Abstract
Stool specimens from 113 adult outpatients with diarrhea in southwestern Nigeria and 63 controls were examined for bacterial and parasitic enteric pathogens. Enterohemorrhagic Escherichia coli (EHEC) (P < 0.02), enteroaggregative E. coli (EAEC) (P < 0.02), and Entamoeba histolytica (P < 0.0002) were significantly associated with diarrhea. Salmonella, Shigella, nontoxigenic Vibrio cholerae, other categories of diarrheagenic E. coli, as well as a variety of helminths were recovered more frequently from the stools of patients than from the stools of controls but did not show a significant association with disease. Multiple pathogens were recovered from 36.3% of specimens, and bloody diarrhea was commonly associated with E. histolytica and diarrheagenic E. coli infections. The majority of EHEC isolates were non-O157 strains that carried the stx2 gene. Of the 23 EHEC-infected patients, 12 (52.2%) presented during the 10th week of the study. EHEC strains isolated within this cluster were more likely to hybridize with the enterohemolysin gene probe, to be nonmotile and sorbitol positive, and to fail to agglutinate O157 antisera. Pulsed-field gel electrophoresis demonstrated that the only strains with XbaI profiles that occurred more than once were isolated during the 10th and 11th weeks of the study, suggesting an outbreak. The study has demonstrated that E. histolytica, EHEC, and EAEC are important diarrheal pathogens within the study area and that sporadic and epidemic EHEC infections occur in developing as well as developed countries. Routine surveillance for diarrheagenic E. coli, even only at the tertiary-care level, would be useful in identifying outbreaks and assist in identifying environmental reservoirs and transmission routes.
Diarrhea is an important cause of disease and death among children in developing countries (20). Adult visitors to tropical developing countries frequently experience traveler's diarrhea, caused by agents that are endemic in those countries but to which visitors have not had the opportunity to develop protective immunity (36). Adult residents of developing countries are less likely to have sporadic diarrhea, and when it occurs, it is unlikely to be life threatening. Therefore, diarrheal pathogens in adults residing in developing countries have been the subject of few investigations, and very little is known about the etiologic epidemiology of pathogens other than epidemic Vibrio cholerae and Shigella. Sporadic endemic diarrhea in adults, however, contributes to the loss of productivity in developing countries and increases the risk that pathogens will be passed to susceptible children or visitors. When such infections do occur, they are often treated empirically, even though very little is known about the etiologic agents in this population; and in many cases, they prompt self-medication with antibiotics, which are often available without prescription (33). We conducted a case-control study over an 11-week period to determine the causes of diarrhea among adults visiting the outpatient clinic of a hospital in the town of Ile-Ife in southwest Nigeria.
MATERIALS AND METHODS
Subjects.
The study was conducted in Ile-Ife, Osun State, Nigeria. Permission to conduct the study was granted by the Research and Ethical Committee of the Obafemi Awolowo University teaching hospital complex, and informed consent was sought from the patients and the controls. Stool specimens were collected from consecutive patients with diarrhea (age range, 17 to 54 years) who sought care at the outpatient department of the Obafemi Awolowo University's teaching hospital between 1 August and 16 October 1998. Control subjects, selected during the same period, were 63 apparently healthy individuals with a similar age distribution and from the same area of residence as the patients. They were selected from among the relatives of other, nonstudy patients without suspected infectious diseases (patients with trauma) visiting the hospital and who had had no history of diarrhea in their households for at least 1 month. For the ill patients, a physician diagnosed acute diarrhea on the basis of frequent watery stools (usually more than three daily) for less than 1 week. Patients with concomitant infections were excluded from the study. A total of 113 patients and 63 controls who met the criteria described above and who gave informed consent were included in the study. Neither patients nor controls admitted to having received antibiotic treatment in the week preceding sampling. Most of the subjects were from low-income families and had no access to appropriately treated potable water.
Specimen collection and processing.
The methods used in the investigation included microscopy for erythrocytes and parasites as well as culture for bacterial enteric pathogens. Stool specimens were examined macroscopically for gross blood and mucus. Wet mounts of fresh stool were made in normal saline and were examined for parasites and erythrocytes. Swabs of stool were inoculated onto the surface of MacConkey and eosin methylene blue agars (Oxoid, Basingstoke, England) and streaked for colony isolation. Colonies arising after 24 and 48 h of incubation at 37°C were streaked onto fresh plates and identified by conventional biochemical tests (3). Enteric bacteria were identified biochemically, and four to five Escherichia coli colonies were retained for further examination. Non-E. coli isolates that were recovered from the same host and that showed the same colony morphology and biochemical profile were considered the same isolate. Stocks of each isolate were maintained by cryopreservation.
DNA hybridization.
Fragment probes (Table 1) were used to screen colony blots of all E. coli and Shigella isolates, prepared by using 541 filter paper (Whatman, Maidstone, England). The fragment probes were prepared from plasmids purified by the method of Birnboim and Doly (6) and digested with appropriate restriction endonucleases. All probes were purified by gel extraction and labeled by random priming with [α-32P]dCTP by using a commercially available labeling kit (Amersham Pharmacia Biotech, Piscataway, N.J.) and removing unincorporated nucleotides by passage through Sephadex G50 microcolumns (Amersham Pharmacia Biotech). Hybridization was carried out by standard techniques under high-stringency conditions (37) and with a hybridization buffer of the following composition: 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 0.5% sodium dodecyl sulfate, 10 mM EDTA, 1× Denhardt's solution, and 100 μg of sonicated salmon sperm DNA per ml. The colony blots were hybridized overnight at 65°C, washed with 0.1× SSC-0.1% sodium dodecyl sulfate at 65°C, and exposed to X-ray film at −80°C overnight. Strains that had been characterized in previous studies were used as controls (Table 1).
TABLE 1.
Fragment probes and control strains used for DNA hybridization experiments
| Patho- type | Target gene | Virulence factor encoded by target gene | Fragment probe used | Control strain(s)a | Refer- ence(s) |
|---|---|---|---|---|---|
| EPEC | eae | Intimin (LEE-encoded adhesin) | pCVD443 (HindIII) | E2348/69 (O127:H6)(eae+, bfpd+) | 11, 25 |
| bfpA | Structural subunit of the bundle- forming plus | pMSD207 (EcoRI) | |||
| EHEC | eae | Intimin | pCVD443 (HindIII) | EDL933 (O157:H7)(eae+, hly+, stx1+, stx2+) | 24, 25, 28 |
| hly | Enterohemolysin | pCVD419 (HindIII) | |||
| stx1 | Shiga toxin 1 | pNN110-18 (SmaI-PstI) | EDL933cu (eae+, hly-stx1+, stx2+) | ||
| stx2 | Shiga toxin 2 | pJN37-19 (BamHI) | |||
| ETEC | elt | LT | pCVD 403 (BamHI) | H10407 (LT+, STh+, STp+) | 12, 19 |
| esth | ST | pCVD 427 (EcoRI) | |||
| lngA | Longus pilus | pOG140 (EcoRV) | |||
| EIEC | inv | Invasion plasmid | pSF55 (HindIII) | EL37 (inv+, sen+) | 18 |
| sen | Shigella enterotoxin 2 | pJS26 (HindIII) | 27 | ||
| EAEC | pAA | Aggregative adherence plasmid | pCVD432 (EcoRI-PstI) | 17-2 (CVD432+, aggA+), 042 (CVD432+, aaf+, set+) | 4, 9, 14, 26 |
| aggA | Aggregative adherence fimbria I | pJPN61 (SspI) | EI37 (sen+) | ||
| aafA | Aggregative adherence fimbria II | pJC2 (EcoRI) | |||
| set | Shigella enterotoxin 1 | pSET (SmaI) | |||
| sen | Shigella enterotoxin 2 | pJS26 (HindIII) | |||
| DAEC | daaC | Diffuse adhesin | pSLM852 (PstI) | C1845 (daaC+) | 5 |
HEp-2 adherence tests.
The HEp-2 adherence test method originally described by Cravioto (7), with slight modifications (40), was used for all E. coli isolates. Bacteria were grown overnight in Luria broth without shaking. HEp-2 cells were grown overnight to 50% confluence in Dulbecco's modified Eagle medium (DMEM; Gibco BRL, Gaithersburg, Md.) containing penicillin, streptomycin, and 10% fetal bovine serum on eight-well chamber slides (Labtek, Scottsvalley, Calif.). The HEp-2 cells were washed three times with phosphate-buffered saline (PBS), and the medium was replaced with DMEM containing 1% mannose. A bacterial suspension (10 μl) was added to each well, and the slides were incubated at 37°C in 5% CO2 for 3 h. The monolayers were washed three times with PBS, fixed with 70% methanol, and stained with Giemsa. Strains that adhered to the monolayers were recorded as adhering in localized, diffuse, or aggregative patterns. Enteropathogenic E. coli (EPEC) strain E2348/69, enteroaggregative E. coli (EAEC) strain 042, and diffusely adherent E. coli (DAEC) strain C1845 were used as positive controls. Nonpathogenic strains HS4 and DH5α were used as negative controls.
Serotyping.
Serological typing of enterohemorrhagic E. coli (EHEC) isolates was carried out by slide agglutination and was confirmed by tube agglutination (35) with commercially available rabbit anti-O157, anti-O26, and anti-O111 immune sera.
Statistical analysis.
The recovery of pathogens from subjects with diarrhea and controls was compared by a two-tailed chi-square test and Fisher's exact test (2).
RESULTS
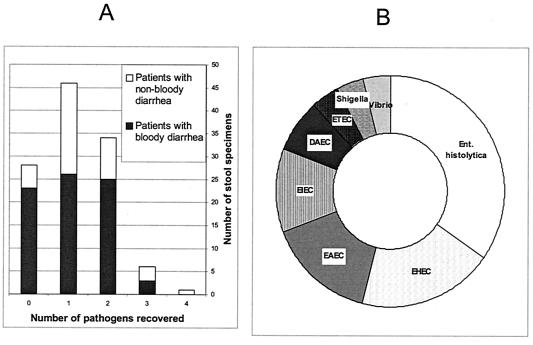
A wide range of bacterial pathogens were detected during the study (Table 2); but only three of these, Entamoeba histolytica, EHEC, and EAEC, were significantly associated with diarrhea (P < 0.05). Gross blood or erythrocytes were encountered in 77 (68.1%) of specimens from patients with diarrhea. The high proportion of bloody diarrhea cases is unsurprising, since in a previous survey (30) we observed that adults in the locality rarely visit a health institution when they have diarrhea unless they perceive the diarrhea as being serious, usually if blood is present. Analyses of the results with respect to the etiology of bloody diarrhea were complicated, since multiple pathogens were identified in 28 (36.4%) specimens from the patients who presented with bloody diarrhea (Fig. 1). As shown in Fig. 1, however, the principal pathogens recovered from 26 patients in whom only one agent was identified were E. histolytica, EHEC, EAEC, and enteroinvasive E. coli (EIEC). No pathogen was identified in specimens from 23 (29.9%) patients with bloody diarrhea and 5 (13.9%) patients with nonbloody diarrhea. As we did not culture for Campylobacter, nor did we screen for enteric viruses, we cannot rule out these infectious etiologies in these patients.
TABLE 2.
Enteric pathogens identified from stool specimens
| Pathogen | No. (%) of subjects
|
P value | |
|---|---|---|---|
| Diarrhea cases (n = 113) | Controls (n = 63) | ||
| Bacteria | |||
| EHEC | 23 (20.4) | 4 (6.4) | 0.013 |
| EPEC | 0 | 1 (1.6) | NSa |
| ETEC | 10 (8.8) | 2 (3.2) | NS |
| EIEC | 7 (6.2) | 1 (1.6) | NS |
| EAEC | 18 (15.9) | 2 (3.2) | 0.011 |
| DAEC | 12 (10.6) | 5 (7.9) | NS |
| Shigella | 8 (7.1) | 2 (3.6) | NS |
| Salmonella enterica serovar Typhi | 2 (1.8) | 0 | NS |
| Salmonella enterica | 3 (2.7) | 0 | NS |
| Yersinia enterocolitica | 0 | 1 (1.6) | NS |
| V. cholerae (non-O1) | 1 (0.9) | 0 | NS |
| Parasites | |||
| E. histolytica | 40 (35.4) | 6 (9.5) | 0.0002 |
| Helminthsb | 6 (5.3) | 3 (4.8) | NS |
NS, not significant (P > 0.05).
Roundworm, flatworm, tapeworm, and hookworm.
FIG. 1.
Pathogen recovery and bloody diarrhea. (A) Recovery of multiple pathogens from diarrheal stool specimens; (B) pathogens recovered from bloody diarrhea specimens from which a single agent was recovered.
The properties of the diarrheagenic E. coli organisms isolated in the course of the study were examined in some detail by using virulence locus probes and HEp-2 adherence (Table 3). EHEC strains carry the locus for enterocyte effacement (LEE) pathogenicity island and genes encoding one or more Shiga toxins. All the EHEC isolates in this study carried genes for intimin (eae, contained within LEE) and Shiga toxin 2 (stx2), and most hybridized to the probe for the virulence plasmid-encoded enterohemolysin. O157 EHEC isolates and sorbitol nonfermenters represented less than a third of the EHEC isolates. There were no eae-negative, stx-positive isolates. Only one EPEC strain, which hybridized to probes for eae and bfp but not to probes for stx1 or stx2, was recovered from a control subject. Strains that hybridized with the probes for elt or esth were categorized as enterotoxigenic E. coli (ETEC). The majority of ETEC isolates were heat-labile enterotoxin (LT) positive. Only two strains, both from controls, hybridized with probes for both heat-stable enterotoxin (ST) and LT, and these were the only strains that hybridized to the lngA probe. EIEC strains were identified by hybridization to the inv probe. All the EIEC isolates so detected also hybridized with the sen probe for Shigella enterotoxin 2. EAEC strains were identified by their ability to adhere to HEp-2 monolayers in an aggregative pattern. Seventy percent of the EAEC isolates hybridized to the CVD 432 probe, a much higher proportion than was seen in a pediatric case-control study conducted in the same region (31). There was considerable heterogeneity of the EAEC isolates with respect to virulence gene content. Strains that showed diffuse adherence on HEp-2 cell monolayers and that did not fall into any of the other categories were classified as DAEC. Fifteen (88%) of these isolates hybridized with the daaC probe.
TABLE 3.
Properties of diarrheagenic E. coli isolates
| Pathotype and property | No. (%) of isolates from:
|
||
|---|---|---|---|
| Patients | Controls | Total | |
| EHECa | |||
| eae | 24 | 4 | 28 (100) |
| hly | 20 | 1 | 21 (75) |
| stx1 | 0 | 0 | 0 |
| stx2 | 20 | 4 | 24 (85.7) |
| O157 antigen | 5 | 0 | 5 (17.9) |
| O26 antigen | 2 | 2 | 4 (14.2) |
| O111 antigen | 6 | 0 | 6 (21.4) |
| Negative or late sorbitol fermentation | 6 | 1 | 7 (25) |
| Localized adherence | 2 | 0 | 2 (7.1) |
| Diffuse adherence | 11 | 3 | 14 (50) |
| EAECb | |||
| CVD432 | 12 | 2 | 14 (56) |
| set | 7 | 0 | 7 (28) |
| sen | 3 | 1 | 4 (16) |
| AAF/I | 8 | 1 | 9 (36) |
| AAF/II | 12 | 0 | 12 (48) |
| daaC | 2 | 0 | 2 (8) |
| ETECc | |||
| ST only | 3 | 0 | 3 (27.3) |
| LT only | 6 | 0 | 6 (54.5) |
| LT+ST+lngA | 0 | 2 | 2 (18.1) |
| EIECd | |||
| inv | 7 | 2 | 9 (100) |
| sen | 7 | 2 | 9 (100) |
| Diffuse adherence | 5 | 0 | 5 (55.6) |
| DAECe | |||
| daaC | 10 | 5 | 15 (88.2) |
Twenty-four EAEC isolates were obtained from 23 individuals (two EHEC strains were recovered from 1 patient); 4 isolates were obtained from the controls.
A total 23 EAEC isolates were obtained from 20 individuals (two EAEC strains were recovered from 3 patients); 2 isolates were obtained from controls.
Nine isolates were obtained from patients, and two isolates were obtained from controls.
Seven isolates were obtained from patients, and two isolates were obtained from controls.
Twelve isolates were obtained from patients, and five isolates were obtained from controls.
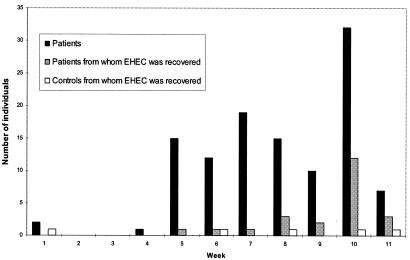
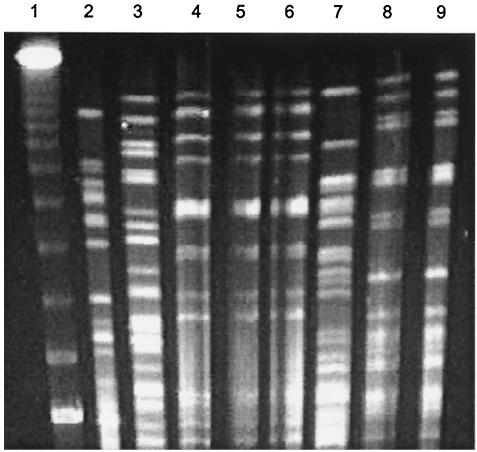
We noted that diarrhea cases were not spread evenly over the 11-week study period (Fig. 2). Of all the pathogens recovered, only EHEC was isolated at a higher concentration during any specific week. Twelve of the EHEC strains isolated from patients were recovered during week 10. We examined XbaI-digested restriction profiles of genomic DNA from all EHEC isolates by pulsed-field gel electrophoresis. Only two profiles were seen more than once, and these were for strains isolated during the 10th and 11th weeks of the study (Fig. 3), suggesting that an outbreak occurred within that time period.
FIG. 2.
Weekly diarrhea cases and recovery of EHEC.
FIG. 3.
Pulsed-field gel electrophoresis of XbaI-digested genomic DNA from selected EHEC isolates showing the two patterns that were seen more than once (lanes 4 to 6 and lanes 8 and 9). The EHEC isolates represented on the gel were isolated during week 6 (lane 2), week 10 (lanes 3 to 7), and week 11 (lanes 8 and 9) of the study. Lane 1, bacteriophage lambda ladder (Bio-Rad).
DISCUSSION
This study suggests that E. histolytica, EHEC, and EAEC are important diarrheal pathogens among adults in southwest Nigeria. E. histolytica was by far the most frequently encountered pathogen and was also commonly found in association with bloody diarrhea. This parasite was also identified in specimens from 9.5% of controls and appears to be highly endemic within the study area.
Several outbreaks and sporadic cases of EHEC have been reported from North America, Europe, Japan, and Australia. In developing countries, with the exception of those in South America, very little is known about the epidemiology of EHEC. There have been very few reports of sporadic EHEC cases in sub-Saharan Africa, outside South Africa (1, 10, 15, 29, 32, 39). In many of these reports, the methods used to detect EHEC were nonspecific or insufficiently sensitive to identify all EHEC isolates, particularly non-O157 strains. Three large EHEC outbreaks have been documented in Africa. The first occurred in Swaziland in 1992 and is probably the largest outbreak of E. coli O157 infection to occur anywhere (13, 21). In a second outbreak, which occurred in the Central African Republic, steamed meat pies (kanda) made out of zebu meat were implicated as the source of infection (16). More recently, a large outbreak occurred in Cameroon, a country on the eastern border of Nigeria (8). A few features were common to all these outbreaks: they occurred in small or remote areas, and there were concomitant or preceding outbreaks of diarrheal disease due to other enteric pathogens that could have masked the outbreak (8, 13). Transmission may have been food borne or waterborne, but person-to-person transmission magnified the outbreaks. Particularly noteworthy is the fact that EHEC was confirmed only with external laboratory assistance. It is highly probable that other EHEC outbreaks that occur within the region are missed.
This study has demonstrated that EHEC causes diarrhea within the study area, both in sporadic and in outbreak contexts. This is the second case-control study that we have conducted in the area and that has identified EHEC (32). The presence of this pathogen presents a potential treatment problem, since the differential diagnosis for bloody diarrhea is Shigella or amoebic dysentery, and both of these are treated empirically with antibiotics (13). Antibiotic therapy can lead to a poor prognosis (by precipitating hemolytic-uremic syndrome) in EHEC-infected individuals, particularly those carrying strains that produce Shiga toxin 2, such as individuals identified in this study (22, 42, 43). The incidence of hemolytic-uremic syndrome in the study area is not known; however, it is important that physicians be made aware of the possible occurrence and the means for the diagnosis and management of this condition. As we also recovered E. histolytica, Shigella, and Salmonella spp., a basis for antibiotic therapy of bloody diarrhea remains; and in the absence of suitable diagnostic aids, prescribers are faced with a dilemma. The incidence of EHEC infection was much greater in this study of adults than in a previous study in the same locality, which focused on pediatric diarrhea (32). This observation is in agreement with the report that there was a higher attack rate in adults than children during the EHEC outbreak in Cameroon (8). These data suggest that adults may be more exposed to infection than children and that, as was supposed previously, cross protection from previous EPEC and/or Shigella infection may not occur.
EAEC is emerging as a significant diarrheal agent worldwide (34). As demonstrated in this and previous work, EAEC appears to be endemic within the study population and other locations in sub-Saharan Africa (17, 38). As is typical for EAEC (31, 41), the strains belonging to this category that were identified in this study were heterogeneous with respect to virulence gene content. In addition to acute and persistent diarrhea, EAEC can cause malnutrition and growth defects in children. The development of diagnosis and treatment protocols for this pathogen is therefore essential to lowering the burden of disease in this and other parts of the world.
E. histolytica is known to be waterborne, but the exact modes of transmission of EHEC and EAEC within the study population are unclear. In Western countries, the most common vehicle for EHEC transmission is undercooked beef; however, meat is generally consumed in overcooked form in southwest Nigeria. Future studies should therefore focus on identifying the risk factors and transmission routes for these two emerging pathogens within the study area. Microbiological surveillance and diagnosis of infections caused by EHEC, EAEC, and related organisms require significant boosting of the present diagnostic capabilities of laboratories within the region. If such diagnostic resources were available, even only in tertiary-care institutions, they could be instrumental in identifying outbreaks, such as the one that occurred in week 10 of this study. The use of selective-diagnostic media or simple agglutination tests to identify sorbitol-negative or O157 E. coli is not a reliable option since most of the EHEC strains identified in this study would not have been detected by these means.
This study has identified two emergent categories of diarrheagenic E. coli as causes of diarrhea in adults in a sub-Saharan African setting. Both agents can bring about severely debilitating or long-term consequences in young children, features that make it imperative that infected persons be identified and that the pathogens be eradicated to prevent their spread to more vulnerable populations. The inability of laboratories in the study area and in most of sub-Saharan Africa to detect these pathogens poses problems for treatment and disease control.
Acknowledgments
This work was funded by grants from the Dan Charitable Fund (to I.N.O.), National Institutes of Health grants AI21657 and DK58957 (to J.B.K.), and International Program in the Chemical Sciences grant NIG01 (to A.L. and I.N.O.).
We thank A. O. Aboderin and Diana Gomez for clinical and technical assistance, Michele Trucksis for assistance with pulsed-field gel electrophoresis, and Jorge Giron and Alfredo Torres for helpful comments.
REFERENCES
- 1.Akinyemi, K. O., A. O. Oyefolu, B. Opere, V. A. Otunba-Payne, and A. O. Oworu. 1998. Escherichia coli in patients with acute gastroenteritis in Lagos, Nigeria. East Afr. Med. J. 75:512-515. [PubMed] [Google Scholar]
- 2.Armitage, P., and G. Berry. 1987. Statistical methods in medical research. Blackwell Scientific Publications, Oxford, United Kingdom.
- 3.Barrow, G., and R. Feltham. 1993. Cowan and Steel's manual for the identification of medical bacteria, 3rd ed. Cambridge University Press, Cambridge, United Kingdom.
- 4.Baudry, B., S. J. Savarino, P. Vial, J. B. Kaper, and M. M. Levine. 1990. A sensitive and specific DNA probe to identify enteroaggregative Escherichia coli, a recently discovered diarrheal pathogen. J. Infect. Dis. 161:1249-1251. [DOI] [PubMed] [Google Scholar]
- 5.Bilge, S., C. Clausen, W. Lau, and S. Moseley. 1989. Molecular characterization of a fimbrial adhesin, F1845, mediating diffuse adherence of diarrhea-associated Escherichia coli to HEp-2 cells. J. Bacteriol. 171:4281-4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birnboim, H. C., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cravioto, A., R. Gross, S. Scotland, and B. Rowe. 1979. An adhesive factor found in strains of Escherichia coli belonging to the traditional enteropathogenic serotypes. Curr. Microbiol. 3:95-99. [Google Scholar]
- 8.Cunin, P., E. Tedjouka, Y. Germani, C. Ncharre, R. Bercion, J. Morvan, and P. M. Martin. 1999. An epidemic of bloody diarrhea: Escherichia coli O157 emerging in Cameroon? Emerg. Infect. Dis. 5:285-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czeczulin, J. R., S. Balepur, S. Hicks, A. Phillips, R. Hall, M. H. Kothary, F. Navarro-Garcia, and J. P. Nataro. 1997. Aggregative adherence fimbria II, a second fimbrial antigen mediating aggregative adherence in enteroaggregative Escherichia coli. Infect. Immun. 65:4135-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dadie, A., T. Karou, N. Adom, A. Kette, and M. Dosso. 2000. Isolation of enteric pathogeic agents in Cote d'Ivoire: Escherichia coli O157:H7 and enteroaggregative E. coli. Bull. Soc. Pathol. Exot. 93:95-96. [PubMed] [Google Scholar]
- 11.Donnenberg, M. S., J. A. Giron, J. P. Nataro, and J. B. Kaper. 1992. A plasmid-encoded type IV fimbrial gene of enteropathogenic Escherichia coli associated with localized adherence. Mol. Microbiol. 6:3427-3437. [DOI] [PubMed] [Google Scholar]
- 12.Echeverria, P., D. N. Taylor, J. Seriwatana, and C. Moe. 1987. Comparative study of synthetic oligonucleotide and cloned polynucleotide enterotoxin gene probes to identify enterotoxigenic Escherichia coli. J. Clin. Microbiol. 25:106-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Effler, E., M. Isaäcson, L. Arntzen, R. Heenan, P. Canter, T. Barrett, L. Lee, C. Mambo, W. Levine, A. Zaidi, and P. M. Griffin. 2001. Factors contributing to the emergence of Escherichia coli O157 in Africa. Emerg. Infect. Dis. 7:812-819. [DOI] [PMC free article] [PubMed]
- 14.Fasano, A., F. R. Noriega, D. R. Manerval, S. Chanasongcram, R. Russell, S. Guandalini, and M. M. Levine. 1995. Shigella enterotoxin 1: an enterotoxin of Shigella flexneri 2a active in rabbit small intestine in vivo and in vitro. J. Clin. Investig. 95:2853-2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Germani, Y., P. Minssart, M. Vohito, S. Yassibanda, P. Glaziou, D. Hocquet, P. Berthelemy, and J. Morvan. 1998. Etiologies of acute, persistent, and dysenteric diarrheas in adults in Bangui, Central African Republic, in relation to human immunodeficiency virus serostatus. Am. J. Trop. Med. Hyg. 59:1008-1014. [DOI] [PubMed] [Google Scholar]
- 16.Germanii, Y., B. Soro, M. Vohito, O. Morel, and J. Morvan. 1997. Enterohaemorrhagic Escherichia coli in the Central African Republic. Lancet 349:1670. [DOI] [PubMed] [Google Scholar]
- 17.Geyid, A., O. Olsvik, and A. Ljungh. 1998. Virulence properties of Escherichia coli isolated from Ethiopian patients with acute or persistent diarrhoea. Ethiop. Med. J. 36:123-139. [PubMed] [Google Scholar]
- 18.Gomes, T. A., M. R. Toledo, L. R. Trabulsi, P. K. Wood, and J. G. Morris, Jr. 1987. DNA probes for identification of enteroinvasive Escherichia coli. J. Clin. Microbiol. 25:2025-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gómez-Duarte, O. G., A. Ruiz-Tagle, D. C. Gomez, G. I. Viboud, K. G. Jarvis, J. B. Kaper, and J. A. Girón. 1999. Identification of lngA, the structural gene of longus type IV pilus of enterotoxigenic Escherichia coli. Microbiology 145:1809-1816. [DOI] [PubMed] [Google Scholar]
- 20.Guerrant, R. L., J. M. Hughes, N. L. Lima, and J. Crane. 1990. Diarrhea in developed and developing countries: magnitude, special settings, and etiologies. Rev. Infect. Dis. 12(Suppl. 1):S41-S50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isaacson, M., P. H. Canter, P. Effler, L. Arntzen, P. Bomans, and R. Heenan. 1993. Haemorrhagic colitis epidemic in Africa. Lancet 341:961. [DOI] [PubMed] [Google Scholar]
- 22.Kimmitt, P. T., C. R. Harwood, and M. R. Barer. 2000. Toxin gene expression by Shiga toxin-producing Escherichia coli: the role of antibiotics and the bacterial SOS response. Emerg. Infect. Dis. 6:458-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levine, M. M., E. J. Bergquist, D. R. Nalin, D. H. Waterman, R. B. Hornick, C. R. Young, and S. Sotman. 1978. Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet i:1119-1122. [DOI] [PubMed] [Google Scholar]
- 24.Levine, M. M., J. G. Xu, J. B. Kaper, H. Lior, V. Prado, B. Tall, J. Nataro, H. Karch, and K. Wachsmuth. 1987. A DNA probe to identify enterohemorrhagic Escherichia coli of O157:H7 and other serotypes that cause hemorrhagic colitis and hemolytic uremic syndrome. J. Infect. Dis. 156:175-182. [DOI] [PubMed] [Google Scholar]
- 25.McDaniel, T. K., K. G. Jarvis, M. S. Donnenberg, and J. B. Kaper. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. USA 92:1664-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nataro, J. P., Y. Deng, D. R. Maneval, A. L. German, W. C. Martin, and M. M. Levine. 1992. Aggregative adherence fimbriae I of enteroaggregative Escherichia coli mediate adherence to HEp-2 cells and hemagglutination of human erythrocytes. Infect. Immun. 60:2297-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nataro, J. P., J. Seriwatana, A. Fasano, D. R. Maneval, L. D. Guers, F. Noriega, F. Dubovsky, M. M. Levine, and J. G. Morris, Jr. 1995. Identification and cloning of a novel plasmid-encoded enterotoxin of enteroinvasive Escherichia coli and Shigella strains. Infect. Immun. 63:4721-4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newland, J. W., and R. J. Neill. 1988. DNA probes for Shiga-like toxins I and II and for toxin-converting bacteriophages. J. Clin. Microbiol. 26:1292-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogunsanya, T. I., V. O. Rotimi, and A. Adenuga. 1994. A study of the aetiological agents of childhood diarrhoea in Lagos, Nigeria. J. Med. Microbiol. 40:10-14. [DOI] [PubMed] [Google Scholar]
- 30.Okeke, I. N. 2003. Antibiotic use and resistance in developing countries, p. 132-139. In S. L. Knobler, S. M. Lemon, M. Najafi, and T. Burroughs (ed.), The resistance phenomenon in microbes and disease vectors. Implications for human health strategies for containment. National Academies Press, Washington, D.C. [PubMed]
- 31.Okeke, I. N., A. Lamikanra, J. Czeczulin, F. Dubovsky, J. B. Kaper, and J. P. Nataro. 2000. Heterogeneous virulence of enteroaggregative Escherchia coli strains isolated from children in Southwest Nigeria. J. Infect. Dis. 181:252-260. [DOI] [PubMed] [Google Scholar]
- 32.Okeke, I. N., A. Lamikanra, H. Steinrück, and J. B. Kaper. 2000. Characterization of Escherichia coli strains from cases of childhood diarrhea in provincial southwestern Nigeria. J. Clin. Microbiol. 38:7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okeke, I. N., A. Lamikanra, and R. Edelman. 1999. Socioeconomic and behavioral factors leading to acquired bacterial resistance to antibiotics in developing countries. Emerg. Infect. Dis. 5:18-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okeke, I. N., and J. P. Nataro. 2001. Enteroaggregative Escherichia coli. Lancet Infect. Dis. 1:304-313. [DOI] [PubMed] [Google Scholar]
- 35.Ørskov, I., and F. Ørskov. 1984. Serotyping of Escherichia coli. Methods Microbiol. 14:43-112. [Google Scholar]
- 36.Sack, R. B. 1990. Travelers' diarrhea: microbiologic basis for prevention and treatment. Rev. Infect. Dis. 12:S59-S63. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 38.Sang, W. K., J. O. Oundo, J. K. Mwituria, P. G. Waiyaki, M. Yoh, T. Iida, and T. Honda. 1997. Multidrug-resistant enteroaggregative Escherichia coli associated with persistent diarrhea in Kenyan children. Emerg. Infect. Dis. 3:373-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sang, W. K., S. M. Saidi, H. Yamamoto, T. Ezaki, T. Iida, M. Yoh, and T. Honda. 1996. Haemorrhagic colitis due to Escherichia coli O157:H7 in Kenya. J. Trop. Pediatr. 42:118-119. [DOI] [PubMed] [Google Scholar]
- 40.Vial, P. A., J. J. Mathewson, H. L. DuPont, L. Guers, and M. M. Levine. 1990. Comparison of two assay methods for patterns of adherence to HEp-2 cells of Escherichia coli from patients with diarrhea. J. Clin. Microbiol. 28:882-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vila, J., M. Vargas, I. R. Henderson, J. Gascon, and J. P. Nataro. 2000. Enteroaggregative Escherichia coli virulence factors in traveler's diarrhea strains. J. Infect. Dis. 182:1780-1783. [DOI] [PubMed] [Google Scholar]
- 42.Wong, C. S., S. Jelacic, R. L. Habeeb, S. L. Watkins, and P. I. Tarr. 2000. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N. Engl. J. Med. 342:1930-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang, X., A. D. McDaniel, L. E. Wolf, G. T. Keusch, M. K. Waldor, and D. W. Acheson. 2000. Quinolone antibiotics induce Shiga toxin-encoding bacteriophages, toxin production, and death in mice. J. Infect. Dis. 181:664-670. [DOI] [PubMed] [Google Scholar]