Abstract
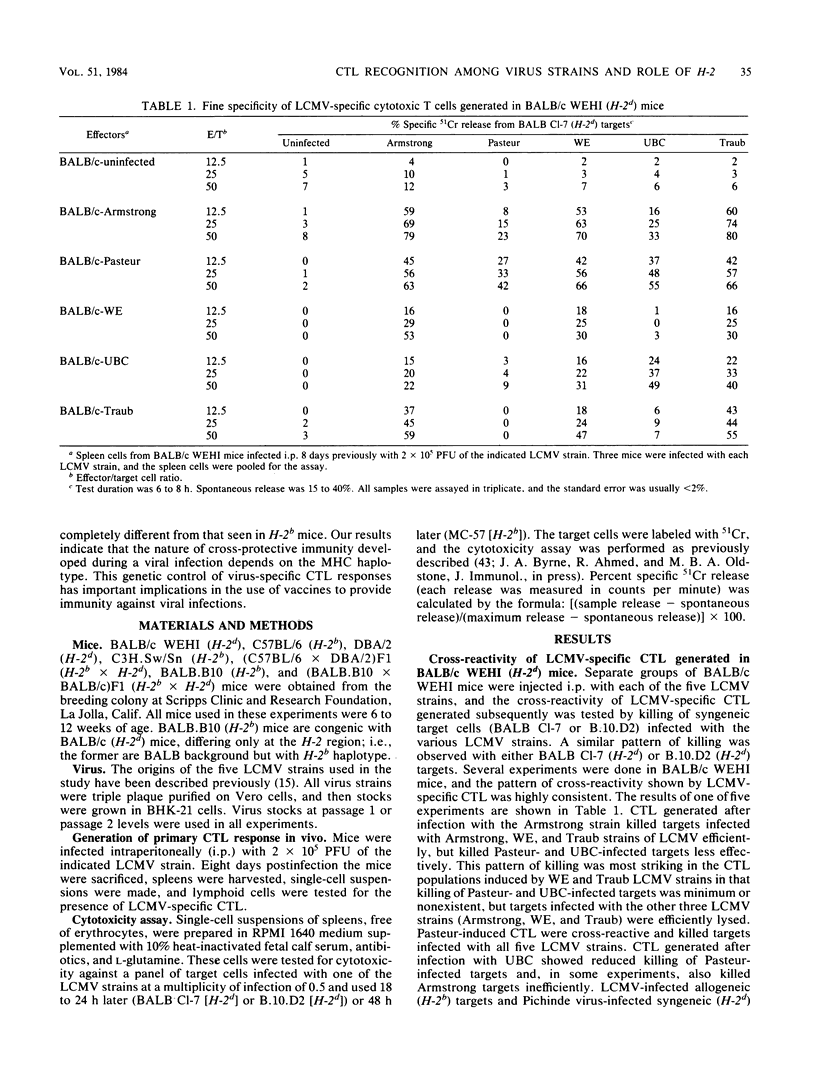
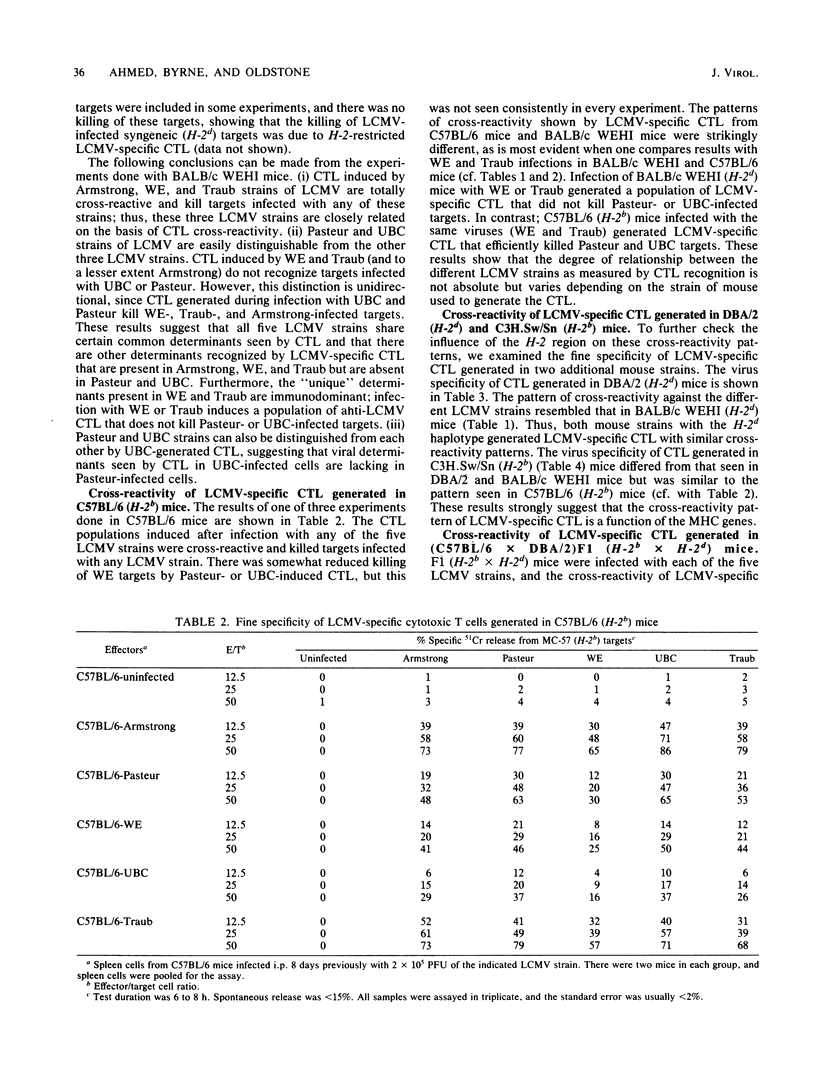
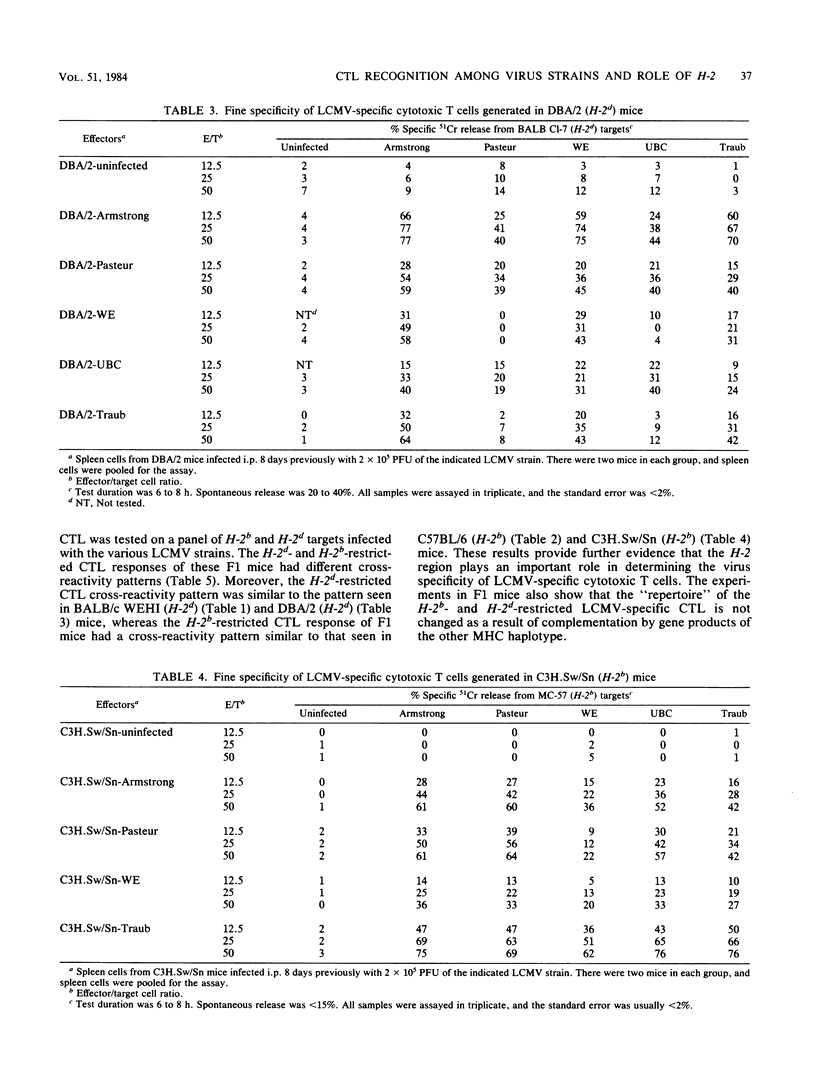
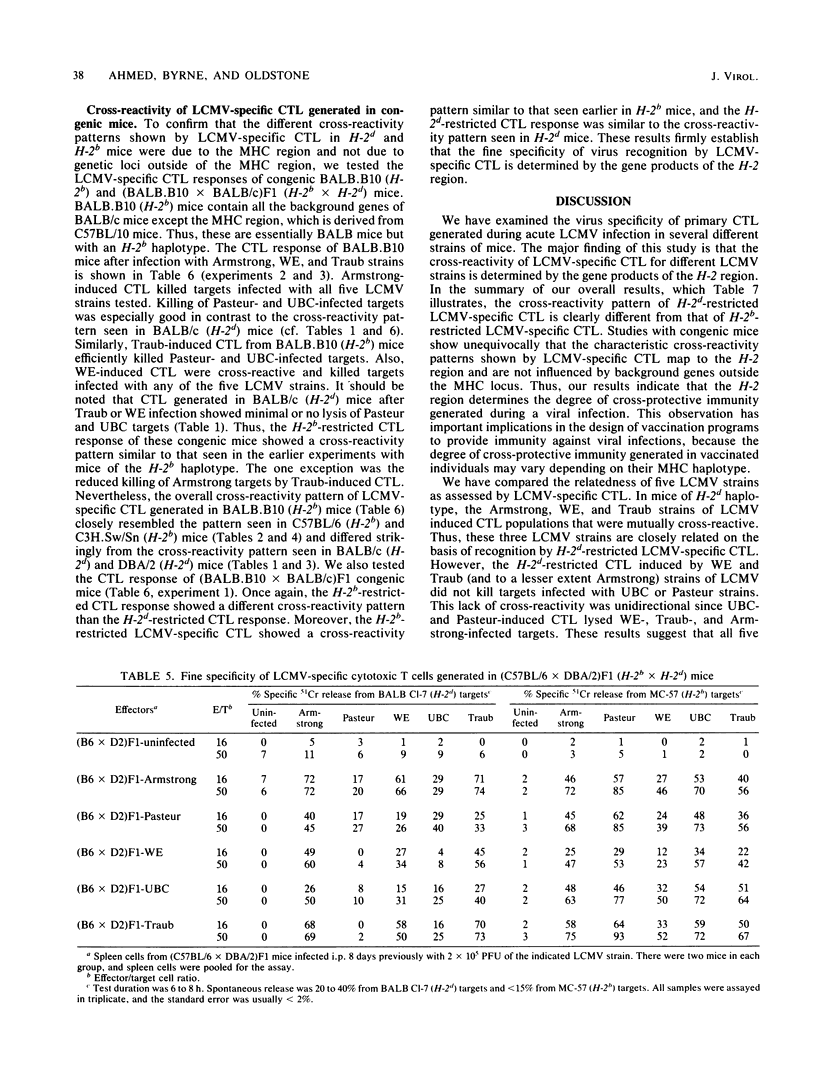
We have compared the relatedness of five different strains of lymphocytic choriomeningitis virus (LCMV) as assessed by LCMV-specific cytotoxic T lymphocytes (CTL). Several different mouse strains were injected with each of the five LCMV strains, and the cross-reactivity of virus-specific CTL generated during the acute infection was tested by killing on a panel of target cells infected with the various LCMV strains. We found that the cross-reactivity pattern of LCMV-specific CTL generated in mice of H-2d haplotype (BALB/c WEHI and DBA/2) was strikingly different from that in mice of H-2b haplotype (C57BL/6 and C3H.Sw/Sn), suggesting that the fine specificity of LCMV-specific CTL is a function of the H-2 region. The characteristic cross-reactivity patterns were also observed in (C57BL/6 X DBA/2)F1 mice, demonstrating that the repertoire of the H-2b- and H-2d-restricted LCMV-specific CTL is not changed as a result of complementation by gene products of the other major histocompatibility haplotype. Studies with congenic BALB.B10 and (BALB.B10 X BALB/c)F1 mice firmly established that the characteristic cross-reactivity patterns of LCMV-specific CTL map to the H-2 region and are not influenced by background genes outside the major histocompatibility locus. These results suggest that LCMV determinants seen in the context of H-2d-restricting elements are different from those seen in the context of H-2b-restricting elements. Moreover, our studies show that CTL can be used as probes for dissecting differences among various LCMV strains, but the degree of relatedness between the different LCMV strains is not absolute when measured by CTL recognition. Since the H-2 region regulates the fine specificity of CTL generated during LCMV infection in its natural host, the degree of cross-protective immunity developed during a viral infection apparently depends on the major histocompatibility haplotype. The importance of these findings lies in understanding susceptibility or resistance of various host populations to viral infections and in designing vaccination programs to provide immunity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ada G. L., Leung K. N., Ertl H. An analysis of effector T cell generation and function in mice exposed to influenza A or Sendai viruses. Immunol Rev. 1981;58:5–24. doi: 10.1111/j.1600-065x.1981.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Benacerraf B., Germain R. N. The immune response genes of the major histocompatibility complex. Immunol Rev. 1978;38:70–119. doi: 10.1111/j.1600-065x.1978.tb00385.x. [DOI] [PubMed] [Google Scholar]
- Benacerraf B., McDevitt H. O. Histocompatibility-linked immune response genes. Science. 1972 Jan 21;175(4019):273–279. doi: 10.1126/science.175.4019.273. [DOI] [PubMed] [Google Scholar]
- Bennink J. R., Yewdell J. W., Gerhard W. A viral polymerase involved in recognition of influenza virus-infected cells by a cytotoxic T-cell clone. Nature. 1982 Mar 4;296(5852):75–76. doi: 10.1038/296075a0. [DOI] [PubMed] [Google Scholar]
- Biddison W. E., Hansen T. H., Levy R. B., Doherty P. C. Involvement of H-2L gene products in virus-immune T-cell recognition. Evidence for an H-2L-restricted T-cell response. J Exp Med. 1978 Dec 1;148(6):1678–1686. doi: 10.1084/jem.148.6.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V. T cell response to viral and bacterial infection. Transplant Rev. 1974;19(0):56–88. doi: 10.1111/j.1600-065x.1974.tb00128.x. [DOI] [PubMed] [Google Scholar]
- Braciale T. J., Andrew M. E., Braciale V. L. Heterogeneity and specificity of cloned lines of influenza-virus specific cytotoxic T lymphocytes. J Exp Med. 1981 Apr 1;153(4):910–923. doi: 10.1084/jem.153.4.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier M. J., Lewicki H. A., Tomori O., Oldstone M. B. Monoclonal antibodies to lymphocytic choriomeningitis and pichinde viruses: generation, characterization, and cross-reactivity with other arenaviruses. Virology. 1981 Aug;113(1):73–85. doi: 10.1016/0042-6822(81)90137-9. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Welsh R. M., Dutko F. J., Oldstone M. B. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- Cole G. A., Nathanson N., Prendergast R. A. Requirement for theta-bearing cells in lymphocytic choriomeningitis virus-induced central nervous system disease. Nature. 1972 Aug 11;238(5363):335–337. doi: 10.1038/238335a0. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Biddison W. E., Bennink J. R., Knowles B. B. Cytotoxic T-cell responses in mice infected with influenza and vaccinia viruses vary in magnitude with H-2 genotype. J Exp Med. 1978 Aug 1;148(2):534–543. doi: 10.1084/jem.148.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P. C., Dunlop M. B., Parish C. R., Zinkernagel R. M. Inflammatory process in murine lymphocytic choriomeningitis is maximal in H-2K or H-2D compatible interactions. J Immunol. 1976 Jul;117(1):187–190. [PubMed] [Google Scholar]
- Dutko F. J., Oldstone M. B. Genomic and biological variation among commonly used lymphocytic choriomeningitis virus strains. J Gen Virol. 1983 Aug;64(Pt 8):1689–1698. doi: 10.1099/0022-1317-64-8-1689. [DOI] [PubMed] [Google Scholar]
- Finberg R., Benacerraf B. Induction, control and consequences of virus specific cytotoxic T cells. Immunol Rev. 1981;58:157–180. doi: 10.1111/j.1600-065x.1981.tb00353.x. [DOI] [PubMed] [Google Scholar]
- Finberg R., Spriggs D. R., Fields B. N. Host immune response to reovirus: CTL recognize the major neutralization domain of the viral hemagglutinin. J Immunol. 1982 Nov;129(5):2235–2238. [PubMed] [Google Scholar]
- Finberg R., Weiner H. L., Fields B. N., Benacerraf B., Burakoff S. J. Generation of cytolytic T lymphocytes after reovirus infection: role of S1 gene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):442–446. doi: 10.1073/pnas.76.1.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flyer D. C., Burakoff S. J., Faller D. V. Cytotoxic T lymphocyte recognition of transfected cells expressing a cloned retroviral gene. 1983 Oct 27-Nov 2Nature. 305(5937):815–818. doi: 10.1038/305815a0. [DOI] [PubMed] [Google Scholar]
- Hale A. H., Ruebush M. J., Lefrançois L. Cross-reactive anti-vesicular stomatitis virus (VSV) cytotoxic T lymphocytes recognize the major surface glycoprotein. Eur J Immunol. 1981 May;11(5):434–436. doi: 10.1002/eji.1830110517. [DOI] [PubMed] [Google Scholar]
- Kurrle R., Röllinghoff M., Wagner H. H-2-linked murine cytotoxic T cell responses specific for sendai virus-infected cells. Eur J Immunol. 1978 Dec;8(12):910–912. doi: 10.1002/eji.1830081216. [DOI] [PubMed] [Google Scholar]
- LEVINE B. B., OJEDA A., BENACERRAF B. STUDIES ON ARTIFICIAL ANTIGENS. III. THE GENETIC CONTROL OF THE IMMUNE RESPONSE TO HAPTEN-POLY-L-LYSINE CONJUGATES IN GUINEA PIGS. J Exp Med. 1963 Dec 1;118:953–957. doi: 10.1084/jem.118.6.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. L., Askonas B. A. Biological properties of an influenza A virus-specific killer T cell clone. Inhibition of virus replication in vivo and induction of delayed-type hypersensitivity reactions. J Exp Med. 1981 Aug 1;154(2):225–234. doi: 10.1084/jem.154.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marker O., Volkert M. Studies on cell-mediated immunity to lymphocytic choriomeningitis virus in mice. J Exp Med. 1973 Jun 1;137(6):1511–1525. doi: 10.1084/jem.137.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt H. O., Deak B. D., Shreffler D. C., Klein J., Stimpfling J. H., Snell G. D. Genetic control of the immune response. Mapping of the Ir-1 locus. J Exp Med. 1972 Jun 1;135(6):1259–1278. doi: 10.1084/jem.135.6.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt H. O., Sela M. Genetic control of the antibody response. I. Demonstration of determinant-specific differences in response to synthetic polypeptide antigens in two strains of inbred mice. J Exp Med. 1965 Sep 1;122(3):517–531. doi: 10.1084/jem.122.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mims C. A., Blanden R. V. Antiviral action of immune lymphocytes in mice infected with lymphocytic choriomeningitis virus. Infect Immun. 1972 Nov;6(5):695–698. doi: 10.1128/iai.6.5.695-698.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskophidis D., Lehmann-Grube F. The immune response of the mouse to lymphocytic choriomeningitis virus. III. Differences of numbers of cytotoxic T lymphocytes in spleens of mice of different strains. Cell Immunol. 1983 Apr 15;77(2):279–289. doi: 10.1016/0008-8749(83)90028-x. [DOI] [PubMed] [Google Scholar]
- Pedersen I. R. Structural components and replication of arenaviruses. Adv Virus Res. 1979;24:277–330. doi: 10.1016/s0065-3527(08)60396-6. [DOI] [PubMed] [Google Scholar]
- Rosenthal K. L., Zinkernagel R. M. Inability of mice to generate cytotoxic T lymphocytes to vesicular stomatitis virus restricted to H-2Kk or H-2Dk. J Immunol. 1981 Feb;126(2):446–451. [PubMed] [Google Scholar]
- Schmitt-Verhulst A. M., Shearer G. M. Bifunctional major histocompatibility-linked genetic regulation of cell-mediated lympholysis to trinitrophenyl-modified autologous lymphocytes. J Exp Med. 1975 Oct 1;142(4):914–927. doi: 10.1084/jem.142.4.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L. A., Vitiello A., Klinman N. R. T-cell and B-cell responses to viral antigens at the clonal level. Annu Rev Immunol. 1983;1:63–86. doi: 10.1146/annurev.iy.01.040183.000431. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Skehel J. J. Influenza A specific cytotoxic T-cell clones that do not recognize viral glycoproteins. Nature. 1982 Dec 16;300(5893):655–657. doi: 10.1038/300655a0. [DOI] [PubMed] [Google Scholar]
- Vitiello A., Sherman L. A. Recognition of influenza-infected cells by cytolytic T lymphocyte clones: determinant selection by class I restriction elements. J Immunol. 1983 Oct;131(4):1635–1640. [PubMed] [Google Scholar]
- Webster R. G., Laver W. G., Air G. M., Schild G. C. Molecular mechanisms of variation in influenza viruses. Nature. 1982 Mar 11;296(5853):115–121. doi: 10.1038/296115a0. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Althage A., Cooper S., Kreeb G., Klein P. A., Sefton B., Flaherty L., Stimpfling J., Shreffler D., Klein J. Ir-genes in H-2 regulate generation of anti-viral cytotoxic T cells. Mapping to K or D and dominance of unresponsiveness. J Exp Med. 1978 Aug 1;148(2):592–606. doi: 10.1084/jem.148.2.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974 Apr 19;248(5450):701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Rosenthal K. L. Experiments and speculation on antiviral specificity of T and B cells. Immunol Rev. 1981;58:131–155. doi: 10.1111/j.1600-065x.1981.tb00352.x. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Welsh R. M. H-2 compatibility requirement for virus-specific T cell-mediated effector functions in vivo. I. Specificity of T cells conferring antiviral protection against lymphocytic choriomeningitis virus is associated with H-2K and H-2D. J Immunol. 1976 Nov;117(5 Pt 1):1495–1502. [PubMed] [Google Scholar]


