Abstract
A 190-base-pair DNA-RNA hybrid containing the Moloney murine leukemia virus origin of plus-strand DNA synthesis was constructed and used as a source of template-primer for the reverse transcriptase in vitro. Synthesis was shown to initiate precisely at the known plus-strand origin. The observation that some of the origin fragments retained ribonucleotide residues on their 5' ends suggests that the primer for chain initiation is an RNA molecule left behind by RNase H during the degradation of the RNA moiety of the DNA-RNA hybrid. If the RNase H is responsible for creating the correct primer terminus, then it must possess a specific endonucleolytic activity capable of recognizing the sequence in the RNA where plus strands are initiated. The 16-base RNase A-resistant fragment which spans the plus-strand origin can also serve as a source of the specific plus-strand primer RNA. Evidence is presented that some of the plus-strand origin fragments synthesized in the endogenous reaction contain 5' ribonucleotides, suggesting that specific RNA primers for plus-strand initiation may be generated during reverse transcription in vivo as well.
Full text
PDF

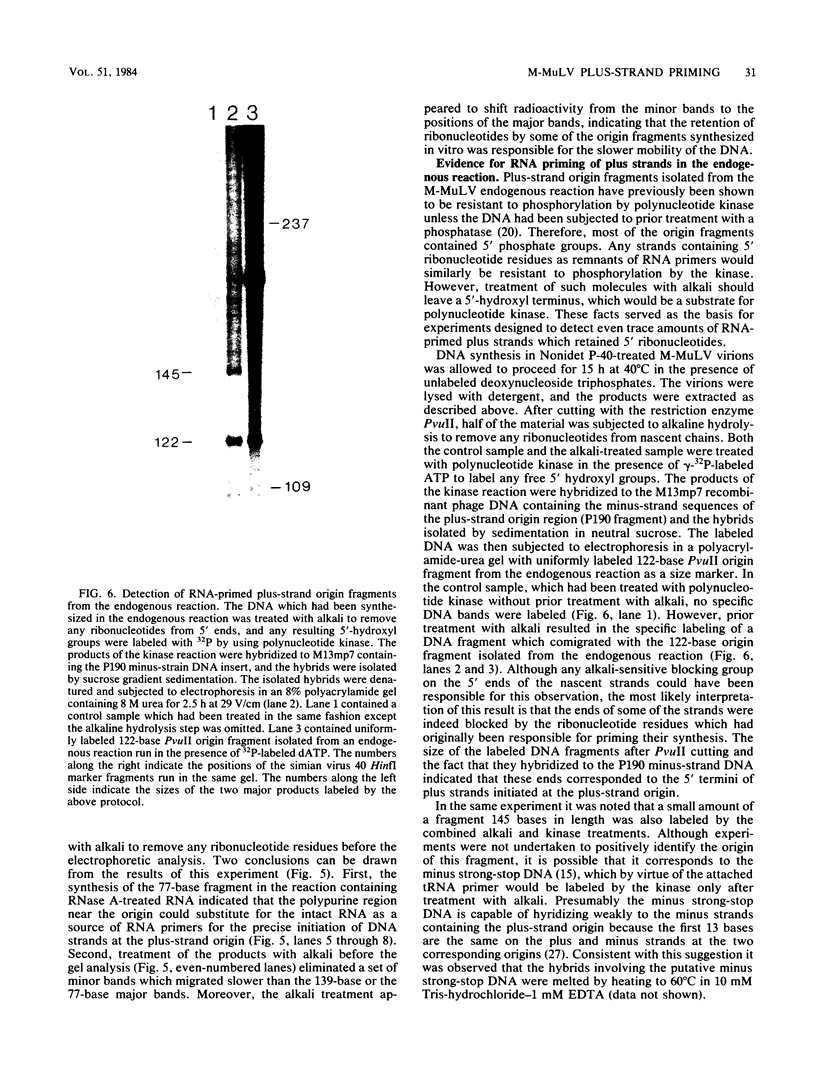


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Baltimore D., Smoler D. F. Association of an endoribonuclease with the avian myeloblastosis virus deoxyribonucleic acid polymerase. J Biol Chem. 1972 Nov 25;247(22):7282–7287. [PubMed] [Google Scholar]
- Baltimore D., Smoler D. Primer requirement and template specificity of the DNA polymerase of RNA tumor viruses. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1507–1511. doi: 10.1073/pnas.68.7.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Been M. D., Champoux J. J. Cutting of M13mp7 phage DNA and excision of cloned single-stranded sequences by restriction endonucleases. Methods Enzymol. 1983;101:90–98. doi: 10.1016/0076-6879(83)01007-1. [DOI] [PubMed] [Google Scholar]
- Champoux J. J., Gilboa E., Baltimore D. Mechanism of RNA primer removal by the RNase H activity of avian myeloblastosis virus reverse transcriptase. J Virol. 1984 Mar;49(3):686–691. doi: 10.1128/jvi.49.3.686-691.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champoux J. J., McConaughy B. L. Priming of superhelical SV40 DNA by Escherichia coli RNA polymerase for in vitro DNA synthesis. Biochemistry. 1975 Jan 28;14(2):307–316. doi: 10.1021/bi00673a017. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Dierks P., Parsons J. T., Faras A. J. RNase H hydrolysis of the 5' terminus of the avian sarcoma virus genome during reverse transcription. Nature. 1978 Mar 9;272(5649):181–184. doi: 10.1038/272181a0. [DOI] [PubMed] [Google Scholar]
- Darlix J. L., Bromley P. A., Spahr P. F. Extensive in vitro transcription of rous sarcoma virus RNA by avian myeloblastosis virus DNA polymerase and concurrent activation of the associated RNase H. J Virol. 1977 Sep;23(3):659–668. doi: 10.1128/jvi.23.3.659-668.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich R., Moelling K. Effect of viral RNase H on the avian sarcoma viral genome during early transcription in vitro. J Virol. 1979 Sep;31(3):630–638. doi: 10.1128/jvi.31.3.630-638.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard G. F. Mechanism of action of Moloney murine leukemia virus RNA-directed DNA polymerase associated RNase H (RNase H I). Biochemistry. 1981 Jan 20;20(2):256–265. doi: 10.1021/bi00505a005. [DOI] [PubMed] [Google Scholar]
- Gilboa E., Goff S., Shields A., Yoshimura F., Mitra S., Baltimore D. In vitro synthesis of a 9 kbp terminally redundant DNA carrying the infectivity of Moloney murine leukemia virus. Cell. 1979 Apr;16(4):863–874. doi: 10.1016/0092-8674(79)90101-6. [DOI] [PubMed] [Google Scholar]
- Gilboa E., Mitra S. W., Goff S., Baltimore D. A detailed model of reverse transcription and tests of crucial aspects. Cell. 1979 Sep;18(1):93–100. doi: 10.1016/0092-8674(79)90357-x. [DOI] [PubMed] [Google Scholar]
- Goulian M., Goulian S. H., Codd E. E., Blumenfield A. Z. Properties of oligodeoxynucleotides that determine priming activity with Escherichia coli deoxyribonucleic acid polymerase I. Biochemistry. 1973 Jul 17;12(15):2893–2901. doi: 10.1021/bi00739a019. [DOI] [PubMed] [Google Scholar]
- Grandgenett D. P., Gerard G. F., Green M. Ribonuclease H: a ubiquitous activity in virions of ribonucleic acid tumor viruses. J Virol. 1972 Dec;10(6):1136–1142. doi: 10.1128/jvi.10.6.1136-1142.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseltine W. A., Kleid D. G., Panet A., Rothenberg E., Baltimore D. Ordered transcription of RNA tumor virus genomes. J Mol Biol. 1976 Sep 5;106(1):109–131. doi: 10.1016/0022-2836(76)90303-x. [DOI] [PubMed] [Google Scholar]
- Kung H. J., Fung Y. K., Majors J. E., Bishop J. M., Varmus H. E. Synthesis of plus strands of retroviral DNA in cells infected with avian sarcoma virus and mouse mammary tumor virus. J Virol. 1981 Jan;37(1):127–138. doi: 10.1128/jvi.37.1.127-138.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mitra S. W., Chow M., Champoux J., Baltimore D. Synthesis of murine leukemia virus plus strong stop DNA initiates at a unique site. J Biol Chem. 1982 Jun 10;257(11):5983–5986. [PubMed] [Google Scholar]
- Mitra S. W., Goff S., Gilboa E., Baltimore D. Synthesis of a 600-nucleotide-long plus-strand DNA by virions of Moloney murine leukemia virus. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4355–4359. doi: 10.1073/pnas.76.9.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J. C., Dobkin C., Spiegelman S. RNA primer used in synthesis of anticomplementary DNA by reverse transcriptase of avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1316–1320. doi: 10.1073/pnas.77.3.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mölling K., Bolognesi D. P., Bauer H., Büsen W., Plassmann H. W., Hausen P. Association of viral reverse transcriptase with an enzyme degrading the RNA moiety of RNA-DNA hybrids. Nat New Biol. 1971 Dec 22;234(51):240–243. doi: 10.1038/newbio234240a0. [DOI] [PubMed] [Google Scholar]
- Olsen J. C., Watson K. F. Avian retrovirus RNA-directed DNA synthesis by purified reverse transcriptase. Covalent linkage of RNA to plus strand DNA. Biochem Biophys Res Commun. 1980 Dec 31;97(4):1376–1383. doi: 10.1016/s0006-291x(80)80019-2. [DOI] [PubMed] [Google Scholar]
- Olsen J. C., Watson K. F. Reverse transcription of avian myeloblastosis virus 35S RNA. Early synthesis of plus strand DNA of discrete size in reconstructed reactions. Nucleic Acids Res. 1982 Feb 11;10(3):1009–1027. doi: 10.1093/nar/10.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omer C. A., Faras A. J. Mechanism of release of the avian rotavirus tRNATrp primer molecule from viral DNA by ribonuclease H during reverse transcription. Cell. 1982 Oct;30(3):797–805. doi: 10.1016/0092-8674(82)90284-7. [DOI] [PubMed] [Google Scholar]
- Panganiban A. T., Temin H. M. The terminal nucleotides of retrovirus DNA are required for integration but not virus production. Nature. 1983 Nov 10;306(5939):155–160. doi: 10.1038/306155a0. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Shoemaker C., Goff S., Gilboa E., Paskind M., Mitra S. W., Baltimore D. Structure of a cloned circular Moloney murine leukemia virus DNA molecule containing an inverted segment: implications for retrovirus integration. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3932–3936. doi: 10.1073/pnas.77.7.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. K., Cywinski A., Taylor J. M. Initiation of plus-strand DNA synthesis during reverse transcription of an avian retrovirus genome. J Virol. 1984 Jan;49(1):200–204. doi: 10.1128/jvi.49.1.200-204.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge J., Hughes S. H. Polypurine tract adjacent to the U3 region of the Rous sarcoma virus genome provides a cis-acting function. J Virol. 1982 Aug;43(2):482–488. doi: 10.1128/jvi.43.2.482-488.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanstrom R., Varmus H. E., Bishop J. M. The terminal redundancy of the retrovirus genome facilitates chain elongation by reverse transcriptase. J Biol Chem. 1981 Feb 10;256(3):1115–1121. [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- Varmus H. E. Form and function of retroviral proviruses. Science. 1982 May 21;216(4548):812–820. doi: 10.1126/science.6177038. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Heasley S., Kung H. J., Oppermann H., Smith V. C., Bishop J. M., Shank P. R. Kinetics of synthesis, structure and purification of avian sarcoma virus-specific DNA made in the cytoplasm of acutely infected cells. J Mol Biol. 1978 Mar 25;120(1):55–82. doi: 10.1016/0022-2836(78)90295-4. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Baltimore D. Purification of the RNA-directed DNA polymerase from avian myeloblastosis virus and its assay with polynucleotide templates. Methods Enzymol. 1974;29:125–130. doi: 10.1016/0076-6879(74)29015-3. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Meuth N. L., Baltimore D. Covalent linkage between ribonucleic Acid primer and deoxyribonucleic Acid product of the avian myeloblastosis virus deoxyribonucleic Acid polymerase. J Virol. 1972 Oct;10(4):622–627. doi: 10.1128/jvi.10.4.622-627.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson K. F., Schendel P. L., Rosok M. J., Ramsey L. R. Model RNA-directed DNA synthesis by avian myeloblastosis virus DNA polymerase and its associated RNase H. Biochemistry. 1979 Jul 24;18(15):3210–3219. doi: 10.1021/bi00582a004. [DOI] [PubMed] [Google Scholar]