Abstract
Pain symptoms in several chronic pain disorders in women, including irritable bowel syndrome, fluctuate with the menstrual cycle suggesting a gonadal hormone component. In female rats, estrogens modulate visceral sensitivity although the underlying mechanism(s) are unknown. In the present study the effects of 17-β estradiol on N-methyl-D-aspartate (NMDA) receptor signaling of colorectal nociceptive processing in the spinal cord were examined. Estrogen receptor alpha and the NR1 subunit of the NMDA receptor are co-expressed in dorsal horn neurons, supporting a direct action of estradiol on NMDA receptors. Intrathecal administration of the NMDA receptor antagonist D(−)-2-amino-5-phosphonopetanoic acid (APV) dose-dependently attenuated the visceromotor response with greater potency in ovariectomized (OVx) rats compared to OVx with estradiol replacement (E2) rats. Estradiol significantly increased protein expression of NR1 in the lumbosacral spinal cord compared to OVx rats. Colorectal distention significantly increased phosphorylation of NR1ser-897, a PKA phosphorylation site on the NR1 subunit in E2, but not OVx rats. Intrathecal administration of a PKA inhibitor significantly attenuated the visceromotor response, decreased NR1 phosphorylation and increased the potency of APV to attenuate the visceromotor response compared to vehicle-treated E2 rats. These data suggest that estradiol increases spinal processing of visceral nociception by increasing NMDA receptor NR1 subunit expression and increasing site specific receptor phosphorylation on the NR1 subunit contributing to an increase in NMDA receptor activity.
Keywords: colorectal distention, visceromotor response, gonadal hormones, estrogen receptor, NMDA receptor, spinal cord, visceral nociception, PKA, estradiol
Introduction
Pain arising from visceral organs is one of the most common forms of pain in the clinical setting, and one of the most frequent reasons why patients seek medical attention (Cervero and Laird 1999). In general, women are more sensitive to pain than men and functional bowel disorders, such as irritable bowel syndrome (IBS) are 2–3 times more prevalent in women (Berkley 1997;Heitkemper and Jarrett 2001;Mayer et al. 2004). The severity of pain symptoms in women with IBS fluctuates with the menstrual cycle suggesting female gonadal hormone(s) modulate pain processing (Houghton et al. 2002;Palomba et al. 2005).
Estrogenic influences on visceral sensitivity have been reported in animal studies. Escape responses to vaginal and uterine distention change across different stages of the estrous cycle (Bradshaw et al. 1999). The threshold to evoke a visceromotor response or pressor response to colorectal distention (CRD) is lowest in proestrus (Sapsed-Byrne et al. 1996). Compared to intact rats, ovariectomy reduces the magnitude of the CRD-evoked visceromotor response and the response of dorsal horn neurons, which is reversed by estradiol replacement (Ji et al. 2003b;Ji et al. 2005). These data suggest estrogens play an important role in modulating visceral nociceptive processing although the underlying mechanisms are not well understood.
Studies suggest that NMDA receptors partially mediate acute or transient visceral pain in humans (Willert et al. 2004;Strigo et al. 2005) and visceral nociception in animals (Kolhekar and Gebhart 1994;Coutinho et al. 1996;Olivar and Laird 1999;McRoberts et al. 2001;Zhang et al. 2004). Systemic and intrathecal NMDA receptor antagonists attenuate CRD-induced spinal Fos expression, the visceromotor response and the response of dorsal horn neurons to noxious and innocuous CRD (Zhai and Traub 1999;Ji and Traub 2001;Traub et al. 2002). These data suggest the involvement of spinal NMDA receptors in the signaling of acute visceral stimuli.
Gonadal steroid hormones modulate NMDA receptor activity in several areas of the brain (Gazzaley et al. 1996;Woolley et al. 1997;El Bakri et al. 2004;Romeo et al. 2005). Sex hormones alter glutamatergic transmission in the brain by regulating expression of glutamate receptors (Diano et al. 1997;D'Souza et al. 2003;Romeo et al., 2005) or by post translational modification (Gazzaley et al., 1996). Estrogens increase NMDA receptor-mediated hypothalamic neuron excitability (Kow et al. 2005), Ca2+ influx through NMDA receptors in hippocampal cultures (Nilsen et al. 2002) and activate cAMP-response element-binding protein (CREB) signaling via an increase in intracellular Ca2+ (Zhao et al. 2005). Since there are some commonalities in mechanisms underlying NMDA receptor-mediated plasticity in the hippocampus and spinal cord, there may be hormonal modulation of NMDA receptor activity in the spinal cord (Ji et al. 2003a).
The purpose of the present study was to determine the underlying mechanism(s) for estradiol modulation of NMDA receptor-mediated colorectal nociceptive processing in the spinal cord. Some of these data were previously reported in abstract form (Tang et al. 2005;Traub et al. 2007).
Methods
Two groups of Sprague-Dawley rats were used in this study. One group was ovariectomized rats (235–275 g; OVx) purchased from Harlan (Frederick, MD). The second group was OVx rats given estradiol replacement (E2: 50 µg 17-β-estradiol -3 benzoate dissolved in 100 µl of safflower oil, given s.c. 48 hrs prior to testing). Rats were used between 10 and 20 days following OVx surgery. This time was sufficient to reduce the plasma estradiol concentration below 5 pg/ml (Ji et al., 2003b), but too short to result in OVx-induced hyperalgesia (Sanoja and Cervero 2005) or decreases in NMDA receptor expression (Adams et al. 2001).
Rats were double-housed in a room maintained at 25 °C under 12 h-12 h alternating light-dark cycle with free access to food and water. All experimental protocols were approved by the University of Maryland Dental School Institutional Animal Care and Use Committee and adhered to guidelines for experimental pain in animals published by the International Association for the Study of Pain.
Double immunofluorescent staining
Rats were overdosed with Nembutal and perfused through the heart with saline followed by 500 ml ice cold 4% paraformaldehyde in 0.1 M phosphate buffer. The lumbosacral (LS) spinal cord segments were removed, placed in fresh fixative overnight and transferred to 30% sucrose for 24–48 h. Twenty micron transverse sections were cut on a cryostat. Free floating sections were first incubated in Goat anti-NR1 (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA) followed by donkey-anti goat conjugated to Alexa fluor 594 (1:1,000, Molecular Probes, Carlsbad, CA). Sections were then incubated in rabbit-anti-ERα (1:1,000, Upstate Biotechnology, Lake Placid, NY) followed by donkey-anti-rabbit IgG conjugated to Alexa fluor 488 (1:1,000, Molecular Probes, Carlsbad, CA). Sections were mounted on gelatin subbed slides and coverslipped with Prolong Gold (Molecular Probes, Carlsbad, CA).
For semiquantitative analysis, sections were examined at 400× using a Nikon microscope equipped for epifluorescence and filter sets for simultaneous viewing of Alexa fluor 488 and 594 as well as filters for viewing each fluorophore individually.
Fluorescence images were taken as 25~30 optical sections with 0.4 or 1 µm (with 63 and 20X, respectively) thickness with a charge-coupled device based digital camera installed on an Axiovert200M microscope with a 63×, 1.3 NA lens or 20X, 0.8NA lens, and an Apotome coupled to Axiovision software (Zeiss). Images were reconstituted as a projected image by the software. JPEG images were opened in Adobe Photoshop CS 8.0 to merge multiple images into a montage.
Behavioral experiments
Rats were anesthetized with halothane and placed in a head holder, the atlanto-occipital membrane was exposed and an intrathecal (i.t.) catheter (PE32 tubing, ReCathCo, Allison Park, PA) was inserted 7.8 cm so the tip was at the level of the lumbosacral spinal segments. Electromyogram (EMG) electrodes made from Teflon-coated 32 gauge stainless steel wire (Cooner Wire Company, Chatsworth, CA) were stitched into the ventrolateral abdominal wall. The electrode leads were tunneled subcutaneously and exteriorized with the intrathecal catheter at the back of the neck (Traub et al., 2002). After the surgery, rats were individually housed and tested 5–7 days later. Rats were fasted for 18–24 hrs prior to testing. Water was available ad libitum.
On the day of the experiment, rats were briefly sedated with halothane in order to place a 5–6 cm latex balloon attached to Tygon tubing through the anus into the descending colon and rectum. The secured end of the balloon was at least 1 cm proximal to the external anal sphincter. Rats were then loosely restrained in Plexiglas tubes with free access to water. Isobaric colorectal distention was maintained by a pressure controller–timing device (Bioengineering, University of Iowa, Iowa City, IA). Starting 30 min after discontinuing the halothane, rats were given 4 trials of colorectal distention (each trial was 6 distentions to 80 mmHg, 20 sec duration, 3 min interstimulus interval) to achieve a stable response. The mean of the last two trials constituted the baseline response to CRD. After establishing the baseline visceromotor response, D(−)-2-amino-5-phosphonopetanoic acid (APV; Sigma, 0.1, 1, 10, 30, 100 nmol, dissolved in normal saline, pH 7.2) was intrathecally injected (10 µl) followed by a 5 µl saline flush. Rats were tested 15, 45, 90 and 150 min after drug administration. Only one dose of APV was given per animal (n = 4–8 per group).
The EMG recordings were collected with a CED 1401 plus and analyzed using Spike 2 for Windows software (Cambridge Electronic Design, Cambridge, UK). The EMG was rectified, and the area under the curve (AUC) for the 20 sec before distention was subtracted from the AUC during the 20 sec distention. The baseline response between groups differed necessitating converting values to percent inhibition in order to compare the effects of APV between experimental groups. The % inhibition of APV was obtained according to the formula: % inhibition = [post drug AUC-baseline AUC] / [0-baseline AUC] × 100. The data are expressed as mean ± SEM.
Data were analyzed in Sigmastat 3.0 using t-test, two-way ANOVA, or one or two way ANOVA for repeated measures as appropriate. If an ANOVA term was significant, multiple comparisons were performed using a Student-Newman-Kuels, p<0.05 was considered significant.
The PKA inhibitors Rp-8-Br-cAMP (Calbiochem, 200 nmol dissolved in saline (Miletic et al. 2004;Yang et al. 2004)), H89 (Calbiochem, 10 nmol, dissolved in 10% DMSO; (Wu et al. 2007)) or the PKC inhibitor chelerythrine chloride (Sigma, 30 nmol dissolved in saline; (Jones and Sorkin 2005)) were injected intrathecally (10 µl) followed by a 5 µl saline flush. Control rats were injected with the corresponding vehicle. The visceromotor response to CRD (80 mmHg, 30 sec duration, 90 sec interstimulus interval) was recorded for 30 min commencing 10 min after intrathecal injection (n = 6–8 per group). On completion of the last distention, the rats were overdosed with Nembutal and spinal cord removed for Western blot analysis.
Western blots
Rats were briefly sedated with halothane to insert the distention balloon into the descending colon and rectum and then loosely restrained in tubes. After 30 minutes of recovery, rats either stayed in the tube (control) or were distended for another 30 minutes (80 mmHg, 30 sec duration, 90 sec interstimulus interval) (n = 4–5 per group). Immediately after that, rats were overdosed with Nembutal (100 mg/kg) and decapitated. The spinal cord was removed by hydraulic extrusion from the spinal canal with 10 ml ice cold saline. The L6 to S2 region of the spinal cord, identified by the lumbar enlargement, was isolated. The spinal cord was cut into dorsal and ventral halves through the central canal. The dorsal spinal cord tissue was snap frozen and kept at −80 °C until use. The tissue was sonicated in RIPA buffer containing (50 mM Tris-HCl, pH 8.0; 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.5% deoxycholic acid, 0.1% SDS, 1 mM Na3VO4, 1 U/ml aprotinin, 20 µg/ml leupetin, and 20 µg/ml pepstatin A). The homogenate was centrifuged at 14,000 g for 10 minutes at 4 °C and the supernatant was collected. The protein concentration was measured using the Bradford method. Protein samples were loaded 25–40 µg per lane and separated on a 7.5% SDS-PAGE gel and transferred to a nitrocellulose membrane. After blocking with 5% bovine serum albumin (BSA) or skim milk in Tris-buffered saline with Tween-20 (TBST) for 1 hour, the membrane was incubated with primary antibody for p-NR1 897, p-NR1 896 (1:1000, Upstate Biotechnology, Lake Placid, NY), or NR1 (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA) and β-actin (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA) at 4 °C overnight. The membrane was then washed with TBST for 30 minutes and incubated for 1 hr with a HRP conjugated secondary antibody (1:3000, Santa Cruz Biotechnology, Santa Cruz, CA). The membrane was washed with TBST for 30 minutes before the bands were viewed using HRP chemiluminescence and film. Band density was determined using ImageJ software (NIH). The blots probed with pNR1 antibody were further incubated in stripping buffer (Pierce Biotechnology, Inc. IL) for 30 min at 50 °C and reprobed with anti-NR1 (1:1000, Cell Signalling technology, Danvers, MA) antibody.
Results
Colocalization of ERα with NMDA receptor in the spinal cord
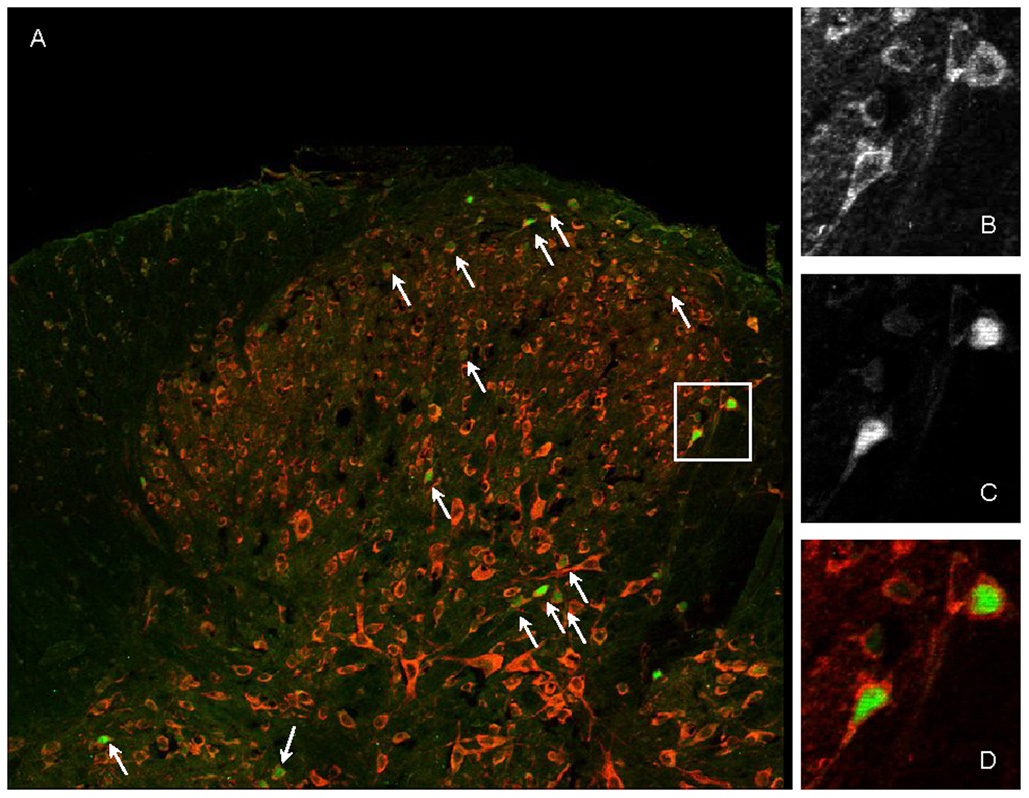
To determine whether there is an anatomical basis for direct modulation of spinal NMDA receptors by estrogens, we used double immunofluorescent labeling to detect ERα and the NR1 subunit of the NMDA receptor in dorsal horn neurons in the LS spinal cord. NR1 was extensively expressed in the dorsal and ventral spinal cord (Figure 1). Consistent with previous reports (Amandusson et al. 1995;Williams and Papka 1996) ERα immunoreactive nuclei were most densely observed in the superficial dorsal horn, the neck of the dorsal horn including the sacral parasympathetic nucleus, lamina X and the dorsal gray commissure. Several hundred ERα immunolabeled neurons in sections from 2 OVx and 2 E2 treated rats were examined at 400×. Most ERα labeled neurons had very clear nuclei labeled for ERα with label for NR1 in the surrounding cytoplasm (see Figure 1). In instances where double labeling was not apparent with the dual filter (mainly lamina II where neurons have large nuclei relative to the cell body), the neuron was examined with filters for the individual fluorophores. In every case where there was a nucleus labeled for ERα there was surrounding cytoplasm labeled for NR1. These data indicate that estrogen receptor α is 100% colocalized with NR1 (although many NR1 labeled cells did not colocalize ERα) and that ERα is anatomically located to directly modulate NMDA receptor activity in spinal dorsal horn neurons.
Figure 1. Colocalization of ERα and NR1 in the dorsal spinal cord.
A: Low power photomontage (4 images using a 20× objective) showing double labeled cells (arrows) in the dorsal horn. NR1 is labeled red, ERα is labeled green. B,C,D: High power (60× objective) images of the cells in the box in A. B: NR1; C: ERα; D: merged image.
Estradiol modulates NMDA receptor activity
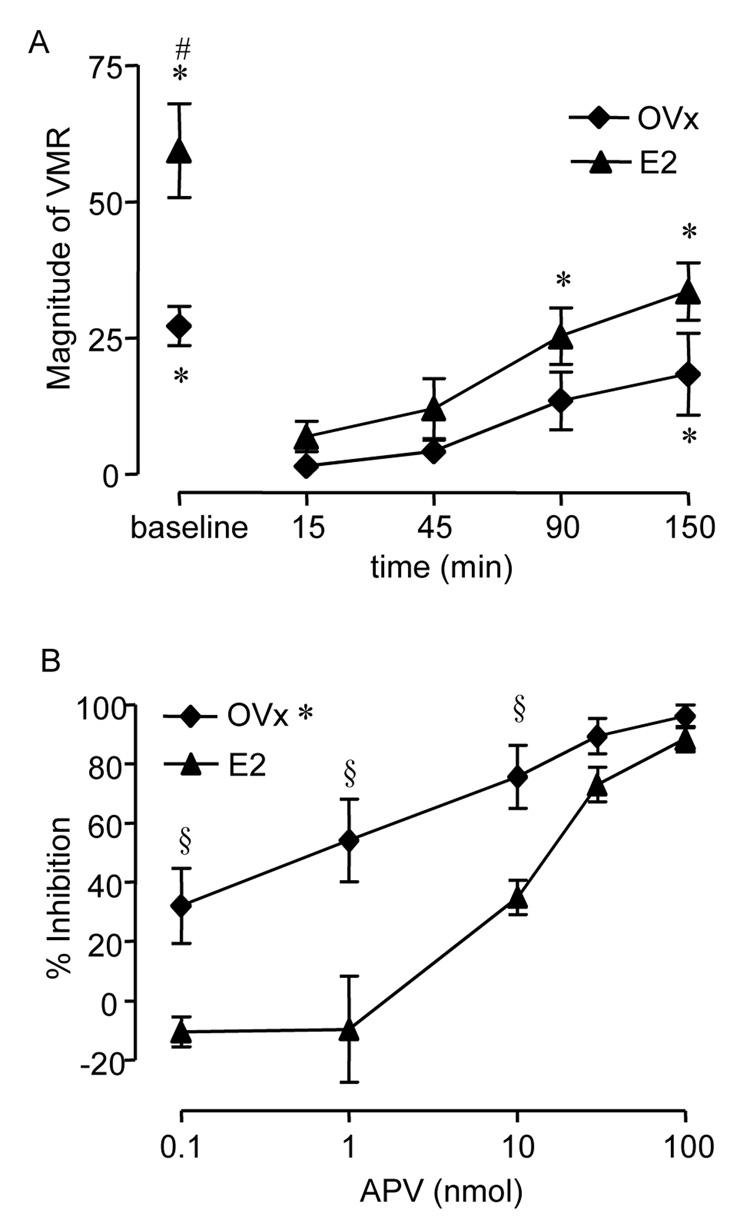
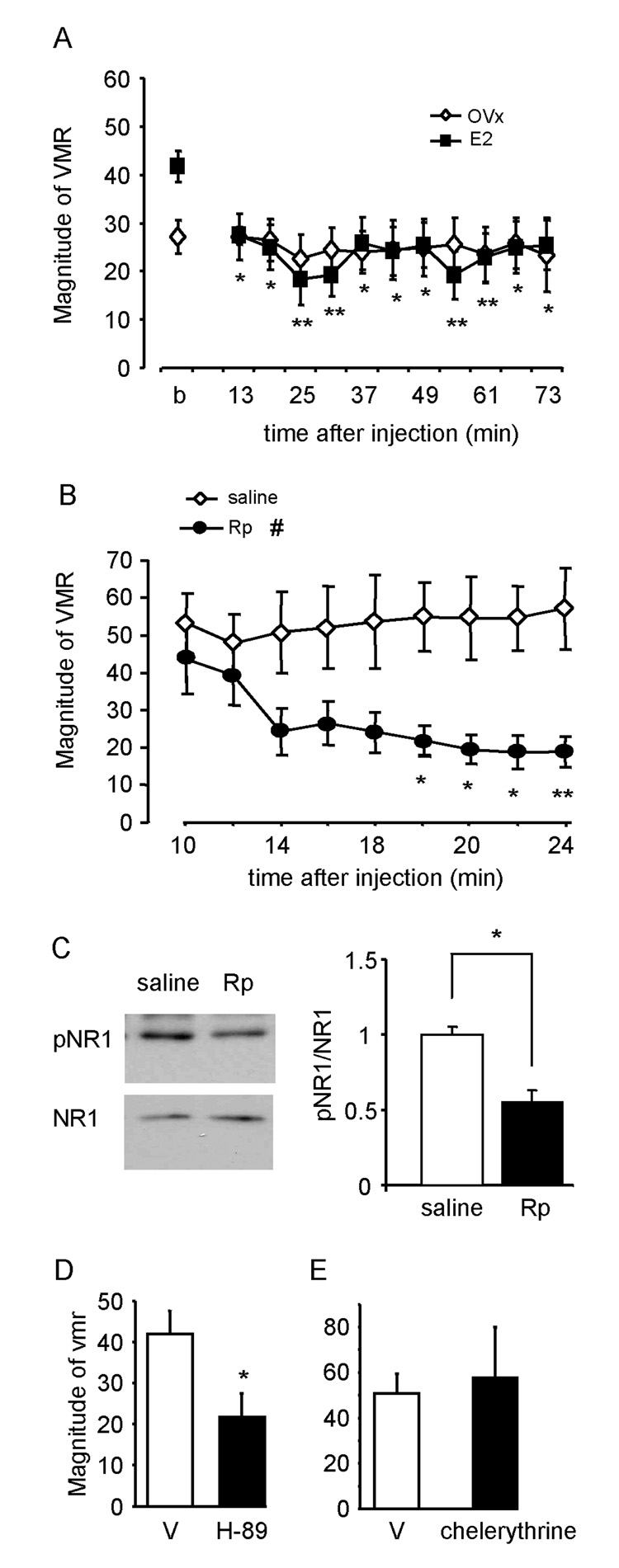
Our previous reports that OVx decreases the magnitude of the visceromotor response and estradiol replacement restores the response to levels observed in intact female rats (Ji et al., 2003b;Ji et al., 2005) along with the present observation that estrogen receptor α colocalizes with NMDA receptors in dorsal horn neurons suggest that estradiol can modulate the visceromotor response to CRD by altering NMDA receptor activity. To test this hypothesis, the effects of intrathecal injection of APV on the visceromotor response to CRD were compared in OVx and E2 rats. The baseline response to noxious CRD was significantly greater in E2 rats compared to OVx rats (t-test, p<0.01; Figure 2A). The peak inhibition of the visceromotor response by APV occurred 15 min after drug administration in both groups (ANOVA, p<0.005; Figure 2A). Since the baseline response to CRD was different between the two groups, the data were transformed to % inhibition to compare the effects of APV at 15 min post drug administration. There was no effect of 0.1 nmol APV on the visceromotor response in E2 rats, but it produced approximately 30% inhibition of the visceromotor response in the OVx rats (Figure 2 B). Increasing doses of APV dose-dependently attenuated the visceromotor response to CRD in both groups with greater potency of APV at lower doses in OVx rats (Two-way ANOVA: p<0.001; Interaction p<0.05; Figure 2 B). The visceromotor response was virtually eliminated by 100 nmol APV in both groups.
Figure 2. The effect of intrathecal APV on the visceromotor response (VMR) to CRD in OVx and E2 rats.
A: Time course of attenuation of the visceromotor response following 100 nmol APV. The peak effect occurred starting 15 min following APV administration. # p<0.01 vs. OVx (baseline). * p<0.001 vs. 15 min post APV for each treatment group. B: % inhibition of the visceromotor response as a function of dose of APV. * p<0.001 vs. E2. § p<0.01 vs. same dose in E2 rats.
Estradiol modulation of the NR1 subunit of the NMDA receptor
We tested two mechanisms through which estradiol could modulate NMDA receptor activity: changing NMDA receptor expression and modulating NMDA receptor phosphorylation.
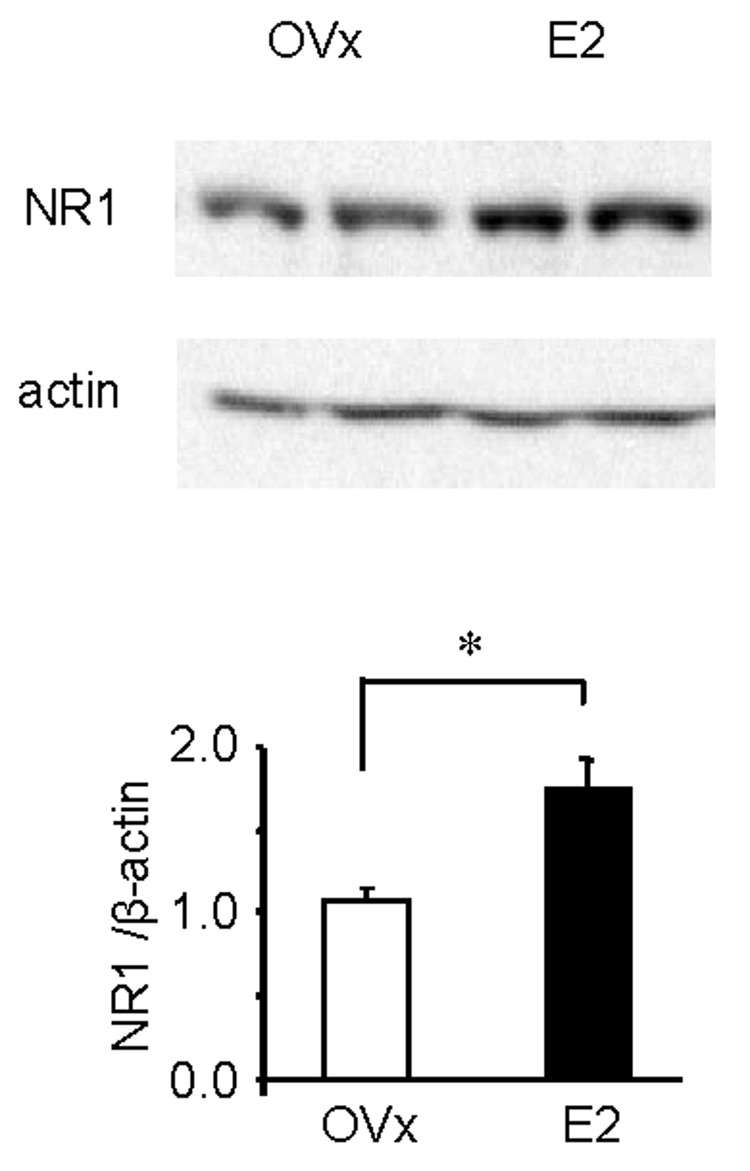
First, the expression of the NR1 subunit of the NMDA receptor in the LS spinal cord in OVx and E2 rats was compared. Estradiol increased NR1 protein expression by 62% compared to OVx rats (p<0.001; Figure 3).
Figure 3. Total NR1 protein expression in estradiol treated rats is significantly greater than OVx rats.
A: representative Western blot showing the NR1 band from two OVx and two E2 rats. β-actin was used as the loading control. B: quantified Western blot data. Values represent the NR1/actin ratio normalized to OVx rats. * p<0.001.
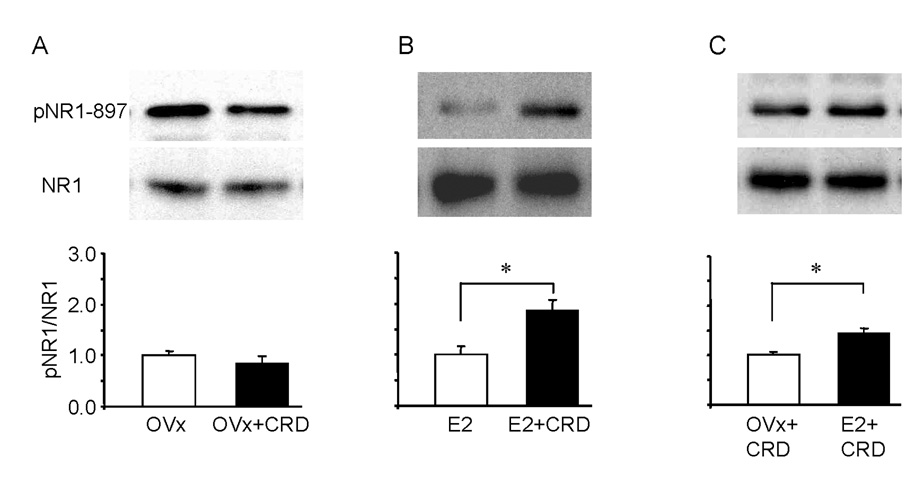
Phosphorylation of two serine residue sites, 896 and 897, on the C-terminal of NR1 are increased in the spinal cord after noxious stimulation on the hindpaw (Zou et al. 2000;Brenner et al. 2004). To determine whether noxious CRD could induce changes in the level of NR1 subunit phosphorylation (pNR1) in LS spinal cord and whether there is a differential expression of pNR1 in OVx and E2 treated animals, rats received colorectal distention or rats were restricted without colonic distention for 30 minutes before the spinal cord was taken for Western blot analysis. Following noxious CRD, pNR1-ser897 was significantly increased in E2 (t-test, p<0.01), but not OVx rats compared with their non-distended controls (Figure 4A, B), resulting in significantly greater levels of pNR1 in E2 rats (t-test, p<0.001; Figure 4C). In both groups the total NR1 level was not affected by distention. CRD did not induce a change in the level of phosphorylation of the ser-896 in either group (data not shown). These data suggest that estradiol mediated phosphorylation of NR1-ser897 could account for the difference in NMDA receptor mediated visceral sensitivity in E2 rats compared to OVx rats.
Figure 4.
Phosphorylation of NR1 ser-897 (pNR1) is increased in the LS spinal dorsal horn in E2, but not OVx rats, following CRD (A,B). Representative Western blots are shown in the upper panels and quantified results in the lower panels. Values represent the pNR1/NR1 ratio normalized to nondistended rats. * p<0.01. C: NR1 ser-897 phosphorylation was significantly greater in E2 rats than OVx rats following CRD. Values represent the pNR1/NR1 ratio normalized to OVx + CRD rats. NR1 was used as the loading control in all groups. * p<0.001.
PKA but not PKC inhibitors attenuate the effect of estradiol
Phosphorylation of the ser-896 and 897 residues of the NR1 subunit are catalyzed by PKC and PKA, respectively (Tingley et al. 1997). Results from the Western blot experiments indicate that the phosphorylation of NR1-ser897, but not NR1-ser896, is significantly increased after CRD in E2, but not OVx rats. To further determine the role of PKA in estradiol modulation of NMDA receptor-mediated colorectal nociceptive processing in the spinal cord, the PKA inhibitor, Rp-8-Br-cAMP (200 nmol), was injected into the LS spinal cord in E2 or OVx rats. After establishing a stable baseline response, Rp-8-Br-cAMP was intrathecally injected and the visceromotor response to CRD was recorded 10 minutes later. Rp-8-Br-cAMP caused a significant attenuation of the visceromotor response in E2 rats commencing 13 minutes after the injection (Figure 5A; One way RM ANOVA, p<0.001). The response remained constant during the next 60 minutes. By 76 minutes after Rp-8-Br-cAMP, the visceromotor response returned towards the baseline level. Rp-8-Br-cAMP had no significant effect in OVx rats over time (Figure 5A; One way RM ANOVA, p=0.9). Overall, Rp-8-Br-cAMP induced significantly greater inhibition of the visceromotor response in E2 rats than that of OVx rats (51% vs. 13%, t-test, p<0.05).
Figure 5. Intrathecally administered PKA inhibitors attenuated the effect of estradiol.
A: the time course of the effect of intrathecally injected Rp-8-Br-cAMP (200 nmol) on the visceromotor response (VMR) to CRD in OVx and E2 rats. b: baseline. * p<0.05, **p<0.01 vs. baseline response in E2 rats. B: the visceromotor response to CRD was atttenuated by Rp-8-Br-cAMP (Rp), but not vehicle in E2 rats. # p<0.05 vs. saline treated group. * p<0.05, ** p<0.01 vs. same time point in saline group. C: representative Western blots (left) and quantification of Western blot data (right) showing Rp-8-Br-cAMP attenuated the phosphorylation of NR1 ser-897 in the rats shown in B. Values represent the pNR1/NR1 ratio normalized to saline treated rats. * p<0.01. D, E: the peak attenuation of the visceromotor response by intrathecal H-89 (D) or chelerythrine chloride (E) compared to the appropriate vehicle (V). * p<0.05 vs. vehicle.
In a separate group of E2 rats, the visceromotor response was recorded starting 10 minutes after the intrathecal injection of Rp-8-Br-cAMP or vehicle. Thirty minutes later, the LS spinal cord was taken for Western blots. As expected, the visceromotor response was significantly attenuated by Rp-8-Br-cAMP (Figure 5B). Western blots from tissue taken from these animals show Rp-8-Br-cAMP decreased the phosphorylation of NR1-ser897 compared with vehicle treated rats (Figure 5C). Intrathecal injection of the PKA inhibitor H89 (10 nmol) also significantly attenuated the visceromotor response compared with vehicle-treated E2 rats confirming the role of PKA in visceral nociceptive processing in E2 rats. The PKC inhibitor, chelerythrine choride (30 nmol), had no effect on the visceromotor response compared to vehicle treated rats (Figure 5 E).
PKA inhibitor potentiates the effect of APV in E2 rats
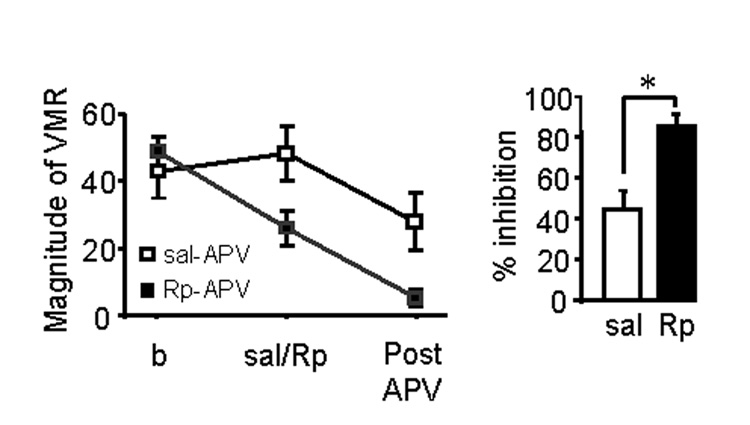
Behavioral and Western blot data implicate estradiol-mediated phosphorylation of NMDA receptors as a mechanism to modulate colorectal nociceptive processing in the spinal cord. To further confirm this hypothesis, the effect of the PKA inhibitor Rp-8-Br-cAMP on the potency of APV was examined in E2 rats. Rp-8-Br-cAMP (200 nmol) or saline was intrathecally injected 20 minutes prior to intrathecal injection of APV (10 nmol) in E2 rats. In saline pretreated E2 rats, APV attenuated the visceromotor response by 44%. In the Rp-8-Br-cAMP pretreated E2 rats, APV was significantly more effective, attenuating the visceromotor response by 85% (Figure 6. t-test, p<0.01). The shift in the effect of APV induced by inhibiting PKA activity further supports our hypothesis that estradiol increases sensitivity to visceral stimuli by modulating the activity of NMDA receptors via PKA mediated phosphorylation.
Figure 6. Intrathecally injected Rp-8-Br-cAMP (Rp) potentiates the effect of APV in E2 rats.
A: the time course of the effect of intrathecally injected Rp-8-Br-cAMP (200 nmol) on the visceromotor response to CRD in E2 rats. * p<0.05 vs. 10 minutes. B: the magnitude of the visceromotor response before any intrathecal drug injection (b), following pretreatment with saline or Rp-8-Br-cAMP (Sal/Rp) and post APV. C: The percent inhibition of the visceromotor response by intrathecally injected APV in saline or Rp-8-Br-cAMP pretreated rats. * p<0.01.
Discussion
The present study demonstrates that: 1) There is a colocalization of ERα with NMDA receptors in LS spinal dorsal horn neurons; 2) Spinal NMDA receptor activity is modulated by estradiol; 3) An increase in the expression of NMDA receptors and CRD-evoked phosphorylation of the NR1 subunit of the NMDA receptor by PKA in estradiol treated rats contributes to the differential activity of spinal NMDA receptors in modulating visceral nociceptive processing.
We have previously reported that the magnitude of the visceromotor response (a suprathreshold response) does not fluctuate with the estrous cycle (Ji et al. 2006), although the threshold to evoke the visceromotor response does fluctuate across the estrous cycle (Sapsed-Byrne et al., 1996;Holdcroft et al. 2000). These data and our previous reports that E2 replacement reverses the antinociceptive effect of ovariectomy (Ji et al., 2003b;Ji et al., 2005), suggests female gonadal hormones modulate visceral nociceptive processing. The contradictory findings in intact cycling rats indicate that other factors in addition to estrogens contribute to nociceptive processing in intact female rats. In fact, we and others have reported antinociceptive effects of progesterone (Ren et al. 2000;Ji et al., 2005). Since the increase in estradiol and progesterone coincide during the rat estrous cycle, the pronociceptive effect of estradiol and the antinociceptive effect of progesterone in the CRD model may obscure each other reducing fluctuations during the course of the estrous cycle. Therefore, in the present study, only OVx and OVx plus E2 rats were examined to determine potential mechanisms underlying the pronociceptive effect of estradiol.
A growing body of evidence suggests gonadal hormones (especially estrogens) play a critical role in modulating pain sensitivity, but the exact site of action is not clear. The present data support estradiol modulation of NMDA receptor activity in the spinal dorsal horn as a mechanism for modulation of visceral nociception in females. Although estradiol was administered systemically in the present study, intrathecal E2 facilitates the visceromotor response in OVx rats to a similar degree as systemic E2 supporting a spinal site of action (Tang et al. 2006).
Spinal NMDA receptors contribute to the processing of somatic (Woolf and Thompson 1991;Ren et al. 1992) and visceral (Kolhekar and Gebhart 1994;Coutinho et al., 1996;Zhai and Traub 1999;Olivar and Laird 1999;Ji and Traub 2001;McRoberts et al., 2001;Traub et al., 2002;Zhang et al., 2004) nociceptive stimuli. The activity of NMDA receptors can be modulated by several physiological/pathophysiological factors. For example, inflammation or nerve injury in somatic tissue increases NMDA receptor subunit phosphorylation, expression and pain-related behaviors (Zou et al., 2000;Guo et al. 2004;Gao et al. 2005;Iwata et al. 2007). Recent studies in some brain areas suggest the activity of NMDA receptors can also be modulated by gonadal hormones. Estradiol increases NMDA receptor-mediated excitatory postsynaptic potentials and long-term potentiation in the hippocampus (Foy et al. 1999). In the CA1 region of the hippocampus, estradiol increases NMDA receptor binding in gonadectomized females, but not in gonadectomized males, which is possibly caused by a greater number of ERα-labeled dendritic spines in females than males (Romeo et al., 2005). ERα colocalizes with NMDA receptors in hypothalamic neurons suggesting a direct effect of estradiol on NMDA receptors (Chakraborty et al. 2003). In the present study we detected colocalization of ERα and NMDA receptors in spinal dorsal horn neurons in the superficial dorsal horn, deeper laminae and lamina X, providing an anatomical framework for estradiol to modulate NMDA receptors in areas of the spinal cord where neurons respond to noxious visceral stimulation.
The mechanism through which estradiol modulates NMDA receptor activity is not well understood. One hypothesis is estradiol upregulates NMDA receptor expression increasing total receptor activity. In the present study, we observed estradiol replacement increased the total amount of NR1 protein expressed in LS spinal cord. This increase is consistent with findings in the CA1 and dentate gyrus that immunofluorescence intensity of NR1 was significantly increased by estradiol replacement in OVx rats (Gazzaley et al., 1996). Although no change in NR1 mRNA levels was observed by the same investigative group, a later report showed an increase in NR1 and NR2B mRNA in the hippocampal CA1 region with estradiol replacement (Cyr et al. 2001). Since the NR1 subunit is an obligatory component of NMDA receptors, the greater expression of NR1 in E2 rats may reflect an increase in the number of NMDA receptors that contribute to increased receptor binding in E2 treated rats (Romeo et al., 2005). This increase in the number of receptors could lead to attenuation of the potency of NMDA receptor antagonists as observed in the present study.
Alternatively, estradiol could increase NMDA receptor phosphorylation increasing receptor activity. Phosphorylation of the NR1 subunit increases NMDA receptor trafficking into synapses (Scott et al. 2003) and neuronal activity by increasing Ca2+ influx (Lan et al. 2001;Bird et al. 2005). In hippocampal and hypothalamic neurons, estradiol increases NMDA receptor current and neuronal excitability through phosphorylation of the NR1 and NR2 subunits (Bi et al. 2000;Kow et al., 2005). This phosphorylation can occur through estradiol mediated increases in PKC and PKA activity and modulation of NMDA receptor binding (Qiu et al. 2003;Romeo et al., 2005). Consistent with this hypothesis we observed an increase in phosphorylation of ser-897 of the NR1 subunit in E2, but not OVx rats after visceral stimulation. Intrathecal administration of PKA inhibitors in E2 rats significantly attenuated the phosphorylation of NR1, decreased the magnitude of the visceromotor response and increased the potency of APV. These data support our hypothesis that estradiol increases responses to nociceptive visceral stimuli by modulating NMDA receptor activity.
An alternative, though not mutually exclusive hypothesis that remains to be tested is that spinal estradiol increases glutamate release from primary afferent terminals in the spinal cord. Estrogen receptors are expressed in primary afferent neurons (Papka et al. 1997;Taleghany et al. 1999) as are NMDA receptors (Liu et al. 1994;Carlton et al. 1998;McRoberts et al., 2001;Chaban et al. 2004) and estradiol modulates primary afferent excitability (Robbins et al. 1992;Chaban et al. 2003;Liu et al. 2005;Hucho et al. 2006;McRoberts et al. 2007). Colonic inflammation increases NMDA receptor activity in colonic afferents (Li et al. 2006), but it is unknown if estradiol mediates this increase in primary afferent NMDA receptor activity.
In conclusion, the present study demonstrates that estradiol increases spinal colorectal nociceptive processing by modulating NMDA receptor activity. Possible mechanisms include increasing the expression of NMDA receptors and changing the level of subunit phosphorylation.
Acknowledgments
Thanks to Dr. Hiroaki Misono for help with the photomicroscopy, and Janet O’Brien and Sangeeta Pandya for technical assistance. Supported by R01 NS 37424.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Adams MM, Oung T, Morrison JH, Gore AC. Length of postovariectomy interval and age, but not estrogen replacement, regulate N-methyl-D-aspartate receptor mRNA levels in the hippocampus of female rats. Exp.Neurol. 2001;170:345–356. doi: 10.1006/exnr.2001.7716. [DOI] [PubMed] [Google Scholar]
- Amandusson A, Hermanson O, Blomqvist A. Estrogen receptor-like immunoreactivity in the medullary and spinal dorsal horn of the female rat. Neurosci.Lett. 1995;196:25–28. doi: 10.1016/0304-3940(95)11828-k. [DOI] [PubMed] [Google Scholar]
- Berkley KJ. Sex differences in pain. Behav.Brain Sci. 1997;20:371–380. doi: 10.1017/s0140525x97221485. [DOI] [PubMed] [Google Scholar]
- Bi R, Broutman G, Foy MR, Thompson RF, Baudry M. The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus. Proc.Natl.Acad.Sci.U.S.A. 2000;97:3602–3607. doi: 10.1073/pnas.060034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird GC, Lash LL, Han JS, Zou X, Willis WD, Neugebauer V. Protein kinase A-dependent enhanced NMDA receptor function in pain-related synaptic plasticity in rat amygdala neurones. J Physiol. 2005;564:907–921. doi: 10.1113/jphysiol.2005.084780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw HB, Temple JL, Wood E, Berkley KJ. Estrous variations in behavioral responses to vaginal and uterine distention in the rat. Pain. 1999;82:187–197. doi: 10.1016/S0304-3959(99)00049-4. [DOI] [PubMed] [Google Scholar]
- Brenner GJ, Ji RR, Shaffer S, Woolf CJ. Peripheral noxious stimulation induces phosphorylation of the NMDA receptor NR1 subunit at the PKC-dependent site, serine-896, in spinal cord dorsal horn neurons. Eur.J.Neurosci. 2004;20:375–384. doi: 10.1111/j.1460-9568.2004.03506.x. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Zhou ST, Coggeshall RE. Evidence for the interaction of glutamate and NK1 receptors in the periphery. Brain Res. 1998;790:160–169. doi: 10.1016/s0006-8993(97)01471-6. [DOI] [PubMed] [Google Scholar]
- Cervero F, Laird JM. Visceral pain. Lancet. 1999;353:2145–2148. doi: 10.1016/S0140-6736(99)01306-9. [DOI] [PubMed] [Google Scholar]
- Chaban VV, Li J, Ennes HS, Nie J, Mayer EA, McRoberts JA. N-methyl-D-aspartate receptors enhance mechanical responses and voltage-dependent Ca2+ channels in rat dorsal root ganglia neurons through protein kinase C. Neuroscience. 2004;128:347–357. doi: 10.1016/j.neuroscience.2004.06.051. [DOI] [PubMed] [Google Scholar]
- Chaban VV, Mayer EA, Ennes HS, Micevych PE. Estradiol inhibits atp-induced intracellular calcium concentration increase in dorsal root ganglia neurons. Neuroscience. 2003;118:941–948. doi: 10.1016/s0306-4522(02)00915-6. [DOI] [PubMed] [Google Scholar]
- Chakraborty TR, Ng L, Gore AC. Colocalization and hormone regulation of estrogen receptor alpha and N-methyl-D-aspartate receptor in the hypothalamus of female rats. Endocrinology. 2003;144:299–305. doi: 10.1210/en.2002-220749. [DOI] [PubMed] [Google Scholar]
- Coutinho SV, Meller ST, Gebhart GF. Intracolonic zymosan produces visceral hyperalgesia in the rat that is mediated by spinal NMDA and nonNMDA receptors. Brain Res. 1996;736:7–15. doi: 10.1016/0006-8993(96)00661-0. [DOI] [PubMed] [Google Scholar]
- Cyr M, Ghribi O, Thibault C, Morissette M, Landry M, Di Paolo T. Ovarian steroids and selective estrogen receptor modulators activity on rat brain NMDA and AMPA receptors. Brain Res.Brain Res.Rev. 2001;37:153–161. doi: 10.1016/s0165-0173(01)00115-1. [DOI] [PubMed] [Google Scholar]
- D'Souza DN, Harlan RE, Garcia MM. Modulation of glutamate receptor expression by gonadal steroid hormones in the rat striatum. Brain Res.Bull. 2003;59:289–292. doi: 10.1016/s0361-9230(02)00885-7. [DOI] [PubMed] [Google Scholar]
- Diano S, Naftolin F, Horvath TL. Gonadal steroids target AMPA glutamate receptor-containing neurons in the rat hypothalamus, septum and amygdala: a morphological and biochemical study. Endocrinology. 1997;138:778–789. doi: 10.1210/endo.138.2.4937. [DOI] [PubMed] [Google Scholar]
- El Bakri NK, Islam A, Zhu S, Elhassan A, Mohammed A, Winblad B, et al. Effects of estrogen and progesterone treatment on rat hippocampal NMDA receptors: relationship to Morris water maze performance. J.Cell Mol.Med. 2004;8:537–544. doi: 10.1111/j.1582-4934.2004.tb00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17beta-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J.Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- Gao X, Kim HK, Chung JM, Chung K. Enhancement of NMDA receptor phosphorylation of the spinal dorsal horn and nucleus gracilis neurons in neuropathic rats. Pain. 2005;116:62–72. doi: 10.1016/j.pain.2005.03.045. [DOI] [PubMed] [Google Scholar]
- Gazzaley AH, Weiland NG, McEwen BS, Morrison JH. Differential regulation of NMDAR1 mRNA and protein by estradiol in the rat hippocampus. J.Neurosci. 1996;16:6830–6838. doi: 10.1523/JNEUROSCI.16-21-06830.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Wei F, Zou S, Robbins MT, Sugiyo S, Ikeda T, et al. Group I metabotropic glutamate receptor NMDA receptor coupling and signaling cascade mediate spinal dorsal horn NMDA receptor 2B tyrosine phosphorylation associated with inflammatory hyperalgesia. J Neurosci. 2004;24:9161–9173. doi: 10.1523/JNEUROSCI.3422-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitkemper MM, Jarrett M. Gender differences and hormonal modulation in visceral pain. Curr.Pain Headache.Rep. 2001;5:35–43. doi: 10.1007/s11916-001-0008-z. [DOI] [PubMed] [Google Scholar]
- Holdcroft A, Sapsed-Byrne S, Ma D, Hammal D, Forsling ML. Sex and oestrous cycle differences in visceromotor responses and vasopressin release in response to colonic distention in male and female rats anesthetized with halothane. Br.J.Anaesth. 2000;85:907–910. doi: 10.1093/bja/85.6.907. [DOI] [PubMed] [Google Scholar]
- Houghton LA, Lea R, Jackson N, Whorwell PJ. The menstrual cycle affects rectal sensitivity in patients with irritable bowel syndrome but not healthy volunteers. Gut. 2002;50:471–474. doi: 10.1136/gut.50.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucho TB, Dina OA, Kuhn J, Levine JD. Estrogen controls PKCepsilon-dependent mechanical hyperalgesia through direct action on nociceptive neurons. Eur.J.Neurosci. 2006;24:527–534. doi: 10.1111/j.1460-9568.2006.04913.x. [DOI] [PubMed] [Google Scholar]
- Iwata H, Takasusuki T, Yamaguchi S, Hori Y. NMDA receptor 2B subunit-mediated synaptic transmission in the superficial dorsal horn of peripheral nerve-injured neuropathic mice. Brain Res. 2007;1135:92–101. doi: 10.1016/j.brainres.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003a;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Ji Y, Murphy AZ, Traub RJ. Estrogen Modulates the Visceromotor Reflex and Responses of Spinal Dorsal Horn Neurons to Colorectal Stimulation in the Rat. J.Neurosci. 2003b;23:3908–3915. doi: 10.1523/JNEUROSCI.23-09-03908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Murphy AZ, Traub RJ. Sex differences in morphine induced analgesia of visceral pain are supraspinally and peripherally mediated. Am J Physiol Regul Integr Comp Physiol. 2006:R307–R314. doi: 10.1152/ajpregu.00824.2005. [DOI] [PubMed] [Google Scholar]
- Ji Y, Tang B, Traub RJ. Estrogen increases and progesterone decreases behavioral and neuronal responses to colorectal distention following colonic inflammation in the rat. Pain. 2005;117:433–442. doi: 10.1016/j.pain.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Ji Y, Traub RJ. Spinal NMDA receptors contribute to neuronal processing of acute noxious and nonnoxious colorectal stimulation in the rat. J.Neurophysiol. 2001;86:1783–1791. doi: 10.1152/jn.2001.86.4.1783. [DOI] [PubMed] [Google Scholar]
- Jones TL, Sorkin LS. Activated PKA and PKC, but not CaMKIIalpha, are required for AMPA/Kainate-mediated pain behavior in the thermal stimulus model. Pain. 2005;117:259–270. doi: 10.1016/j.pain.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Kolhekar R, Gebhart GF. NMDA and quisqualate modulation of visceral nociception in the rat. Brain Res. 1994;651:215–226. doi: 10.1016/0006-8993(94)90700-5. [DOI] [PubMed] [Google Scholar]
- Kow LM, Easton A, Pfaff DW. Acute estrogen potentiates excitatory responses of neurons in rat hypothalamic ventromedial nucleus. Brain Res. 2005;1043:124–131. doi: 10.1016/j.brainres.2005.02.068. [DOI] [PubMed] [Google Scholar]
- Lan JY, Skeberdis VA, Jover T, Grooms SY, Lin Y, Araneda RC, et al. Protein kinase C modulates NMDA receptor trafficking and gating. Nat Neurosci. 2001;4:382–390. doi: 10.1038/86028. [DOI] [PubMed] [Google Scholar]
- Li J, McRoberts JA, Ennes HS, Trevisani M, Nicoletti P, Mittal Y, et al. Experimental colitis modulates the functional properties of NMDA receptors in dorsal root ganglia neurons. Am.J.Physiol Gastrointest.Liver Physiol. 2006;291:G228. doi: 10.1152/ajpgi.00097.2006. [DOI] [PubMed] [Google Scholar]
- Liu B, Eisenach JC, Tong C. Chronic estrogen sensitizes a subset of mechanosensitive afferents innervating the uterine cervix. J Neurophysiol. 2005;93:2167–2173. doi: 10.1152/jn.01012.2004. [DOI] [PubMed] [Google Scholar]
- Liu H, Wang H, Sheng M, Jan LY, Jan YN, Basbaum AI. Evidence for presynaptic N-methyl-D-aspartate autoreceptors in the spinal cord dorsal horn. Proc.Natl.Acad.Sci.U.S.A. 1994;91:8383–8387. doi: 10.1073/pnas.91.18.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Berman S, Chang L, Naliboff BD. Sex-based differences in gastrointestinal pain. Eur J Pain. 2004;8:451–463. doi: 10.1016/j.ejpain.2004.01.006. [DOI] [PubMed] [Google Scholar]
- McRoberts JA, Coutinho SV, Marvizon JC, Grady EF, Tognetto M, Sengupta JN, et al. Role of peripheral N-methyl-D-aspartate (NMDA) receptors in visceral nociception in rats. Gastroenterology. 2001;120:1737–1748. doi: 10.1053/gast.2001.24848. [DOI] [PubMed] [Google Scholar]
- McRoberts JA, Li J, Ennes HS, Mayer EA. Sex-dependent differences in the activity and modulation of N-methyl-d-aspartic acid receptors in rat dorsal root ganglia neurons. Neuroscience. 2007 doi: 10.1016/j.neuroscience.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miletic G, Hanson EN, Savagian CA, Miletic V. Protein kinase A contributes to sciatic ligation-associated early activation of cyclic AMP response element binding protein in the rat spinal dorsal horn. Neurosci Lett. 2004;360:149–152. doi: 10.1016/j.neulet.2004.02.060. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Chen S, Brinton RD. Dual action of estrogen on glutamate-induced calcium signaling: mechanisms requiring interaction between estrogen receptors and src/mitogen activated protein kinase pathway. Brain Res. 2002;930:216–234. doi: 10.1016/s0006-8993(02)02254-0. [DOI] [PubMed] [Google Scholar]
- Olivar T, Laird JM. Differential effects of N-methyl-D-aspartate receptor blockade on nociceptive somatic and visceral reflexes. Pain. 1999;79:67–73. doi: 10.1016/S0304-3959(98)00152-3. [DOI] [PubMed] [Google Scholar]
- Palomba S, Orio F, Jr, Manguso F, Russo T, Falbo A, Lombardi G, et al. Leuprolide acetate treatment with and without coadministration of tibolone in premenopausal women with menstrual cycle-related irritable bowel syndrome. Fertil.Steril. 2005;83:1012–1020. doi: 10.1016/j.fertnstert.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Papka RE, Srinivasan B, Miller KE, Hayashi S. Localization of estrogen receptor protein and estrogen receptor messenger RNA in peripheral autonomic and sensory neurons. Neuroscience. 1997;79:1153–1163. doi: 10.1016/s0306-4522(97)00076-6. [DOI] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, et al. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K, Hylden JL, Williams GM, Ruda MA, Dubner R. The effects of a non-competitive NMDA receptor antagonist, MK-801, on behavioral hyperalgesia and dorsal horn neuronal activity in rats with unilateral inflammation. Pain. 1992;50:331–344. doi: 10.1016/0304-3959(92)90039-E. [DOI] [PubMed] [Google Scholar]
- Ren K, Wei F, Dubner R, Murphy A, Hoffman GE. Progesterone attenuates persistent inflammatory hyperalgesia in female rats: involvement of spinal NMDA receptor mechanisms. Brain Res. 2000;865:272–277. doi: 10.1016/s0006-8993(00)02267-8. [DOI] [PubMed] [Google Scholar]
- Robbins A, Berkley KJ, Sato Y. Estrous cycle variation of afferent fibers supplying reproductive organs in the female rat. Brain Res. 1992;596:353–356. doi: 10.1016/0006-8993(92)91572-v. [DOI] [PubMed] [Google Scholar]
- Romeo RD, McCarthy JB, Wang A, Milner TA, McEwen BS. Sex Differences in Hippocampal Estradiol-Induced N-Methyl-D-Aspartic Acid Binding and Ultrastructural Localization of Estrogen Receptor-Alpha. Neuroendocrinology. 2005;81:391–399. doi: 10.1159/000089557. [DOI] [PubMed] [Google Scholar]
- Sanoja R, Cervero F. Estrogen-dependent abdominal hyperalgesia induced by ovariectomy in adult mice: A model of functional abdominal pain. Pain. 2005;118:243–253. doi: 10.1016/j.pain.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Sapsed-Byrne S, Ma D, Ridout D, Holdcroft A. Estrous cycle phase variations in visceromotor and cardiovascular responses to colonic distension in the anesthetized rat. Brain Res. 1996;742:10–16. doi: 10.1016/s0006-8993(96)00989-4. [DOI] [PubMed] [Google Scholar]
- Scott DB, Blanpied TA, Ehlers MD. Coordinated PKA and PKC phosphorylation suppresses RXR-mediated ER retention and regulates the surface delivery of NMDA receptors. Neuropharmacology. 2003;45:755–767. doi: 10.1016/s0028-3908(03)00250-8. [DOI] [PubMed] [Google Scholar]
- Strigo IA, Duncan GH, Catherine Bushnell M, Boivin M, Wainer I, Rodriguez Rosas ME, et al. The effects of racemic ketamine on painful stimulation of skin and viscera in human subjects. Pain. 2005;113:255–264. doi: 10.1016/j.pain.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Taleghany N, Sarajari S, DonCarlos LL, Gollapudi L, Oblinger MM. Differential expression of estrogen receptor alpha and beta in rat dorsal root ganglion neurons. J.Neurosci.Res. 1999;57:603–615. [PubMed] [Google Scholar]
- Tang B, Ji Y, Traub RJ. Estrogen alters NMDA receptor signaling of colorectal pain in the rat. Soc.for Neuroscience. 2005 abstract viewer:294.4. [Google Scholar]
- Tang B, Ji Y, Traub RJ. Estrogen-induced facilitation of visceral nociceptive processing in the rat is spinally mediated. Soc.for Neuroscience. 2006 abstract viewer:346.5. [Google Scholar]
- Tingley WG, Ehlers MD, Kameyama K, Doherty C, Ptak JB, Riley CT, et al. Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-D-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J.Biol.Chem. 1997;272:5157–5166. doi: 10.1074/jbc.272.8.5157. [DOI] [PubMed] [Google Scholar]
- Traub RJ, Ji Y, Tang B. Estrogen increases NMDA receptor expression and phosphorylation modulating visceral pain in the rat. American Pain Society abstracts. 2007 http://www.ampainsoc.org/db2/abstract/2007/view?poster_id=3080#655. [Google Scholar]
- Traub RJ, Zhai QZ, Ji Y, Kovalenko M. NMDA receptor antagonists attenuate noxious and nonnoxious colorectal distention-induced Fos expression and the visceromotor reflex. Neurosci. 2002;113:205–211. doi: 10.1016/s0306-4522(02)00170-7. [DOI] [PubMed] [Google Scholar]
- Willert R, Woolf C, Hobson A, Delaney C, Thompson D, Aziz Q. The development and maintenance of human visceral pain hypersensitivity is dependent on the N-methyl-d-aspartate receptor. Gastroenterology. 2004;126:683–692. doi: 10.1053/j.gastro.2003.11.047. [DOI] [PubMed] [Google Scholar]
- Williams SJ, Papka RE. Estrogen receptor-immunoreactive neurons are present in the female rat lumbosacral spinal cord. J.Neurosci Res. 1996;46:492–501. doi: 10.1002/(SICI)1097-4547(19961115)46:4<492::AID-JNR11>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Thompson SWN. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J.Neurosci. 1997;17:1848–1859. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Su G, Ma L, Zhang X, Lei Y, Lin Q, et al. The role of c-AMP-dependent protein kinase in spinal cord and post synaptic dorsal column neurons in a rat model of visceral pain. Neurochem.Int. 2007;50:710–718. doi: 10.1016/j.neuint.2007.01.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HW, Hu XD, Zhang HM, Xin WJ, Li MT, Zhang T, et al. Roles of CaMKII, PKA, and PKC in the induction and maintenance of LTP of C-fiber-evoked field potentials in rat spinal dorsal horn. J.Neurophysiol. 2004;91:1122–1133. doi: 10.1152/jn.00735.2003. [DOI] [PubMed] [Google Scholar]
- Zhai QZ, Traub RJ. The NMDA receptor antagonist MK-801 attenuates c-Fos expression in the lumbosacral spinal cord following repetitive noxious and nonnoxious colorectal distention. Pain. 1999;83:321–329. doi: 10.1016/s0304-3959(99)00116-5. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang X, Westlund KN. Restoration of spontaneous exploratory behaviors with an intrathecal NMDA receptor antagonist or a PKC inhibitor in rats with acute pancreatitis. Pharmacol Biochem.Behav. 2004;77:145–153. doi: 10.1016/j.pbb.2003.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Chen S, Ming Wang J, Brinton RD. 17[beta]-estradiol induces Ca2+ influx, dendritic and nuclear Ca2+ rise and subsequent cyclic AMP response element-binding protein activation in hippocampal neurons: A potential initiation mechanism for estrogen neurotrophism. Neuroscience. 2005;132:299–311. doi: 10.1016/j.neuroscience.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Zou X, Lin Q, Willis WD. Enhanced phosphorylation of NMDA receptor 1 subunits in spinal cord dorsal horn and spinothalamic tract neurons after intradermal injection of capsaicin in rats. J.Neurosci. 2000;20:6989–6997. doi: 10.1523/JNEUROSCI.20-18-06989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]