Abstract
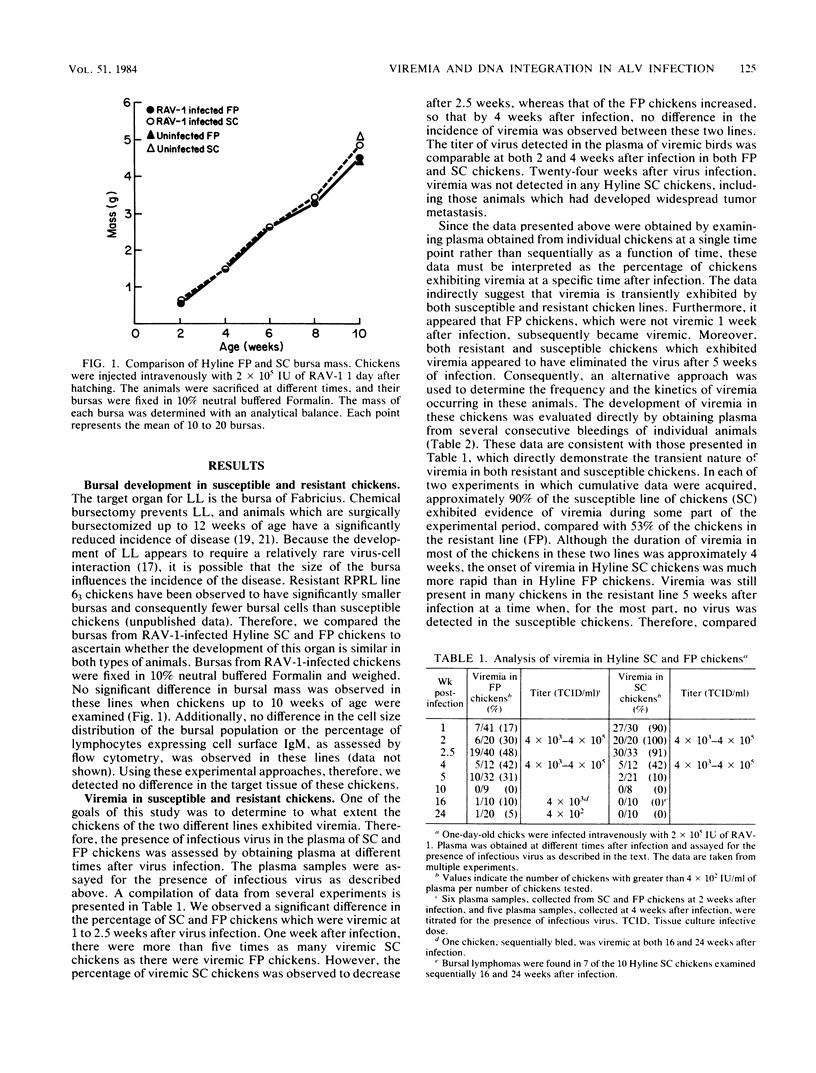
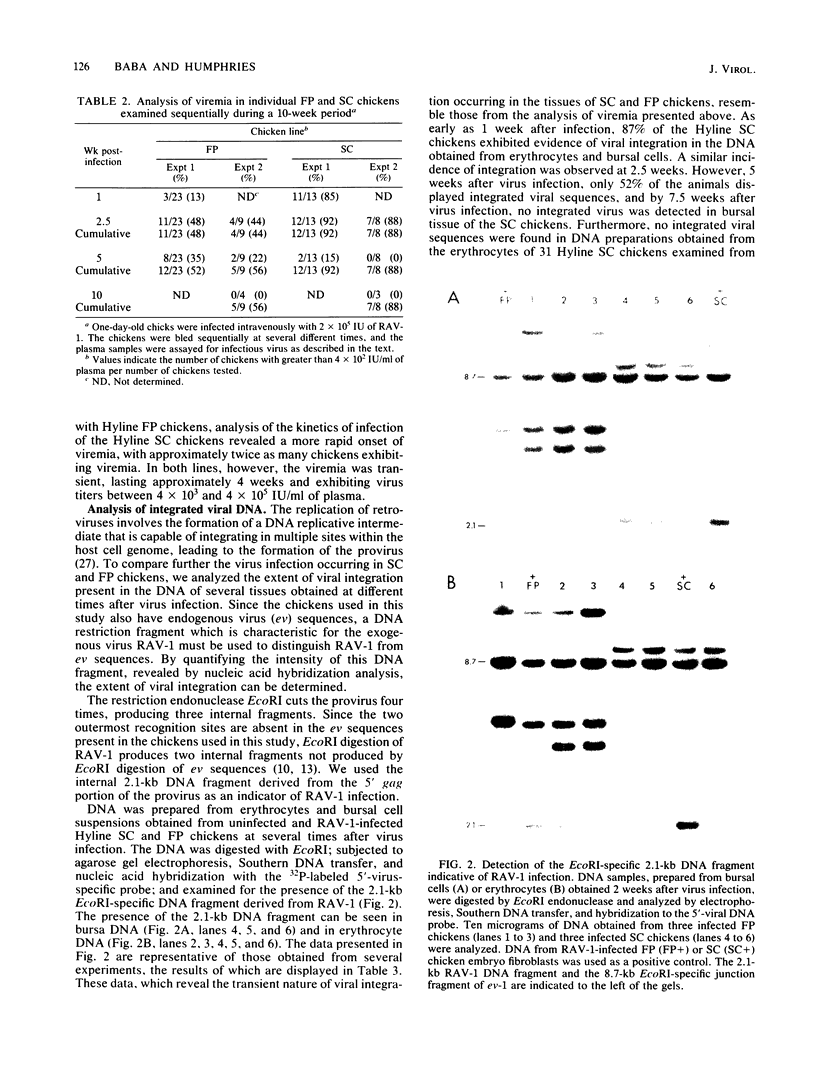
Avian leukosis viruses induce lymphoid leukosis, a lymphoma which develops within the bursa of Fabricius several months after virus infection. Chickens from the Hyline SC and FP lines are, respectively, susceptible and resistant to avian leukosis virus-induced lymphoid leukosis. We examined plasma and cellular DNA obtained from avian leukosis virus-infected chickens for the presence of viremia and integrated viral sequences to determine whether the extent of virus infection is comparable in individuals of both lines. A less than twofold difference in the frequency of viremia was detected between chickens of the two different lines. Although the analysis of plasma samples, which were obtained at different times postinfection, demonstrated that the duration of viremia was comparable in both susceptible and resistant chickens, the onset of the viremia observed in susceptible chickens generally preceded by 1 week that observed in resistant chickens. Moreover, integrated viral sequences were detected in approximately 90% of the SC and 40% of the FP chickens. The appearance of infectious virus in the plasma was, in general, associated with the presence of integrated viral sequences in both the bursal cells and the erythrocytes obtained from the same chicken. The presence of both the viremia and the integrated viral DNA sequences was transient, suggesting a mechanism for the elimination of virus-infected cells in both susceptible and resistant chickens. Furthermore, at 5 weeks postinfection no integrated exogenous viral sequences were detected in splenic lymphocytes obtained from either chicken line, regardless of whether these chickens were viremic or had integrated viral sequences detectable in other tissues. Our results indicate that extensive avian leukosis virus replication occurs in approximately 50% of the FP and 100% of the SC chickens. Although it appears that the viral infection spreads more quickly in the SC chickens, our results afford no obvious explanation of the resistance to the development of lymphoma exhibited by FP chickens.
Full text
PDF

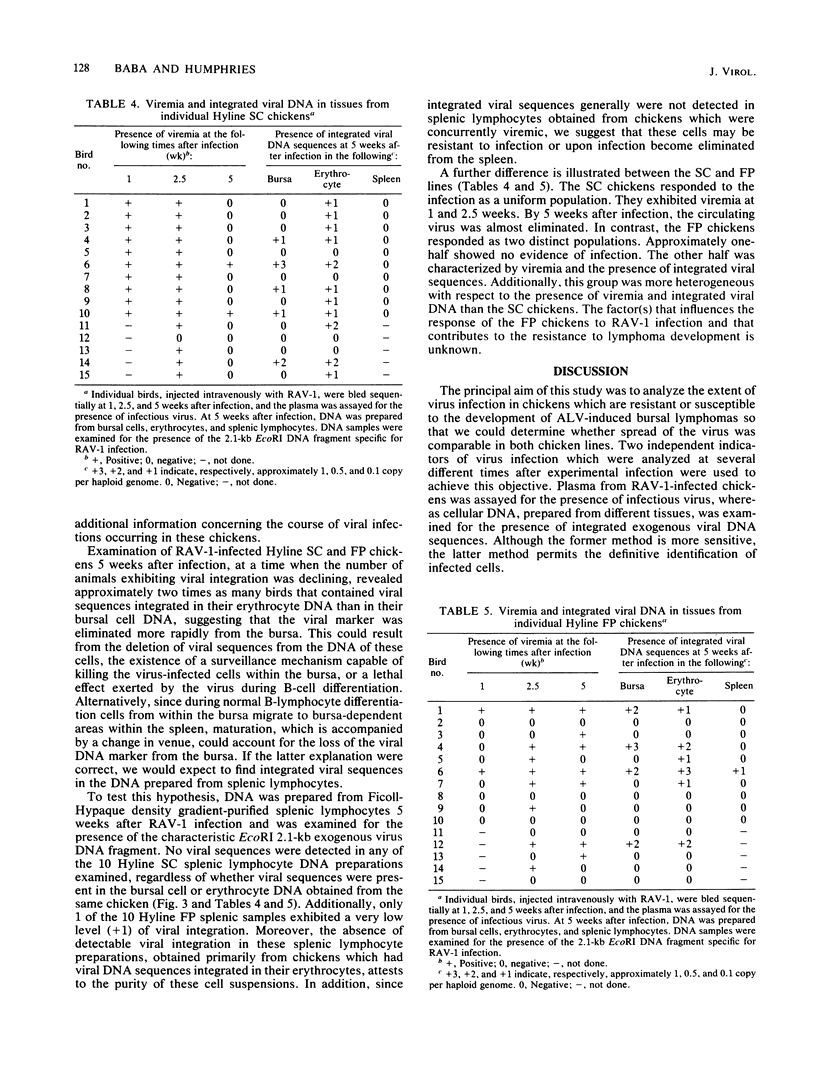


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F., Rosenberg N., Lewis S., Thomas E., Baltimore D. Organization and reorganization of immunoglobulin genes in A-MULV-transformed cells: rearrangement of heavy but not light chain genes. Cell. 1981 Dec;27(2 Pt 1):381–390. doi: 10.1016/0092-8674(81)90421-9. [DOI] [PubMed] [Google Scholar]
- Cooper G. M., Neiman P. E. Transforming genes of neoplasms induced by avian lymphoid leukosis viruses. Nature. 1980 Oct 16;287(5783):656–659. doi: 10.1038/287656a0. [DOI] [PubMed] [Google Scholar]
- Cooper G. M., Neiman P. E. Two distinct candidate transforming genes of lymphoid leukosis virus-induced neoplasms. Nature. 1981 Aug 27;292(5826):857–858. doi: 10.1038/292857a0. [DOI] [PubMed] [Google Scholar]
- Cooper M. D., Cain W. A., Van Alten P. J., Good R. A. Development and function of the immunoglobulin producing system. I. Effect of bursectomy at different stages of development on germinal centers, plasma cells, immunoglobulins and antibody production. Int Arch Allergy Appl Immunol. 1969;35(3):242–252. [PubMed] [Google Scholar]
- Cooper M. D., Lawton A. R., Kincade P. W. A two-stage model for development of antibody-producing cells. Clin Exp Immunol. 1972 May;11(1):143–149. [PMC free article] [PubMed] [Google Scholar]
- Cooper M. D., Purchase H. G., Bockman D. E., Gathings W. E. Studies on the nature of the abnormality of B cell differentiation in avian lymphoid leukosis: production of heterogeneous IgM by tumor cells. J Immunol. 1974 Oct;113(4):1210–1222. [PubMed] [Google Scholar]
- DeLorbe W. J., Luciw P. A., Goodman H. M., Varmus H. E., Bishop J. M. Molecular cloning and characterization of avian sarcoma virus circular DNA molecules. J Virol. 1980 Oct;36(1):50–61. doi: 10.1128/jvi.36.1.50-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fialkow P. J. Clonal origin of human tumors. Biochim Biophys Acta. 1976 Oct 12;458(3):283–321. doi: 10.1016/0304-419x(76)90003-2. [DOI] [PubMed] [Google Scholar]
- Fung Y. K., Fadly A. M., Crittenden L. B., Kung H. J. Avian lymphoid leukosis virus infection and DNA integration in the preleukotic bursal tissues: a comparative study of susceptible and resistant lines. Virology. 1982 Jun;119(2):411–421. doi: 10.1016/0042-6822(82)90100-3. [DOI] [PubMed] [Google Scholar]
- Goubin G., Goldman D. S., Luce J., Neiman P. E., Cooper G. M. Molecular cloning and nucleotide sequence of a transforming gene detected by transfection of chicken B-cell lymphoma DNA. Nature. 1983 Mar 10;302(5904):114–119. doi: 10.1038/302114a0. [DOI] [PubMed] [Google Scholar]
- Halpern M. S., Ewert D. L., Flores L. J., Crittenden L. B. The influence of the ev 3 locus on the inducibility of serum antibody reactivity for envelope glycoprotein group-specific determinants. Virology. 1983 Jul 30;128(2):502–504. doi: 10.1016/0042-6822(83)90278-7. [DOI] [PubMed] [Google Scholar]
- Hughes S. H., Toyoshima K., Bishop J. M., Varmus H. E. Organization of the endogenous proviruses of chickens: implications for origin and expression. Virology. 1981 Jan 15;108(1):189–207. doi: 10.1016/0042-6822(81)90538-9. [DOI] [PubMed] [Google Scholar]
- Humphries E. H., Allen R., Glover C. Clonal analysis of the integration and expression of endogenous avian retroviral DNA acquired by exogenous viral infection. J Virol. 1981 Aug;39(2):584–596. doi: 10.1128/jvi.39.2.584-596.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel B. G., Hayward W. S., Robinson H. L., Fang J., Astrin S. M. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell. 1981 Feb;23(2):323–334. doi: 10.1016/0092-8674(81)90128-8. [DOI] [PubMed] [Google Scholar]
- Neiman P., Payne L. N., Weiss R. A. Viral DNA in bursal lymphomas induced by avian leukosis viruses. J Virol. 1980 Apr;34(1):178–186. doi: 10.1128/jvi.34.1.178-186.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne G. S., Courtneidge S. A., Crittenden L. B., Fadly A. M., Bishop J. M., Varmus H. E. Analysis of avian leukosis virus DNA and RNA in bursal tumours: viral gene expression is not required for maintenance of the tumor state. Cell. 1981 Feb;23(2):311–322. doi: 10.1016/0092-8674(81)90127-6. [DOI] [PubMed] [Google Scholar]
- Peterson R. D., Purchase H. G., Burmester B. R., Cooper M. D., Good R. A. Relationships among visceral lymphomatosis, bursa of Fabricius, and bursa-dependent lymphoid tissue of the chicken. J Natl Cancer Inst. 1966 Apr;36(4):585–598. doi: 10.1093/jnci/36.4.585. [DOI] [PubMed] [Google Scholar]
- Purchase H. G., Gilmour D. G. Lymphoid leukosis in chickens chemically bursectomized and subsequently inoculated with bursa cells. J Natl Cancer Inst. 1975 Oct;55(4):851–855. doi: 10.1093/jnci/55.4.851. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robinson H. L., Pearson M. N., DeSimone D. W., Tsichlis P. N., Coffin J. M. Subgroup-E avian-leukosis-virus-associated disease in chickens. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1133–1141. doi: 10.1101/sqb.1980.044.01.122. [DOI] [PubMed] [Google Scholar]
- Roussel M., Saule S., Lagrou C., Rommens C., Beug H., Graf T., Stehelin D. Three new types of viral oncogene of cellular origin specific for haematopoietic cell transformation. Nature. 1979 Oct 11;281(5731):452–455. doi: 10.1038/281452a0. [DOI] [PubMed] [Google Scholar]
- Sheiness D., Bishop J. M. DNA and RNA from uninfected vertebrate cells contain nucleotide sequences related to the putative transforming gene of avian myelocytomatosis virus. J Virol. 1979 Aug;31(2):514–521. doi: 10.1128/jvi.31.2.514-521.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Varmus H. E. Form and function of retroviral proviruses. Science. 1982 May 21;216(4548):812–820. doi: 10.1126/science.6177038. [DOI] [PubMed] [Google Scholar]
- Vogt P. K., Ishizaki R. Patterns of viral interference in the avian leukosis and sarcoma complex. Virology. 1966 Nov;30(3):368–374. doi: 10.1016/0042-6822(66)90115-2. [DOI] [PubMed] [Google Scholar]