Abstract
The Platelia Candida-specific antigen and antibody assays (Bio-Rad Laboratories) were used to test serial serum samples from seven neutropenic adult patients with hematological malignancies who had developed systemic Candida tropicalis infections. The diagnosis of candidiasis was based on a positive blood culture (all seven patients) and the isolation of C. tropicalis from a normally sterile site (six patients). All patients received early antifungal therapy with amphotericin B and/or an azole derivative and had successful outcomes. When the combined assays were applied to sera collected at different time points before and after the first positive blood culture, all patients tested positive. In six patients, at least one positive test was obtained with sera collected, on average, 5 days (range, 2 to 10 days) prior to the first positive blood culture, while blood cultures were constantly negative. High and persistent mannanemias were detected in all patients during the neutropenic period. In five patients, an increased antibody response was detected when the patients recovered from aplasia. Controls consisted of 48 serum samples from 12 febrile neutropenic patients with aspergillosis (n = 4), bacteremia (n = 4), or no evidence of infection (n = 4). A low level of mannanemia was detected in only one serum sample, and none showed significant Candida antibody titers. Our data thus confirm the value of the combined detection of mannanemia and antimannan antibodies in individuals at risk of candidemia and suggest that in neutropenic patients, an approach based on the regular monitoring of both markers could contribute to the earlier diagnosis of C. tropicalis systemic infection.
Treatment of patients with hematological malignancies, particularly those with acute myeloblastic leukemia, has evolved toward the use of increasingly aggressive antineoplastic regimens and autologous or allogeneic bone marrow or peripheral blood stem cell transplantation (26). These therapeutic approaches induce severe neutropenia and have resulted in an increased incidence of bacterial and fungal infections (1, 12, 19, 25, 47). The rate of nosocomial fungemia has increased dramatically over the past decade. Candida species account for 10 to 15% of all hospital-acquired bloodstream pathogens. Autopsy studies have shown that the incidences of fungal infections are 15 to 25% among patients with leukemia or those undergoing bone marrow transplant and 10% among those with lymphoma (4, 27). Systemic candidiasis is associated with long hospital stays and mortality rates of 18 to 70% (25). A shift in the spectrum of infecting species has also occurred; and non-Candida albicans species of Candida, although they may have a lower intrinsic pathogenic potential than C. albicans, are now identified in more than 45% of episodes of hematogenously disseminated candidiasis (30, 44).
The main risk factors for candidemia are the degree and duration of neutropenia; prior colonization with Candida spp.; mucosal barrier disruption following cytotoxic chemotherapy or irradiation; prolonged use of broad-spectrum antibiotics, particularly glycopeptides; the number of antibiotics received; and mucosal colonization by Candida (46). These risk factors, which serve to identify individuals at high risk of developing candidemia, are shared by a large number of patients. Moreover, the clinical features of systemic candidiasis are nonspecific, making the early diagnosis of systemic candidiasis difficult (38, 45). Histopathology- or culture-based examination of sterile body sites is often not feasible in practice, and for reasons that remain unclear, culture of blood for fungi, even when it is performed daily, has a poor sensitivity (9, 24). As a consequence, the diagnosis of candidemia is generally established at a late stage, or even by autopsy, in a considerable number of cases, which accounts for its poor prognosis (8, 13).
In order to overcome these difficulties, several groups have focused on the development of biological tests based on the detection of either antibodies to Candida proteins or polysaccharides or Candida components such as mannan (32, 48), glucan (28), arabinitol (41), or nucleic acids (15, 21) in body fluids under the assumption that these molecules would prove to be early specific markers of disseminated infection. Among these putative markers, mannan is a major component of the Candida cell wall, both quantitatively and qualitatively. Extensive studies of this polysaccharide have demonstrated its role as a potent modulator of innate and adaptive immunity (24, 31, 36, 40). Mannan induces a strong antibody response toward a large repertoire of oligomannose epitopes. Some of these antibodies may be protective to the host, while others may not. In this context, a new diagnostic approach has recently been proposed by our group, based on the combined detection of mannan and antimannan antibodies in patients at risk of developing candidiasis. This strategy is based on the detection of mannan and antimannan antibodies by two distinct immunoenzymatic assays (the Platelia Candida-specific antigen [Ag] and Platelia Candida-specific antibody [Ab] tests; Bio-Rad Laboratories, Marnes-la Coquette, France). Retrospective studies based on the analysis of more than 500 serum samples from 130 patients with candidemia treated in different hospital wards and 150 control serum samples from patients without candidemia showed that the combined use of these tests had a 93% specificity and an 80% sensitivity in the diagnosis of infections caused by the most pathogenic Candida species. Among the main conclusions of these retrospective studies was the fact that regular serum sampling was critical to achieving an early diagnosis (36, 37, 49).
We recently investigated a pseudoepidemic of Candida tropicalis infections that occurred in a cohort of seven adult neutropenic patients with lymphoblastic or myeloid leukemia undergoing myeloablative treatment. The availability of serial serum samples together with complete clinical and biological records gave us the opportunity to assess the Platelia tests for the detection of infections caused by C. tropicalis, a species emerging as one of the main causative agents of candidemia in patients with malignancies (3, 20).
MATERIALS AND METHODS
Patients.
Between June 1998 and December 2000, a cohort of consecutive adult patients with hematological malignancies was enrolled in a prospective study aimed at evaluating the value of serial screening for Aspergillus galactomannan in patients at high risk of invasive aspergillosis. All patients received myeloablative treatment that induced neutropenia (polymorphonuclear leukocyte count, <500/μl). During the neutropenia that followed chemotherapy, patients were hospitalized in single reverse isolation rooms or in laminar airflow-protected rooms. Chest X rays were systematically taken in these rooms twice a week. The axillary temperature was measured every 3 h. Microbiological monitoring included the culture of blood on a daily basis (six samples per week) and the retrieval of samples from the throat and nose and the collection of urine and stool samples twice a week. Serum samples were collected on admission to the hospital and were also collected, together with blood samples for culture, at the onset of the first neutropenic febrile episode and daily thereafter while the patients remained neutropenic or when there was a clinical suspicion of fungal infection. When the first febrile episode (axillary temperature, >38°C for longer than 3 h) occurred, a β-lactam antibiotic plus an aminoglycoside was used as empirical therapy after microbiological sampling and chest X ray. Antibiotic therapy was adapted later according to the culture results. When cultures were negative and fever persisted, vancomycin or teicoplanin was added at 48 h. Amphotericin B was administered when either the fever persisted for 24 to 48 h following the addition of a glycopeptide or a subsequent episode of fever occurred in a patient with ongoing neutropenia. The antifungal therapy was also adapted according to the culture results or when renal failure occurred. In both cases, an azole drug or liposomal amphotericin B was prescribed.
During this survey seven patients (four females and three males; mean age, 51.8 ± 14.2 years) developed systemic C. tropicalis infection, confirmed by a positive blood culture and subsequent mycological examination of cerebrospinal fluid or urine specimens, liver or spleen biopsy specimens, cutaneous nodules, or aqueous humor, depending on the clinical symptoms. The clinical and mycological findings are summarized in Table 1, together with the nature, duration, and dose of antifungal treatment. This cluster of C. tropicalis infections probably resulted from a microepidemic, which is under investigation.
TABLE 1.
Underlying diseases and mycological culture findings for infected patientsa
| Patient no. (age [yr], sex) | Hematological malignancy | Other diagnostic site of Candida isolation or infection | Antifungal therapy (dose) and duration | Outcome at day 30 | Treatment and comments |
|---|---|---|---|---|---|
| 1 (71, F) | AML | Day 15, Abelcet (300 mg/day) + itraconazole (400 mg/day) for 5 weeks | Survived | Day 17, chemotherapy; day 15, invasive pulmonary aspergillosis | |
| 2 (48, M) | Refractory anemia with an excess of blasts | Urine, throat, cutaneous (biopsy specimen) | Day 0, AMB (1 mg/kg of body wt/day); day 2, Abelcet (300 mg/day) + fluconazole (1,200 mg/day) + 5FC (7.5 g/day); day 14, itraconazole (200 mg/day) + 5 FC (7.5 g/day); >day 30, itraconazole (200 mg/day) | Survived | Day 10, chemotherapy; >day 0, cutaneous metastasis and myosite |
| 3 (61, M) | AML | Cutaneous, renal, endophthalmitis | Day 1, AMB (1 mg/kg); day 0 to day 9, Abelcet (300 mg/day) + itraconazole (60 mg/day); day 10 to day 22, itraconazole (600 mg/day); day 23 to day 50, voriconazole (400 mg/day) i.v.; day 50 to day 70, voriconazole (400 mg/day) per os | Survived | Day 19, chemotherapy; day 1, invasive pulmonary aspergillosis |
| 4 (40, F) | ALL | Cutaneous, renal, occular, liver, spleen (biopsy specimens) | Day 0 to day 22, abelcet (300 mg per day); day 23 to day 94, Ambisome® (400 mg per day) | Survived | Day 7, chemotherapy |
| 5 (70, F) | AML, breast cancer | Cutaneous (biopsy specimen) | Day 0 to day 25: AMB and then Abelcet (total dose, 7,180 mg) | Survived (recovery) | Day 6, chemotherapy |
| 6 (28, M) | AML | Septicemic metastasis | Day 2 to day 21, AMB (1 mg/kg per day) + fluconazole (1,200 mg/day) per os >day 21, itraconazole (600 mg/day) per os | Survived (recovery) | Day 12, chemotherapy; day 10, septicemia due to E. faecium and S. epidermidis |
| 7 (55, F) | ALL | Cerebrospinal fluid, cutaneous (biopsy specimen) | Day −2 to day 0, AMB (60 mg/day) i.v.; Day 1 to day 7, fluconazole (800 mg/day) per os + Abelcet (300 mg/day) i.v.; >day 7, Ambisome (300 mg/day) | Survived | Day 22, chemotherapy; day 7, infection relapse with isolation of C. tropicalis from blood and liver biopsy specimen |
C. tropicalis was isolated from the blood of all patients. Abbreviations: F, female; M, male; AML, acute myeloid leukemia; ALL, acute lymphocytic leukemia; AMB, amphotericin B; 5FC, flucytosine; i.v., intravenous injection. Abelcet and Ambisome are liposomal amphotericin B.
A total of 83 serum samples were collected from these patients (average number of serum samples per patient, 11.2 ± 5.6). The samples were stored at −80°C, transferred in dry ice, and tested in a blinded fashion.
Controls consisted of 12 febrile neutropenic adult patients hospitalized in the same hematology unit (4 patients with invasive aspergillosis, 4 patients with bacterial septicemia, and 4 patients with no evidence of infection). Four serum samples were collected from each of the control patients before (n = 2), during (n = 1), and after (n = 1) the period of aplasia. Altogether, 48 control serum samples were available for testing.
Mycological investigations.
Blood samples for culture and specimens from clinically infected foci were collected according to established clinical guidelines (7). Ten milliliters of blood was inoculated in a BACTEC Plus aerobic/F bottle and incubated for up to 14 days with the BACTEC 9240 blood culture system (Becton Dickinson, Le Pont de Claix, France). Other clinical samples were cultured at 30°C on Sabouraud dextrose agar containing gentamicin (40 mg/liter) and chloramphenicol (0.4 g/liter). The yeast isolates were identified by the presence or absence of germ tube formation at 37°C in human serum and by the API 32C identification system (Bio-Mérieux, Marcy l'Etoile, France) (11).
Detection of anti-C. albicans mannan antibodies in human sera.
Antibodies to C. albicans mannan were detected by the commercially available Platelia Candida-specific Ab test (Bio-Rad Laboratories) (36). Microtiter plates were sensitized with C. albicans cell wall mannan, which was extracted and purified from C. albicans strain VW32 grown in bioreactors by standard protocols (10). Enzyme immunoassay reactions were performed with the BEP III automate (Dade-Behring Laboratories, Paris, France). Each set of tests included a standard curve, which was obtained by using serial twofold dilutions of a pool of sera strongly reacting with yeast mannan. For individual serum samples, 100 μl of serum diluted 1/6,400 was applied to each well, and the plate was incubated at 37°C for 1 h. After the plate was washed, 100 μl of horseradish peroxidase-conjugated anti-human immunoglobulin was added, and the mixture was incubated for 1 h at 37°C. After intensive washing, the reactions were revealed by 30 min of incubation in the dark with 200 μl of tetramethylbenzidine solution, and the absorbance (λ = 450 and 620 nm) was then measured. The results are reported as arbitrary units (AU) in relation to the standard curve. Ten AU was considered indicative of candidiasis (37).
Detection of mannanemia.
Mannanemia was detected by the commercially available Platelia Candida-specific Ag test (Bio-Rad Laboratories). This test involves the monoclonal antibody (MAb) designated EBCA1, which recognizes a repetitive epitope present in Candida mannan (42). The minimal epitope of this MAb has been shown to correspond to α-linked mannopentaose of the C. albicans VW32 mannan acid-stable domain. This epitope is also present on numerous C. albicans mannoproteins (18). Because of the repetitive nature of this epitope, MAb EBCA1 is used as both the antigen-capture and the detection antibody. Microtiter plates were sensitized with MAb EBCA1 in an industrial setting.
Three-hundred microliters of patient serum was denatured with 100 μl of EDTA treatment solution, boiled for 3 min, and then centrifuged at 10,000 × g for 10 min. Fifty microliters of supernatant was mixed in EBCA1-coated wells with 50 μl of horseradish peroxidase-conjugated EBCA1. After incubation for 90 min at 37°C, the plates were washed thoroughly and the reaction was revealed by incubation with 200 μl of tetramethylbenzidine solution for 30 min in the dark. The optical density was read at λ equal to 450 and 620 nm on a PR2100 reader (Bio-Rad Laboratories). Each experiment included a calibration curve, which was made with a pool of normal human serum supplemented with known concentrations of mannan ranging from 0.1 to 2 ng/ml. Reactions were performed in duplicate. For mannanemia levels exceeding 2 ng/ml (above the range of the calibration curve), a precise determination of the antigen concentration, which is important for patient monitoring, was performed systematically. The test was repeated after dilution of the patient serum 1/5 in negative control serum.
RESULTS
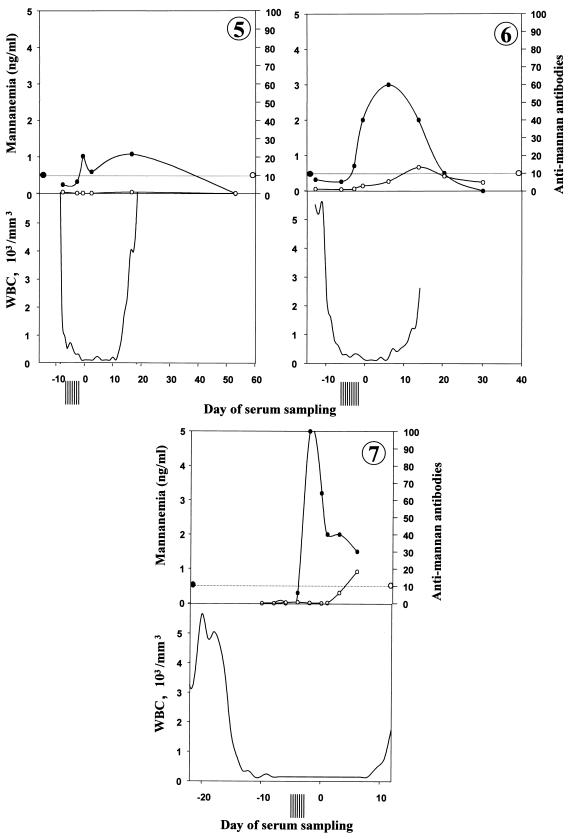
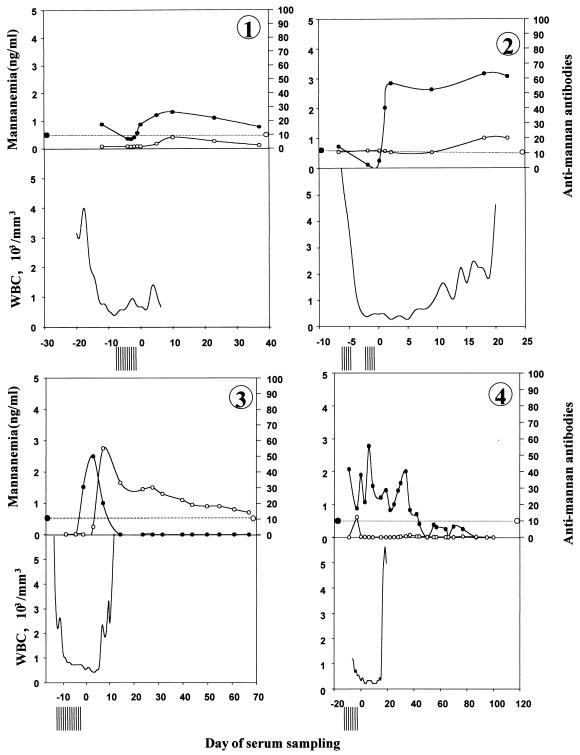
The clinical and antifungal therapy data for the patients with C. tropicalis bloodstream infections are shown in Table 1. Day 0 corresponds to the date when the first blood sample positive by culture was drawn (i.e., an average of 2 to 3 days before fungal growth was obtained). Patients 1 and 3 had concurrent aspergillosis, as suggested by the high serum Aspergillus galactomannan titers, computed tomography scans, and isolation of Aspergillus fumigatus from bronchoalveolar fluid. Patient 7 also presented with septicemia due to Enterococcus faecium and Staphylococcus epidermidis. All patients with C. tropicalis infection included in this study survived at day 30. Figures 1 to 7 show the evolution of mannanemia, antimannan antibodies, leukocyte counts, and negative blood cultures for patients 1 to 7, respectively. As described above, day 0 corresponds to the date when the first blood sample positive by culture was collected. Altogether, a mean of eight culture-negative blood samples were collected before the first culture-positive blood sample was collected. Interestingly, during the same period, significant mannanemia was detected in sera obtained 12, 7, 2, 9, 2, and 2 days before day 0 in patients 1, 2, 3, 4, 6, and 7, respectively. Peaks of mannanemia often coincided with low titers of antimannan antibodies and vice versa. For example, patient 4 presented a significant antimannan antibody response as the level of antigenemia decreased at day −2. This was followed by a drop in antibody titers as the antigen titers increased at day 0. Finally, antibodies remained undetectable during the neutropenic period, whereas the level of antigenemia was high. In all patients, high levels of circulating mannan, which generally ranged from 1 to 3 ng/ml, were detected. The level of mannanemia reached 5 ng/ml in patient 7. The duration of mannanemia (defined as a level >0.5 ng/ml) ranged from 8 to 51 days. Finally, the mannanemia kinetics generally resolved to nonsignificant values after effective antifungal treatment. The decrease in the levels of circulating antigens was generally faster when an antimannan antibody response developed at the end of aplasia. Such a seroconversion was observed in patients 3, 4, 6, and 7. Patient 4 developed an early antibody response, with the antibodies rapidly complexed by the high levels of antigen.
FIG. 1.
Kinetics of circulating mannan (•) and antimannan antibodies (○) in sequentially drawn sera from seven adult neutropenic patients (panels 1 to 7, respectively) with systemic candidiasis. The evolution of mannanemia and antimannan antibodies (the levels of which are given in AU) is shown, together with leukocyte (WBC) counts (the bottom part of each figure) and the number of negative blood cultures (vertical bars preceding day 0), as a function of time in relation to day 0, which represents the day on which the first blood sample positive for C. tropicalis by culture was taken. The horizontal dotted line represents the cutoff values of the tests (0.5 ng/ml for the mannanemia test and 10 AU for the antimannan antibody test).
The specificities of the Platelia Candida-specific Ag and Platelia Candida-specific Ab tests have been assessed in previous studies by analyzing control sera from hospitalized patients. In addition to these previous evaluations, 12 patients without candidemia from the same cohort who presented with fever were included as controls in the present study. The clinical data for these patients, together with the results of the Platelia Candida-specific Ag and Platelia Candida-specific Ab tests, are shown in Table 2. Only 1 of the 48 control serum samples displayed a positive cutoff value by the Platelia Candida-specific Ag test, and none gave a positive Platelia Candida-specific Ab test result.
TABLE 2.
Clinical characteristics of control subjectsa
| Patient no. (age [yr], sex) | Hematological malignancy | Infection | Duration of neutropenia (days) | Platelia test results
|
Treatment | |
|---|---|---|---|---|---|---|
| Antigen (ng/ml) | Antibody (AU) | |||||
| C1 (45, F) | AML | Septicemia (Pseudomonas fluorescens) | 6 | 0.14, 0.11, 0.11, 0.14 | 0, 0, 0, 0 | Autologous stem cell transplantation |
| C2 (54, M) | NHL | Septicemia (S. epidermidis) | 11 | 0.15, 0.14, 0.13, 0.13 | 5.5, 5.4, 4.7, 4.4 | Autologous stem cell transplantation |
| C3 (21, M) | AML | Septicemia (S. epidermidis) | 32 | 0.14, 0.17, 0.11, 0.2 | 0, 0, 0, 0 | Chemotherapy |
| C4 (60, M) | NHL | Septicemia (Pseudomonas aeruginosa) | 7 | 0.13, 0.12, 0.12, 0.47 | 0, 0, 0, 2.4 | Autologous stem cell transplantation |
| C5 (41, M) | AML | Invasive aspergillosis (A. fumigatus) | 14 | 0.13, 0.1, 0.19, 0.19 | 0.7, 0.4, 0.9, 2 | Chemotherapy |
| C6 (66, M) | AML | Invasive aspergillosis (A. fumigatus) | 18 | 0.16, 0.18, 0.15, 0.44 | 0.5, 1.6, 2.4, 0.95 | Chemotherapy |
| C7 (7, F) | AML | Invasive aspergillosis (A. fumigatus) | 32 | 0.18, 0.1, 0.22, 0.5b | 0, 0.7, 0.9, 2.9 | Chemotherapy |
| C8 (8, M) | NHL | Invasive aspergillosis (A. fumigatus) | 57 | 0.16, 0.15, 0.17, 0.35 | 0, 0, 0, 0.5 | Chemotherapy |
| C9 (58, F) | Myeloma | None | 17 | 0.31, 0.16, 0.27, 0.2 | 0, 0, 0, 0 | Chemotherapy |
| C10 (42, M) | Myelodysplastic syndrome in transformation | None | 6 | 0.14, 0.25, 0, 0.17 | 3.5, 2.5, 1.7, 3.8 | Chemotherapy |
| C11 (75, M) | Myeloma | None | 5 | 0.17, 0.1, 0.19, 0.12 | 9, 9.3, 6, 7.2 | Autologous stem cell transplantation |
| C12 (57, M) | LMC | None | 9 | 0.17, 0.15, 0.16, 0.14 | 0, 0, 0, 0.3, 0.2 | Chemotherapy |
Abbreviations: F, female; M, male; AML, acute myeloid leukemia; ALL, acute lymphocytic leukemia; NHL, non-Hodgkin's lymphoma; LMC, chronic myeloid leukemia.
The value of 0.5 was above the cutoff value, indicating a positive result.
DISCUSSION
Candidiasis is the leading cause of invasive fungal infection in patients with malignant hematological disorders (particularly those with acute myeloid leukemia) and bone marrow transplant recipients (29, 35, 44). Prolonged neutropenia associated with the treatment of hematological malignancies is a major risk factor predisposing these patients to invasive candidiasis (1, 20, 23). The growing incidence of systemic candidiasis has a strong impact on overall rates of mortality (13, 43). Moreover, the increased morbidity as a result of candidemia generates important extra costs related to longer hospital stays and prophylactic, empirical, or curative antifungal treatment (33). While the availability of new antifungal drugs such as echinocandins (39) and new azoles (16) is a promising step toward improving outcomes for patients with systemic candidiasis, a definitive and early diagnostic approach is imperative to avoid delays in the initiation of treatment for infected patients and to prevent unnecessary therapy in high-risk individuals who are only colonized (38).
Several organizations, including the European Organization for the Research and Treatment of Cancer/Mycosis Study Group, have focused on this problem and have generated specific sets of criteria for case definition. The specificities of these definitions, based on host and clinical characteristics, can be improved by incorporating culture-based and non-culture-based microbiological criteria (2, 22). Culture-based criteria include positive biopsy or histopathology findings as irrefutable evidence of infection, but this may involve potentially life-threatening procedures and may result in a delay in the initiation of potentially life-saving therapy. In contrast, blood cultures are easy to perform, and all groups agree about the initiation of antifungal therapy following the isolation of Candida species from a blood culture (7, 8, 34). However, it has long been recognized that this “gold standard” reference microbiological diagnostic procedure lacks sensitivity, for reasons that remain obscure (43). Therefore, blood cultures cannot be relied on as the only approach to the diagnosis of systemic candidiasis. A considerable amount of work has been devoted to the development of non-culture-based methods based on the detection of fungal components (28, 37, 48) or metabolites (41) to complement blood cultures.
Almost all methods recently described in high-quality microbial research journals attain the theoretical goal of higher sensitivities than blood culture (9, 48). However, no consensus has been reached about the use of these tests in the management of patients with invasive candidiasis. This is related to the lack of prospective studies on well-stratified patients that take into account the highly complex and polymorphic pathophysiologies of patients (17). The results of tests for the detection of yeast DNA by PCR obtained recently are promising in terms of sensitivity, specificity, and identification at the species level (15, 21). Apart from amplification methods that can detect very small quantities of fungal DNA, another strategy consists of the detection of major fungal molecules. Present methods of antigen detection have very high levels of sensitivity, and these continue to improve (9, 37, 38, 48). The major antigen of Candida species is mannan. Among the immunomodulatory properties that influence the pathophysiology of infection (31), Candida mannan and mannoconjugates that share the same oligomannose epitopes are potent immunogens and lead to the production of different classes of antibodies which may in some cases be protective (6, 14, 24). The repertoire of Candida oligomannose epitopes expressed on mannan, mannoproteins, and glycolipids may elicit an antibody response; and these epitopes may circulate after their release from fungal cells during the infection process (24, 32). In a previous study of circulating epitopes consisting of a sequence of α-linked mannose residues (18), a balance was observed between accelerated clearance in the presence of antibodies and an apparent down-regulation of antibody synthesis when high levels of circulating epitopes were present (37). Therefore, a new strategy consisting of the combined measurement of mannanemia and an antibody response was developed, based on two automated sensitive standardized enzyme immunoassays, the Platelia Candida-specific Ag and Platelia Candida-specific Ab tests. Retrospective studies showed that the sensitivities of the Platelia tests ranged from 80 to 100% for infections caused by the most pathogenic Candida species, C. albicans, Candida glabrata, and C. tropicalis, which represent up to 80% of clinical isolates recovered, depending on the hospital ward (36, 37, 49).
All C. tropicalis infections investigated in the present study were detected by a positive blood culture. Early systemic antifungal therapy was initiated as soon as a systemic fungal infection was suspected; and all seven patients were alive 30 days after the onset of candidemia, including patients 1, 3, and 6, in whom mixed infections had been detected. The clustering of cases over a comparatively short period of time, together with the rapid occurrence of embolic lesions in six of the seven patients, suggests a common source of infection. The molecular relatedness of the strains is under investigation (5). All seven patients were included in a prospective evaluation of serum Aspergillus galactomannan levels at the time of C. tropicalis infection. Serial serum samples were thus available, which allowed a thorough evaluation of the two Platelia tests under the recommended conditions (i.e., screening of a series of samples starting before the onset of infection). Previous studies have reported good specificities and negative predictive values for the Platelia tests (37). These findings were confirmed by including relevant controls (sera from febrile patients treated in the same ward and either infected or not infected with A. fumigatus or bacteria).
In all patients except patient 5, significant mannanemia was detected between 12 and 2 days before the first positive blood culture, a period when blood cultures were constantly negative. In patient 5, the first positive Platelia Candida-specific Ag test was obtained on day 0. In most patients, significant mannanemia persisted for several weeks. Mannanemia was detected early in patient 4, who finally presented with histologically and culture-proven hepatosplenic candidiasis. In this respect, Prella et al. (M. Prella, J. Bille, M. Pugnale, M. Cavassini, C. Durussel, M. Knaup, B. Duvoisin, M. P. Glauser, and T. Calandra, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. 847, 2001) recently reported on a series of 12 patients with confirmed hepatosplenic candidiasis; 11 of these patients presented with positive mannanemia and/or antimannan antibodies by the Platelia Candida-specific tests an average of 18 days prior to the appearance of radiological signs. This is an interesting finding, since the diagnosis of hepatosplenic candidiasis is generally difficult to establish.
Patient 4 also presented a sharp peak in antimannan antibody titers. However, the antibody response was transient and rapidly became undetectable when the antigen titer again increased at day 0, a profile consistent with immune complex formation. Significant antibody responses were detected in patients 2 and 3 for several weeks. In these two patients, as well as in patients 6 and 7, recovery from aplasia was associated with an increase in antimannan antibody levels. No significant antibody responses were observed in the controls. As a complement to the antigen levels, which were significantly higher during the period of aplasia (P < 0.02 by Student's t test), detection of antibodies at levels that mirror the level of antigenemia are also indicative of tissue invasion. The results of the present study suggest that the inclusion of regular serological surveillance for mannanemia and anti-Candida mannan antibodies in patients would complement blood culture for the early detection of candidiasis in at-risk patients.
Analysis of the contribution of serology to the diagnosis of candidiasis is often hampered by the small number of serum samples available and the irregularity of sampling. The availability of a sample collected before the beginning of infection is also necessary, since rising titers are highly indicative of an increasing fungal burden. The samples collected during the present study met all these requirements. Altogether, the correlation of serological and microbiological findings with clinical and radiological data allowed a precise evaluation of the role of the combined Platelia tests in the detection of C. tropicalis systemic infection in neutropenic patients.
Acknowledgments
We are grateful to Valerie Hopwood and Annie Robinet for helpful comments and help with manuscript editing. We thank also Laurence Richard and Nadine François for valuable technical assistance.
REFERENCES
- 1.Anaissie, E. J., J. H. Rex, O. Uzun, and S. Vartivarian. 1998. Predictors of adverse outcome in cancer patients with candidemia. Am. J. Med. 104:238-245. [DOI] [PubMed] [Google Scholar]
- 2.Ascioglu, S., J. H. Rex, B. de Pauw, J. E. Bennett, J. Bille, F. Crokaert, D. W. Denning, J. P. Donnelly, J. E. Edwards, Z. Erjavec, D. Fiere, O. Lortholary, J. Maertens, J. F. Meis, T. F. Patterson, J. Ritter, D. Selleslag, P. M. Shah, D. A. Stevens, and T. J. Walsh. 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 34:7-14. [DOI] [PubMed] [Google Scholar]
- 3.Bodey, G. 1993. Candidiasis. Pathogenesis, diagnosis, and treatment, 2nd ed. Raven Press, New York, N.Y. 420.
- 4.Bodey, G., B. Bueltmann, W. Duguid, D. Gibbs, H. Hanak, M. Hotchi, G. Mall, P. Martino, F. Meunier, S. Milliken, et al. 1992. Fungal infections in cancer patients: an international autopsy survey. Eur. J. Clin. Microbiol. Infect. Dis. 11:99-109. [DOI] [PubMed] [Google Scholar]
- 5.Bougnoux, M. E., S. Morand, and C. d'Enfert. 2002. Usefulness of multilocus sequence typing for characterization of clinical isolates of Candida albicans. J. Clin. Microbiol. 40:1290-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casadevall, A., A. Cassone, F. Bistoni, J. E. Cutler, W. Magliani, J. W. Murphy, L. Polonelli, and L. Romani. 1998. Antibody and/or cell-mediated immunity, protective mechanisms in fungal disease: an ongoing dilemma or an unnecessary dispute? Med. Mycol. 36(Suppl. 1):95-105. [PubMed] [Google Scholar]
- 7.Denning, D. W., E. G. Evans, C. C. Kibbler, M. D. Richardson, M. M. Roberts, T. R. Rogers, D. W. Warnock, R. E. Warren, et al. 1997. Guidelines for the investigation of invasive fungal infections in haematological malignancy and solid organ transplantation. Eur. J. Clin. Microbiol. Infect. Dis. 16:424-436. [DOI] [PubMed] [Google Scholar]
- 8.Edwards, J. E., G. P. Bodey, R. A. Bowden, T. Buchner, B. E. de Pauw, S. G. Filler, M. A. Ghannoum, M. Glauser, R. Herbrecht, C. A. Kauffman, S. Kohno, P. Martino, F. Meunier, T. Mori, M. A. Pfaller, J. H. Rex, T. R. Rogers, R. H. Rubin, J. Solomkin, C. Viscoli, T. J. Walsh, and M. White. 1997. International Conference for the Development of a Consensus on the Management and Prevention of Severe Candidal Infections. Clin. Infect. Dis. 25:43-59. [DOI] [PubMed] [Google Scholar]
- 9.Erjavec, Z., and P. E. Verweij. 2002. Recent progress in the diagnosis of fungal infections in the immunocompromised host. Drug Resist. Update 5:3-10. [DOI] [PubMed] [Google Scholar]
- 10.Faille, C., D. W. Mackenzie, J. C. Michalski, and D. Poulain. 1992. Evaluation of an enzyme immunoassay using neoglycolipids constructed from Candida albicans oligomannosides to define the specificity of anti- mannan antibodies. Eur. J. Clin. Microbiol. Infect. Dis. 11:438-446. [DOI] [PubMed] [Google Scholar]
- 11.Freydiere, A. M., R. Guinet, and P. Boiron. 2001. Yeast identification in the clinical microbiology laboratory: phenotypical methods. Med. Mycol. 39:9-33. [DOI] [PubMed] [Google Scholar]
- 12.Gaytan-Martinez, J., E. Mateos-Garcia, E. Sanchez-Cortes, J. Gonzalez-Llaven, L. J. Casanova-Cardiel, and J. L. Fuentes-Allen. 2000. Microbiological findings in febrile neutropenia. Arch. Med. Res. 31:388-392. [DOI] [PubMed] [Google Scholar]
- 13.Groll, A. H., P. M. Shah, C. Mentzel, M. Schneider, G. Just-Nuebling, and K. Huebner. 1996. Trends in the postmortem epidemiology of invasive fungal infections at a university hospital. J. Infect. 33:23-32. [DOI] [PubMed] [Google Scholar]
- 14.Han, Y., M. H. Riesselman, and J. E. Cutler. 2000. Protection against candidiasis by an immunoglobulin G3 (IgG3) monoclonal antibody specific for the same mannotriose as an IgM protective antibody. Infect. Immun. 68:1649-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hebart, H., J. Loeffler, L. Kanz, and H. Einsele. 2000. Molecular methods in the diagnosis of infections in the immunocompromised host. Curr. Opin. Infect. Dis. 13:355-359. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman, H. L., and R. C. Rathbun. 2002. Review of the safety and efficacy of voriconazole. Expert Opin. Investig. Drugs 11:409-429. [DOI] [PubMed] [Google Scholar]
- 17.Hyams, K. C. 1998. Developing case definitions for symptom-based conditions: the problem of specificity. Epidemiol. Rev. 20:148-156. [DOI] [PubMed] [Google Scholar]
- 18.Jacquinot, P. M., Y. Plancke, B. Sendid, G. Strecker, and D. Poulain. 1998. Nature of Candida albicans-derived carbohydrate antigen recognized by a monoclonal antibody in patient sera and distribution over Candida species. FEMS Microbiol. Lett. 169:131-138. [DOI] [PubMed] [Google Scholar]
- 19.Jarvis, W. R. 1995. Epidemiology of nosocomial fungal infections, with emphasis on Candida species. Clin. Infect. Dis. 20:1526-1530. [DOI] [PubMed] [Google Scholar]
- 20.Kontoyiannis, D. P., I. Vaziri, H. A. Hanna, M. Boktour, J. Thornby, R. Hachem, G. P. Bodey, and I. I. Raad. 2001. Risk factors for Candida tropicalis fungemia in patients with cancer. Clin. Infect. Dis. 33:1676-1681. [DOI] [PubMed] [Google Scholar]
- 21.Loeffler, J., K. Schmidt, H. Hebart, U. Schumacher, and H. Einsele. 2002. Automated extraction of genomic DNA from medically important yeast species and filamentous fungi by using the MagNA Pure LC system. J. Clin. Microbiol. 40:2240-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maertens, J., J. Verhaegen, K. Lagrou, J. Van Eldere, and M. Boogaerts. 2001. Screening for circulating galactomannan as a noninvasive diagnostic tool for invasive aspergillosis in prolonged neutropenic patients and stem cell transplantation recipients: a prospective validation. Blood 97:1604-1610. [DOI] [PubMed] [Google Scholar]
- 23.Maertens, J., M. Vrebos, and M. Boogaerts. 2001. Assessing risk factors for systemic fungal infections. Eur. J. Cancer Care 10:56-62. [DOI] [PubMed] [Google Scholar]
- 24.Martinez, J. P., M. L. Gil, J. L. Lopez-Ribot, and W. L. Chaffin. 1998. Serologic response to cell wall mannoproteins and proteins of Candida albicans. Clin. Microbiol. Rev. 11:121-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martino, P., C. Girmenia, A. Micozzi, R. Raccah, G. Gentile, M. Venditti, and F. Mandelli. 1993. Fungemia in patients with leukemia. Am. J. Med. Sci. 306:225-232. [DOI] [PubMed] [Google Scholar]
- 26.Martino, R., and M. Subira. 2002. Invasive fungal infections in hematology: new trends. Ann. Hematol. 81:233-243. [DOI] [PubMed] [Google Scholar]
- 27.Meyers, J. D. 1990. Fungal infections in bone marrow transplant patients. Semin. Oncol. 17:10-13. [PubMed] [Google Scholar]
- 28.Miyazaki, T., S. Kohno, K. Mitsutake, S. Maesaki, K. Tanaka, N. Ishikawa, and K. Hara. 1995. Plasma (1->3)-β-d-glucan and fungal antigenemia in patients with candidemia, aspergillosis, and cryptococcosis. J. Clin. Microbiol. 33:3115-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pagano, L., A. Antinori, A. Ammassari, L. Mele, A. Nosari, L. Melillo, B. Martino, M. Sanguinetti, F. Equitani, F. Nobile, M. Carotenuto, E. Morra, G. Morace, and G. Leone. 1999. Retrospective study of candidemia in patients with hematological malignancies. Clinical features, risk factors and outcome of 76 episodes. Eur. J. Haematol. 63:77-85. [DOI] [PubMed] [Google Scholar]
- 30.Pfaller, M. A. 1996. Nosocomial candidiasis: emerging species, reservoirs, and modes of transmission. Clin. Infect. Dis. 22:S89-S94. [DOI] [PubMed] [Google Scholar]
- 31.Podzorski, R. P., G. R. Gray, and R. D. Nelson. 1990. Different effects of native Candida albicans mannan and mannan-derived oligosaccharides on antigen-stimulated lymphoproliferation in vitro. J. Immunol. 144:707-716. [PubMed] [Google Scholar]
- 32.Ponton, J., and G. Quindos. 2002. Non-culture-based diagnostics, p. 393-425. In R. A. Calderone (ed.), Candida and candidiasis. American Society for Microbiology, Washington, D.C.
- 33.Rentz, A. M., M. T. Halpern, and R. Bowden. 1998. The impact of candidemia on length of hospital stay, outcome, and overall cost of illness. Clin. Infect. Dis. 27:781-788. [DOI] [PubMed] [Google Scholar]
- 34.Rex, J. H., T. J. Walsh, J. D. Sobel, S. G. Filler, P. G. Pappas, W. E. Dismukes, J. E. Edwards, et al. 2000. Practice guidelines for the treatment of candidiasis. Clin. Infect. Dis. 30:662-678. [DOI] [PubMed] [Google Scholar]
- 35.Rossetti, F., D. L. Brawner, R. Bowden, W. G. Meyer, H. G. Schoch, L. Fisher, D. Myerson, R. C. Hackman, H. M. Shulman, G. E. Sale, et al. 1995. Fungal liver infection in marrow transplant recipients: prevalence at autopsy, predisposing factors, and clinical features. Clin. Infect. Dis. 20:801-811. [DOI] [PubMed] [Google Scholar]
- 36.Sendid, B., J. L. Poirot, M. Tabouret, A. Bonnin, D. Caillot, D. Camus, and D. Poulain. 2002. Combined detection of mannanaemia and antimannan antibodies as a strategy for the diagnosis of systemic infection caused by pathogenic Candida species. J. Med. Microbiol. 51:433-442. [DOI] [PubMed] [Google Scholar]
- 37.Sendid, B., M. Tabouret, J. L. Poirot, D. Mathieu, J. Fruit, and D. Poulain. 1999. New enzyme immunoassays for sensitive detection of circulating Candida albicans mannan and antimannan antibodies: useful combined test for diagnosis of systemic candidiasis. J. Clin. Microbiol. 37:1510-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevens, D. A. 2002. Diagnosis of fungal infections: current status. J. Antimicrob. Chemother. 49(Suppl. 1):11-19. [DOI] [PubMed] [Google Scholar]
- 39.Stone, E. A., H. B. Fung, and H. L. Kirschenbaum. 2002. Caspofungin: an echinocandin antifungal agent. Clin. Ther. 24:351-377. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki, S. 1997. Immunochemical study on mannans of genus Candida. I. Structural investigation of antigenic factors 1, 4, 5, 6, 8, 9, 11, 13, 13b and 34. Curr. Top. Med. Mycol. 8:57-70. [PubMed] [Google Scholar]
- 41.Switchenko, A. C., C. G. Miyada, T. C. Goodman, T. J. Walsh, B. Wong, M. J. Becker, and E. F. Ullman. 1994. An automated enzymatic method for measurement of d-arabinitol, a metabolite of pathogenic Candida species. J. Clin. Microbiol. 32:92-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trinel, P. A., C. Faille, P. M. Jacquinot, J. C. Cailliez, and D. Poulain. 1992. Mapping of Candida albicans oligomannosidic epitopes by using monoclonal antibodies. Infect. Immun. 60:3845-3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verweij, P. E., and J. F. Meis. 2000. Microbiological diagnosis of invasive fungal infections in transplant recipients. Transplant. Infect. Dis. 2:80-87. [DOI] [PubMed] [Google Scholar]
- 44.Viscoli, C., C. Girmenia, A. Marinus, L. Collette, P. Martino, B. Vandercam, C. Doyen, B. Lebeau, D. Spence, V. Krcmery, B. De Pauw, and F. Meunier. 1999. Candidemia in cancer patients: a prospective, multicenter surveillance study by the Invasive Fungal Infection Group (IFIG) of the European Organization for Research and Treatment of Cancer (EORTC). Clin. Infect. Dis. 28:1071-1079. [DOI] [PubMed] [Google Scholar]
- 45.Warnock, D. W. 1998. Fungal infections in neutropenia: current problems and chemotherapeutic control. J. Antimicrob. Chemother. 41(Suppl. D):95-105. [DOI] [PubMed] [Google Scholar]
- 46.Wenzel, R. P. 1995. Nosocomial candidemia: risk factors and attributable mortality. Clin. Infect. Dis. 20:1531-1534. [DOI] [PubMed] [Google Scholar]
- 47.Wingard, J. R., and H. L. Leather. 2001. Empiric antifungal therapy for the neutropenic patient. Oncology (Huntington) 15:351-363. [PubMed] [Google Scholar]
- 48.Yeo, S. F., and B. Wong. 2002. Current status of nonculture methods for diagnosis of invasive fungal infections. Clin. Microbiol. Rev. 15:465-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yera, H., B. Sendid, N. Francois, D. Camus, and D. Poulain. 2001. Contribution of serological tests and blood culture to the early diagnosis of systemic candidiasis. Eur. J. Clin. Microbiol. Infect. Dis. 20:864-870. [DOI] [PubMed] [Google Scholar]