Abstract
We reported that several HIV protease inhibitors (HIV-PIs) interfere with the endoproteolytic processing of two farnesylated proteins, yeast a-factor and mammalian prelamin A. We proposed that these drugs interfere with prelamin A processing by blocking ZMPSTE24, an integral membrane zinc metalloproteinase known to play a critical role in its processing. However, because all of the drug inhibition studies were performed with cultured fibroblasts or crude membrane fractions rather than on purified enzyme preparations, no definitive conclusions could be drawn. Here, we purified Ste24p, the yeast ortholog of ZMPSTE24, and showed that its enzymatic activity was blocked by three HIV-PIs (lopinavir, ritonavir, and tipranavir). A newer HIV-PI, darunavir, had little effect on Ste24p activity. None of the HIV-PIs had dramatic effects on the enzymatic activity of purified Ste14p, the prenylprotein methyltransferase. These studies strongly support our hypothesis that HIV-PIs block prelamin A processing by directly affecting the enzymatic activity of ZMPSTE24, and in this way they may contribute to lipodystrophy in individuals undergoing HIV-PI treatment.
Keywords: ZMPSTE24, Ste24p, HIV-PI, lamins, Ste14p, lipodystrophy, protease, methyltransferase
Many eukaryotic proteins, such as the mating pheromone a-factor in Saccharomyces cerevisiae, the Ras proteins in both yeast and mammals, and the nuclear lamins in mammals, terminate with a CaaX motif (in which the “C” is cysteine, “a” is often an aliphatic amino acid, and “X” is one of several different amino acids) [1]. The CaaX motif triggers a series of posttranslational modifications. First, the cysteine is farnesylated or geranylgeranylated by cytosolic protein prenyltransferases. Second, the last three amino acids of the protein (i.e., the “aaX”) are clipped off and released. This reaction is generally carried out by RCE1, a membrane endoprotease of the endoplasmic reticulum (ER); however, in the case of a-factor in yeast and prelamin A in mammals, this step can be carried out by both RCE1 and a membrane zinc metalloproteinase of the ER, designated Ste24p in yeast and ZMPSTE24 in mammals [2–4]. Finally, the newly exposed isoprenylcysteine is methylated by an integral membrane methyltransferase, designated Ste14p in yeast and ICMT in mammals [5,6]. These reactions leave the protein with a carboxyl-terminal prenylcysteine α-carboxyl methyl ester.
At least two farnesylated proteins, a-factor in yeast and prelamin A in mammals, undergo additional endoproteolytic processing. In the case of a-factor, the first seven amino acids of the protein are removed by Ste24p, which render the protein susceptible to a final endoproteolytic processing reaction by Axl1p [2,7,8]. In the case of prelamin A, ZMPSTE24 cleaves off the last 15 amino acids of the protein, including the farnesylcysteine methyl ester, releasing mature lamin A [9]. ZMPSTE24 and Ste24p are orthologs. Mammalian ZMPSTE24 faithfully carries out both the carboxyl- and amino-terminal endoproteolytic processing steps in the maturation of yeast a-factor, and the two enzymes exhibit high degrees of amino acid identity/similarity and share the classic features of a zinc metalloprotease [10–12].
Several mutations in LMNA, the gene encoding prelamin A and lamin C, cause lipodystrophy, and a similar phenotype has been noted in patients treated with HIV-PIs [13,14]. Furthermore, individuals with mandibuloacral dysplasia (MAD), which is characterized in part by lipodystrophy, contain mutations in ZMPSTE24 [11]. Recently, several groups have shown that HIV-PIs can lead to accumulation of prelamin A in fibroblasts, suggesting that these drugs interfere with the conversion of prelamin A to mature lamin A [15–17]. Coffinier et al. [16,17] found that the prelamin A that accumulated is farnesylated, and additional studies with crude membrane fractions suggested that HIV-PIs might inhibit ZMPSTE24. This conclusion was tentative, however, since no studies were performed with purified enzyme preparations. Moreover, one could have argued that an effect of HIV-PIs on ZMPSTE24 was implausible, given that the HIV-protease is an aspartyl protease and HIV-PIs have never been reported to inhibit a zinc metalloproteinase. For these reasons, we considered it important to examine the effects of HIV-PIs on purified enzyme preparations.
Materials and Methods
Materials
Lopinavir (LPV), ritonavir (RTV), and tipranavir (TPV) were obtained from the NIH AIDS Research and Reference program, Division of AIDS, NIAID, NIH. Darunavir (DRV) was a gift from Dr. Arun Ghosh (Department of Chemistry, Purdue University). n-Dodecyl-β-d-maltopyranoside (DDM) was obtained from Anatrace (Maumee, OH). A farnesylated a-factor peptide (YIIKGVFWDPA(farnesyl)CVIA) was synthesized and purchased from either California Peptide Research (San Diego, CA) or EZBiolab (Westfield, IN). E. coli polar lipid extract was purchased from Avanti Polar Lipids (Alabaster, AL). S-Adenosyl-l-[14C-methyl]-methionine (SAM) was purchased from GE Healthcare (Piscataway, NJ). All other chemicals were obtained from Fisher Scientific (Pittsburgh, PA).
Yeast Strains and Plasmids
A Δste24Δrce1 strain of S. cerevisiae (SM3614) was transformed with pSM1282, a plasmid encoding a His10HA3N-terminally-tagged version of Ste24p or pCHH10m3N-Ste14, a plasmid encoding a His10myc3N-terminally-tagged version of Ste14p [12,18,19]. These strains were designated CH2771 and CH2733, respectively. Both proteins were under the control of the constitutive 3’-phosphoglycerate kinase promoter.
Purification of Ste24p and Ste14p
Microsomes were isolated as previously described [18]. Briefly, CH2733 and CH2771 cells were grown in synthetic complete drop out media (minus uracil) and harvested at 2 OD600. Cell pellets were washed with 50 mM NaN3 and centrifuged for 5 min at 5000 × g. Cells were resuspended in lysis buffer plus inhibitors (300 mM sorbitol, 100 mM NaCl, 6 mM MgCl2, 10 mM Tris-HCl pH 7.5, 1% aprotinin, 2 mM AEBSF-HCl, 1 mM DTT) and incubated for 15 min on ice. Cells were twice frozen on liquid nitrogen and thawed. Cells were then lysed twice by passing the cells through a French press at 18,000 psi. After removing cellular debris by centrifugation at 500 × g, the supernatant fluid was incubated with micrococcal nuclease (Worthington Biochemical, Lakewood, NJ) (50 units/per ml lysate). The lysate was then centrifuged at 150,000 × g for 1 h at 4 °C. Membrane fraction pellets were resuspended in lysis buffer containing inhibitors and 10% glycerol.
Ste14p and Ste24p are multispanning membrane proteins and were purified by methods similar to those described by Anderson et al. [18]. Briefly, CH2733 or CH2771 membranes (125 mg) were solubilized at a final concentration of 5 mg/mL for 1 h at 4 °C in 1% (w:v) DDM and 20 mM imidazole in buffer S containing 300 mM sorbitol, 100 mM NaCl, 6 mM MgCl2, 10 mM Tris-HCl pH 7.5, 1% aprotinin, 2 mM AEBSF-HCl, 1 mM DTT, and 10% glycerol. Nonsolubilized membranes were removed by centrifugation (30 min at 300,000 × g at 4 °C). The supernatant fluid was mixed with Talon metal affinity resin beads (500 uL of a 50% v:v slurry equilibrated with buffer S) (Clontech, Palo Alto, CA) for 1 h at 4 °C. After binding, the beads were washed twice with 5 mL buffer A (buffer S, 1% DDM, 40 mM imidazole), 5 mL of buffer B once (buffer A and 0.5 M KCl), and 5 mL buffer C once (buffer S, 0.1% DDM, 0.5 M KCl, 40 mM imidazole). The protein was then eluted from the beads with 3 mL elution buffer (buffer S containing 1 M imidazole and 0.1% (w:v) DDM). The eluate was added to an Amicon Ultra 30,000 MWCO concentrator and centrifuged 15–20 min at 5000 × g at 4 °C. Protein concentration was determined with the amido black protein assay [20]. Ste24p and Ste14p were reconstituted in liposomes composed of E. coli polar lipid extract by rapidly diluting the proteins 33-fold in 100 mM MES, pH 7.0 containing a ~500-fold lipid:protein ratio (w:w) [18].
Silver Staining and Immunoblot Analysis
Proteins (1 µg for silver staining and 0.1 µg for immunoblotting) were size-fractionated on a 10% SDS-polyacrylamide gel, and either stained with silver or transferred to a 0.2-µm nitrocellulose membrane (Whatman, Florham Park, NJ) for immunoblotting. For silver staining, the gel was fixed in 45% ethanol/9% glacial acetic acid, incubated in 0.125% AgNO3 and developed in 3% NaCO3/1.5% formaldehyde. Percent purity was estimated with ImageJ (National Institutes of Health, Bethesda, MD). Nitrocellulose membranes were blocked with nonfat powdered milk (20% (w:v) in PBS containing 0.05% TWEEN-20) and probed with primary polyclonal antibodies (1:1500 anti-Ste24p or 1:1000 anti-Ste14p) in 5% nonfat powdered milk (w:v) in PBS containing 0.05% TWEEN-20. After washing the membrane, antibody binding was detected with a horseradish peroxidase–labeled goat anti-rabbit IgG (1:10,000) in 5% (w:v) nonfat powdered milk in PBS containing 0.05% TWEEN-20. Signal was detected using the SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, IL).
In Vitro Proteolysis Methyltransferase Coupled Vapor Diffusion Activity Assay
Ste24p activity was measured with a coupled proteolysis-methylation activity assay in the presence of excess Ste14p [16,19,21]. Activity assays were assembled on ice. Purified proteins were incubated with lipid and varying concentrations of HIV-PI or vehicle (DMSO) for 5 min. After adding the a-factor peptide substrate, a mixture of MES pH 7.0/[14C]SAM was added to a final concentration of 100 mM MES and 20 µM [14C]SAM. This rapidly diluted sample (130 μl) was then divided into two 60 μl reactions that contained 0.09 µg Ste24p, 0.19 µg Ste14p, and 5 µM of the peptide substrate. After incubating the reactions for 30 min at 30 °C, reactions were terminated by adding 50 µl 1 M NaOH/1% SDS. The samples were spotted onto filter paper and lodged into the neck of a scintillation vial containing 10 ml BioSafe II scintillation fluid (RPI, Mount Prospect, IL), and [14C]methanol was allowed to diffuse into the fluid for 2.5 h at room temperature. Radioactivity was quantified in a Packard Tri-Carb 1600CA liquid scintillation analyzer. Base-releaseable [14C]methanol in reactions containing the HIV-PI was reported as a percent of the DMSO control. The apparent IC50max was calculated with GraphPad Prism 4.0 (San Diego, CA).
Ste14p activity was measured with the same methods except that 40 µM N-acetyl-S-farnesyl-l-cysteine (provided by Dr. Richard A. Gibbs, Purdue University) was used as the substrate.
Results and Discussion
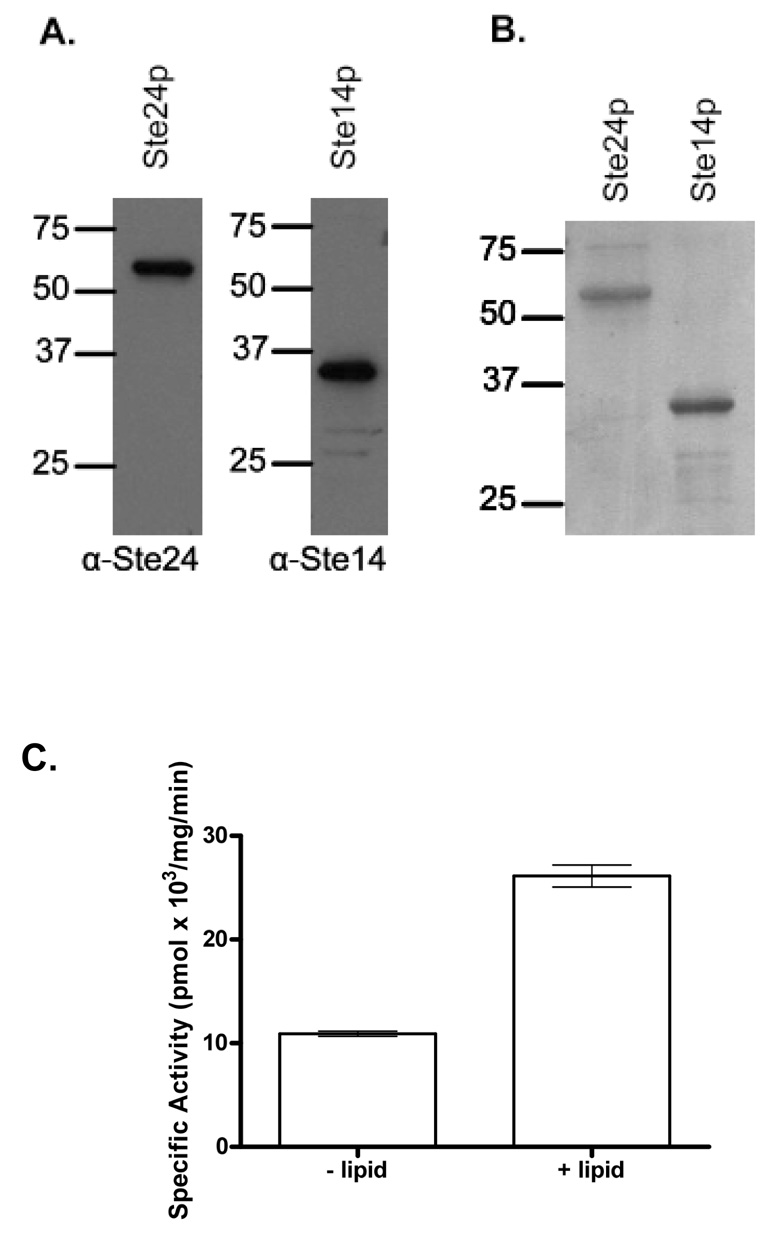
Ste14p and Ste24p, both multispanning integral membrane proteins, were solubilized from crude yeast membrane preparations with 1% (w:v) DDM and isolated by cobalt metal affinity chromatography after removal of the nonsolubilized fraction by ultracentrifugation. Following binding of the solubilized material to the resin and washing as described in Materials and Methods, proteins was eluted from the resin in 0.1% DDM buffer containing 1 M imidazole, pH 8.0. The identity of the purified proteins was established by immunoblotting (Figure 1A), and the purity was assessed on silver stained SDS-polyacrylamide gels (Figure 1B). Both proteins were >90% pure, as judged by ImageJ analysis.
Figure 1. Purification of Ste24p and Ste14p in 1% DDM.
A. Immunoblots of the purified proteins with antibodies specific for Ste24p and Ste14p. Ste24p was detected with the rabbit polyclonal antibody SM215 specific for amino acids 223–306 of Ste24p (1:1500 dilution) [8]; Ste14p was detected with the rabbit polyclonal antibody SM168 that recognizes amino acids 197–239 of Ste14p (1:1000) [26]. B. Silver-stained SDS-polyacrylamide gel of purified Ste24p (1 µg) and Ste14p (1 µg). Both were >90% pure, as judged by ImageJ analysis. C. Effect of reconstitution by rapid dilution in polar lipid extract on the activity of Ste24p. Ste24p and Ste14p (0.09 µg and 0.19 µg, respectively) were either rapidly diluted 33-fold in 100 mM MES, pH 7.0 (− lipid) or reconstituted in 150 µg E. coli polar lipid extract by 33-fold rapid dilution (+ lipid) and the specific activity was determined by the in vitro coupled endoproteolysis-methylation vapor-diffusion activity assay. Data is the result of three independent experiments, with each point performed in duplicate. Error bars represent SEM.
Ste24p activity was measured using a coupled endoproteolysis-methylation assay with an a-factor substrate containing the C-terminal –aaX sequence (YIIKGVFWDPA(farnesyl)CVIA) in the presence of an excess of purified Ste14p [19]. In this assay, the purified Ste24p releases the last three amino acids from the farnesylated peptide, exposing a farnesylcysteine that subsequently undergoes carboxylmethylation by Ste14p. Methylation was assessed by quantifying base-releasable [14C]methanol in a vapor diffusion assay [18,22]. In order for the purified proteins to exhibit their maximal enzymatic activities, they needed to be reconstituted into E. coli polar lipid extract by rapid dilution. Upon reconstitution in E. coli lipid, the protein activity was enhanced (Figure 1C).
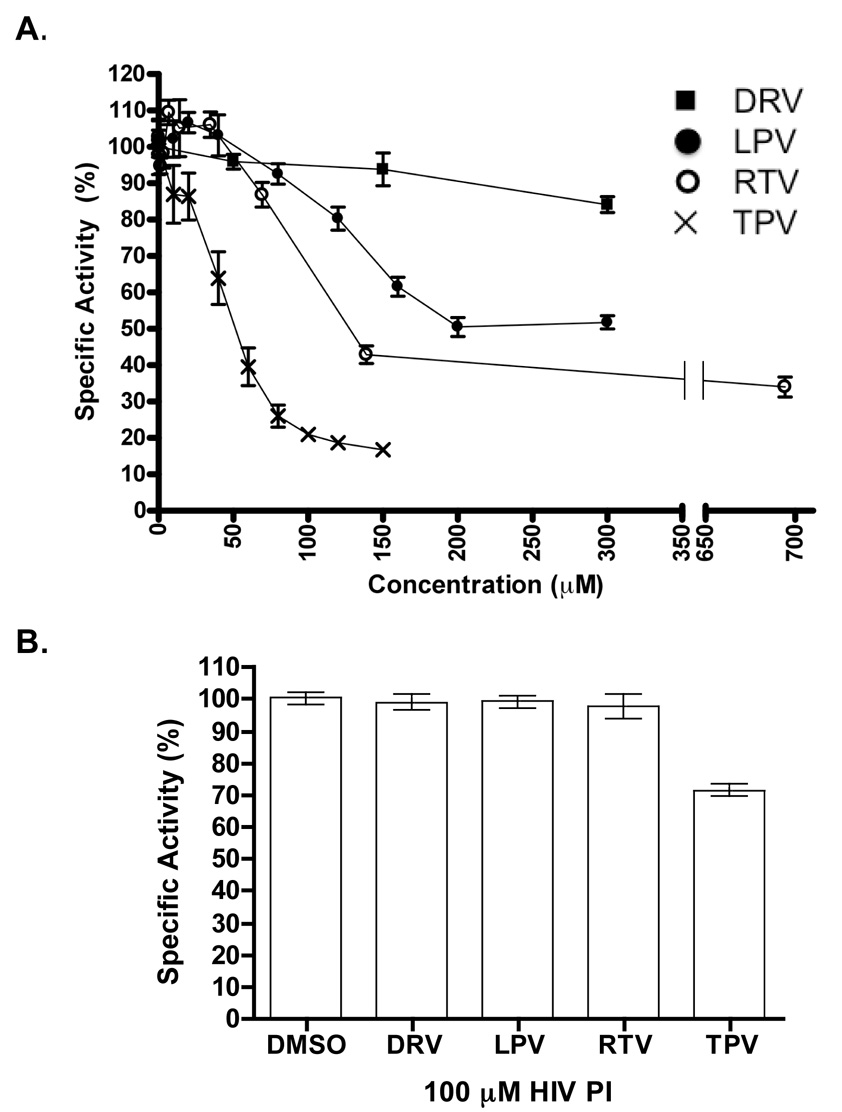
Ste24p activity was inhibited by several commonly prescribed HIV-PIs (Figure 2A). Because the inhibition curves do not approach a specific activity of zero, perhaps due to the presence of excess lipid, an apparent IC50max was calculated by defining the bottom of each inhibition curve as zero. Under the conditions used in these assays, the extent of inhibition was greatest with tipranavir (apparent IC50max of 33.3 ± 3.4 µM), followed by ritonavir (apparent IC50max of 93.6 ± 12.6 µM) and lopinavir (apparent IC50max of 125.6 ± 11.4 µM) (Figure 2A). Darunavir had little effect on Ste24p activity (Figure 2A). Assessing the effects of the HIV-PIs on Ste24p activity with the coupled endoprotease-methylation assay requires that these drugs have little effect on Ste14p activity. Indeed, none of the drugs tested had any effect on Ste14p activity, except TPV, which showed some inhibition at 100 μM (Figure 2B).
Figure 2. Effect of HIV-PIs on the enzymatic activity of pure Ste24p and Ste14p.
A. Effect of HIV-PIs on purified Ste24p activity. HIV-PIs darunavir (■) (0–300 µM), lopinavir (●) (0–300 µM), ritonavir (○) (0–695 µM), and tipranavir (×) (0–150 µM) were each incubated with Ste24p and Ste14p for 5 min before adding the peptide substrate and reconstituting the enzymatic activities by rapid dilution in lipid. Activity was detected using the coupled endoproteolysis-methylation vapor-diffusion activity assay. Data is reported as the percent of vehicle activity (DMSO). Each curve displays the data obtained from 3–5 independent experiments. Error bars show SEM. B. Effects of HIV-PIs on purified Ste14p activity. Ste14p was incubated with each HIV-PI (100 µM) for 5 min before adding AFC and reconstitution by rapid dilution in the presence of lipid. Activity is detected by the vapor diffusion activity assay and is reported as percent of the activity found in the DMSO control. The data shown is the result of three independent experiments (mean ± SEM).
Purification of both Ste24p and Ste14p in the same detergent made it possible to create an ideal assay for Ste24p activity. When using crude membrane extracts, one is forced to add a large excess of membranes to make sure that all of the prenylprotein substrates are methylated. When one uses pure proteins, the amount of Ste14p and its enzymatic activity are known. Most importantly, the use of highly purified enzymes reduces the chance that the peptide substrate will be cleaved by another protein in the crude membrane extract.
Our protocol used to purify Ste14p and Ste24p improved upon an earlier procedure by the use of cobalt versus nickel affinity resin and the addition of an ultracentrifugation step to remove nonsolubilized membranes [19]. Thus, the purified material that we generated was not contaminated with yeast membranes containing active Ste24p. The older protocol also used a 1% (w:v) DDM elution buffer, which reduced the ability to dilute the protein below the critical micellar concentration. In our revised protocol, we use a 0.1% (w:v) DDM solution to elute the purified protein. Another improvement in the current protocol was the use of 30,000 MWCO concentrators, allowing us to generate 100-fold more concentrated enzyme preparations. The final change we made was to add a step in which we reconstituted the concentrated proteins into liposomes by rapid dilution, thereby returning the enzymes to a lipid environment and increasing their activity.
In early studies using crude membrane fractions, we suggested that HIV-PIs might inhibit ZMPSTE24 activity [16,17]. However, the possibility that the observed inhibition in the coupled proteolysis methylation assay was due to effects on another enzyme remained. The current studies with purified enzymes established that these drugs block Ste24p activity (Figure 2A). These results were used to compare the inhibition data of pure Ste24p to that of ZMPSTE24 contained in crude membrane fractions [16,17]. Importantly, the order of potency of the different HIV-PIs was similar for mouse ZMPSTE24 in the crude membrane system and purified Ste24p in the reconstituted yeast assay system. TPV was the best inhibitor, LPV and RTV had intermediate inhibitory effects and DRV did not inhibit in either enzyme assay system. We emphasize that the actual amounts of the inhibitor available to the enzyme in both crude membranes and the reconstituted enzyme system are not known, and thus the calculated IC50 and IC50max values are estimates and specific to each experimental system. Importantly, LPV, RTV and DRV did not affect the activity of Ste14p, the prenylprotein methyltransferase, and TPV inhibited Ste14p to a relatively small extent. Together, the current studies confirm our previous results suggesting a direct effect of HIV-PIs on ZMPSTE24 activity. LPV, RTV, and TPV all blocked endoproteolysis by purified Ste24p, and the data correlated well with results obtained with ZMPSTE24 in crude membranes [16,17].
In humans and mouse, prelamin A is generally efficiently cleaved by ZMPSTE24 to yield mature lamin A. When cleavage is blocked, farnesylated and carboxylmethylated prelamin A accumulates and causes progeroid phenotypes, prominent among which are lipodystrophy. This problem occurs for individuals with Hutchinson-Gilford Progeria Syndrome (HGPS) who have a mutant form of lamin A deleted for the ZMPSTE24 cleavage site. Similarly individuals with recessive mutations that diminish ZMPSTE24 activity have the disorder MAD, also characterized by lipodystrophy [11,23,24]. Lipodystrophy is also known to occur in some HIV patients undergoing long-term treatment with HIV-PIs [14,25]. The work presented here and elsewhere strongly suggests that this side effect is at least in part likely due to the direct inhibition of ZMPSTE24 proteolytic activity by some HIV-PIs [15–17]. Notably DRV is not inhibitory, warranting consideration of additional non-inhibitory HIV-PIs for use in therapeutic cocktails for HIV [17]. The reason that HIV-PIs, which are aspartyl protease inhibitors, potently inhibit the zinc metalloproteinase ZMPSTE24 is not understood and remains to be further investigated.
Acknowledgments
We thank Dr. Arun K. Ghosh (Purdue University) for kindly supplying darunavir. We also like thank Dr. Richard A. Gibbs (Purdue University) for supplying N-acetyl-S-farnesyl-l-cysteine. This work was supported by NIH grants AR050200, HL76839, and CA099506 to SY and GM41223 to SM.
Abbreviations
- HIV
human immunodeficiency virus
- PI
protease inhibitor
- DRV
darunavir
- LPV
lopinavir
- RTV
ritonavir
- TPV
tipranavir
- ICMT
isoprenylcysteine carboxyl methyltransferase
- DDM
N-dodecyl-β-D-maltopyranoside
- MES
2-(N-morpholino)-ethanesulfonic acid
- PBS
phosphate buffered saline
- AFC
N-acetyl-S-farnesyl-l-cysteine
- MAD
mandibuloacryl dysplasia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clarke S. Protein isoprenylation and methylation at carboxyl-terminal cysteine residues. Annu Rev Biochem. 1992;61:355–386. doi: 10.1146/annurev.bi.61.070192.002035. [DOI] [PubMed] [Google Scholar]
- 2.Fujimura-Kamada K, Nouvet FJ, Michaelis S. A novel membrane-associated metalloprotease, Ste24p, is required for the first step of NH2-terminal processing of the yeast a-factor precursor. J Cell Biol. 1997;136:271–285. doi: 10.1083/jcb.136.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyartchuk VL, Ashby MN, Rine J. Modulation of ras and a-factor function by carboxyl-terminal proteolysis. Science. 1997;275:1796–1800. doi: 10.1126/science.275.5307.1796. [DOI] [PubMed] [Google Scholar]
- 4.Trueblood CE, Boyartchuk VL, Picologlou EA, Rozema D, Poulter CD, Rine J. The CaaX proteases, Afc1p and Rce1p, have overlapping but distinct substrate specificities. Mol Cell Biol. 2000;20:4381–4392. doi: 10.1128/mcb.20.12.4381-4392.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hrycyna CA, Sapperstein SK, Clarke S, Michaelis S. The Saccharomyces cerevisiae STE14 gene encodes a methyltransferase that mediates C-terminal methylation of a-factor and RAS proteins. EMBO J. 1991;10:1699–1709. doi: 10.1002/j.1460-2075.1991.tb07694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hrycyna CA, Clarke S. Farnesyl cysteine C-terminal methyltransferase activity is dependent upon the STE14 gene product in Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:5071–5076. doi: 10.1128/mcb.10.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adames N, Blundell K, Ashby MN, Boone C. Role of yeast insulin-degrading enzyme homologs in propheromone processing and bud site selection. Science. 1995;270:464–467. doi: 10.1126/science.270.5235.464. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt WK, Tam A, Michaelis S. Reconstitution of the Ste24p-dependent N-terminal proteolytic step in yeast a-factor biogenesis. J Biol Chem. 2000;275:6227–6233. doi: 10.1074/jbc.275.9.6227. [DOI] [PubMed] [Google Scholar]
- 9.Corrigan DP, Kuszczak D, Rusinol AE, Thewke DP, Hrycyna CA, Michaelis S, Sinensky MS. Prelamin A endoproteolytic processing in vitro by recombinant ZMPSTE24. Biochem J. 2005;387:129–138. doi: 10.1042/BJ20041359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung GK, Schmidt WK, Bergo MO, Gavino B, Wong DH, Tam A, Ashby MN, Michaelis S, Young SG. Biochemical studies of ZMPSTE24-deficient mice. J Biol Chem. 2001;276:29051–29058. doi: 10.1074/jbc.M102908200. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal AK, Fryns JP, Auchus RJ, Garg A. Zinc metalloproteinase, ZMPSTE24, is mutated in mandibuloacral dysplasia. Hum Mol Genet. 2003;12:1995–2001. doi: 10.1093/hmg/ddg213. [DOI] [PubMed] [Google Scholar]
- 12.Tam A, Nouvet FJ, Fujimura-Kamada K, Slunt H, Sisodia SS, Michaelis S. Dual roles for Ste24p in yeast a-factor maturation: NH2-terminal proteolysis and COOH-terminal CaaS processing. J Cell Biol. 1998;142:635–649. doi: 10.1083/jcb.142.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agarwal AK, Garg A. Genetic basis of lipodystrophies and management of metabolic complications. Annu Rev Med. 2006;57:297–311. doi: 10.1146/annurev.med.57.022605.114424. [DOI] [PubMed] [Google Scholar]
- 14.Chen D, Misra A, Garg A. Clinical review 153: Lipodystrophy in human immunodeficiency virus-infected patients. J Clin Endocrinol Metab. 2002;87:4845–4856. doi: 10.1210/jc.2002-020794. [DOI] [PubMed] [Google Scholar]
- 15.Caron M, Auclair M, Sterlingot H, Kornprobst M, Capeau J. Some HIV protease inhibitors alter Lamin A/C maturation and stability, Srebp-1 nuclear localization and adipocyte differentiation. Aids. 2003;17:2437–2444. doi: 10.1097/00002030-200311210-00005. [DOI] [PubMed] [Google Scholar]
- 16.Coffinier C, Hudon SE, Farber EA, Chang SY, Hrycyna CA, Young SG, Fong LG. HIV protease inhibitors block the zinc metalloproteinase ZMPSTE24 and lead to an accumulation of prelamin A in cells. Proc Natl Acad Sci U S A. 2007;104:13432–13437. doi: 10.1073/pnas.0704212104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coffinier C, Hudon SE, Lee R, Farber EA, Nobumori C, Miner JH, Andres DA, Spielmann HP, Hrycyna CA, Fong LG, Young SG. A potent HIV protease inhibitor, darunavir, does not inhibit ZMPSTE24 or lead to an accumulation of farnesyl-prelamin A in cells. J Biol Chem. 2008;283:9797–9804. doi: 10.1074/jbc.M709629200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson JL, Frase H, Michaelis S, Hrycyna CA. Purification, functional reconstitution, and characterization of the Saccharomyces cerevisiae isoprenylcysteine carboxylmethyltransferase Ste14p. J Biol Chem. 2005;280:7336–7345. doi: 10.1074/jbc.M410292200. [DOI] [PubMed] [Google Scholar]
- 19.Tam A, Schmidt WK, Michaelis S. The multispanning membrane protein ste24p catalyzes CaaX proteolysis and NH2-terminal processing of the yeast a-factor precursor. J Biol Chem. 2001;276:46798–46806. doi: 10.1074/jbc.M106150200. [DOI] [PubMed] [Google Scholar]
- 20.Schaffner W, Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973;56:502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- 21.Hrycyna CA, Wait SJ, Backlund PS, Jr., Michaelis S. Yeast ste14 methyltransferase, expressed as trpe-ste14 fusion protein in escherichia coli, for in vitro carboxylmethylation of prenylated polypeptides. Methods Enzymol. 1995;250:251–266. doi: 10.1016/0076-6879(95)50077-4. [DOI] [PubMed] [Google Scholar]
- 22.Ota IM, Clarke S. Enzymatic methylation of 23-29-kda bovine retinal rod outer segment membrane proteins. Evidence for methyl ester formation at carboxyl-terminal cysteinyl residues. J Biol Chem. 1989;264:12879–12884. [PubMed] [Google Scholar]
- 23.Miyoshi Y, Akagi M, Agarwal AK, Namba N, Kato-Nishimura K, Mohri I, Yamagata M, Nakajima S, Mushiake S, Shima M, Auchus RJ, Taniike M, Garg A, Ozono K. Severe mandibuloacral dysplasia caused by novel compound heterozygous ZMPSTE24 mutations in two Japanese siblings. Clin Genet. 2008;73:535–544. doi: 10.1111/j.1399-0004.2008.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shackleton S, Smallwood DT, Clayton P, Wilson LC, Agarwal AK, Garg A, Trembath RC. Compound heterozygous ZMPSTE24 mutations reduce prelamin A processing and result in a severe progeroid phenotype. J Med Genet. 2005;42:e36. doi: 10.1136/jmg.2004.029751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carr A, Samaras K, Burton S, Law M, Freund J, Chisholm DJ, Cooper DA. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. Aids. 1998;12:F51–F58. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Romano JD, Schmidt WK, Michaelis S. The Saccharomyces cerevisiae prenylcysteine carboxyl methyltransferase Ste14p is in the endoplasmic reticulum membrane. Mol Biol Cell. 1998;9:2231–2247. doi: 10.1091/mbc.9.8.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]