent with studies in mouse lymphoid lines that demonstrated BiP can bind transiently to other heavy chain domains (Kaloff and Haas, 1995), but that the CH1 domain is responsible for the stable interaction with BiP (Hendershot et al., 1987), which results in the retention of unassembled heavy chains (Morrison, 1978; Seligmann et al., 1979). This conclusion was further supported by data obtained when the CH1 domain mutant was coexpressed with a BiP ATPase mutant (G37) that acts as a kinetic trap for substrate proteins (Hendershot et al., 1996). In the presence of mutant BiP expression, there was an increase in the amount of BiP coprecipitating with the CH1-deleted heavy chain and a small decrease in its secretion (Figure 1B). This is consistent with BiP interacting transiently with some of the other heavy chain domains (Kaloff and Haas, 1995) and distinguishing BiP’s interaction with the CH1 domain (continuous) from that with the other Ig domains (transient). The fact that mutant BiP expression does not completely block the secretion of the CH1-deleted heavy chain demonstrates that the other domains on most of the chains must fold independently of BiP and that only a small portion of them binds to BiP. Thus, it appears that COS cells functionally recapitulate the binding of BiP to Ig heavy chains and provide a suitable system for studying this interaction.
Figure 1.
Expression of the CH1-deleted γ heavy chain. (A) COS cells transfected with cDNAs encoding WT γ or γΔCH1 heavy chains were metabolically labeled for 2 h. Cell lysates (γ) and culture supernatants (γm) were prepared and reacted with a goat anti- human γ heavy chain-specific antiserum. Immune complexes were precipitated with protein A-Sepharose beads and analyzed by SDS-PAGE under reducing conditions. (B) COS cells were cotransfected with cDNA for γΔCH1 heavy chains together with either WT or ATPase mutant (G37) hamster BiP. Cells were metabolically labeled for 45 min in the presence of DTT, washed, and chased in complete media devoid of DTT for 2 h. Cell lysates were prepared and immunoprecipitated with either anti-γ or anti-BiP antisera, and culture supernatants were reacted with anti-γ (γm). Immune complexes were reduced and analyzed by SDS-PAGE.
Can a Two-Domain Heavy Chain Be Created for Folding Studies That Mimics a Full-Length γ?
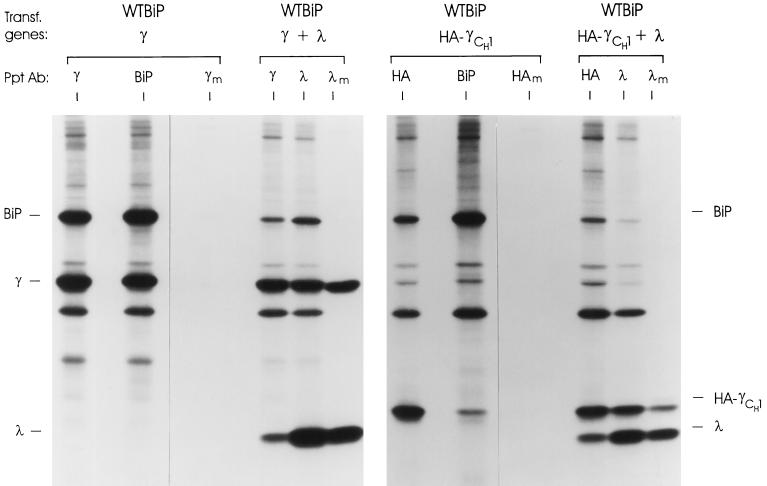
To analyze the interaction of BiP with the CH1 domain of free heavy chains, we wanted to be able to determine the folding status of this domain. One way to do this is to monitor the oxidation status of the domain, which is dependent on it folding correctly and can be assessed by the mobility of the protein on nonreducing gels (Hendershot et al., 1996; Hellman et al., 1999). However, full-length Ig heavy chains form disulfide-linked dimers that migrate at ∼110 kDa, making it difficult to monitor the folding of individual domains. Additionally, any changes in folding that might occur upon assembly with LCs could not be monitored by this type of analysis, since the LCs are covalently attached to the heavy chains via disulfide bonds. Thus, we constructed a simpler “heavy chain” for folding studies. We produced a two-domain heavy chain with a stop codon immediately 3′ of the CH1 coding region (γCH1), thereby removing the hinge region that includes the cysteine residues involved in heavy chain dimerization and in covalent attachment of LC. Although this truncated heavy chain could be coprecipitated with BiP, the anti-γ antiserum was unable to recognize it (our unpublished results), making it impossible to monitor secretion of the smaller free heavy chain. When λ LCs were also coexpressed, we found that the truncated heavy chain could combine with LC (albeit noncovalently) and be secreted, but again it was impossible to determine the efficiency of this reaction. To facilitate the immunoprecipitation studies of the truncated heavy chain, we added an epitope from the influenza hemagglutinin (HA) protein to the C terminus of the truncated heavy chain (HA-γCH1). cDNAs encoding either WT heavy chain or the truncated HA-γCH1 protein were coexpressed with either WT hamster BiP or λ LCs. In the absence of LC expression, both the full-length and truncated heavy chains were retained in the cell (Figure 2). In both cases, BiP was coprecipitated with the heavy chains. Care must be taken in overinterpreting the relative signals in the various immunoprecipitation reactions. Less labeled BiP is coprecipitated with the truncated heavy chains, in part, because the steady state level of these mutant heavy chains is lower than the full-length γ chain. This is due to a combination of their somewhat lower rate of synthesis and their reduced stability (our unpublished results). Additionally, the amount of full-length heavy chain found in the anti-BiP lane is not a clear indication of their coprecipitation with BiP, because these heavy chains bind directly to protein A-Sepharose beads. The truncated heavy chains do not bind to protein A because the CH3 domain is no longer present. When these two heavy chains were coexpressed with LCs, we found that in both cases they readily assembled with the LC and were secreted (Figure 2). Thus, the epitope-tagged truncated heavy chain behaved like a full-length heavy chain in these assays, making them a reasonable model to analyze the folding status of the CH1 domain in free, nontransported heavy chains.
Figure 2.
Construction and analysis of truncated γ heavy chain. Cells were cotransfected with cDNAs for the indicated genes and metabolically labeled for 3 h. Lysates were prepared and immunoprecipitated with the indicated antiserum. Culture supernatants (m) were collected and immunoprecipitated with the indicated antiserum (λm and HAm). Isolated proteins were reduced and analyzed by SDS-PAGE.
Is the BiP-bound CH1 Domain Folded or Unfolded?
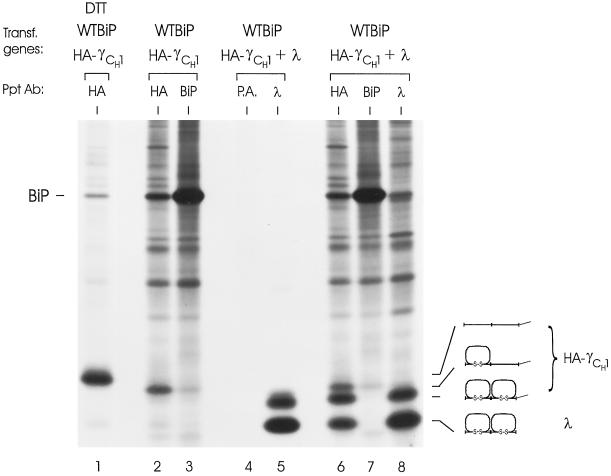
To understand the reason for stable BiP binding to the CH1 domain on free heavy chains, we determined whether BiP remains bound to the CH1 domain because this domain is unable to fold in the absence of LC or, alternatively, whether BiP is also capable of binding hydrophobic faces on folded proteins. We reasoned that a folded CH1 domain might possess exposed hydrophobic patches before assembly with LC. The oxidation status of an Ig domain can readily be assessed by examining its mobility on a nonreducing SDS gel. As these domains fold and are oxidized, their mobility increases such that it is possible to distinguish between two-domain chains with neither, one, or both domains folded (Hendershot et al., 1996). The HA-tagged truncated heavy chain was labeled in the presence of DTT to provide a marker for its mobility as a completely reduced protein (Figure 3, lane 1). WT hamster BiP was cotransfected into the cells to ensure that BiP levels were not limiting. The truncated heavy chains labeled in the absence of DTT clearly migrated faster than the fully reduced chains (lanes 2 and 3). It was not immediately clear whether the faster mobility was due to one or both domains possessing disulfide bonds. Therefore, the mobility of the free truncated heavy chain was compared with that of its fully assembled and secreted counterpart, which should be completely oxidized. Three Ig species were detected in cells expressing both LC and HA-γCH1 when the anti-HA antibody was used (lane 6). The slowest species comigrated with the free truncated heavy chain, the fastest represented the λLC, and the intermediate band represented the mature, fully oxidized HA-γCH1. This species coprecipitated with LC (lane 8) but not BiP (lane 7) and was secreted noncovalently associated with the LC (lane 5). These data indicate that a single domain (CH1) remains unfolded in free heavy chains that have not assembled with LC, thus providing a persistent site for BiP interaction. Furthermore, they suggest that LCs are somehow involved in the folding of the CH1 domain.
Figure 3.
Folding status of the CH1 domain in unassembled truncated γ heavy chains. COS cells were transfected with cDNAs encoding the indicated genes. One dish expressing WT BiP and the truncated heavy chain was labeled in the presence of DTT to provide a marker for completely reduced heavy chain (lane 1). After 3 h of labeling, all dishes were washed twice and lysed in the presence of NEM and apyrase. The cell lysates (lanes 2, 3, 6, 7, and 8) and culture supernatant (lanes 4 and 5) were immunoprecipitated with the indicated antibodies or protein A-Sepharose alone (P.A.) and analyzed by SDS-PAGE under nonreducing conditions. The mobilities of the various folding intermediates are indicated.
What Are the LC Structural Requirements for Inducing BiP Release and CH1 Domain Folding?
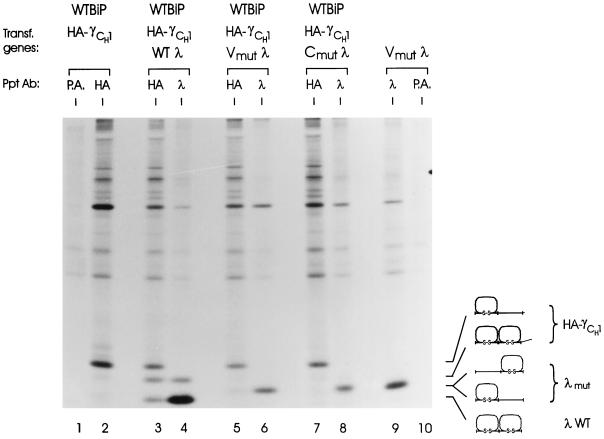
Because BiP associates with the variable domain of the LC during the course of its folding (Hellman et al., 1999), we determined whether it was essential that both domains of the LC be able to fold before it could “help” the CH1 domain fold or whether two partially folded heavy and light chains could interact and aid the folding of each other’s corresponding domain. We made use of two λ LC mutants that were unable to complete the folding of either their V or C domain due to pair-wise mutation of the cysteine residues involved in intradomain disulfide bond formation (Hellman et al., 1999). Because these mutant LCs fold only a single domain, they migrate slower than the WT λ LC on nonreducing gels (Figure 4, lane 9). The truncated heavy chain was expressed alone (to serve as a marker for the partially folded molecule), or together with WT, Vmut, or Cmut λ LC. The Vmut LC was expressed alone to serve as a marker for the partially folded LC. While expression of the WT LC induced folding and assembly of approximately half of the truncated heavy chains (Figure 4, lanes 3 and 4), neither of the LC mutants was able to associate stably with HA-γCH1 or induce its folding (Figure 4). When the same analysis was repeated under reducing conditions, we detected a very small amount of both the V and C domain mutants coprecipitating with the truncated heavy chain (our unpublished results). The absence of signals representing coprecipitation on the nonreducing gel suggests that these complexes must be nonproductive aggregates that do not enter the gel under nonreducing conditions. This might explain the weaker signal that is observed for both heavy and light chains (compare lanes 3, 5, and 7 for heavy chain and lanes 6, 8, and 9 for LC) in the lanes corresponding to LC mutants. These data show that both LC domains must be capable of folding for the LC to contribute to BiP release and folding of the CH1 domain.
Figure 4.
LC folding requirements for H-L assembly and CH1 domain folding. COS cells were transfected with cDNAs for the indicated genes. After 3 h of labeling, cell lysates were prepared in the presence of NEM and apyrase and then reacted with the indicated antibodies or protein A-Sepharose alone (P.A.). Immunoprecipitated proteins were analyzed by SDS-PAGE under nonreducing conditions. The mobilities of the various folding intermediates of both the heavy and light chain are shown to the right.
Can an Isolated, Folded CL Domain Bind to and Allow the CH1 Domain to Fold as Might Occur in the pre-B Cell Ig Receptor?
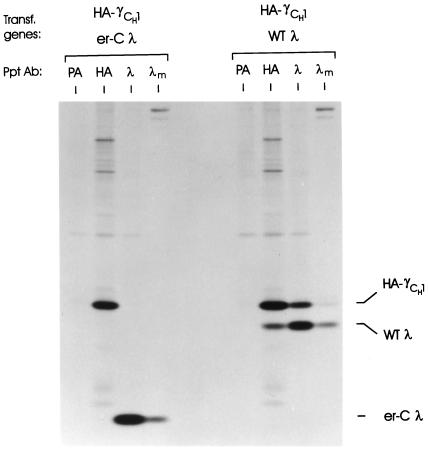
The failure of the V and C mutant LCs to assist CH1 domain folding could be due to the fact that each possesses an unfolded domain that might interfere with the process. Since the V and C domains of the surrogate LC of the pre-B cell Ig receptor are synthesized as separate proteins (VpreB and λ5), we determined whether an isolated C domain could interact with and allow the CH1 domain to fold. We used a LC construct in which the ER-targeting signal sequence was directly spliced to the λI constant domain. Cells were transfected with HA-γCH1 and either WT λ or the λ CL domain alone (er-Cλ). While assembly of WT λ with the truncated heavy chain was readily observed in coprecipitation experiments (Figure 5), there was no indication that the isolated CL domain was able to combine with the truncated heavy chain, as neither protein coprecipitated with the other. When the culture supernatants of both transfectants were examined, we found that the isolated C domain could be secreted like the WT λ, but unlike the WT λ it did not allow secretion of HA-γCH1 (Figure 5). Thus, it appears that a LC composed of a folded V and C domain is required for both final folding of the heavy chain and HL assembly.
Figure 5.
Evaluation of the ability of an ER-targeted CL domain to bind to heavy chains. COS cells were cotransfected with the truncated heavy chain and either WT λ or the ER-targeted λ constant domain (er-Cλ). Cells were metabolically labeled for 3 h, and cell lysates were prepared and reacted with anti-λ, anti-HA, or protein A-Sepharose alone (PA). Culture supernatants were immunoprecipitated with anti-λ (λm). Precipitated proteins were analyzed by SDS-PAGE under reducing conditions.
How Do LCs Facilitate CH1 Domain Folding?
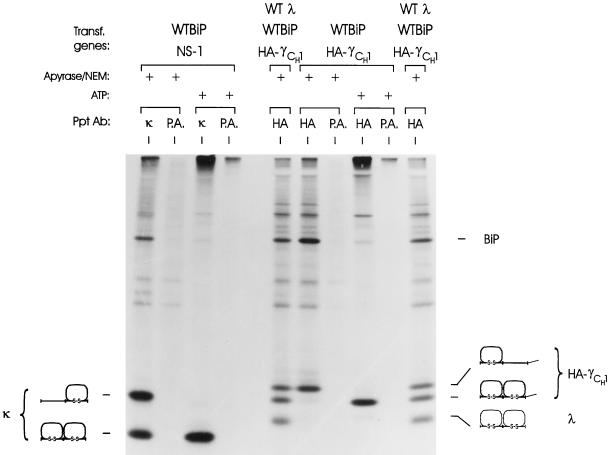
Our data clearly indicate that the CH1 domain is not folded until it assembles with a LC. We envisioned two models to explain these data. First, the LC could associate with the heavy chain and in some way displace BiP from the complex, allowing the heavy chain to fold. Second, BiP might constantly cycle off the CH1 domain, but in the absence of the LC the CH1 domain could be unable to fold and therefore rebind to BiP. The latter possibility implies that the folded LC may provide a scaffold on which the CH1 domain folds. To distinguish between these possibilities, we asked if the CH1 domain could fold if BiP was released in vitro. Similar studies have been done with the NS-1 κ LC, which binds BiP and is not secreted. If cell lysates or immune complexes are treated with ATP, BiP is released and the NS-1 LC folds (Knittler et al., 1995). The NS-1 LC was used as a control in our experiments. As reported previously, we found that at least half of the κ LC isolated in the presence of the alkylating agent NEM (to block postlysis oxidation) and absence of ATP is incompletely oxidized and bound to BiP (Figure 6, lane 1). However, if ATP is added, BiP is released and the LC folds completely (Figure 6, lane 3). This is completely consistent with the previous study in which the LC was isolated from lymphoid cells (Knittler et al., 1995). When the truncated heavy chain was examined in the same way, we found that the partially folded chain (Figure 6, lane 6) was able to fold completely in the absence of LC if BiP was released (Figure 6, lane 8). Cells triply transfected with BiP, HC, and LC served as a control for the migration of the folding intermediates (lanes 5 and 10). These data demonstrate that the CH1 domain is not intrinsically unable to fold. Instead, the heavy chain appears to be stably bound to BiP via a CH1 domain that is unable to fold until a LC is synthesized and available to associate with the heavy chain.
Figure 6.
ATP-mediated in vitro release of BiP from NS-1 LCs and truncated heavy chains and the effect on their folding. COS cells were cotransfected with cDNAs for WT BiP and either NS-1 LC or truncated heavy chain and metabolically labeled for 1 h. Cells were either lysed in the presence of apyrase and NEM or cell lysates were prepared without these agents and incubated with ATP. The resulting lysates were immunoprecipitated with the indicated immune reagents. A dish of COS cells that were triply transfected with WT λ, WT BiP, and the truncated heavy chain was labeled and immunoprecipitated with the anti-HA monoclonal antibody to serve as a control for the migration of partially and completely folded heavy chain (lanes 5 and 10). The samples were analyzed by SDS-PAGE under nonreducing conditions. The migration of BiP and each folding intermediate is indicated to the right.
DISCUSSION
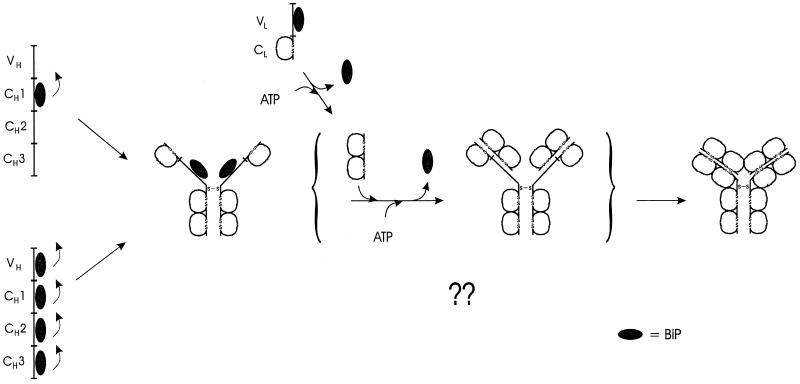
The data provided here reveal the mechanism by which heavy chains remain continuously associated with BiP until assembly with completely folded LC is achieved. In this way, both the LC and BiP facilitate proper development and functioning of the B cell repertoire, as they ensure that incompletely assembled heavy chains remain in the ER. Simply described, the mechanism involves three steps. First, BiP interacts with the CH1 domain, a domain that remains unfolded in free heavy chains. Second, a LC either “catches” the heavy chain when BiP has cycled off, thereby preventing BiP from rebinding, or associates with the BiP–heavy chain complex and “triggers” BiP release. Finally, the CH1 domain is able to fold and assemble stably with the LC, yielding a transport-competent and functional Ig molecule (Figure 7).
Figure 7.
Schematic of the intermediates in H2L2 Ig assembly and role of BiP in the process. Although BiP can associate transiently with other Ig heavy chain domains, the CH1 domain is the primary site of BiP binding that is responsible for the retention of unassembled heavy chains. Unlike the other heavy chain domains, the CH1 domain remains unfolded and unoxidized in the absence of LCs. Synthesis and binding of a folding competent LC to the heavy chain promotes BiP release by an undefined mechanism and allows the CH1 domain to fold. The completely folded and assembled Ig molecule is now transport competent.
Our data argue that complete folding of the heavy chain is dependent on its assembly with LC and not that assembly of Ig molecules is dependent on the proper folding of the individual subunits. Assembly-dependent folding of Ig molecules was previously proposed by Haas and co-workers (Kaloff and Haas, 1995; Leitzgen et al., 1997). Using a CH1-deleted heavy chain, they demonstrated that BiP could bind to other Ig heavy-chain domains, but that this interaction was transient and restricted to unoxidized heavy chains (Kaloff and Haas, 1995). They hypothesized that domain pairing between the two heavy chains promoted their folding and BiP release and that BiP remained bound to the CH1 domain because it does not homodimerize but instead pairs with the CL domain of LC. They further speculated that pairing with LCs would be required to allow the CH1 domain to fold. The data we present provide direct support for their central hypothesis that folding of Ig domains is dependent on assembly. However, our data further reveal that the role of LC is not to act as a scaffold, on which the CH1 domain can fold, but instead serves to release BiP from the heavy chain, since this domain is perfectly capable of folding if BiP is released in vitro. Although the structure of all Ig domains is extremely similar, the CH1 domain is clearly distinguished from the other Ig domains in its requirements for folding. Most of the other domains appear to fold rapidly and independently of BiP, because expression of the BiP mutants did not greatly affect the folding or secretion of the CH1-deleted heavy chain (Figure 1B). In the case of the CH2 and CH3 domains, this may relate to their ability to homodimerize as mentioned above (Kaloff and Haas, 1995). However, the VH domain should also not homodimerize on an unassembled heavy chain, and yet this domain is efficiently folded and oxidized in our truncated heavy chain. Additionally, the observation that some heavy chains with CH3 domain deletions can be secreted as HL molecules (Hendershot et al., 1987) suggests that domain pairing may not be a strict requirement for the folding of all Ig domains.
We were somewhat surprised to find that only LCs possessing two folded domains could successfully induce the folding of the CH1 domain. It is possible that the VH and VL domains pair and form a “semistable” interaction that allows the CL domain to contact the CH1 domain and in some way displace BiP. Haas’s laboratory (Leitzgen et al., 1997) has shown that folding of the VL domain of two mutant, nonsecreted LCs is impaired and can be aided by their heterodimerization with a VH domain during heavy chain assembly. They suggested that the inability of these mutant LCs to be secreted is due to their inability to form homodimers. In the absence of heavy chains, most LCs homodimerize and can be secreted (Ma et al., 1990), lending support to this hypothesis. However, another study (Dul et al., 1996) identified a number of LCs that are apparently secreted as monomers, making it unclear whether assembly is generally essential for LC folding. If the heavy and light chains do serve to “cross” fold each other, our data from the LC mutants suggest that assembled intermediates that do not yield successfully folded products must be rather unstable and fall apart quickly. The requirement for a LC that is capable of folding both of its domains to fold the CH1 domain would be particularly useful in controlling the assembly of the pre-B cell Ig receptor where each LC domain is provided by a separate protein (i.e. VpreB and λ5) (Sakaguchi and Melchers, 1986; Kudo and Melchers, 1987). This would also provide an additional control for monitoring the variable region, which can undergo extensive mutation during Ig repertoire development, to ensure that a LC with an improperly folded variable region does not assemble with heavy chains and allow them to be transported. We hypothesize that in pre-B cells either the surrogate LC complex assembles first and then binds to the heavy chain or VpreB and λ5 bind separately, but only if both bind to the heavy chain would BiP be displaced and the CH1 domain allowed to fold. The failure of our ER-targeted CL domain to combine with the truncated heavy chain might argue against this second model. However, we cannot rule out the possibility that a solitary CL domain can interact nonproductively with the CH1 domain and is either too transient or too weak to withstand the conditions of cell lysis and immunoprecipitation.
The mechanism by which LC releases BiP from the heavy chain is at the heart of controlling the integrity of the Ig assembly process. Most of the current data available on mechanisms of molecular chaperone action come from in vitro studies and suggest that they act to aid protein folding by continually binding and releasing proteins in a progressively folded state (Bukau and Horwich, 1998). Importantly, our data demonstrate that the CH1 domain is capable of folding independent of LC if BiP is released (Figure 6). Therefore, if BiP cycling does occur, it must be faster than the rate of CH1 domain folding, since we found no evidence for the intracellular accumulation of completely folded CH1 domain.
Hsp70 family members are thought to associate with proteins in an ATP-bound state. The nucleotide is rapidly hydrolyzed to ADP which “locks” the hsp70 protein more stably to the unfolded protein. This hydrolysis is catalyzed by a DnaJ-like protein (Liberek et al., 1991). Exchange of ATP then allows release of the unfolded protein. In bacteria and mitochondria this nucleotide exchange occurs through the action of a GrpE protein (Liberek et al., 1991). In mammalian cytosol, several proteins have been identified that play a role in regulating nucleotide exchange. An hsp70-interacting protein (Hip) prevents release of ADP, thus stabilizing the cytosolic hsp70s in an ADP-bound state (Hohfeld et al., 1995). Two other proteins, an hsp70 organizing protein (Hop) (Gross and Hessefort, 1996; Frydman and Hohfeld, 1997) and Bag-1 (Hohfeld and Jentsch, 1997), have been shown to facilitate nucleotide exchange in vitro. Perhaps a DnaJ and/or Hip protein are part of the BiP–heavy chain complex in the ER, which would keep BiP in an ADP-bound form and thus more stably bound to the CH1 domain. Interaction of the LC with this complex could diminish its affinity for an ADP-stabilizing component. Alternatively, the LC may bring another protein (like Hop or Bag-1) to the BiP–heavy chain complex that would promote nucleotide exchange. Our ability to release BiP with ATP after the cells have been lysed could be explained if the association of a DnaJ- or Hip-like protein was unstable to detergent lysis or if large amounts of exogenously added ATP were sufficient to drive nucleotide exchange. While such speculations are reasonable in light of what is known about the action of hsp70 proteins, it is important to note that no ER homologues for Hip, Hop, or Bag-1 have been identified in mammalian cells. An ER-targeted, DnaJ-like protein was identified in mouse cells recently (Brightman et al., 1995), but no functional data have been reported for this protein.
In conclusion, we have provided a clearer understanding of the mechanism(s) ensuring that only completely assembled Ig molecules leave the ER. We have demonstrated that heavy chains do not fold completely in the ER until they assemble with LCs. Because the Ig domain is one of the more commonly used structures in proteins, it is very conceivable that assembly-dependent folding of a protein subunit is not unique to the Ig molecule but represents a commonly used quality-control mechanism to ensure that only properly assembled multimeric proteins are transported from the ER.
ACKNOWLEDGMENTS
This work was supported by NIH grant GM-54068, the Cancer Center CORE grant CA-21765, and the American Lebanese Syrian Associated Charities of St. Jude Children’s Research Hospital. J.W.B. was supported by NIH grant F32 GM-18443.
REFERENCES
- Amzel LM, Poljak RJ. Three-dimensional structure of immunoglobulins. Annu Rev Immunol. 1979;48:961–997. doi: 10.1146/annurev.bi.48.070179.004525. [DOI] [PubMed] [Google Scholar]
- Blond-Elguindi S, Cwirla SE, Dower WJ, Lipshutz RJ, Sprang SR, Sambrook JF, Gething MJ. Affinity panning of a library of peptides displayed on bacteriophages reveals the binding specificity of BiP. Cell. 1993;75:717–728. doi: 10.1016/0092-8674(93)90492-9. [DOI] [PubMed] [Google Scholar]
- Bole DG, Hendershot LM, Kearney JF. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol. 1986;102:1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt CR, Morrison SL, Birshtein BK, Milcarek C. Loss of a consensus splice signal in a mutant immunoglobulin gene eliminates the CH1 domain exon from the mRNA. Mol Cell Biol. 1984;4:1270–1277. doi: 10.1128/mcb.4.7.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightman SE, Blatch GL, Zetter BR. Isolation of a mouse cDNA encoding MTJ1, a new murine member of the DnaJ family of proteins. Gene. 1995;153:249–254. doi: 10.1016/0378-1119(94)00741-a. [DOI] [PubMed] [Google Scholar]
- Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- Dul JL, Aviel S, Melnick J, Argon Y. Ig light chains are secreted predominantly as monomers. J Immunol. 1996;157:2969–2975. [PubMed] [Google Scholar]
- Frydman J, Hohfeld J. Chaperones get in touch: the Hip-Hop connection. Trends Biochem Sci. 1997;22:87–92. doi: 10.1016/s0968-0004(97)01005-0. [DOI] [PubMed] [Google Scholar]
- Gardner AM, Aviel S, Argon Y. Rapid degradation of an unassembled immunoglobulin light chain is mediated by a serine protease and occurs in a pre-Golgi compartment. J Biol Chem. 1993;268:25940–25947. [PubMed] [Google Scholar]
- Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Goto Y, Hamaguchi K. Unfolding and refolding of the reduced constant fragment of the immunoglobulin light chain. J Mol Biol. 1982;156:911–926. doi: 10.1016/0022-2836(82)90147-4. [DOI] [PubMed] [Google Scholar]
- Gross M, Hessefort S. Purification and characterization of a 66-kDa protein from rabbit reticulocyte lysate which promotes the recycling of hsp 70. J Biol Chem. 1996;271:16833–16841. doi: 10.1074/jbc.271.28.16833. [DOI] [PubMed] [Google Scholar]
- Haas IG, Wabl M. Immunoglobulin heavy chain binding protein. Nature. 1983;306:387–389. doi: 10.1038/306387a0. [DOI] [PubMed] [Google Scholar]
- Hellman R, Vanhove M, Lejeune A, Stevens FJ, Hendershot LM. The in vivo association of BiP with newly synthesized proteins is dependent on their rate and stability of folding and simply on the presence of sequences that can bind to BiP. J Cell Biol. 1999;144:21–30. doi: 10.1083/jcb.144.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot L, Bole D, Kohler G, Kearney JF. Assembly and secretion of heavy chains that do not associate posttranslationally with immunoglobulin heavy chain-binding protein. J Cell Biol. 1987;104:761–767. doi: 10.1083/jcb.104.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot L, Wei J, Gaut J, Melnick J, Aviel S, Argon Y. Inhibition of immunoglobulin folding and secretion by dominant negative BiP ATPase mutants. Proc Natl Acad Sci USA. 1996;93:5269–5274. doi: 10.1073/pnas.93.11.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot LM, Wei J-Y, Gaut JR, Lawson B, Freiden PJ, Murti KG. In vivo expression of mammalian BiP ATPase mutants causes disruption of the endoplasmic reticulum. Mol Biol Cell. 1995;6:283–296. doi: 10.1091/mbc.6.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the PCR. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Hohfeld J, Jentsch S. GrpE-like regulation of the hsc70 chaperone by the antiapoptotic protein BAG-1 [published erratum appears in EMBO J 1998 Feb 2;17(3):847] EMBO J. 1997;16:6209–6216. doi: 10.1093/emboj/16.20.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohfeld J, Minami Y, Hartl FU. Hip, a novel cochaperone involved in the eukaryotic Hsc70/Hsp40 reaction cycle. Cell. 1995;83:589–598. doi: 10.1016/0092-8674(95)90099-3. [DOI] [PubMed] [Google Scholar]
- Kaloff CR, Haas IG. Coordination of immunoglobulin chain folding and immunoglobulin chain assembly is essential for the formation of functional IgG. Immunity. 1995;2:629–637. doi: 10.1016/1074-7613(95)90007-1. [DOI] [PubMed] [Google Scholar]
- Knittler MR, Dirks S, Haas IG. Molecular chaperones involved in protein degradation in the endoplasmic reticulum: quantitative interaction of the heat shock cognate protein BiP with partially folded immunoglobulin light chains that are degraded in the endoplasmic reticulum. Proc Natl Acad Sci USA. 1995;92:1764–1768. doi: 10.1073/pnas.92.5.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo A, Melchers F. A second gene, VpreB in the lambda 5 locus of the mouse, which appears to be selectively expressed in preB lymphocytes. EMBO J. 1987;6:2267–2272. doi: 10.1002/j.1460-2075.1987.tb02500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitzgen K, Knittler MR, Haas IG. Assembly of immunoglobulin light chains as a prerequisite for secretion. A model for oligomerization-dependent subunit folding. J Biol Chem. 1997;272:3117–3123. doi: 10.1074/jbc.272.5.3117. [DOI] [PubMed] [Google Scholar]
- Liberek K, Marszalek J, Ang D, Georgopoulos C, Zylicz M. Escherichia coli DnaJ and GrpE heat shock proteins jointly stimulate ATPase activity of DnaK. Proc Natl Acad Sci USA. 1991;88:2874–2878. doi: 10.1073/pnas.88.7.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilie H, Rudolph R, Buchner J. Association of antibody chains at different stages of folding: prolyl isomerization occurs after formation of quaternary structure. J Mol Biol. 1995;248:190–201. doi: 10.1006/jmbi.1995.0211. [DOI] [PubMed] [Google Scholar]
- Liu AY, Mack PW, Champion CI, Robinson RR. Expression of mouse::human immunoglobulin heavy-chain cDNA in lymphoid cells. Gene. 1987;54:33–40. doi: 10.1016/0378-1119(87)90344-1. [DOI] [PubMed] [Google Scholar]
- Ma J, Kearney JF, Hendershot LM. Association of transport-defective light chains with immunoglobulin heavy chain binding protein. Mol Immunol. 1990;27:623–630. doi: 10.1016/0161-5890(90)90004-j. [DOI] [PubMed] [Google Scholar]
- Morrison SL. Murine heavy chain disease. Eur J Immunol. 1978;8:194–199. doi: 10.1002/eji.1830080311. [DOI] [PubMed] [Google Scholar]
- Sakaguchi N, Melchers F. Lambda 5, a new light-chain-related locus selectively expressed in preB lymphocytes. Nature. 1986;324:579–582. doi: 10.1038/324579a0. [DOI] [PubMed] [Google Scholar]
- Seligmann M, Mihaesco E, Preud’homme J-L, Danon F, Brouet J-C. Heavy chain disease:current findings and concepts. Immunol Rev. 1979;48:145–167. doi: 10.1111/j.1600-065x.1979.tb00302.x. [DOI] [PubMed] [Google Scholar]
- Sitia R, Neuberger M, Alberini C, Bet P, Fra A, Valetti C, Williams G, Milstein C. Developmental regulation of IgM secretion: the role of the carboxy-terminal cysteine. Cell. 1990;60:781–790. doi: 10.1016/0092-8674(90)90092-s. [DOI] [PubMed] [Google Scholar]
- Skowronek MH, Hendershot LM, Haas IG. The variable domain of nonassembled Ig light chains determines both their half-life and binding to BiP. Proc Natl Acad Sci USA. 1998;95:1574–1578. doi: 10.1073/pnas.95.4.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J-Y, Gaut JR, Hendershot LM. In vitro dissociation of BiP:peptide complexes requires a conformational change in BiP after ATP binding but does not require ATP hydrolysis. J Biol Chem. 1995;270:26677–26682. doi: 10.1074/jbc.270.44.26677. [DOI] [PubMed] [Google Scholar]