Abstract
To study the growing trend of extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae in pediatric intensive care units (PICUs), 88 nonrepetitive ESBL-producing isolates were prospectively collected and analyzed by molecular methods during a 16-month period. The emergence and dissemination of ESBL-producing Enterobacteriaceae in PICUs are the consequence of the clonal dissemination of a few epidemic strains along with the horizontal transmission of resistance gene-carrying plasmids among bacterial organisms.
Since the initial description of extended-spectrum β-lactamase (ESBL) production by Klebsiella pneumoniae and Escherichia coli in the 1980s (1, 10), strains of enterobacteria that are resistant to extended-spectrum cephalosporins are increasingly being recognized (3, 6). ESBL-producing enterobacteria have been frequently implicated in outbreaks in pediatric intensive care units (PICUs) and neonatal intensive care units (NICUs) (2, 9, 11). We started to screen for ESBL-producing enterobacteria by using the method recommended by the NCCLS since September 2000. In our hospital, we found a marked increase in the number of the ESBL-producing enterobacteria in the PICUs and NICUs. To further delineate the mode of dissemination of ESBLs, we collected clinical isolates of ESBL-positive members of the family Enterobacteriaceae from the ICUs during the period of September 2000 to December 2001. The relatedness of these isolates and the types of ESBLs was investigated.
In the Chang Gung Children's Hospital at Kweishan, there are one PICU and four NICUs. PICU 2L admitted all critical pediatric patients beyond the neonatal stage. NICUs 3L1, 3L2, 5L1, and 5L2 admitted only neonatal and premature patients. 3L1 and 3L2 were separate units located in the same floor, and so were 5L1 and 5L2. Pediatricians and nursing staff members could move freely among these ICUs, provided regulations of hospital infection control were obeyed. A geographically independent ward, Taipei PICU (TPICU), is located in the Taipei branch of Chang Gung Memorial Hospital, which is about 20 kilometers away from Chang Gung Children's Hospital at Kweishan.
A total of 88 nonrepetitive ESBL-producing isolates of E. coli (n = 16), Enterobacter cloacae (n = 26), and K. pneumoniae (n = 46) were collected from specimens from various sources during the study period. All isolates were screened for the presence of ESBLs by the disk diffusion method with cefotaxime (30 μg) and ceftazidime (30 μg), and the results were confirmed by the phenotypic confirmatory test recommended by the NCCLS (14, 15).
In the present study, the genetic relatedness of the clinical isolates was investigated by the method of infrequent restriction site PCR (IRS-PCR) (19). Isolates with identical banding patterns or pattern difference of fewer than four bands were arbitrarily assigned to the same genotypes or subtypes of an existing genotype. Isolates with more than four band differences were considered different and assigned to different genotypes.
To determine the ESBL types, three primer sets previously described for the detection of the blaTEM, blaSHV, and blaCTX-M genes were used in the amplification procedure (5, 13, 16). PCR products were purified with Microcon PCR centrifugal filter devices (Millipore) and sequenced by an ABI 377 automatic sequencer (Perkin-Elmer Applied Biosystems). A search for homologous sequences in the GenBank database was done with the FASTA software through the internet.
The presence of plasmids was checked by the modified alkaline lysis method described earlier (8). DNA-DNA hybridization was performed by the method of Southern (18), except that the probe for the target β-lactamase gene was prepared from a blaSHV-12 or blaCTX-M-3 PCR product and labeled with digoxigenin-11-dUTP (Roche).
As shown in Table 1, a total of 88 nonrepetitive ESBL-producing isolates were collected during the study period. CTX-M-3 (52 isolates) was the most prevalent type of ESBL identified, followed in the order of frequency by SHV-12 (35 isolates). SHV-12 was present in all isolates of E. cloacae, and CTX-M-3 was found coexisting with SHV-12 in three E. cloacae isolates. CTX-M-3 was also the most common type of ESBL produced by E. coli (81%) and K. pneumoniae (78%). These were the most common ESBLs found with the published TEM, SHV, and CTX-M sets of primers. Other ESBLs, including OXY-1, TEM-31, and SHV-2, -2a, -5, and -12, were found in sporadic isolates of E. coli and K. pneumoniae (Table 1). Eleven representative isolates of E. coli, 10 of E. cloacae, and 13 of K. pneumoniae were examined for their plasmid patterns. All of the isolates tested carried one or multiple large plasmids greater than 95 kb in size. DNA-DNA hybridization with a blaSHV-12 or blaCTX-M-3 PCR product as the probe showed that these bla genes were located on the large plasmids in the majority of the representative isolates. Only two isolates of E. coli, two of E. cloacae, and one of K. pneumoniae did not carry plasmid-mediated bla.
TABLE 1.
Number and distribution of various ESBLs among strains of E. cloacae, E. coli, and K. pneumoniae
| Bacteria | Total no. | No. of different ESBLs
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| SHV-12 | SHV-2 | SHV-2a | SHV-5 | CTX-M-3 | CTX-M-14 | TEM-31 | OXY-1 | ||
| E. cloacae | 26 | 26 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| E. coli | 16 | 3 | 0 | 0 | 1 | 13 | 0 | 0 | 0 |
| K. pneumoniae | 46 | 6 | 4 | 3 | 1 | 36 | 1 | 2 | 1 |
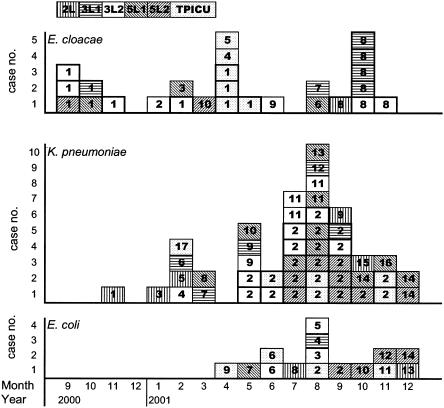
Results of the genotyping and their relationship to the ICUs where the clinical isolates were collected are shown in Fig. 1. Molecular typing identified 17, 10, and 14 different genotypes among K. pneumoniae, E. cloacae, and E. coli isolates, respectively. Two predominant genotypes, types 1 and 8, were found in 18 (69%) of the 26 E. cloacae isolates. Isolates with genotype 8 were mainly derived from NICUs 3L1 and 3L2, while genotype 1 was from the TPICU and NICU 3L2. The most common genotype found in K. pneumoniae isolates (23 of 46, 50%) was genotype 2, most of which were collected from 3L2, 5L2, and the TPICU. Diverse genotypes were found among the 16 E. coli isolates.
FIG. 1.
Incidence of ESBL-producing clinical isolates of E. cloacae, E. coli, and K. pneumoniae in the six ICUs studied between September 2000 and December 2001. Every square represents one isolate, and genotypes are shown as arabic numbers. Different ICUs are differentiated by different patterns.
Infection by ESBL-producing gram-negative bacteria has become a serious problem in Taiwan. According to a report from a medical center in northern Taiwan, the proportion of isolates of K. pneumoniae exhibiting ESBL phenotypes has increased progressively from 3.4% in 1993 to 10.3% in 1997 (7). From 1998 through 2000, several reports from different hospitals in Taiwan showed that ESBL production accounts for 8 to 30% of the total number of clinical isolates of K. pneumoniae (4, 7, 12, 17, 23, 25), and SHV-5 and SHV-12 were the most prevalent ESBLs. In addition, four novel β-lactamases (CMY-8, SHV-25, SHV-26, and IMP-8) were identified in 2000 in Taiwan (4, 12, 17, 23, 24). Among the ESBL-producing E. coli isolates, which accounted for 1.6 to 6.7% of the total number of E. coli isolates, strains expressing CTX-M-3 and CMY-2 were disseminated in Taiwan (25).
In the present study, the most common types of ESBL identified among clinical isolates were SHV-12 and CTX-M-3. SHV-12 was present in all isolates of E. cloacae, while CTX-M-3 was predominant in E. coli and K. pneumoniae. The ESBL genes were located on the large plasmids in most of the isolates. Previous studies have shown that bacteria could acquire resistance from other organisms through the exchange of genetic materials, such as plasmids, in vivo (19, 22). While clonal spread could not entirely explain the rapid and wide dissemination of ESBLs among different species of the family Enterobacteriaceae, our results provide evidence that the increase in ESBL-producing Enterobacteriaceae in hospitals is in part due to the widespread dissemination of resistance plasmids.
Clonal spread was detected by the molecular typing method in the present study. Two distinct strains of E. cloacae and one of K. pneumoniae were prevalent within the PICUs, as demonstrated by IRS-PCR. Genotyping is very important when epidemic strains are resistant to many antimicrobial agents. Pulsed-field gel electrophoresis is considered the most reliable molecular typing method. IRS-PCR, the method used in this study, is simpler and less time-consuming. It has been applied in the investigation of outbreaks caused by many different bacterial organisms, including K. pneumoniae, Serratia marcescens, and Salmonella enteritidis (20, 21, 26). By use of this method, we were able to identify major genotypes of K. pneumoniae and E. cloacae circulating in the hospital wards studied.
In conclusion, the increase in the number of ESBL-producing isolates of Enterobacteriaceae in PICUs is due to the occurrence of multiple consecutive outbreaks of infections associated with ESBL-producing bacteria. The dissemination of ESBL-producing Enterobacteriaceae is a consequence of the clonal expansion of a few epidemic strains and the spread of resistance plasmids among bacterial organisms. Since the resistance displayed by bacteria reflects the environment in which the organism thrives, immediate action, including reinforcement of infection control measures, should be taken to prevent further spread of the resistant bacteria.
Acknowledgments
This work was supported in part by grants CMRP798 (to T.L.W.) and CMRP1313 (to C.H.C.) from Chang Gung Memorial Hospital, Taoyuan, Taiwan.
REFERENCES
- 1.Bauernfeind, A., and G. Horl. 1987. Novel R-factor-borne β-lactamase conferring resistance to cephalosporins. Infection 15:257-259. [DOI] [PubMed] [Google Scholar]
- 2.Bingen, E. H., P. Desjardins, G. Arlet, F. Bourgeois, P. Mariani-Kurkdjian, N. Y. Lambert-Zechovsky, E. Denamur, A. Philippon, and J. Elison. 1993. Molecular epidemiology of plasmid spread among extended broad-spectrum β-lactamase-producing Klebsiella pneumoniae isolates in a pediatric hospital. J. Clin. Microbiol. 31:179-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bush, K., and G. A. Jacoby. 1997. Nomenclature of TEM β-lactamases. J. Antimicrob. Chemother. 39:1-3. [DOI] [PubMed] [Google Scholar]
- 4.Chang, F. Y., L. K. Siu, C. P. Fung, M. H. Huang, and M. Ho. 2001. Diversity of SHV and TEM β-lactamases in Klebsiella pneumoniae: gene evolution in northern Taiwan and two novel β-lactamases, SHV-25 and SHV-26. Antimicrob. Agents Chemother. 45:2407-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gniadkowski, M., I. Schneider, A. Palucha, R. Jungwirth, B. Mikiewicz, and A. Bauernfeind. 1998. Cefotaxime-resistant Enterobacteriaceae isolates from a hospital in Warsaw, Poland: identification of a new CTX-M-3 cefotaxime-hydrolyzing β-lactamase that is closely related to the CTX-M-1/MEN-1 enzyme. Antimicrob. Agents Chemother. 42:827-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacoby, G. A., and A. A. Medeiros. 1991. More extended-spectrum β-lactamases. Antimicrob. Agents Chemother. 35:1697-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jan, I. S., P. R. Hsueh, L. J. Teng, S. W. Ho, and K. T. Luh. 1998. Antimicrobial susceptibility testing for Klebsiella pneumoniae isolates resistant to extended-spectrum beta-lactam antibiotics. J. Formos. Med. Assoc. 97:661-666. [PubMed] [Google Scholar]
- 8.Kado, C. I., and S. T. Liu. 1981. Rapid procedure for detection and isolation of large and small plasmids. J. Bacteriol. 145:1365-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim, Y. K., H. Pai, H. J. Lee, S. E. Park, E. H. Choi, J. Kim, J. H. Kim, and E. C. Kim. 2002. Bloodstream infections by extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in children: epidemiology and clinical outcome. Antimicrob. Agents Chemother. 46:1481-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knothe, H., P. Shah, V. Kremery, M. Antal, and S. Mitsuhashi. 1983. Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection 11:315-317. [DOI] [PubMed] [Google Scholar]
- 11.Lebessi, E., H. Dellagrammaticas, P. T. Tassios, L. S. Tzouvelekis, S. Ioannidou, M. Foustoukou, and N. J. Legakis. 2002. Extended-spectrum β-lactamase-producing Klebsiella pneumoniae in a neonatal intensive care unit in the high-prevalence area of Athens, Greece. J. Clin. Microbiol. 40:799-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu, P. Y., J. C. Tung, S. C. Ke, and S. L. Chen. 1998. Molecular epidemiology of extended-spectrum β-lactamase-producing Klebsiella pneumoniae isolates in a district hospital in Taiwan. J. Clin. Microbiol. 36:2759-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mabilat, C., and S. Goussard. 1993. PCR detection and identification of genes for extended-spectrum β-lactamases, p. 553-559. In D. H. Persing, T. F. Smith, F. C. Tenover, and T. J. White (ed.). Diagnostic molecular microbiology: principles and applications. American Society for Microbiology, Washington, D.C.
- 14.NCCLS. 2001. Performance standards for antimicrobial susceptibility testing, 11th supplement. M100-S11, vol. 21, no. 1. NCCLS, Wayne, Pa.
- 15.NCCLS. 2001. Performance standards for antimicrobial disk susceptibility tests. Approved standard M2-A6. NCCLS, Wayne, Pa.
- 16.Rasheed, J. K., C. Jay, B. Metchock, F. Berkowitz, L. Weigel, J. Crellin, C. Steward, B. Hill, A. A. Medeiros, and F. C. Tenover. 1997. Evolution of extended-spectrum β-lactam resistance (SHV-8) in a strain of Escherichia coli during multiple episodes of bacteremia. Antimicrob. Agents Chemother. 41:647-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siu, L.-K., P. L. Lu, P.-R. Hsueh, F. M. Lin, S.-C. Chang, K.-T. Luh, M. Ho, and C.-Y. Lee. 1999. Bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a pediatric oncology ward: clinical features and identification of different plasmids carrying both SHV-5 and TEM-1 genes. J. Clin. Microbiol. 37:4020-4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Southern, E. M. 1975. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J. Mol. Biol. 98:503-517. [DOI] [PubMed] [Google Scholar]
- 19.Su, L.-H., C.-H. Chiu, C. Chu, M.-H. Wang, J.-H. Chia, and T.-L. Wu. 2003. In vivo acquisition of ceftriaxone resistance in Salmonella enterica serotype Anatum. Antimicrob. Agents Chemother. 47:563-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su, L.-H., C.-H. Chiu, T.-L. Wu, C. Chu, J.-H. Chia, A. J. Kuo, C. C. Lee, C. F. Sun, and J. T. Ou. 2002. Molecular epidemiology of Salmonella enterica serovar Enteritidis isolated in Taiwan. Microbiol. Immunol. 46:833-840. [DOI] [PubMed] [Google Scholar]
- 21.Su, L.-H., S. H. Leu, Y. P. Chiu, J.-H. Chia, A. J. Kuo, C. F. Sun, T. Y. Lin, and T.-L. Wu. 2000. Molecular investigation of two clusters of nosocomial bacteraemia caused by multiresistant Klebsiella pneumoniae using pulsed-field gel electrophoresis and infrequent-restriction-site PCR. J. Hosp. Infect. 46:110-117. [DOI] [PubMed] [Google Scholar]
- 22.Winokur, P. L., D. L. Vonstein, L. J. Hoffman, E. K. Uhlenhopp, and G. V. Doern. 2001. Evidence for transfer of CMY-2 AmpC β-lactamase plasmids between Escherichia coli and Salmonella isolates from food animals and humans. Antimicrob. Agents Chemother. 45:2716-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan, J.-J., S. M. Wu, S.-H. Tsai, J.-J. Wu, and I.-J. Su. 2000. Prevalence of SHV-12 among clinical isolates of Klebsiella pneumoniae producing extended-spectrum β-lactamases and identification of a novel AmpC enzyme (CMY-8) in southern Taiwan. Antimicrob. Agents Chemother. 44:1438-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan, J.-J., W.-C. Ko, and J.-J. Wu. 2001. Identification of a plasmid encoding SHV-12, TEM-1, and a variant of IMP-2 metallo-β-lactamase, IMP-8, from a clinical isolate of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 45:2368-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan, J.-J., W.-C. Ko, S.-H. Tsai, H.-M. Wu, Y.-T. Lin, and J.-J. Wu. 2000. Dissemination of CTX-M-3 and CMY-2 β-lactamases among clinical isolates of Escherichia coli in southern Taiwan. J. Clin. Microbiol. 38:4320-4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoo, J.-H., J.-H. Choi, W.-S. Shin, D.-H. Huh, Y.-K. Cho, K.-M. Kim, M.-Y. Kim, and M.-W. Kang. 1999. Application of infrequent-restriction-site PCR to clinical isolates of Acinetobacter baumannii and Serratia marcescens. J. Clin. Microbiol. 37:3108-3112. [DOI] [PMC free article] [PubMed] [Google Scholar]