Myanmar is one of the 22 high-burden countries for tuberculosis (TB) (1). A previous study showed that a sizable number of Mycobacterium tuberculosis isolates are resistant to first-line anti-TB drugs in Yangon, Myanmar (5). The object of the present study was to determine if certain clones of M. tuberculosis predominated and to ascertain whether there were similarities to strains from neighboring areas.
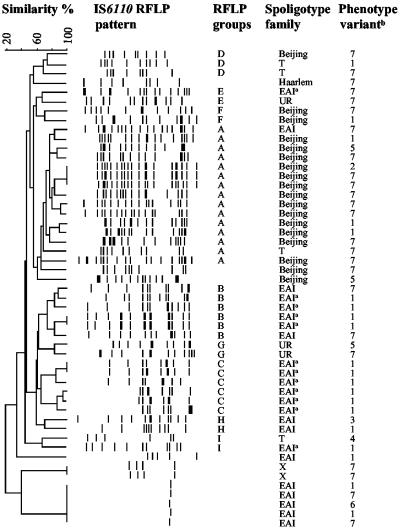
Of 864 patients who attended a referral clinic of the National TB Program in Yangon in July 2000, 202 were diagnosed with pulmonary TB based on medical history, clinical signs, two smear-positive sputum samples, and a chest X-ray. Of these, approximately half were new cases (no previous TB treatment) and isolates from 51 cases were available for the present study. All isolates belonged to the M. tuberculosis complex (AccuProbe Mycobacterium tuberculosis complex test; Gen-Probe, San Diego, Calif.). Standard biochemical assays (thiophen-2-carboxylic acid hydrazide [TCH] susceptibility test, niacin accumulation test, nitrate reduction test, and pyrazinamidase activity) were performed for phenotyping (4). Spoligotyping was performed with a commercial kit as recommended by the manufacturer (Isogen Bioscience BV, Maarssen, The Netherlands). IS6110 restriction fragment length polymorphism (RFLP) analyses were performed as previously described (8). The phenotype variants, spoligotype families, and IS6110 RFLP dendrogram of the isolates are shown in Fig. 1. The majority of the isolates belonged phenotypically to the classical and Asian human M. tuberculosis variants as previously defined by Collins et al. (2). The spoligotype families were defined in accordance with Sebban et al. (6), but three isolates did not correspond to any of the defined families. When the RFLP patterns of the isolates with >65% Dice coefficient similarity were examined, a criterion used previously to discern IS6110 groups (9), nine groups (A to I) could be discriminated.
FIG. 1.
Dendrogram of 51 isolates produced following Dice and unweighted pair group method with arithmetic average analysis of IS6110 RFLP and distribution of the isolates according to spoligotype family and phenotype variants. Spoligotype families are according to Sebban et al. (6). UR, previously unreported spoligotype families. a, shared type 292; b, variant 1, Asian human M. tuberculosis variant (susceptible to TCH, positive niacin accumulation, nitrate reduction, and pyrazinamidase activity tests); variant 7, classical human M. tuberculosis variant (resistant to TCH, positive niacin accumulation, nitrate reduction, and pyrazinamidase activity tests); variants 2 to 6, variants between classical and Asian human M. tuberculosis strains.
The Beijing (n = 18) and East African Indian (EAI) (n = 23) were the most prevalent spoligotype families, but our study could not reveal if transmission was due to the occurrence of a recent outbreak or was a reflection of selective advantages for these genotypes in this setting. Our finding is in line with other studies showing a predominant presence of the Beijing family strains in Asian countries (3). Twelve out of the 23 EAI spoligotype family isolates belonged to shared type 292. The predominant presence of one particular spoligotype pattern of the EAI family has not previously been reported even though the EAI family has been spreading in India and East Africa (7).
Fifteen of the 18 Beijing family isolates belonged to RFLP group A, whereas the EAI family isolates were distributed among RFLP groups A, B, C, E, H, and I. Moreover, the Beijing family isolates belonged predominately to the classical human M. tuberculosis variant while the EAI family isolates belonged predominately to the Asian human M. tuberculosis variant (P = 0.02, chi-square test). These differences suggest that the two spoligotype families may have evolved differently in Myanmar and that the majority of the Beijing family strains seem to belong to a distinct genetic lineage. The high heterogeneity of the RFLP patterns of the isolates indicates, however, that several strains contribute to the TB endemicity in Yangon.
Acknowledgments
This study was supported by the University of Bergen and Haukeland University Hospital, Bergen, Norway.
We thank Christophe Sola, Nalin Rastogi, and coworkers (Unité de la Tuberculose et des Mycobactéries, Institut Pasteur de Guadeloupe, Guadeloupe) for sharing their extensive spoligotype database with us.
REFERENCES
- 1.Blled, D., C. Watt, and C. Dye. 2001. Global TB control. World Health Organization, Geneva, Switzerland.
- 2.Collins, C. H., M. D. Yates, and J. M. Grange. 1982. Subdivision of Mycobacterium tuberculosis into five variants for epidemiological purposes: methods and nomenclature. J. Hyg. 89:235-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glynn, J. R., J. Whiteley, P. J. Bifani, K. Kremer, and D. van Soolingen. 2002. Worldwide occurrence of Beijing/W strains of Mycobacterium tuberculosis: asystematic review. Emerg. Infect. Dis. 8:843-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grange, J., and I. deKantor. 1996. Guidelines for speciation within the Mycobacterium tuberculosis complex, 2nd ed. World Health Organization, Geneva, Switzerland.
- 5.Phyu, S., T. Ti, R. Jureen, T. Hmun, H. Myint, A. Htun, H. M. Grewal, and B. Bjorvatn. 2003. Drug-resistant Mycobacterium tuberculosis among new tuberculosis patients, Yangon, Myanmar. Emerg. Infect. Dis. 9:274-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sebban, M., I. Mokrousov, N. Rastogi, and C. Sola. 2002. A data-mining approach to spacer oligonucleotide typing of Mycobacterium tuberculosis. Bioinformatics 18:235-243. [DOI] [PubMed] [Google Scholar]
- 7.Sola, C., I. Filliol, M. C. Gutierrez, I. Mokrousov, V. Vincent, and N. Rastogi. 2001. Spoligotype database of Mycobacterium tuberculosis: biogeographic distribution of shared types and epidemiologic and phylogenetic perspectives. Emerg. Infect. Dis. 7:390-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Embden, J. D. A., M. D. Cave, J. T. Crawford, J. W. Dale, K. D. Eisenach, B. Gicquel, P. Hermans, C. Martin, R. McAdam, T. M. Shinnick, and P. M. Small. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warren, R. M., S. L. Sampson, M. Richardson, G. D. Van Der Spuy, C. J. Lombard, T. C. Victor, and P. D. van Helden. 2000. Mapping of IS6110 flanking regions in clinical isolates of Mycobacterium tuberculosis demonstrates genome plasticity. Mol. Microbiol. 37:1405-1416. [DOI] [PubMed] [Google Scholar]