Abstract
Stress-mediated loss of synaptogenesis in the hippocampus appears to play a role in depressive and mood disorders. However, little is known about the effect of stress/depression on the plasticity and survival of cortical neurons. In this report, we have examined whether chronic stress increases the vulnerability of neurons in the rat cortex. We have used a chronic unpredictable mild stress (CMS) as a rat model of depression. CMS (5 weeks treatment) produced anedonia and increased corticosterone levels. These effects were accompanied by a detectable increase in caspase-3 positive neurons in the cerebral cortex, suggesting apoptosis. Desipramine, a well known antidepressant, reversed the pro-apoptotic effect of CMS. These results suggest that antidepressants may reduce the pathological changes seen in stress-induced depressive disorders.
Keywords: Caspase-3, chronic mild stress (CMS), depression, desipramine (DMI), cerebral cortex
Although depression has been associated with impaired neurotransmitter function, particularly in the noradrenergic and serotonergic systems [8, 20], the monoamine hypothesis no longer provides a satisfactory explanation of the mode of action of antidepressant agents or the underlying pathology of depression. Indeed, the last two decades have seen a proliferation of studies examining alternative mechanisms to elucidate the therapeutic efficacy of antidepressants. These include the effect of antidepressants on neurogenesis in adult hippocampus of rodents [25, 38] and non-human primates [35], as well as cortical synaptic strength in vitro [3].
Postmortem brains of patients with major depression exhibit loss of neuronal and glial density [11, 19, 32, 34]. In addition, stress, a risk factor for depression in humans, evokes in animals dendritic shrinkage and cell loss within the hippocampus [15, 27]. Loss of neurons is seen also in animal models of stress that mimic human depression [15]. The hippocampus appears to be particularly sensitive to stress stimuli in both animals and humans as this brain area undergoes selective volume reduction and dendritic retraction. Thus, it has been suggested that depression may be associated with decreased hippocampal plasticity, and that antidepressants may prevent the neuronal atrophy seen in depressed patients. However, relatively little is known about the effect of stress or depression on cortical neurons. In this study, we examined whether depression affects neuronal survival in the cerebral cortex. To induce depression in rats, we used an animal model of depression termed chronic unpredictable mild stress (CMS). This model, which has already been characterized and validated by a number of investigators [29, 30, 37, 40], relies on the fact that rats receiving unpredictable chronic stressful stimuli develop core symptoms of major depression, including anhedonia, decreased place preference conditioning and impaired grooming response. We report a novel effect of CMS on cortical plasticity which is reversed by chronic antidepressant treatments.
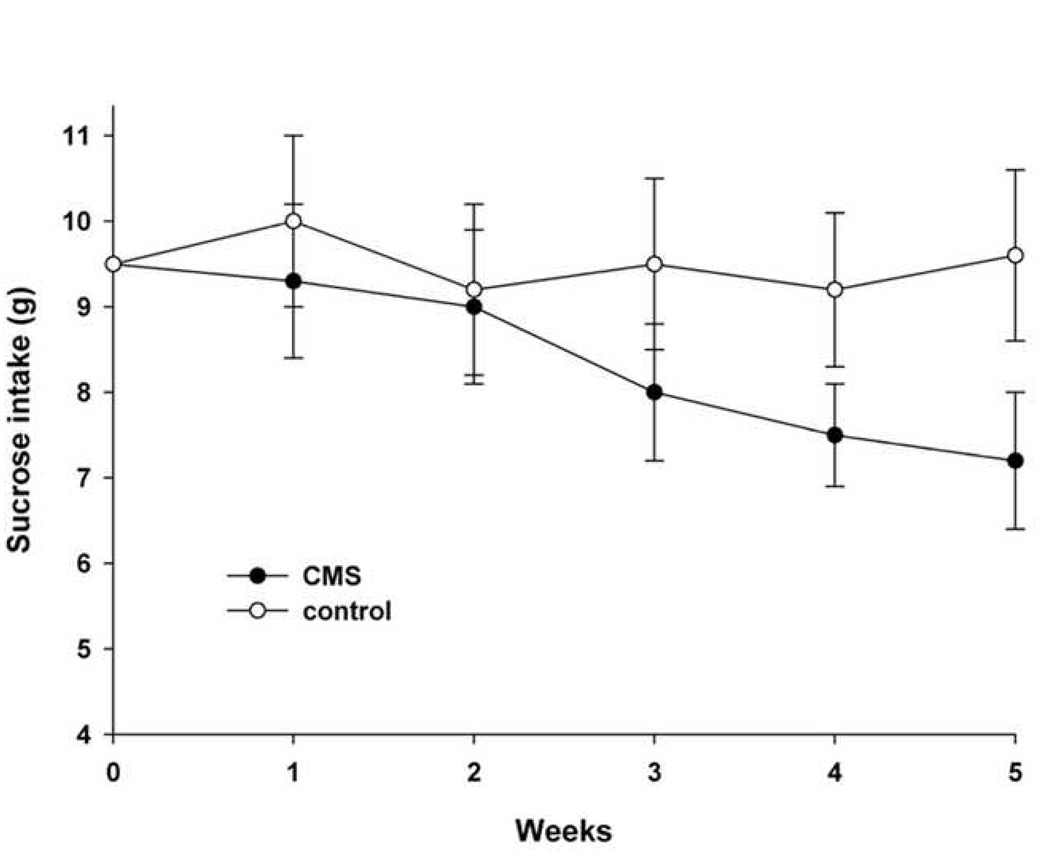
Adult male Sprague-Dawley rats (180–250 g; Taconic, Germantown, NY) were housed three per cage in a temperature-controlled environment with a 12 hr light/dark cycle. All handling procedures were in accordance with the National Institute of Health Guide for the care and use of laboratory animals and were approved by the Georgetown University Institutional Animal Care and Use Committee. Rats were trained to consume 1% (w/v) sucrose solution before starting the CMS protocol. Training consisted of three 1-hr tests (Monday, Wednesday and Friday), in which animals could select between two preweighted bottles, one with 1% sucrose solution and one with tap water, after 20 hr food and water deprivation. Animals were then randomly assigned to control and stressed groups. For 5 weeks, rats received no CMS or daily stressor stimuli according to Table 1. This procedure was adapted from Moreau et al. [30]. Stressor stimuli were applied to the CMS group at a different time each day to elicit unpredictability. Moreover, CMS rats were handled with gloves (Stainless-Steel Mesh Gloves, A5151, Fisher Scientific) to induce an additional undesirable confinement. Both food and water were removed the night before the sucrose test which was conducted once a week (Wednesday). Animals were then allowed to drink 1% sucrose solution or tap water, and tested for increased preference for the sweet solution by monitoring the amount of sucrose consumed in a 1-hr test. Consumption of sucrose fell in the CMS group starting at 3 weeks of stress (Fig. 1). By 4 weeks of CMS, sucrose intake was further reduced, and it remained low when the animals were tested the following week (Fig. 1). This is not due to the inability of CMS animals to drink, because the amount of tap water consumption did not decrease throughout the experiments (week 1= 1.9 ± 0.5 g, week 5 = 2.0 ± 0.4 g). These data confirm previous observations that CMS causes a reduction in the consumption of palatable sucrose solution and validate the notion that CMS is a useful animal model for the study and understanding of the pathophysiological mechanisms underlying depressive states [5, 40].
Table 1.
CMS schedule
| Morning | Afternoon | |
|---|---|---|
| Monday | Restricted movements* 1 hr | Restricted movements 1 hr; overnight illumination |
| Tuesday | Restricted movements 1 hr | Restricted movements 1 hr; overnight food and water deprivation |
| Wednesday | Access to restricted food (1 gram) for 2 hr | Restricted movements 1 hr; overnight water deprivation |
| Thursday | 1 hr exposure to empty bottle; restricted movements 1 hr | Restricted movements; group-housed in soiled cage overnight |
| Friday | Restricted movements 1 hr | Reverse light-dark cycle through the weekend |
Restricted movements consisted of placing rats into a mouse cage (24W×10L×9H cm) for 1 hr.
Figure 1. CMS decreases consumption of sucrose.
Rats were trained to drink a palatable solution of 1% sucrose for one week (0) and then were assigned to receive CMS or no CMS (control) as described in Table 1 for the indicated weeks. Sucrose consumption was measured in a 1-hr test by weighing preweighed bottles. Control rats were not subjected to any of the stressors except for water deprivation 20 hr prior to each sucrose intake test. Data are the mean ± standard deviation (n=10 each group). Differences in sucrose consumption were analyzed using a two-way ANOVA test with group and time as factors. Compared to control, CMS significantly decreases consumption of sucrose by 3 weeks (p<0.05).
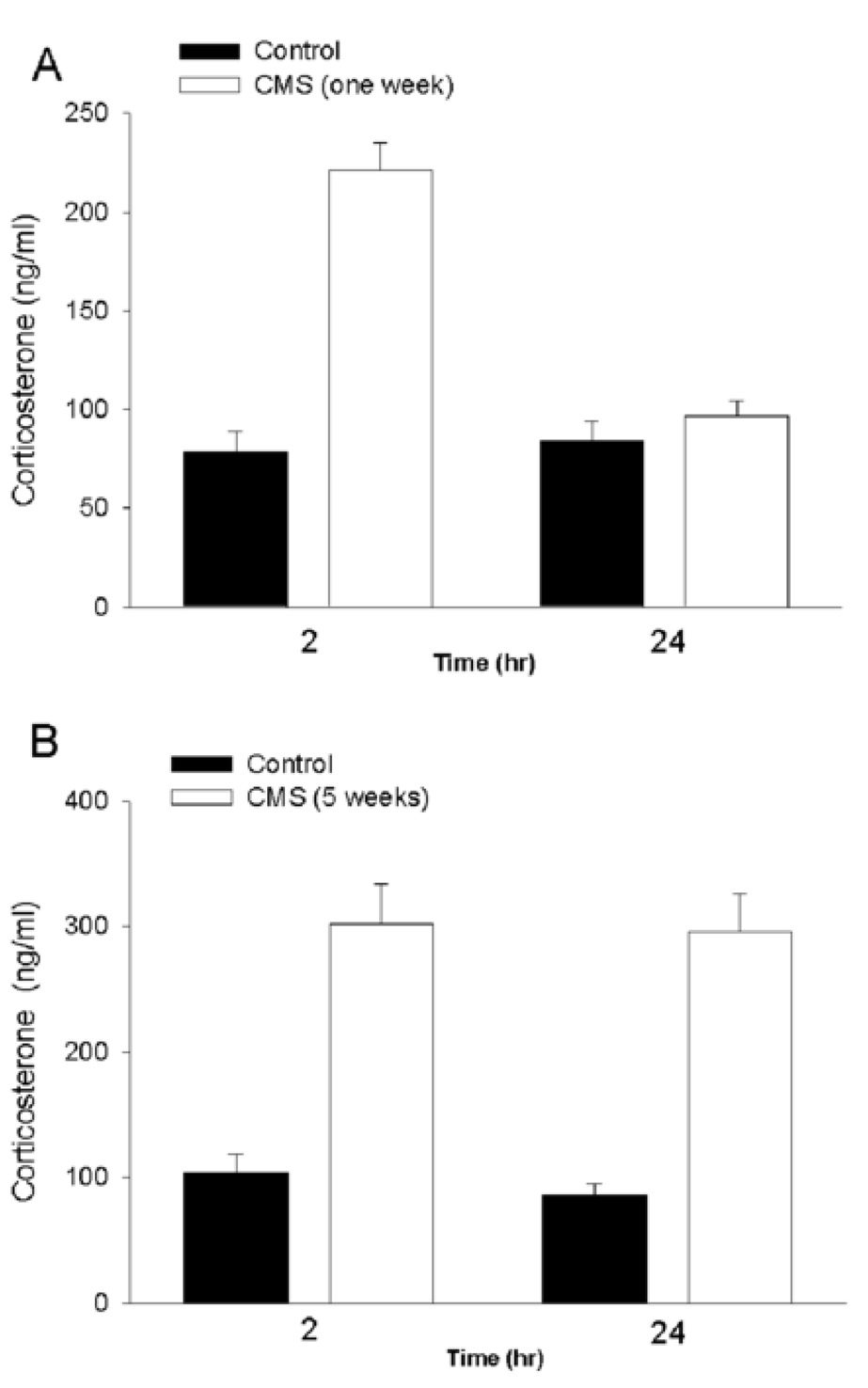
The hypothalamic pituitary adrenal axis (HPA) is an essential component of an individual’s capacity to cope with stress. Excessive stimulation of HPA has been implicated in depression [13, 21]. Glucocorticoid secretion from the adrenal medulla is a valid measurement of HPA activation. Therefore, we determined plasma corticosterone levels in non-CMS and CMS rats. To provide a correlation between HPA activation and anhedonia, corticosterone levels were measured after 1 or 5 weeks of CMS. Animals were sacrificed at 2 and 24 hr after the restrain test on Monday afternoon of the second or sixth week treatment. CMS-treated rats exhibited higher corticosterone levels than controls (Fig. 2). However, corticosterone levels in rats exposed to CMS for 5 weeks (Fig. 2B) were significantly higher than those exposed to CMS for only 1 week (Fig. 2A) at both 2 and 24 hr. These data support a previous suggestion that CMS induces an alteration in the dynamics of HPA axis [14].
Figure 2. Effect of CMS on plasma corticosterone levels.
Rats received CMS for 1 (A) or 5 (B) weeks and were sacrificed at the indicated times after the last stressor. Trunk blood (10 ml) was collected immediately into chilled glass tubes containing heparin. The samples were centrifuged at 4°C at 3000 rpm. The separated plasma was stored at −70°C until assayed for circulating corticosterone. Corticosterone levels were determined by 125I radioimmunoassay using a commercially available reagent kit (MP Biochemicals, LLC, Orangeburg, NY). The assay sensitivity was 21 ng/ml. Data are the mean ± standard error of 10 separate samples. Differences in corticosterone levels were analyzed using a two-way ANOVA with group and time as two factors. Compared to control, CMS significantly increases levels of corticosterone (p<0.05). However, there was also a significant difference in the CMS 1 week group between 2 and 24 hr (p<0.05).
Postmortem brains of depressed patients show DNA fragmentation and neuronal apoptosis, suggesting an enhanced neuronal vulnerability in depression [23]. Neuronal loss and reduced neurogenesis have also been seen in animal models of mood disorders [7, 15, 23]. Neuronal cell death may occur through two mechanisms, an acute form, or necrosis, that occurs rapidly, or the delayed form, apoptosis [41]. The former cannot be prevented efficiently because it tends to be prominent following more extreme conditions such as ischemic insults or mechanical injury. Apoptosis in mammalian cells can be controlled by several pro-apoptotic genes. Among others, cysteine proteases or caspases are crucial for cell death [41]. Caspases are synthesized as inactive pro-enzymes and are activated by proteolytic cleavage. Multiple caspases may activate one another in a sequential cascade manner. For example, caspases such as 8 and 9 are activated early on, whereas caspase-3 is a frequent downstream effector in the cascade. When stimuli activate caspase-3 in neurons, these cells are committed to die [41]. Thus, activation of caspase-3 is essential in neuronal apoptosis.
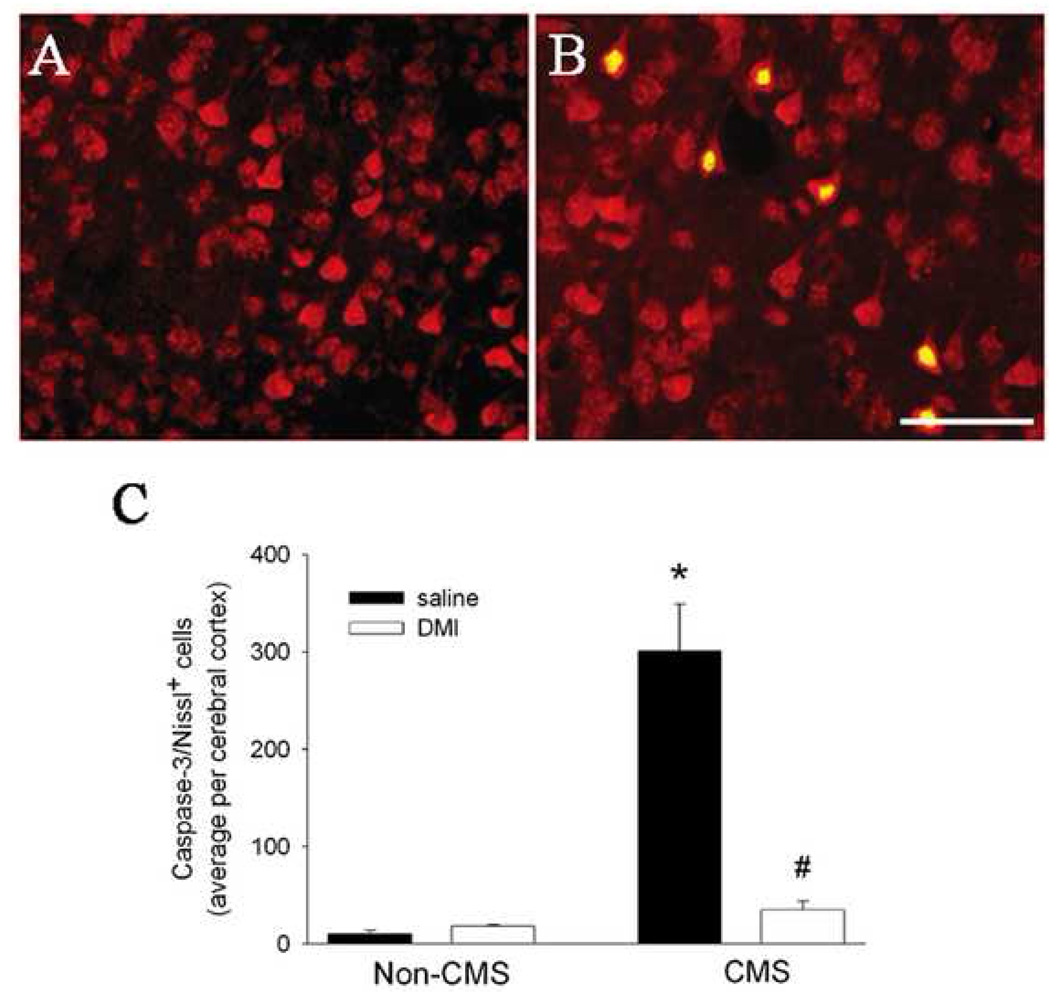
These considerations prompted us to test the hypothesis that CMS may induce caspase-3 dependent apoptotic cell death. Rats received CMS for 5 weeks. At the end of the fifth week, rats were anesthetized and prepared for histological analyses. Serial cross sections throughout the cortex (frontal to occipital) were prepared and stained with an antibody against the activated (cleaved) form of caspase-3, as previously described [2, 33]. Moreover, to examine whether neurons are sensitive to CMS, double labeling was carried out using Nissl staining or NeuN, markers for neuronal cell bodies. To generate a semi quantitative analysis of apoptosis, caspase-3 positive cells in the frontal cortex were counted in serial sections using a 20X objective and MetaMorph® Imaging software (Universal Imaging Corporation™, Downingtown, PA) as previously described [2, 33]. Little or no caspase-3 positive cells were detected in the cortex of control (non-CMS) rats (Figs. 3A and C). Instead, caspase-3 positive cells were observed in CMS rats (Figs. 3B and C). These cells were mostly localized in layer V of the cortex and were Nissl (Fig. 3B) or NeuN positive (data not shown), suggesting neurons. We exclude that CMS evokes apoptosis only in the cortex because we also detected few caspase-3 positive cells in a small portion of the CA1 region of the hippocampus (data not shown). Because this investigation focuses on the effect of CMS on the cerebral cortex, and the effect of chronic stress on the pathophysiology of the hippocampus is well documented (reviewed in [27]), we did not quantify the extent of caspase-3 positive cells in the hippocampus or other brain areas. Nevertheless, our data, together with those showing that chronic stress up-regulates proapoptotic proteins in the cortex [16, 24], are significant because they reveal that the cerebral cortex can also be sensitive to the deleterious effect of stress.
Figure 3. CMS promotes neuronal apoptosis.
Non-CMS and CMS rats received saline or DMI starting at the third week of CMS. Rats were tested for sucrose consumption at 5 weeks. At the beginning of the sixth week, animals were anesthetized with ketamine/xylazine (80/10 mg/kg respectively, i.p.) and then sacrificed by intracardiac perfusion with 4% paraformaldehyde in 0.1M PBS, pH 7.4. The brains were removed and post-fixed in the same fixative for 2 hr, then transferred into a buffered graded sucrose (10, 20, and 30%). Serial coronal sections (16 µm) were prepared throughout the cortex. Sections were double-stained for NeuroTrace™ red fluorescent Nissl Stain, which recognizes neurons, and an antibody against the activated form of caspase-3 (1:100 dilution, Chemicon, Temecula, CA), as previously described [2, 33]. All sections were then mounted using Vectashield mounting medium (Vector Laboratories, Burlingame, CA). A and B: Examples of sections from the frontal cortex of control (A) and CMS (B) rats double stained for Nissl (red) and caspase-3 (green); Yellow=red and green. Caspase-3 positivity is evident in both nuclei and cytoplasm. Bar=100 µm. C: Sections were analyzed with a Zeiss fluorescence microscope Axioplan2 (Carl Zeiss MicroImaging, Inc., Thornwood, NY). The number of caspase-3 positive neurons in the frontal cortex was determined in an area of ~0.24 mm2 each section for a total length of the frontal cortex of ~3.5 mm, using at least 15 sections per animal. Data, expressed as average of positive cells per cerebral (frontal) cortex, are the mean ± standard error of 4 animals per group. *p<0.01 vs control, #p<0.05 vs CMS (one way ANOVA and Pairwise Multiple Comparison Procedures).
The data presented here confirm that chronic stress/depression, in addition to suppressing neurogenesis [23], may also trigger apoptosis [9, 24]. At present, it is unclear why only some populations of neurons are affected by CMS and which mechanisms are responsible for activation of caspase-3. Caspase-3 positive neurons in CMS rats resembled, morphologically, pyramidal cells (Fig. 3B). These neurons are glutamatergic. Low but persistent levels of glutamate or other excitatory amino acids such as N-methyl-d-aspartate (NMDA), have been shown to cause apoptosis [1]. NMDA receptor antagonists have antidepressant-like properties (reviewed in [36]). Thus, NMDA receptors may play a role in the pathological findings described here. On the other hand, the apoptotic neurons seen after CMS may express glucocorticoid receptors. Glucocorticoids have been shown to cause neuronal damage [17]. Moreover, severe, prolonged stress has been shown to induce neuronal death through apoptosis [9, 24]. Thus, the hyperactivity of the HPA axis obtained in rats after 5 weeks CMS may be responsible for the apoptosis seen in some cortical neurons. Apoptosis is also caused by the pro-inflammatory cytokine interleukin 1-β[6] whose expression has been shown to increase by CMS [14]. Exogenous administration of this cytokine produces depressive-like symptoms [28]. Therefore, CMS-induced apoptosis may be the result of glucocorticoids working in concert with inflammatory cytokines and excitatory amino acids. More anatomical and cellular studies aimed at revealing the phenotype of neurons undergoing apoptosis in CMS will help define the proapoptotic mechanisms of CMS.
Recent data have suggested that the therapeutic action of antidepressants may include their effect on neuronal survival. In fact, antidepressants reduce tissue atrophy in depressed patients [39], as well as in animal models of depression [7, 38]. The rat CMS model has been used to test the pharmacological properties of antidepressants (reviewed in [40]). Therefore, we examined whether neuronal apoptosis could be reversed by a chronic treatment with desipramine (DMI). CMS was delivered for two weeks. On Monday of the third week, we started DMI (10 mg/kg; Sigma, St Louis, MO) or saline treatment (daily subcutaneous injection). DMI or saline continued for two more weeks along with CMS. Animals were sacrificed on Monday of the sixth week and their brains prepared for analysis of caspase-3 positive neurons. We observed a decrease in apoptotic neurons in CMS rats receiving DMI when compared to CMS rats treated with saline (Fig. 3C). Taken together, these data suggest that antidepressant clinical efficacy may include both their anti-apoptotic property as well as induction of neurogenesis. It remains to be established whether the anti-apoptotic property of DMI is shared by other antidepressants.
The molecular and cellular mechanisms of neuroprotection by DMI seen in this study remain to be characterized. Neurotrophic factors have been proposed to mediate the therapeutic effect of antidepressants. At least two neurotrophic factors are induced by DMI, brain-derived neurotrophic factor (BDNF) and basic fibroblast growth factor (FGF2) [26, 31]. These neurotrophic factors interfere with the fundamental mechanism of apoptotic cell death. In addition, both neurotrophic factors exhibit properties that are relevant for the clinical efficacy of antidepressants, namely induction of neurogenesis in the hippocampus [4, 22], promotion of neurite branching in cortical neurons and modification of excitatory synapses [12, 18]. Thus, we favor the hypothesis that DMI prevents CMS-mediated apoptosis by inducing the expression of BDNF and FGF2. This suggestion would be in line with the notion that depression decreases synaptic plasticity while antidepressants, by increasing the availability of neurotrophic factors, may overcome a loss of synaptic connections seen in patients [10].
In conclusion, CMS increases the number of caspase-3 positive neurons in the cerebral cortex. As shown previously [16, 24], other proapoptotic markers were seen in the cortex of stressed animals. Thus, cortical neuronal apoptosis should be added to a list of events that have been proposed to explain loss of neuronal function and viability seen in depressive disorders. DMI reduced significantly the effect of CMS, supporting the hypothesis that antidepressants could oppose the stress-induced loss of neuronal network by increasing neuronal survival. Thus, these findings provide additional evidence that depression may be associated with neuronal loss and that pharmacological intervention should be sought to reverse neuronal apoptosis.
Acknowledgments
We would like to thank Dr. C Liu for statistical analysis and D Leader for diligent editorial assistance. Supported by NIH grant NS047977.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- 2.Bachis A, Aden SA, Nosheny RL, Andrews PM, Mocchetti I. Axonal transport of Human Immunodeficiency Virus Type 1 envelope glycoprotein 120 is found in association with neuronal apoptosis. J. Neurosci. 2006;26:6771–6780. doi: 10.1523/JNEUROSCI.1054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bal-Klara A, Bird MM. The effects of various antidepressant drugs on the fine-structure of neurons of the cingulate cortex in culture. Neuroscience. 1990;37:685–692. doi: 10.1016/0306-4522(90)90099-p. [DOI] [PubMed] [Google Scholar]
- 4.Barnabe-Heider F, Miller FD. Endogenously produced neurotrophins regulate survival and differentiation of cortical progenitors via distinct signaling pathways. J Neurosci. 2003;23:5149–5160. doi: 10.1523/JNEUROSCI.23-12-05149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bekris S, Antoniou K, Daskas S, Papadopoulou-Daifoti Z. Behavioural and neurochemical effects induced by chronic mild stress applied to two different rat strains. Behav Brain Res. 2005;161:45–59. doi: 10.1016/j.bbr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Carrasco J, Penkowa M, Giralt M, Camats J, Molinero A, Campbell IL, Palmiter RD, Hidalgo J. Role of metallothionein-III following central nervous system damage. Neurobiol Dis. 2003;13:22–36. doi: 10.1016/s0969-9961(03)00015-9. [DOI] [PubMed] [Google Scholar]
- 7.Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci U S A. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delgado PL. How antidepressants help depression: mechanisms of action and clinical response. J. Clin Psychiatry. 2004;65 Suppl 4:25–30. [PubMed] [Google Scholar]
- 9.DeVries AC, Joh HD, Bernard O, Hattori K, Hurn PD, Traystman RJ, Alkayed NJ. Social stress exacerbates stroke outcome by suppressing Bcl-2 expression. Proc Natl Acad Sci U S A. 2001;98:11824–11828. doi: 10.1073/pnas.201215298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med. 2004;5:11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- 11.Eastwood SL, Harrison PJ. Synaptic pathology in the anterior cingulate cortex in schizophrenia and mood disorders. A review and a Western blot study of synaptophysin, GAP-43 and the complexins. Brain Res Bull. 2001;55:569–578. doi: 10.1016/s0361-9230(01)00530-5. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh A, Carnahan J, Greenberg ME. Requirement for BDNF in activity-dependent survival of cortical neurons. Science. 1994;263:1618–1623. doi: 10.1126/science.7907431. [DOI] [PubMed] [Google Scholar]
- 13.Gillespie CF, Nemeroff CB. Hypercortisolemia and Depression. Psychosom Med. 2005;67:S26–S28. doi: 10.1097/01.psy.0000163456.22154.d2. [DOI] [PubMed] [Google Scholar]
- 14.Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry. 2007 doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- 15.Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psychiatry. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- 16.Haack D, Luu H, Cho J, Chen MJ, Russo-Neustadt A. Exercise reverses chronic stress-induced Bax oligomer formation in the cerebral cortex. Neurosci Lett. 2008;438:290–294. doi: 10.1016/j.neulet.2008.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haynes LE, Griffiths MR, Hyde RE, Barber DJ, Mitchell IJ. Dexamethasone induces limited apoptosis and extensive sublethal damage to specific subregions of the striatum and hippocampus: implications for mood disorders. Neuroscience. 2001;104:57–69. doi: 10.1016/s0306-4522(01)00070-7. [DOI] [PubMed] [Google Scholar]
- 18.Korada S, Zheng W, Basilico C, Schwartz ML, Vaccarino FM. Fibroblast growth factor 2 is necessary for the growth of glutamate projection neurons in the anterior neocortex. J Neurosci. 2002;22:863–875. doi: 10.1523/JNEUROSCI.22-03-00863.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lenze E, Cross D, McKeel D, Neuman RJ, Sheline YI. White matter hyperintensities and gray matter lesions in physically healthy depressed subjects. Am J Psychiatry. 1999;156:1602–1607. doi: 10.1176/ajp.156.10.1602. [DOI] [PubMed] [Google Scholar]
- 20.Leonard BE. Evidence for a biochemical lesion in depression. J. Clin. Psychiatry. 2000;61 Suppl 6:12–17. [PubMed] [Google Scholar]
- 21.Leonard BE. The HPA and immune axes in stress: The involvement of the serotonergic system. Eur Psychiatry. 2005;20:S302–S306. doi: 10.1016/s0924-9338(05)80180-4. [DOI] [PubMed] [Google Scholar]
- 22.Li AJ, Suzuki S, Suzuki M, Mizukoshi E, Imamura T. Fibroblast growth factor-2 increases functional excitatory synapses on hippocampal neurons. Eur J Neurosci. 2002;16:1313–1324. doi: 10.1046/j.1460-9568.2002.02193.x. [DOI] [PubMed] [Google Scholar]
- 23.Lucassen PJ, Heine VM, Muller MB, van der Beek EM, Wiegant VM, De Kloet ER, Joels M, Fuchs E, Swaab DF, Czeh B. Stress, depression and hippocampal apoptosis. CNS & neurological disorders drug targets. 2006;5:531–546. doi: 10.2174/187152706778559273. [DOI] [PubMed] [Google Scholar]
- 24.Lucassen PJ, Vollmann-Honsdorf GK, Gleisberg M, Czeh B, De Kloet ER, Fuchs E. Chronic psychosocial stress differentially affects apoptosis in hippocampal subregions and cortex of the adult tree shrew. Eur J Neurosci. 2001;14:161–166. doi: 10.1046/j.0953-816x.2001.01629.x. [DOI] [PubMed] [Google Scholar]
- 25.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mallei A, Shi B, Mocchetti I. Antidepressant treatments induce the expression of basic fibroblast growth factor in cortical and hippocampal neurons. Mol Pharmacol. 2002;61:1017–1024. doi: 10.1124/mol.61.5.1017. [DOI] [PubMed] [Google Scholar]
- 27.McEwen BS. Physiology and Neurobiology of Stress and Adaptation: Central Role of the Brain. Physiol. Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 28.Merali Z, Brennan K, Brau P, Anisman H. Dissociating anorexia and anhedonia elicited by interleukin-1beta: antidepressant and gender effects on responding for "free chow" and "earned" sucrose intake. Psychopharmacology (Berl) 2003;165:413–418. doi: 10.1007/s00213-002-1273-1. [DOI] [PubMed] [Google Scholar]
- 29.Monleon S, D'Aquila P, Parra A, Simon VM, Brain PF, Willner P. Attenuation of sucrose consumption in mice by chronic mild stress and its restoration by imipramine. Psychopharmacology (Berl) 1995;117:453–457. doi: 10.1007/BF02246218. [DOI] [PubMed] [Google Scholar]
- 30.Moreau JL, Borgulya J, Jenck F, Martin JR. Tolcapone: a potential new antidepressant detected in a novel animal model of depression. Behav Pharmacol. 1994;5:344–350. [PubMed] [Google Scholar]
- 31.Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nolan CL, Moore GJ, Madden R, Farchione T, Bartoi M, Lorch E, Stewart CM, Rosenberg DR. Prefrontal cortical volume in childhood-onset major depression: preliminary findings. Arch Gen Psychiatry. 2002;59:173–179. doi: 10.1001/archpsyc.59.2.173. [DOI] [PubMed] [Google Scholar]
- 33.Nosheny RL, Bachis A, Acquas E, Mocchetti I. Human immunodeficiency virus type 1 glycoprotein gp120 reduces the levels of brain-derived neurotrophic factor in vivo: potential implication for neuronal cell death. Eur J Neurosci. 2004;20:2857–2864. doi: 10.1111/j.1460-9568.2004.03764.x. [DOI] [PubMed] [Google Scholar]
- 34.Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perera TD, Coplan JD, Lisanby SH, Lipira CM, Arif M, Carpio C, Spitzer G, Santarelli L, Scharf B, Hen R, Rosoklija G, Sackeim HA, Dwork AJ. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J. Neurosci. 2007;27:4894–4901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrie RX, Reid IC, Stewart CA. The N-methyl-D-aspartate receptor, synaptic plasticity, and depressive disorder. A critical review. Pharmacol Ther. 2000;87:11–25. doi: 10.1016/s0163-7258(00)00063-2. [DOI] [PubMed] [Google Scholar]
- 37.Sanchez C, Gruca P, Papp M. R-citalopram counteracts the antidepressant-like effect of escitalopram in a rat chronic mild stress model. Behav Pharmacol. 2003;14:465–470. doi: 10.1097/01.fbp.0000087733.21047.60. [DOI] [PubMed] [Google Scholar]
- 38.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 39.Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- 40.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 41.Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]