Abstract
Peroxisome proliferator-activated receptors (PPARs) belong to the nuclear steroid receptor superfamily. Originally, the receptors were identified as critical controllers for several key enzymes that catalyze the oxidation of fatty acids. PPARs consist of three members: PPAR-α, PPAR-β/δ, and PPAR-γ. Among them, PPAR-γ is essential for controlling thermogenesis and adipocyte differentiation. The ligands for PPAR-γ include 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2)—a metabolite from the prostaglandin synthesis pathway, and “glitazones”—drugs utilized in the treatment of patients with diabetes. The precursors for prostaglandins are fatty acids consumed from diet and these precursors have long been postulated to have a regulatory role in immune functions. Emerging evidence indicates that PPAR-γ and its ligands are indeed important for the modulation of immune and inflammatory reactions. In this review, we will spotlight the molecular mechanisms of receptor/ligand function and how they may regulate immune and inflammatory reactions. We also propose that PPAR-γ and its endogenous ligands are participating factors for Type 1/Type 2 T and NK cell differentiation and development. Deciphering the mechanism of action of PPAR-γ and its ligands may lead to a new therapeutic regiment for treatment of diseases involving dysfunction of the immune system.
Keywords: Fatty acids, NK cells, Nuclear hormone receptor, Th1/Th2 differentiation, Transcription
1. Introduction
And take your father and your households, and come unto me: and I will give you the good of the land of Egypt, and ye shall eat the FAT of the land. (Genesis 45:18 King James Bibles of 1611)
A proper intake of dietary fat is very important for mammalian well-being. The consumed fat is converted into essential fatty acids that are building blocks for a variety of cell functions. Diseases associated with malnutrition due to deficiencies in dietary fat, such as infectious diseases, are common in most developing countries. In industrialized countries, major causes of mortality and morbidity are high-fat diet-related diseases such as cardiovascular diseases, obesity, and diabetes. Hormonal systems have evolved in mammals for the regulation of physiological responses to dietary intakes of fatty acids. The key players for such important responses are a family of transcription factors known as peroxisome proliferator-activated receptors (PPARs) that belong to the nuclear hormone receptor superfamily. Ligands for PPARs include long-chain unsaturated fatty acids and their metabolites as well as lipophilic drugs “fibrates” and antidiabetic drugs “glitazones”. They mainly control adipogenesis and thermogenesis by regulation of the expression of a broad range of genes involved in lipid and glucose metabolism and adipocyte differentiation. Recent evidence indicates that PPARs, especially PPAR-γ and the corresponding ligands, play a critical role in immune and inflammatory responses. The purpose of this review is to summarize emerging data on the cellular and molecular mechanism of PPAR-γ function and examine the role of PPAR-γ in the modulation of the immune system.
2. Peroxisome
Originally discovered in the early 1950s, peroxisome was the last “true” subcellular organelle to be detected in eukaryotic cells. The pioneering studies by de Duve and his colleagues in the mid-1960s defined its biochemical properties. Peroxisome can be detected in all human cells except mature red blood cells. Its biological functions, which vary with cell types, include (1) the catabolism and metabolism of hydrogen peroxide, as the name “peroxisome” was originally implied, (2) the β-oxidation of very long-chain fatty acids that cannot be oxidized by mitochondria, and oxidation of prostaglandins, purines, and polyamines, (3) initiation of the biosynthesis of plasmalogens which are present in large amounts in myelin, (4) catabolism of pipecolic, phythanic, and glyoxylic acids (reviewed in Ref. [1]).
Approximately 35–40 years ago, a group of structurally diverse agents was found to promote an increase in the number of hepatic peroxisome in rodents. These agents are collectively named “peroxisome proliferators” (reviewed in Ref. [2]). The increase in the number of peroxisome was found to be associated with an induction of gene expression involved in fatty acid oxidation; thereby a receptor-mediated mechanism of action was postulated. In 1990, Issemann and Green [3] identified and cloned a transcription factor that can regulate genes essential for fatty acid oxidation in the presence of peroxisome proliferators. Characterization of its amino acid sequence revealed that the newly identified transcription factor was a member of the nuclear hormone receptor superfamily. The transcription factor was designated as PPAR-α, the first member of PPAR family. Additional PPAR subtypes, PPAR-γ and PPAR-δ/β, were subsequently identified and cloned (reviewed in Ref. [4]).
3. PPAR and cofactors
PPAR-α is mainly expressed in hepatocytes, enterocytes, smooth muscle cells, endothelial and kidney cells. Brown and white adipocyte tissues are major sites for PPAR-γ expression [5]. Interestingly, the human PPAR-γ gene was first cloned from a bone marrow cDNA library by Greene et al. in 1995 [6]. These investigators identified two transcripts corresponding to a full-length PPAR-γ mRNA and a short form devoid of functional domains. Northern blot analysis revealed that the full-length mRNA was only detected in cultured normal primary bone marrow stromal cells, whereas normal neutrophils and peripheral blood lymphocytes express only the short-form mRNA. Thus, the role of PPAR-γ in the molecular regulation of immune system function was not fully appreciated until recent studies demonstrated that the full-length PPAR-γ was indeed expressed in activated T, B cells and monocytes/macrophages [7–14].
The molecular structures of three PPARs are very similar (reviewed in Ref. [15]). The proteins contain (1) a DNA binding domain (DBD) with high homology (about 80%) among the family members; (2) a ligand binding domain (LBD) which is located at C-termini and shares about 65% homology between the proteins (Fig. 1). The highly conserved structure of the DBD further supports the fact that all three subtypes of PPARs bind to the same response element composed of two hexamer AGGTCA separated by one nucleotide (the PPAR response element [PPRE]). The less conserved LBD permits each PPAR to have its own specific ligands. The large pocket in the LBD revealed by crystal structure analysis explains the observation that PPAR ligands are diverse in structure [16].
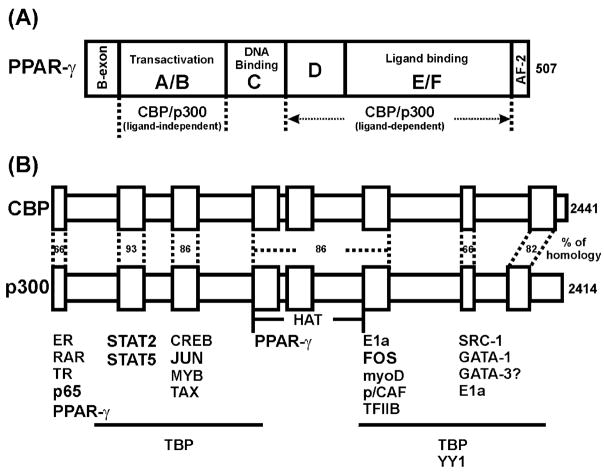
Fig. 1.
PPAR-γ and CBP/p300. Human PPAR-γ is shown as a linear structure to display both DNA and ligand binding domains. The regions involved in the interaction with CBP/p300, either in a ligand-dependent or a ligand-independent manner, are indicated (A). Comparison of the structures of CBP and p300 is shown (B). Homology of amino acid sequence of each interaction domain for both proteins is indicated. Association of CBP/p300 with several transcription factors and cofactors is illustrated at the bottom.
PPARs can interact with other proteins. In an inactive state, PPARs are complexed with nuclear co-repressors (NCo-Rs) that maintain the PPARs as inactive (reviewed in Ref. [17]). Upon ligand binding to PPARs, conformation changes lead to the release of NCo-R from the PPARs. Activated PPARs interact with another member of the nuclear steroid receptor superfamily, the retinoid X receptor (RXR), to form a heterodimer. The heterodimer will bind to PPREs located in the promoter regions of target genes (reviewed in Ref. [15]). The activated PPARs can also recruit other accessory proteins essential for the initiation of gene transcription (reviewed in Ref. [18]). These accessory proteins include nuclear co-activators (N-CoAs) and cyclic-AMP response element binding protein (CREB)-binding protein (CBP)/protein 300 (p300) (Fig. 1) and have multiple functions essential for gene transcription (reviewed in Refs. [19,20]). For example, CBP/p300 not only possesses acetyltransferase activity for histones and nonhistone substrates, but also functions as a molecular scaffold and signal integrator through its association with other transcription factors (Fig. 1), signalling molecules and nuclear hormone receptors such as PPARs [21–25]. PPAR-γ can interact with p300 with or without ligand [25]. In the absence of ligand, the interaction is through the N-terminus of p300, whereas the C-terminus of p300 is crucial for interaction in the presence of ligand (Fig. 1). CBP and p300 are often considered to be functional homologues, but emerging evidence has demonstrated functional difference between the two molecules (reviewed in Ref. [26]).
Many coactivators have been identified, but only steroid receptor coactivator (SRC) family members have been intensively studied and well characterized (reviewed in Ref. [20]). The SRC family of coactivators contains three groups. (1) SRC-1 (N-CoA1) interacts with a wide range of nuclear receptors including PPAR in a ligand-dependent fashion. By interaction with general transcription factors such as TATA binding protein (TBP) and TFIIB, SRC-1 functions as a bridging molecule. In addition, SRC-1 can enhance AP-1-, NF-κB-, and STATs-mediated transcriptional regulation of gene expression [27–29], indicating that SRC-1 can play a role in crosstalk between intracellular signalling pathways. (2) Transcription intermediary factor 2 (TIF2) and GR-interacting protein 1 (GRIP1) belong to the second family of SRC proteins (SRC-2, N-CoA2). TIF2 and GRIP1 represent human and murine analogues, respectively, as they share about 94% homology at their amino acid sequence level. Like SRC-1, TIF2 and GRIP1 interact with GR, ER, and RAR in a ligand-dependent manner. (3) The third group of SRC family members (SRC-3, N-CoA3) include: RAC3, AIB1, TRAM-1, and ACTR (human) and p300/CBP interacting protein (p/CIP) (mouse). Their expression can be increased by hormone treatment. In addition to SRC family of cofactors, another cofactor crucial for PPAR-γ-mediated transcription is PPAR-γ coactivator-1 (PGC-1) [30]. PGC-1 is an essential cofactor for PPAR-γ activity in adaptive thermogenesis. The transcriptional activation of uncoupling protein-1 (UCP-1) by PPAR-γ can be enhanced by PGC-1. Overexpression of PPAR-γ increases interaction between PGC-1 and SRC-1 and CBP/p300. The current understanding of PGC-1 is limited to its role in thermogenesis. Evidently, the interactions among cofactors are important regulatory mechanisms in multiple intracellular signalling pathways involving PPAR-γ.
4. Ligands for PPAR-γ
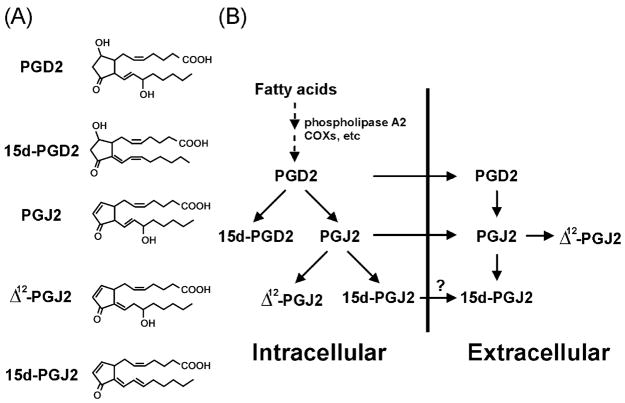
One of the natural ligands for PPAR-γ is 15d-PGJ2 (15-deoxy-Δ12,14-prostaglandin J2) [31,32], the major metabolite from prostaglandin D2 (PGD2) that is derived from sequential metabolism of arachidonic acid (Fig. 2). Upon stimulation, arachidonic acid is released from cell membrane phospholipids catalyzed by phospholipases such as phospholipase A2. After being converted to an unstable endoperoxide intermediate by cyclooxygenases (COXs), subsequent enzymatic reaction yields several structurally related products called eicosanoids, including leukotrienes (LTs) and prostaglandins (PGs) (reviewed in Ref. [33]). PGD2 is one of the major metabolites among PGs in a variety of cells, including monocytes/macrophages (reviewed in Refs. [34,35]). It has been shown that in vitro PGD2 can spontaneously transform into prostaglandins of the J series including PGJ2, Δ12-PGJ2 and 15d-PGJ2. PGJ2 and Δ12-PGJ2 can also be generated in vivo (Fig. 2). However, whether 15d-PGJ2 can be formed in vivo has been debated for sometime (reviewed in Ref. [36]). The recent study by Shibata et al. [37] may be helpful to resolve this issue. Using a murine monoclonal antibody specifically against 15d-PGJ2, these investigators demonstrate that 15d-PGJ2 exists in the cytoplasm of most of the foamy or spindle macrophages in human atherosclerotic plaques. Furthermore, RAW264.7 cells (a mouse macrophage cell line) stimulated with LPS (10 μg/ml) generated intracellular 15d-PGJ2. The extracellular concentrations of 15d-PGJ2 can reach 60–100 nM within 24 h after stimulation. Roughly, about 30% of PGD2 is converted into 15d-PGJ2. The concentrations of 15d-PGJ2 used for most in vitro studies are in a range from 1 to 10 μM. However, the physiological significance of this agent at this concentration range in vivo is questionable (reviewed in Ref. [36]). By using normal phase high-performance liquid chromatography (HPLC) analysis, Maxey et al. [38] found that the preparation of 15d-PGJ2 by chemical decomposition of PGD2 was a mixture of at least five isomers with the same molecular weight. There are substantial differences in the relative potency of these isomers as demonstrated by a PPAR-γ ligand-binding assay. Surprisingly, two isomers present in minor amounts in the preparation have relatively higher activities. Thus, this observation may account for the need of higher concentrations of 15d-PGJ2 for in vitro studies. PPAR-γ needs RXR as its heterodimer partner to fully exert transcription activation and sufficient endogenous ligand(s) for RXR may function as “sensitizers” to reduce the concentrations of PPAR-γ ligands required for receptor activation [39]. The effectiveness of the ligand crossing cellular membrane may also be a factor in determining receptor activation.
Fig. 2.
Synthesis of 15d-PGJ2. The structure of each prostaglandin is shown (A). The pathway for 15d-PGJ2 synthesis is depicted as a schematic representation (B). Question mark indicates that there may be a possible transporter for 15d-PGJ2. COXs: cyclooxygenases.
More recently, Davies et al. [40] discovered another natural ligand for PPAR-γ, azPC (hexadecyl azelaoyl phosphatidylcholine). This oxidized phospholipid can be liberated from a small pool of alky phosphatidylcholines in oxidized low-density lipoprotein (oxLDL) by phospholipase A1. The apparent affinity of azPC for PPAR-γ was found to be around 40 nM that is in the range of concentrations required for rosiglitazone binding to PPAR-γ. Unlike 15d-PGJ2, the entry of azPC into cells is relatively effective. The activation of PPAR-γ by azPC may not need secondary messengers as it may occur through the interaction of azPC with an unknown surface receptor. The expression of the scavenger receptor CD36, encoded by a PPRE-responsive gene, can be induced by azPC. CD36 promotes the uptake of oxLDL and differentiation of macrophages to foam cells. Most strikingly, azPC can also induce COX2, another PPRE-responsive gene, in intercellular adhesion molecule-3 (ICAM-3) bound human monocytes where PPAR-γ expression is strongly induced [41]. These observations put PPAR-γ and azPC in a proinflammatory and proatherosclerotic pathway. Interestingly, 15d-PGJ2, like azPC, can enhance luciferase activity of (COX-2 PPRE)3-luc reporter in the presence of PMA, but unlike azPC, it inhibits the activity of the full-length promoter [41].
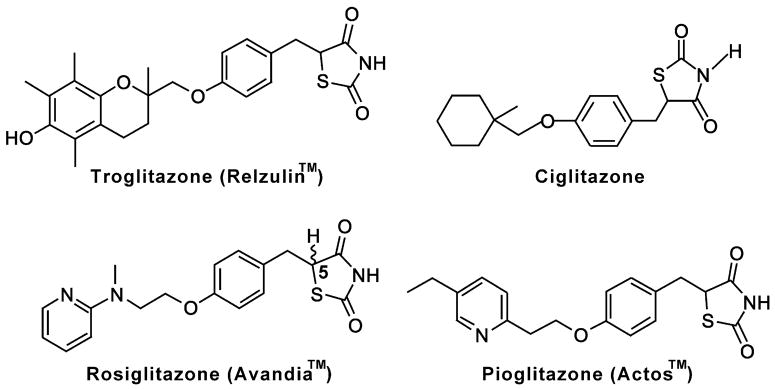
The most intensively studied synthetic PPAR-γ ligands (agonist) are thiazolidinediones (TZDs), a class of drugs termed “glitazones” (Fig. 3). TZDs were originally developed without the knowledge of their molecular targets by screening rodent models of insulin resistance and used as insulin sensitizers for treatment of patients with Type II diabetes [42]. In 1995, Forman et al. [31] and Lenhmann et al. [43] independently found that glitazones were functional ligands for PAPR-γ. Subsequent data prove that PPAR-γ is one of the molecular targets for the therapeutic effects of glitazones ([44], reviewed in Ref. [45]). TZDs approved for clinical applications are troglitazone (Rezulin™), rosiglitazone (Avandia™), and pioglitazone (Actos™). Commercially available TZDs for research purposes only include troglitazone and ciglitazone. The structures of each “glitazone” are very similar, but the side effects in patients are different. For example, short- or long-term use of troglitazone can cause idiosyncratic hepatotoxicity that results in liver failure. Based on this toxicity, troglitazone was suspended for clinical application and withdrawn from the market. Use of rosiglitazone plus insulin has been reported to be associated with some adverse cardiovascular events including cardiac failure.
Fig. 3.
Structures of “glitazones”. The structures of synthetic ligands for PPAR-γ such as troglitazone, ciglitazone, rosiglitazone, and pioglitazone are shown. Corresponding commercial brand names are also indicated.
Several synthetic ligands (agonists) whose structures are not related to glitazones have also been studied (reviewed in Ref. [4]). GW0072 functions more like a modulator than a pure agonist or antagonist for PPAR-γ. The plasticizer BADGE has been shown to function as an antagonist with very weak affinity for PPAR-γ. However, the concentrations required for the antagonist effect of BADGE are very close to the level of its solubility, which makes BADGE a poor antagonist. GW9662 is a potent irreversible PPAR ligand since its interaction with the receptor results in alkylation of an amino acid residue (Cysteine 286 in PPAR-γ) that is conserved in all three receptor subtypes. In cell-based assays, GW9662 shows higher affinity for PPAR-γ than for the other two receptor subtypes with effective concentrations less than 10 μM in multiple cell types. Nonsteroidal anti-inflammatory drugs have also been found to be low-affinity ligands for PPAR-γ and PPAR-α [46]. Intensive studies of these drugs related to anticancer and anti-inflammatory effects have been recently reported [47]. These studies provide a framework for developing new drugs for treatment of cancer and autoimmune diseases.
5. Molecular mechanisms of PPAR-γ and its ligands in the modulation of immune system
15d-PGJ2 appears to function as a modulator of many immune responses [48]. The mechanism by which 15d-PGJ2 enters the cells remains elusive, but it is possible that a transporter system used by other cyclopentanone prostaglandins plays a role [49]. While intracellular 15d-PGJ2, either exogenously transported or endogenously produced, mainly functions as an inhibitor to repress inflammatory cytokine expression, it can also function as an inducer of certain specific proinflammatory factors. The molecular mechanisms underlying these differential effects involve intracellular (PPAR-γ-dependent and/or PPAR-γ-independent) and/or membrane – receptor pathways (Fig. 4).
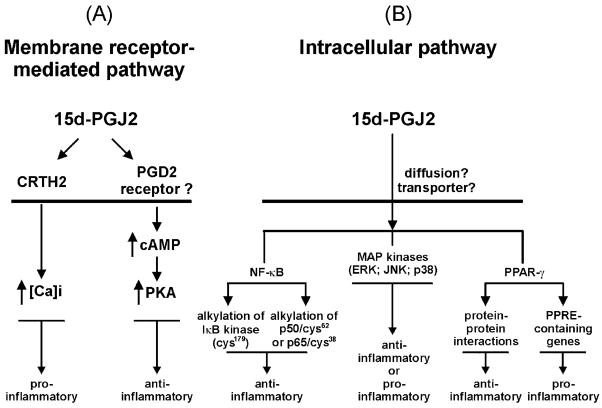
Fig. 4.
Mechanisms of 15d-PGJ2 actions. 15d-PGJ2 may interact with a surface receptor(s) to initiate activation of signalling pathways (A). CRTH2 (chemokine receptor in T helper 2 cell) is expressed in Th2 cells and eosinophils. 15d-PGJ2 can interact with CRTH2 on eosinophils to trigger an increase in intracellular calcium resulting in activation of a signalling cascade. 15d-PGJ2 may additionally bind to a yet undefined PGD2-like receptor on the surface of neutrophils and Type 1 T (or NK) cells to increase cAMP and PKA activity. 15d-PGJ2 can also directly affect intracellular pathways (B) in a PPAR-γ-dependent and/or -independent manner. Intracellular 15d-PGJ2 can be generated either from the endogenous synthesis pathway (Fig. 3) or by entry through an unknown transporter or passive diffusion. 15d-PGJ2 may react with cysteinyl residues in IKKβ and p65 as well as p50 to repress NK-κB-mediated transactivation activity. 15d-PGJ2 can also bind PPAR-γ, and the ligand/receptor complex interacts with RXR to form a heterodimer that recognizes PPRE in the promoter region of target genes leading to active transcription. In addition, PPAR-γ can compete with other transcription factors such as AP-1 and NF-κB for sequestering essential cofactors such as p300/CBP through protein– protein interactions. Furthermore, 15d-PGJ2 affects other signalling pathways, including the MAPK/ERK pathway through undefined mechanisms.
5.1. Intracellular pathways
Intracellular pathways used by 15d-PGJ2 have been suggested to be both PPAR-γ-independent and PPAR-γ-dependent. The effect of 15d-PGJ2 and glitazones on the NF-κB pathway has been intensively studied. First, in a PPAR-γ-independent manner, 15d-PGJ2 (2–5 μM) is able to form Michael adducts with cellular nucleophilic and covalently modify IKKβ (IκB kinase β) through its reactive cyclopentenone moiety [50,51]. Modified IKKβ cannot phosphorylate IκB (inhibitor of NF-κB) and thereby the release of NF-κB is hindered. This leads to the failure of nuclear translocation and subsequent activation of the target genes. Glitazones have no such effects. By the same mechanism, 15d-PGJ2 can also modify the cysteinyl residues located in the DNA-binding domain of NF-κB subunits p65 (cysteine 38) and p50 (cysteine 62), leading to the impairment of DNA binding capability of NF-κB [52]. Secondly, the transcription responses mediated by AP-1, NF-κB, and STAT1 induced by LPS can also be repressed by submicromolar concentrations (0.1–0.5 μM) of 15d-PGJ2 only in the presence of PPAR-γ [7]. PPAR-γ prevents NF-κB molecules from binding to the cognate cis-element in the promoter region of cytokine genes through protein–protein interaction with NF-κB. Analogous to the protein–protein interaction of PPAR-γ with NF-κB molecules, Yang et al. [53] demonstrated that both 15d-PGJ2 and glitazones could inhibit PHA-induced human T-cell proliferation and IL-2 gene expression. The repression results from the interaction of activated PPAR-γ with nuclear factor for activated T cells (NFAT), thereby blocking the binding of NFAT to its cognate cis-element in the IL-2 promoter. Recently, we found that PPAR-γ activated by 15d-PGJ2 competes with a limited quantity of p300/CBP available for transactivation of the IFN-γ promoter [54]. The IFN-γ promoter contains several essential cis-elements for transcription factors including AP-1, NF-κB, and STATs [55]. These data are consistent with previous studies that p300/CBP and SRC-1 are essential for full activation of AP-1, NF-κB, and STATs, as well as other transcription factor-mediated gene promoter activation [20–29] (Fig. 1). Our results suggest that the reduction of availability of these essential factors may be responsible for the inhibition of IFN-γ promoter activity by PPAR-γ activation. Thus, sharing of common coactivators such as CBP/p300 and SRC-1 by nuclear receptors and other transcription factors (e.g., AP-1, NF-κB and STATs) may play an important role in the multiple intracellular signalling pathways [56]. Additionally, the ligand may induce other transcription factors to interact with cis-elements other than traditional PPRE on target genes, as has been demonstrated for synthetic PPAR-α ligands [57,58]. Finally, suppression of the inflammatory immune responses by PPAR-γ activation may also be achieved through induction of apoptosis of immune cells [59,60,80].
On the other hand, 15d-PGJ2 and glitazones can also promote the induction of proinflammatory proteins under certain conditions. The production of TNF-α and IL-6 in LPS-treated db/db mice is enhanced by 15d-PGJ2 and glitazones [61]. In human monocytes, 15d-PGJ2 differentially regulates chemokine gene expression [62]. 15d-PGJ2 inhibits PMA-induced chemokine gene expression, whereas it enhances LPS-induced IL-8 gene expression. 15d-PGJ2 by itself can induce IL-8 gene expression but suppresses MCP-1 gene expression. Similar observation has also been obtained from THP-1-derived macrophages [116]. TCR-activated human T-cells and LPS-treated endothelial cells can also produce more IL-8 when treated with 15d-PGJ2 [63,64]. Other genes, such as CD36 in macrophages [41,65,66] and cyclooxygenase 2 in colon cells [67], are also induced upon 15d-PGJ2 and glitazone treatment. These up-regulating effects of 15d-PGJ2 and glitazones may be partially attributed to a PPAR-γ-dependent pathway in which ligand-bound PPAR-γ interacts with the cognate cis-element (PPRE) in the promoter region of the target genes [41,62,65–67].
Although glitazones are considered as PPAR-γ ligands, they do not always exhibit same transcriptional modification activity as 15d-PGJ2. Emerging evidence indicates that interaction of nuclear receptors with coactivators can occur in a differential manner. For example, 15d-PGJ2, the natural ligand for PPAR-γ, can induce ligand-activated receptor interaction with SRC-1, SRC-2 (TIF2 and AIB1) and p300, while troglitazone, the synthetic ligand for PPAR-γ, does not have these effects [68]. PPAR-γ and RXR have their own preference for interaction with cofactors as well. PPAR-γ has a stronger interaction with CBP/p300, whereas RXR preferentially interacts with TBP (TATA binding protein) [69]. The preferential interaction of cofactors with nuclear receptors activated by specific ligands may partially explain observations in which some genes are potently regulated by 15d-PGJ2-, but not glitazones-, activated PPAR-γ or vice versa.
Disruption of PPAR-γ gene is embryonic lethal [70], which limits the availability of a PPAR-γ knockout mouse model to study the role of this transcription factor in the immune system. A viable PPAR-γ conditional knockout mouse, recently developed by Akiyama et al. [71], appears to offer a remedy. Several findings in this mouse model provide important clues for understanding the molecular roles of glitazones. Troglitazone repressed the expression of ABCA1 gene (the downstream target for PPAR-γ) in both PPAR-γ-deficient and wild-type mice, whereas other glitazones such as rosiglitazone, ciglitazone, and piog litazone had no such effect. These findings further support the previous in vitro observation in which troglitazone, but not rosiglitazone and pioglitazone, suppressed PMA-induced TNF-α production by a human monocyte cell line [113]. Thus, glitazones may have unique functional mechanisms that are not solely mediated by PPREs and the PPAR-γ pathway. Similar observations have been made with fibrates (the synthetic ligands for PPAR-α), which can also function through transcription factor(s) other than PPAR-α and elements unrelated to PPRE on some target genes [57].
Other signalling pathways that may be affected by 15d-PGJ2 and glitazones include mitogen-activated protein kinases (MAPKs). There are three pertinent MAPK pathways: the extracellular signal-regulated kinase (ERK), the p38 MAPK (the 38-kDa MAPK), and the c-Jun N-terminal kinase (JNK) (reviewed in Ref. [72]). In activated human T cells, Harris et al. [63] observed that 15d-PGJ2 can induce IL-8 production through MAPK/ERK and NF-κB independent of PPAR-γ, whereas glitazones have no effect at all. Inhibitors of both MAPK and NF-κB can block 15d-PGJ2-induced IL-8 production. However, in human macrophages, the LPS-induced phosphorylation of ERKs and degradation of IκBα can be inhibited by 15d-PGJ2, leading to the inhibition of LPS-induced production of NO, TNF-α, and thromboxane B2 [73]. In human NK (natural killer) cells, we have observed that 15-PGJ2 inhibits IL-2-induced MAP/ERK kinase activities [54]. This may contribute to the inhibitory effect of 15d-PGJ2 on biological functions of NK cells, including natural killing activity and IFN-γ production [74]. The differential effects of 15d-PGJ2 on the MAPK/ERK pathway are not surprising since similar results were observed in other nonhemopoietic systems [75–78]. In general, 15d-PGJ2 can suppress mitogenic-induced MAPK/ERK, whereas the ligand by itself can marginally induce the kinase activity. Regarding the effects on JNK, 15d-PGJ2 slightly up-regulates JNK activity in a mouse macrophage cell line [52]. In human vascular endothelial cells, high concentrations of troglitazone (10 μM) and 15d-PGJ2 (15 μM) induce c-Jun phosphorylation through activation of JNK [79]. Activation of p38 MAPK by 15d-PGJ2 correlates with its ability to enhance macrophage apoptosis induced by LPS and IFN-γ [80]. Thus, temporal and spatial factors will markedly affect the outcome of a specific signalling pathway.
The biological activity of PPAR-γ can also be regulated by MAP kinases with diverse consequences. Zhang et al. [81] reported that phosphorylation of PPAR-γ by insulin-activated MAPKs led to activation of PPAR-γ in adipocytes. However, Hu et al. [82] demonstrated an inhibited adipogenesis in association with an MAPK-mediated phosphorylation of PPAR-γ. The site of phosphorylation in PPAR-γ is located in the amino-terminal A/B domain of the receptor, and the phosphorylation leads to the reduction of ligand-binding affinity of its carboxyl-terminal LBD domain and subsequent activity of PPAR-γ as a transcription factor [83].
5.2. Membrane–receptor pathway
Since 15d-PGJ2 shares a similar structure with its D series compounds that interact with cell surface receptors, the search for putative surface receptor of 15d-PGJ2 has been an important area of investigation. Utilizing a human HEK 293 cell line stably transfected with human PGD2 receptor, Wright et al. [84] found that 15d-PGJ2 can induce an increase in cAMP, albeit at a 600-fold higher concentration than that was required for PGD2; thereby 15d-PGJ2 has a weak agonist activity on PGD2 receptor. In human neutrophils, Vaidya et al. [85] found that in the presence of a phosphodiesterase inhibitor, the concentration of 15d-PGJ2 required to inhibit oxygen burst and cell adhesion dropped from 1 μM to 150 nM, although the phosphodiesterase inhibitor alone had no effect. Thus, 15d-PGJ2 may affect neutrophil function by elevating intracellular cAMP through a membrane receptor. We have found that treatment of NK 92 cells (a human natural killer cell line) with 15d-PGJ2 can moderately induce protein kinase A (PKA) activity [54], which may partially contribute to the suppressive effect of 15d-PGJ2 on cytokine-induced IFN-γ secretion in NK cells. The concentrations of 15d-PGJ2 used in these studies were in the range that demonstrates anti-inflammatory effects. The results suggest that 15d-PGJ2 may also interact with a surface receptor on NK cells.
Chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2) is a newly identified, seven-transmembrane G protein-coupled PGD2 receptor, structurally similar to the members of FPR (N-formyl peptide receptor) family [86]. Recently, Monneret et al. [87] demonstrated that 15d-PGJ2 is a potent and selective agonist for this cell surface receptor in eosinophils. Interestingly, indomethacin, a weak agonist for PPAR-γ and commonly used for its anti-inflammatory, antipyretic, and analgesic effects, is also an agonist for CRTH2 [88]. The affinities of these two agents for CRTH2 are about two to three orders of magnitude higher than for PPAR-γ. Ligand–receptor interaction stimulates calcium mobilization, actin polymerization, and CD11b expression in eosinophils. These effects could not be observed in monocytes and neutrophils. This observation is the first evidence to support the model that 15d-PGJ2 can function at physiological concentrations and may be more proinflammatory than anti-inflammatory. Thus, as discussed above, the different mechanisms of action of 15d-PGJ2 may be determined by many factors, such as the type of cells, ligand concentrations employed, and the nature of the receptor it may interact.
6. Implications of PPARγ in the development of Type 1/Type 2 immune cells
Given the fact that functional human PPAR-γ was originally cloned from a human bone marrow cDNA library, PPAR-γ and its ligands may also be important in the regulation of differentiation and development of immune cells such as monocytes/macrophages, T cells and NK cells. Based on the profiles of cytokine production, immune cells such as T and NK cells could be classified into two major subsets, i.e., Type 1 and Type 2 (reviewed in Refs. [89–92]). Type 1 T cells produce IFN-γ, and are mainly involved in cell-mediated immune responses, whereas Type 2 T cells produce IL-4, IL-5, and IL-13, and participate in humoral responses. Type 2 cytokines also promote growth and differentiation of mast cells and eosinophils. Regulation of cytokine genes by transcription factors has been proposed as one of the molecular mechanisms for cell polarization (reviewed in Refs. [89–91]), although the expression of these genes such as IFN-γ can also be regulated at the posttranscriptional levels [93]. Identification of a novel transcription factor T-bet (T-box expressed in T cells) has enabled us to further understand IFN-γ expression and Type 1 T-cell lineage differentiation [94]. T-bet belongs to the T-box family of transcription factors that play important roles in developmental regulation processes. Induction of T-bet expression in primary T cells is strongly correlated with Th1 lineage differentiation and IFN-γ expression, although T-bet expression seems to be independent of STAT4-mediated signalling pathway [95]. Expression of IFN-γ in Th1 and NK cells from T-bet knockout mice was dramatically reduced, and these cells produced more Th2 type cytokines such as IL-4 and IL-5 [96]. Analogous to the role of T-bet in Type 1 lineage differentiation and IFN-γ expression, GATA3 (a zinc finger motif transcription factor with WGATAR as its cognate DNA binding sequence) is a key player for Type 2 lineage differentiation and expression of Type 2 cytokines (IL-4, IL-5, and IL-13) [97,98]. GATA3 expression polarizes T cell towards Type 2 lineage and represses IFN-γ expression [99]. The balance between T-bet and GATA3 expression has been proposed to be crucial for Type 1/Type 2 T-cell differentiation [89,100]. IFN-γ production from T and NK cells can be inhibited by both endogenous and synthetic PPAR-γ ligands through either PPAR-γ-dependent or/and -independent pathways [54,101], suggesting that the receptor itself and its ligands, including polyunsaturated fatty acids and eicosanoids, may have a significant impact on balancing immune responses. Furthermore, results from DNA microarray analysis of gene expression show that the expression of PPAR-γ is much higher (about 5- to 8-fold) in Type 2 T (helper or cytotoxic) cells, compared with Type 1 T (helper or cytotoxic) cells [102]. We also observed that PPAR-γ expression is induced in human NK cells under Type 2 culture conditions (IL-4 and anti-IFN-γ antibodies) [54]. Additionally, IL-4 has been shown to be able to inhibit T-bet expression. Whether the inhibitory effect of IL-4 on T-bet expression is related to its induction of PPAR-γ remains to be determined. Noticeably, Jones and Daynes [103] showed in a recent report that PPAR-α suppresses the transcription of T-bet in T cells through its ability to influence redox state within the cells. The suppression of T-bet expression can be reversed in PPAR-α knockout mice, which have a higher level of IFN-γ production than the control littermates. Since PPAR-α is structurally similar to PPAR-γ, it is possible that PPAR-γ may similarly suppress T-bet expression. PPAR-γ can also be regulated by IFN-γ. In adipocytes, IFN-γ mediates the degradation of PPAR-γ through the ubiquitin–proteasome pathway [104]. Such a mechanism may also be applicable to immune cells.
Interestingly, PGD2, the precursor of 15d-PGJ2, is preferentially produced by antigen-stimulated human CD4+ Th2 cells, but not CD4+ Th1, cells [105]. In addition, CRTH2, a cell surface receptor for 15d-PGJ2, is preferentially expressed in Th2 cells [86,106,107]. It is also noteworthy that Glimcher’s laboratory recently identified a set of phospholipase A2 (group V and GXII-2) genes that are preferentially expressed in mouse Th2 cells [108]. It has long been known that free arachidonic acid can be released from cell membrane by phospholipase A2. The free lipids can be further converted into PGD2 and J series of PG through a sequential reaction catalyzed by cyclooxygenases and PGD synthases [109]. PGD synthases are also preferentially expressed in type 2 CD4+ T cells. Contribution of eicosanoid to Type 2 differentiation can be further supported by the findings that bone marrow-derived dendritic cells can produce PGE2, which, during the antigen priming stage, promotes dendritic cells to produce the Type 2 cytokine IL-10 but not the Type 1 cytokine IL-12 [110]. Likewise, activation of PPAR-γ by its ligands dramatically reduces the production of IL-12 in immature human monocyte-derived dendritic cells [111]. A recent study of EAE (experimental allergic encephalomyelitis) mouse model demonstrates that PPAR-γ agonists can reduce production of IL-12 and differentiation of neural antigen-specific Th1 cells, which leads to the repression of EAE pathogenesis [117]. In light of the observations discussed above, we propose that PPAR-γ may be an additional transcription factor required for Type 2 T-cell differentiation (Fig. 5). The endogenous PPAR-γ ligands, 15d-PGJ2 as a metabolite from the arachidonic acid pathway, and azPC derived from phospholipids may also be critical factors for Type 1 and Type 2 T cells (and possibly NK cells) differentiation in vivo. The availability of newly established PPAR-γ conditional knockout mice and T-bet knockout mice will be useful in further clarifying the issue.
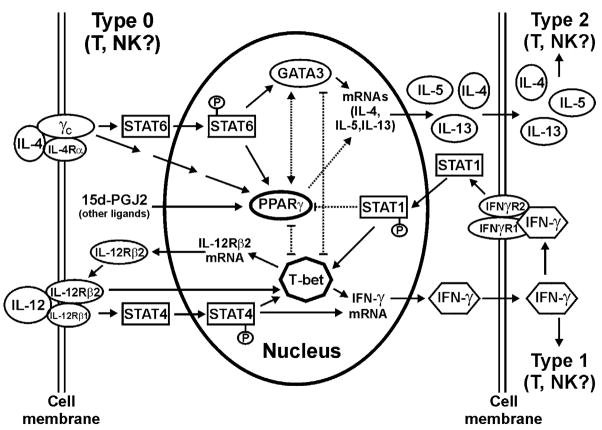
Fig. 5.
PPAR-γ in Type 1/Type 2 T (NK) cell differentiation. Major molecular components involved in Type 1/Type 2 T (NK) cell differentiation are presented. The schematic diagram suggests several possible mechanisms by which PPAR-γ and its endogenous ligands favour Type 2 differentiation. Briefly, interaction of IL-4 with its cognate receptor will induce, either directly or indirectly through STAT6, the expression of genes such as GATA3 and PPAR-γ to increase the production of several cytokines including IL-4 itself, IL-5, and IL-13. These cytokines drive cell differentiation to Type 2 phenotype. Meanwhile, GATA3 and PPAR-γ may also negatively regulate the expression of genes critical for Type 1 differentiation such as T-bet. Binding of IL-12 with its surface receptor triggers activation of a JAK2/STAT4 cascade leading to IFN-γ gene expression. T-bet is the transcription factor whose expression is essential for IFN-γ gene expression in CD4+ T cells and NK cells. Whether T-bet is the downstream target for JAK2/STAT4 pathway remains to be determined. Expression of IFN-γ gene is a hallmark of Type 1 differentiation. Secreted IFN-γ binds to its surface receptor to initiate a JAKs/STAT1 signalling cascade, which promotes ubiquitin–proteasome-mediated PPAR-γ degradation. Thus, reduction of IFN-γ will help maintain the level of PPAR-γ. Meanwhile, PPAR-γ ligands can also block the production of IL-12. Together, the outcomes will favour Type 2 T and/or NK cell differentiation.
7. Conclusions
It is clear that fatty acids consumed from diet have a fundamental role in regulating immune and inflammatory responses mediated by pathways linked to prostaglandin synthesis, phospholipid metabolism, and PPARs. The importance of PPARs in the immune system can further be highlighted by the recent observation that IFN-γ expression in PPAR-α knockout mice is much higher than the control littermates [112]. Thus, further understanding of the molecular mechanisms involved in the regulation of these pathways will facilitate the development of new therapeutic approaches to the treatment and prevention of diseases with underlying immune dysfunction. For example, the development and the activation of allergen-specific Type 2 CD4+ T cells are associated with allergic reaction involving activation of mast cells and release of IgE that results in pathological conditions such as asthma. Additionally, overexpansion of Type 1 CD4+ T cells is frequently found in inflammatory autoimmune diseases such as rheumatoid arthritis, Crohn’s disease, and multiple sclerosis. Indeed, the therapeutic efficacy of PPAR-γ agonists has been tested on the animal models [114,117]. The results show that the compound can attenuate acute and chronic inflammation. However, cautions have been raised for the potential application of 15d-PGJ2 on human subjects due to some of its proinflammatory effects observed from the studies of human cells [62,115]. Nevertheless, given the recent progress in our understanding of PPAR-γ signalling pathway, we expect that the importance of PPAR-γ and its endogenous ligands in the development, maturation, and function of the immune system will be further appreciated through rigorous research.
Acknowledgments
We thank Dr. John Ortaldo and Dr. Ji-Ming Wang for their critical reading of the manuscript. Special thanks to Bill Bere, Stephanie M. Krebs, Anna Mason, and Della Reynolds for their technical support. We also express our appreciation for the encouragement and support from the members of the Laboratory of Experimental Immunology, NCI-Frederick.
References
- 1.de Duve C. The peroxisome in retrospect. Ann NY Acad Sci. 1996;27(804):1–10. doi: 10.1111/j.1749-6632.1996.tb18603.x. [DOI] [PubMed] [Google Scholar]
- 2.de la Iglesia F, McGuire EJ, Haskins JR, Lalwani ND. Structural diversity of peroxisome proliferators and their effects on mammalian liver cells in vivo. Ann NY Acad Sci. 1996;804:310–27. doi: 10.1111/j.1749-6632.1996.tb18625.x. [DOI] [PubMed] [Google Scholar]
- 3.Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–50. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 4.Willson TM, Brown PJ, Sternbach DD, Henke BR. The PPARs: from orphan receptors to drug discovery. J Med Chem. 2000;43:527–50. doi: 10.1021/jm990554g. [DOI] [PubMed] [Google Scholar]
- 5.Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-α, -β, and -γ in the adult rat. Endocrinology. 1996;137:354–66. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- 6.Greene ME, Blumberg B, McBride OW, Yi HE, Kronquist K, Hsieh L, et al. Isolation of the human peroxisome proliferator activated receptor γ cDNA: expression in hematopoietic cells and chromosomal mapping. Gene Expr. 1995;4:281–99. [PMC free article] [PubMed] [Google Scholar]
- 7.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 8.Jiang C, Ting AT, Seed B. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–6. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 9.Huang JT, Welch JS, Ricote M, Binder CJ, Willson TM, Kelly C, et al. Interleukin-4-dependent production of PPAR-γ ligands in macrophages by 12/15-lipoxygenase. Nature. 1999;400:378–82. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- 10.Clark RB, Bishop-Bailey D, Estrada-Hernandez T, Hla T, Puddington L, Padula SJ. The nuclear receptor PPARγ and immunoregulation: PPARγ mediates inhibition of helper T cell responses. J Immunol. 2000;164:1364–71. doi: 10.4049/jimmunol.164.3.1364. [DOI] [PubMed] [Google Scholar]
- 11.Yang XY, Wang LH, Mihalic K, Xiao W, Chen T, Li P, et al. Interleukin (IL)-4 indirectly suppresses IL-2 production by human T lymphocytes via peroxisome proliferator-activated receptor γ activated by macrophage-derived 12/15-lipoxygenase ligands. J Biol Chem. 2002;277:3973–8. doi: 10.1074/jbc.M105619200. [DOI] [PubMed] [Google Scholar]
- 12.Wang P, Anderson PO, Chen S, Paulsson KM, Sjogren HO, Li S. Inhibition of the transcription factors AP-1 and NF-κB in CD4 T cells by peroxisome proliferator-activated receptor gamma ligands. Int Immunopharmacol. 2001;1:803–12. doi: 10.1016/s1567-5769(01)00015-7. [DOI] [PubMed] [Google Scholar]
- 13.Harris SG, Phipps RP. The nuclear receptor PPAR gamma is expressed by mouse T lymphocytes and PPAR-gamma agonist induce apoptosis. Eur J Immunol. 2001;31:1098–105. doi: 10.1002/1521-4141(200104)31:4<1098::aid-immu1098>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 14.Clark RB. The role of PPARs in inflammation and immunity. J Leukoc Biol. 2002;71:388–400. [PubMed] [Google Scholar]
- 15.Rosen ED, Spiegelman B. PPARγ: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem. 2001;276:37731–4. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- 16.Nolte RT, Wisely GB, Westin S, Cobb JE, Lambert MH, Kurokawa R, et al. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-γ. Nature. 1998;395:137–43. doi: 10.1038/25931. [DOI] [PubMed] [Google Scholar]
- 17.Jepsen K, Rosenfeld MG. Biological roles and mechanistic actions of co-repressor complexes. J Cell Sci. 2002;115:689–98. doi: 10.1242/jcs.115.4.689. [DOI] [PubMed] [Google Scholar]
- 18.Rosenfeld MG, Glass CK. Coregulator codes of transcriptional regulation by nuclear receptors. J Biol Chem. 2001;276:36865–8. doi: 10.1074/jbc.R100041200. [DOI] [PubMed] [Google Scholar]
- 19.Vo N, Goodman RH. CREB-binding protein and p300 in transcriptional regulation. J Biol Chem. 2001;276:13505–8. doi: 10.1074/jbc.R000025200. [DOI] [PubMed] [Google Scholar]
- 20.Leo C, Chen JD. The SRC family of nuclear receptor coactivators. Gene. 2000;24:1–11. doi: 10.1016/s0378-1119(00)00024-x. [DOI] [PubMed] [Google Scholar]
- 21.Gerritsen ME, Williams AJ, Neish AS, Moore S, Shi Y, Collins T. CREB-binding protein/p300 are transcriptional coactivators of p65. Proc Natl Acad Sci U S A. 1997;94:2927–32. doi: 10.1073/pnas.94.7.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blobel GA, Nakajima T, Eckner R, Montminy M, Orkin SH. CREB-binding protein cooperates with transcription factor GATA-1 is required for erythroid differentiation. Proc Natl Acad Sci U S A. 1998;95:2061–6. doi: 10.1073/pnas.95.5.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugawara A, Uruno A, Kudo M, Ikeda Y, Sato K, Taniyam Y, et al. Transcription suppression of thromboxane receptor gene by peroxisome proliferator-activated receptor-γ via interaction with Sp1 in vascular smooth muscle cells. J Biol Chem. 2002;277:9676–83. doi: 10.1074/jbc.M104560200. [DOI] [PubMed] [Google Scholar]
- 24.Pfitzner E, Jahne R, Wissler M, Stoecklin E, Groner B. p300/CREB-binding protein enhances the prolactin-mediated transcriptional induction through direct interaction with the trans-activation domain of Stat5, but does not participate in the Stat5-mediated suppression of the glucocorticoid response. Mol Endocrinol. 1998;12:1582–93. doi: 10.1210/mend.12.10.0180. [DOI] [PubMed] [Google Scholar]
- 25.Gelman L, Zhou G, Fajas L, Raspe E, Fruchart JC, Auwerx J. p300 interacts with the N- and C-terminal part of PPARγ2 in a ligand-independent and -dependent manner, respectively. J Biol Chem. 1999;274:7681–8. doi: 10.1074/jbc.274.12.7681. [DOI] [PubMed] [Google Scholar]
- 26.McManus KJ, Hendzel MJ. CBP, a transcriptional coactivator and acetyltransferase. Biochem Cell Biol. 2001;79:253–66. [PubMed] [Google Scholar]
- 27.Lee SK, Kim HJ, Na SY, Kim TS, Choi HS, Im SY, et al. Steroid receptor coactivator-1 coactivates activating protein-1-mediated transactivations through interaction with the c-Jun and c-Fos subunits. J Biol Chem. 1998;273:16651–4. doi: 10.1074/jbc.273.27.16651. [DOI] [PubMed] [Google Scholar]
- 28.Na SY, Lee SK, Han SJ, Choi HS, Im SY, Lee JW. Steroid receptor coactivator-1 interacts with the p50 subunits and co-activate nuclear factor κB-mediated transactivations. J Biol Chem. 1998;273:10831–4. doi: 10.1074/jbc.273.18.10831. [DOI] [PubMed] [Google Scholar]
- 29.Litterst CM, Pfitzner E. Transcriptional activation by STAT6 requires the direct interaction with NCoA-1. J Biol Chem. 2001;276:45713–21. doi: 10.1074/jbc.M108132200. [DOI] [PubMed] [Google Scholar]
- 30.Puigserver P, Adelmant G, Wu Z, Fan M, Xu J, O’Malley B, et al. Activation of PPARgamma coactivator-1 through transcription factor docking. Science. 1999;286:1368–71. doi: 10.1126/science.286.5443.1368. [DOI] [PubMed] [Google Scholar]
- 31.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-deoxy-Δ12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR-γ. Cell. 1995;83:803–12. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 32.Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann J. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell. 1995;83:813–9. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 33.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–5. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 34.Tilley SL, Coffman TM, Koller BH. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J Clin Invest. 2001;108:15–23. doi: 10.1172/JCI13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Straus DS, Glass CK. Cyclopentenone prostaglandins: new insights on biological activities and cellular targets. Med Res Rev. 2001;21:185–210. doi: 10.1002/med.1006. [DOI] [PubMed] [Google Scholar]
- 36.Narumiya S, FitzGerald G. Genetics and pharmacological analysis of prostanoid receptor function. J Clin Invest. 2001;108:25–30. doi: 10.1172/JCI13455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shibata T, Kondo M, Osawa T, Shibata N, Kobayashi M, Uchida K. 15-Deoxy-Δ12,14-prostaglandin J2. J Biol Chem. 2002;277:10459–66. doi: 10.1074/jbc.M110314200. [DOI] [PubMed] [Google Scholar]
- 38.Maxey KM, Hessler E, MacDonald J, Hitchingham L. The natural and composition of 15-deoxy-Δ12,14 PGJ2. Prostaglandins Other Lipid Mediat. 2000;62:15–21. doi: 10.1016/s0090-6980(00)00072-1. [DOI] [PubMed] [Google Scholar]
- 39.Forman BM. The antidiabetic agent LG100754 sensitizes cells to low concentrations of peroxisome proliferator-activated receptor γ ligands. J Biol Chem. 2002;227:12503–6. doi: 10.1074/jbc.C200004200. [DOI] [PubMed] [Google Scholar]
- 40.Davies SS, Pontsler AV, Marthe GK, Harrison KA, Murphy RC, Hinshaw JC, et al. Oxidized alky phospholipids are specific, high affinity peroxisome proliferator-activated receptor γ ligands and agonists. J Biol Chem. 2001;276:16015–23. doi: 10.1074/jbc.M100878200. [DOI] [PubMed] [Google Scholar]
- 41.Pontsler AV, St Hilaire A, Marathe GK, Zimmerman GA, McIntyre TM. Cyclooxygenase-2 is induced in monocytes by peroxisome proliferator activated receptor-γ and oxidized alky phospholipids from oxidized low density lipoprotein. J Biol Chem. 2002;277:13026–9. doi: 10.1074/jbc.M109546200. [DOI] [PubMed] [Google Scholar]
- 42.Hulin B, McCarthy PA, Gibbs EM. The glitazone family of antidiabetic agents. Curr Pharm Des. 1996;2:85–102. [Google Scholar]
- 43.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Wilkison TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPARγ) J Biol Chem. 1995;270:12953–6. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 44.Berger J, Bailey P, Biswas C, Cullinan CA, Doebber TW, Hayes NS, et al. Thiazolidinediones produce a conformational change in peroxisomal proliferator-activated receptor-γ: binding and activation correlated with antidiabetic actions in db/db mice. Endocrinology. 1996;137:4189–95. doi: 10.1210/endo.137.10.8828476. [DOI] [PubMed] [Google Scholar]
- 45.Spiegelman BM. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47:507–14. doi: 10.2337/diabetes.47.4.507. [DOI] [PubMed] [Google Scholar]
- 46.Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA. Peroxisome proliferator-activators α and γ are activated by indomethacin and other nonsteroidal anti-inflammatory drugs. J Biol Chem. 1997;272:3406–10. doi: 10.1074/jbc.272.6.3406. [DOI] [PubMed] [Google Scholar]
- 47.He TC, Chan TA, Vogelstein B, Kinzler KW. PPARδ is an APC-regulated target of nosteroidal antiinflammatory drugs. Cell. 1999;99:335–45. doi: 10.1016/s0092-8674(00)81664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–50. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 49.Narumiya S, Fukushima M. Site and mechanism of growth inhibition by prostaglandins: I Active transport and intracellular accumulation of cyclopentenone prostaglandins, a reaction leading to growth inhibition. J Pharmacol Exp Ther. 1986;239:500–5. [PubMed] [Google Scholar]
- 50.Rossi A, Kapahi P, Natoli G, Takahashi T, Chen Y, Karin M, et al. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IκB kinase. Nature. 2000;403:103–8. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- 51.Castrillo A, Díaz-Guerra MJM, Hortelano S, Martín-Sanz P, Boscá L. Inhibition of IκB kinase and IκB phosphorylation by 15-deoxy-Δ12,14-prostaglandin J2 in activated murine macrophages. Mol Cell Biol. 2000;20:1692–8. doi: 10.1128/mcb.20.5.1692-1698.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Straus DS, Pascual G, Li M, Welch JS, Ricote M, Hsiang CH, et al. 15-Deoxy-Δ12,14-prostaglandin J2 inhibits multiple steps in the NF-κB signalling pathway. Proc Natl Acad Sci U S A. 2000;97:4844–9. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang XY, Wang LH, Chen T, Hodge DR, Resau JH, DaSilva L, et al. Activation of human T lymphocytes is inhibited by peroxisome proliferator-activated receptor γ (PPARγ) agonists. PPARγ co-association with transcription factor NFAT. J Biol Chem. 2000;275:4541–4. doi: 10.1074/jbc.275.7.4541. [DOI] [PubMed] [Google Scholar]
- 54.Zhang X, Young HA. Inhibition of biological functions of natural killer cells by 15-deoxy-Δ12,14-prostaglandin J2 through PPAR-γ-dependent and -independent mechanism. FA-SEB J. 2002;16(5 Pt II):A1082. [Google Scholar]
- 55.Young HA. Regulation of interferon-γ gene expression. J Interferon Cytokine Res. 1996;16:563–8. doi: 10.1089/jir.1996.16.563. [DOI] [PubMed] [Google Scholar]
- 56.Luo G, Yu-Lee L. Stat5b inhibits NFκB-mediated signalling. Mol Endocrinol. 2000;14:114–23. doi: 10.1210/mend.14.1.0399. [DOI] [PubMed] [Google Scholar]
- 57.Zhang X, Chen ZQ, Wang Z, Mohan W, Tam SP. Protein–DNA interaction at a drug-responsive element of the human apolipoprotein A–I gene. J Biol Chem. 1996;271:27152–60. [PubMed] [Google Scholar]
- 58.Cunard R, Ricote M, DiCampli D, Archer DC, Kahn DA, Glass CK, et al. Regulation of cytokine expression by ligands of peroxisome proliferator activated receptors. J Immunol. 2002;168:2795–802. doi: 10.4049/jimmunol.168.6.2795. [DOI] [PubMed] [Google Scholar]
- 59.Harris SG, Phipps RP. Prostaglandin D (2), its metabolite 15-d-PGJ (2), and peroxisome proliferator activated receptor-gamma agonists induce apoptosis in transformed, but not normal, human T lineage cells. Immunology. 2002;105:23–4. doi: 10.1046/j.0019-2805.2001.01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harris SG, Phipps RP. The nuclear receptor PPAR gamma is expressed by mouse T lymphocytes and PPAR gamma agonists induce apoptosis. Eur J Immunol. 2001;1:1098–105. doi: 10.1002/1521-4141(200104)31:4<1098::aid-immu1098>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 61.Thieringer R, Fenyk-Melody JE, Le Grand CB, Shelton BA, Detmers PA, Somers EP, et al. Activation of peroxisome proliferator-activated receptor gamma does not inhibit IL-6 or TNF-alpha responses of macrophages to lipopolysaccharide in vitro or in vivo. J Immunol. 2000;164:1046–54. doi: 10.4049/jimmunol.164.2.1046. [DOI] [PubMed] [Google Scholar]
- 62.Zhang X, Wang JM, Gong WH, Mukaida N, Young HA. Differential regulation of chemokine gene expression by 15-deoxy-Δ12,14-prostaglandin J2. J Immunol. 2001;166:7104–11. doi: 10.4049/jimmunol.166.12.7104. [DOI] [PubMed] [Google Scholar]
- 63.Harris SG, Smith RS, Phipps RP. 15-Deoxy-Δ12,14-PGJ2 induces IL-8 production in human T cells by a mitogen-activated protein kinase pathway. J Immunol. 2002;168:1372–9. doi: 10.4049/jimmunol.168.3.1372. [DOI] [PubMed] [Google Scholar]
- 64.Jozkowicz A, Dulak J, Prager M, Nanobashvili J, Nigisch A, Winter B, et al. Prostaglandin J2 induces synthesis of interleukin-8 by endothelial cells in a PPAR-γ-independent manner. Prostaglandins Other Lipid Mediat. 2001;66:165–77. doi: 10.1016/s0090-6980(01)00155-1. [DOI] [PubMed] [Google Scholar]
- 65.Tontonoz P, Nagy L, Alvarez JGA, Thomazy VA, Evans RM. PPARγ promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–72. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 66.Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM. PPAR-gamma dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med. 2001;7:48–52. doi: 10.1038/83336. [DOI] [PubMed] [Google Scholar]
- 67.Paik JH, Ju JH, Lee JY, Boudreau MD, Hwang DH. Two opposing effects of non-steroidal anti-inflammatory drugs on the expression of the inducible cyclooxygenase. Mediation through different signaling pathways. J Biol Chem. 2000;275:28173–9. doi: 10.1074/jbc.M002329200. [DOI] [PubMed] [Google Scholar]
- 68.Kodera Y, Takeyama K, Murayam A, Suzawa M, Masuhiro Y, Kato S. Ligand type-specific interactions of peroxisome proliferator-activated receptor gamma with transcriptional coactivators. J Biol Chem. 2000;275:33201–4. doi: 10.1074/jbc.C000517200. [DOI] [PubMed] [Google Scholar]
- 69.Schulman IG, Shao G, Heyman RA. Transactivation by retinoid X receptor–peroxisome proliferator-activated receptor-γ (PPARγ) heterodimers: intermolecular synergy requires only the PPARγ hormone-dependent activation function. Mol Cell Biol. 1998;18:3483–94. doi: 10.1128/mcb.18.6.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, et al. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–95. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 71.Akiyama TE, Sakai S, Lambert G, Nicol CJ, Matsusue K, Pimprale S, et al. Conditional disruption of the peroxisome proliferator-activated receptor gamma gene in mice results in lowered expression of ABCA1, ABCG1, and apoE in macrophages and reduced cholesterol efflux. Mol Cell Biol. 2002;22:2607–19. doi: 10.1128/MCB.22.8.2607-2619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rincon M. MAP-kinase signaling pathways in T cells. Curr Opin Immunol. 2001;13:339–45. doi: 10.1016/s0952-7915(00)00224-7. [DOI] [PubMed] [Google Scholar]
- 73.Guyton K, Bond R, Reilly C, Gilkeson G, Halushka P, Cook J. Differential effects of 15-deoxy-delta (12, 14)-prostaglandin J2 and a peroxisome proliferator-activated receptor gamma agonist on macrophage activation. J Leukoc Biol. 2001;69:631–8. [PubMed] [Google Scholar]
- 74.Yu TK, Caudell EG, Smid C, Grimm EA. IL-2 activation of NK cells: involvement of MKK1/2/ERK but not p38 kinase pathway. J Immunol. 2000;164:6244–51. doi: 10.4049/jimmunol.164.12.6244. [DOI] [PubMed] [Google Scholar]
- 75.Goetze S, Kintscher U, Kim S, Meehan MP, Kaneshiro K, Collins AR, et al. Peroxisome proliferator-activated receptor-gamma ligands inhibit nuclear but not cytosolic extracellular signal-regulated kinase/mitogen-activated protein-kinase-regulated steps in vascular smooth muscle cell migration. J Cardiovasc Pharmacol. 2001;38:909–21. doi: 10.1097/00005344-200112000-00013. [DOI] [PubMed] [Google Scholar]
- 76.Takeda K, Ichiki T, Tokunou T, Iino N, Takeshita A. 15-De-oxy-delta 12, 14-prostaglandin J2 and thiazolidinediones activate the MEK/ERK pathway through phosphatidylinositol 3-kinase in vascular smooth muscle cells. J Biol Chem. 2001;276:48950–5. doi: 10.1074/jbc.M108722200. [DOI] [PubMed] [Google Scholar]
- 77.Goetze S, Kim S, Xi XP, Graf K, Yang DC, Fleck E, et al. Troglitazone inhibits mitogenic signaling by insulin in vascular smooth muscle cells. J Cardiovasc Pharmacol. 2000;35:749–57. doi: 10.1097/00005344-200005000-00011. [DOI] [PubMed] [Google Scholar]
- 78.Wilmer WA, Dixon C, Lu L, Hilbelink T, Rovin BH. A cyclo-pentenone prostaglandin activates mesangial MAP kinase independently of PPARgamma. Biochem Biophys Res Commun. 2001;281:57–62. doi: 10.1006/bbrc.2001.4301. [DOI] [PubMed] [Google Scholar]
- 79.Chen NG, Han X. Dual function of troglitazone on ICAM-1 gene expression in human vascular endothelium. Biochem Biophys Res Commun. 2001;282:717–22. doi: 10.1006/bbrc.2001.4628. [DOI] [PubMed] [Google Scholar]
- 80.Hortelano S, Castrillo A, Alvarez AM, Bosca L. Contribution of cyclopentenone prostaglandins to the resolution of inflammation through the potentiation of apoptosis in activated macrophages. J Immunol. 2000;165:6525–31. doi: 10.4049/jimmunol.165.11.6525. [DOI] [PubMed] [Google Scholar]
- 81.Zhang B, Berger J, Zhou G, Elbrecht A, Biswas S, White-Carringotn S, et al. Insulin- and mitogen-activated protein kinase-mediated phosphorylation and activation of peroxisome proliferator-activated receptor-γ. J Biol Chem. 1996;271:31771–4. doi: 10.1074/jbc.271.50.31771. [DOI] [PubMed] [Google Scholar]
- 82.Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipo-genesis through MAP kinase-mediated phosphorylation of PPAR-γ. Science. 1996;274:2100–3. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- 83.Shao D, Rangwal SM, Bailey ST, Krakow SL, Reginato MJ, Lazar MA. Interdomain communication regulating ligand binding by PPAR-gamma. Nature. 1998;396:377–80. doi: 10.1038/24634. [DOI] [PubMed] [Google Scholar]
- 84.Wright DH, Metters KM, Abramovitz M, Ford-Hutchinson AW. Characterization of the recombinant human prostanoid DP receptor and identification of L-644,698, a novel selective DP agonist. Br J Pharmacol. 1998;123:1317–24. doi: 10.1038/sj.bjp.0701708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vaidya S, Somers EP, Wright SD, Detmers PA, Bansal VS. 15-Deoxy-Δ12,14-prostaglandin J2 inhibits the β2 intergrin-dependent oxidative burst: involvement of a mechanism distinct from peroxisome proliferator-activated receptor γ ligation. J Immunol. 1999;163:6187–92. [PubMed] [Google Scholar]
- 86.Nagata K, Tanaka K, Ogawa K, Kemmotsu K, Imai T, Yoshie O, et al. Selective expression of a novel surface molecule by human Th2 cells in vivo. J Immunol. 1999;162:1278–86. [PubMed] [Google Scholar]
- 87.Monneret G, Li H, Vasilescu J, Rokach J, Powell WS. 15-Deoxy-Δ12,14-prostaglandin D2 and J2 are potent activators of human eosinophils. J Immunol. 2002;168:3563–9. doi: 10.4049/jimmunol.168.7.3563. [DOI] [PubMed] [Google Scholar]
- 88.Hirai H, Tanaka K, Takano S, Ichimasa M, Nakamura M, Nagata K. Cutting edge: agonistic effect of indomethacin on a prostaglandin D2 receptor, CRTH2. J Immunol. 2002;168:981–5. doi: 10.4049/jimmunol.168.3.981. [DOI] [PubMed] [Google Scholar]
- 89.Rengarajan J, Szabo SJ, Glimcher LH. Transcriptional regulation of Th1/Th2 polarization. Immunol Today. 2000;21:479–83. doi: 10.1016/s0167-5699(00)01712-6. [DOI] [PubMed] [Google Scholar]
- 90.Peritt D, Robertson S, Gri G, Showe L, Aste-Amezaga M, Trinchieri G. Differentiation of human NK cells into NK1 and NK2 subsets. J Immunol. 1998;161:5821–4. [PubMed] [Google Scholar]
- 91.Loza MJ, Perussia B. Final steps of natural killer cell maturation: a model for type 1–type-2 differentiation? Nat Immunol. 2001;2:917–24. doi: 10.1038/ni1001-917. [DOI] [PubMed] [Google Scholar]
- 92.Colonna M. Can we apply the Th1–Th2 paradigm to all lymphocytes? Nat Immunol. 2001;2:899–900. doi: 10.1038/ni1001-899. [DOI] [PubMed] [Google Scholar]
- 93.Hodge DL, Martinez A, Julias JG, Taylor LS, Young HA. Regulation of nuclear gamma interferon gene expression by interleukin 12 (IL-12) and IL-2 represents a novel form of posttranscriptional control. Mol Cell Biol. 2002;22:1742–53. doi: 10.1128/MCB.22.6.1742-1753.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–69. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 95.Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, et al. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292:1907–10. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 96.Szabo SJ, Sulliivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in Th1 lineage commitment and IFN-γ production in CD4 and CD8 T cells. Science. 2002;295:338–42. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 97.Farrar JD, Ouyang W, Löhning M, Assenmacher M, Radbruch A, Kanagawa O, et al. An instructive component in T helper cell type 2 (Th2) development mediated by GATA-3. J Exp Med. 2001;193:643–50. doi: 10.1084/jem.193.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ranganath S, Murphy KM. Structure and specificity of GA-TA proteins in Th2 development. Mol Cell Biol. 2001;21:2716–25. doi: 10.1128/MCB.21.8.2716-2725.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee HJ, Takemoto N, Kurata H, Kamogawa Y, Miyatake S, O’Garra A, et al. GATA-3 induces T helper cell type 2 (Th2) cytokine expression and chromatin remodelling in committed Th1 cells. J Exp Med. 2000;192:105–15. doi: 10.1084/jem.192.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Farrar JD, Asnagli H, Murphy KM. T helper subset development: roles of instruction, selection, and transcription. J Clin Invest. 2002;109:431–5. doi: 10.1172/JCI15093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Marx N, Kehrle B, Kohlhammer K, Grub M, Koenig W, Hombach V, et al. PPAR activators as antiinflammatory mediators in human T lymphocytes: implications for atherosclerosis and transplantation-associated arteriosclerosis. Circ Res. 2002;90:703–10. doi: 10.1161/01.res.0000014225.20727.8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chtanova T, Kemp RA, Sutherland APR, Ronchese F, Mackay CR. Gene microarrays reveal extensive differential gene expression in both CD4+ and CD8+ type 1 and type 2 T cells. J Immunol. 2001;167:3057–63. doi: 10.4049/jimmunol.167.6.3057. [DOI] [PubMed] [Google Scholar]
- 103.Jones DC, Daynes RA. The nuclear hormone receptor PPARα’s ability to control T-bet transcription is due to its ability to regulate redox homeostasis within T cells. FASEB J. 2002;16(4 Part I):A700. [Google Scholar]
- 104.Floyd ZE, Stephens JM. Interferon-γ-mediated activation and ubiquitin–proteasome dependent degradation of PPARγ in adipocytes. J Biol Chem. 2002;277:4062–8. doi: 10.1074/jbc.M108473200. [DOI] [PubMed] [Google Scholar]
- 105.Tanaka K, Ogawa K, Sugamura K, Nakamura M, Takano S, Nagata K. Cutting edge: differential production of prostaglandin D2 by human helper T cell subsets. J Immunol. 2000;164:2277–80. doi: 10.4049/jimmunol.164.5.2277. [DOI] [PubMed] [Google Scholar]
- 106.Hirai H, Tanaka K, Yoshie O, Ogawa K, Kenmotsu K, Takamori Y, et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cell, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Med. 2001;193:255–61. doi: 10.1084/jem.193.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Abe H, Takeshita T, Nagata K, Arita T, Endo Y, Fujita T, et al. Molecular cloning, chromosome mapping and characterization of the mouse CRTH2 gene, a putative member of the leukocyte chemoattractant receptor family. Gene. 1999;227:71–7. doi: 10.1016/s0378-1119(98)00599-x. [DOI] [PubMed] [Google Scholar]
- 108.Ho IC, Arm JP, Bingham CO, III, Choi A, Austen F, Glimcher LH. A novel group of phospholipase A2s preferentially expressed in type 2 helper T cells. J Biol Chem. 2001;276:18321–6. doi: 10.1074/jbc.M008837200. [DOI] [PubMed] [Google Scholar]
- 109.Rocca B, FitzGerald GA. Cyclooxygenases and prostaglandins: shaping up the immune response. Int Immunopharmacol. 2002;2:603–30. doi: 10.1016/s1567-5769(01)00204-1. [DOI] [PubMed] [Google Scholar]
- 110.Harizi H, Juzan M, Pitard V, Moreau JF, Gualde N. Cyclo-oxygenase-2-issued prostaglandin E2 enhances the production of endogenous IL-10, which down-regulates dendritic cell functions. J Immunol. 2002;168:2255–63. doi: 10.4049/jimmunol.168.5.2255. [DOI] [PubMed] [Google Scholar]
- 111.Kalinski P, Hilkens CM, Snijders A, Snijdewint FG, Kapsenberg ML. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol. 1997;159:28–35. [PubMed] [Google Scholar]
- 112.Jones DC, Ding X, Daynes RA. Nuclear receptor peroxisome proliferator-activated receptor α (PPARα) is expressed in resting murine lymphocytes. J Biol Chem. 2002;277:6838–45. doi: 10.1074/jbc.M106908200. [DOI] [PubMed] [Google Scholar]
- 113.Naitoh T, Kitahara M, Tsuruzoe N. The effect of activation of peroxisome proliferator-activated receptor gamma (PPARγ) on human monocyte function: PPARγ ligands do not inhibit tumor necrosis factor-alpha release in human monocytic cell line THP-1. Cell Biol Toxicol. 2000;16:131–5. doi: 10.1023/a:1007694110835. [DOI] [PubMed] [Google Scholar]
- 114.Cuzzocrea S, Wayman NS, Mazzon E, Dugo L, Paola RD, Serraino I, et al. The cyclopentenone prostaglandin 15-de-oxy-Δ12,14-prostaglandin J2 attenuates the development of acute and chronic inflammation. Mol Pharmacol. 2002;6:997–1007. doi: 10.1124/mol.61.5.997. [DOI] [PubMed] [Google Scholar]
- 115.Bureau F, Desmet C, Mélotte D, Jaspar F, Volanti C, Vander-plasschen A, et al. A proinflammatory role for the cyclopen-tenone prostaglandins at low micromolar concentrations: oxidative stress-induced extracellular signal-regulated kinase activation without NF-κB inhibition. J Immunol. 2002;168:5318–25. doi: 10.4049/jimmunol.168.10.5318. [DOI] [PubMed] [Google Scholar]
- 116.Fu Y, Luo N, Lopes-Virella MF. Upregulation of interleukin-8 expression by prostaglandin D2 metabolite 15-deoxy-delta12, 14 prostaglandin J2 (15d-PGJ2) in human THP-1 macrophages. Atherosclerosis. 2002;160:11–20. doi: 10.1016/s0021-9150(01)00541-x. [DOI] [PubMed] [Google Scholar]
- 117.Natarajan C, Bright JJ. Peroxisome proliferator-activated receptor-gamma agonists inhibit experimental allergic encephalomyelitis by blocking IL-12 production, IL-12 signalling and Th1 differentiation. Genes Immun. 2002;3:59–70. doi: 10.1038/sj.gene.6363832. [DOI] [PubMed] [Google Scholar]