Abstract
In polarized HepG2 hepatoma cells, sphingolipids are transported to the apical, bile canalicular membrane by two different transport routes, as revealed with fluorescently tagged sphingolipid analogs. One route involves direct, transcytosis-independent transport of Golgi-derived glucosylceramide and sphingomyelin, whereas the other involves basolateral to apical transcytosis of both sphingolipids. We show that these distinct routes display a different sensitivity toward nocodazole and cytochalasin D, implying a specific transport dependence on either microtubules or actin filaments, respectively. Thus, nocodazole strongly inhibited the direct route, whereas sphingolipid transport by transcytosis was hardly affected. Moreover, nocodazole blocked “hyperpolarization,” i.e., the enlargement of the apical membrane surface, which is induced by treating cells with dibutyryl-cAMP. By contrast, the transcytotic route but not the direct route was inhibited by cytochalasin D. The actin-dependent step during transcytotic lipid transport probably occurs at an early endocytic event at the basolateral plasma membrane, because total lipid uptake and fluid phase endocytosis of horseradish peroxidase from this membrane were inhibited by cytochalasin D as well. In summary, the results show that the two sphingolipid transport pathways to the apical membrane must have a different requirement for cytoskeletal elements.
INTRODUCTION
Elements of the cytoskeleton, such as the actin microfilaments and tubulin-based microtubules, have an important role in maintaining cell structure and in mediating trafficking of intracellular membranes (Mays et al., 1994). Polarized cells, which have distinct apical and basolateral plasma membrane domains, are known to use cytoskeletal filaments to facilitate vesicular transport between different membrane compartments. The role of microtubules in vesicular transport in polarized cells has been studied in some detail. Disruption of microtubules with agents such as nocodazole or colchicine especially inhibits protein trafficking from the trans-Golgi network to the apical domain, whereas trafficking to the basolateral domain is not or only little affected (Eilers et al., 1989; Parczyk et al., 1989; Breitfeld et al., 1990; Matter et al., 1990; van Zeijl and Matlin, 1990; Gilbert et al., 1991). Microtubules also facilitate the transport of vesicles from the basolateral to the apical domain by transcytosis; by contrast, they do not appear to function in the transcytotic transport pathway in the opposite direction (Breitfeld et al., 1990; Hunziker et al., 1990; Matter et al., 1990). During transcytosis, the microtubule-dependent step is probably located after endocytic uptake, because in both polarized epithelial cells such as Madin-Darby canine kidney (MDCK) cells and nonpolarized cells such as baby hamster kidney cells, endocytosis is not inhibited by microtubule inhibitors (Hunziker et al., 1990; Matter et al., 1990; Kok et al., 1992). However, in hepatocytes, receptor-mediated uptake of the asialoglycoprotein receptor (Harada et al., 1995) and fluid phase uptake of horseradish peroxidase (HRP) (Sakisaka et al., 1988) have been reported to be inhibited by colchicine.
The involvement of actin filaments in vesicular trafficking is less well defined. Rearrangement of the actin cytoskeleton is supposed to play an important role in the establishment of cell polarity (Drubin and Nelson, 1996). In polarized epithelial cells, a dense network of actin filaments is found under the apical surface of simple epithelial cells. In hepatocytes, an accumulation of actin filaments is observed around the bile canaliculi, representing the apical membrane domain of these cells. The actin network is linked to a bundle of actin filaments that forms the core of the microvilli, which cover the apical surface. It has been proposed that the actin network plays a role in targeting Golgi-derived vesicles to the apical domain, probably via the actin-binding motor protein myosin-1 (Fath and Burgess, 1993; Fath et al., 1994). Indeed, it was shown that partial disassembly of the actin cytoskeleton triggers exocytosis (Muallem et al., 1995). Still, an extensive disruption of the actin or microvillar organization results in an inhibition of apically directed vesicles (Muallem et al., 1995) or an accumulation of such vesicles in the subapical region of the cell (Costa de Beauregard et al., 1995). However, no consensus has been reached yet, because it has also been reported that cytochalasin D (cytD), a drug that disrupts actin filaments, does not affect trafficking from the Golgi to either the basolateral or the apical plasma membrane domain (Salas et al., 1986; Parczyk et al., 1989).
By contrast, several studies have reported that trafficking along the endocytic pathway is sensitive to this drug. Evidence obtained with different cell types has shown that both clathrin-dependent and clathrin-independent endocytosis are inhibited by cytD (Sandvig and van Deurs, 1990; Gottlieb et al., 1993; Chazaud et al., 1994; Jackman et al., 1994; Parton et al., 1994; Durrbach et al., 1996; Maples et al., 1997). In polarized cells, actin-dependent endocytosis has only been reported to take place at the apical domain (Gottlieb et al., 1993; Jackman et al., 1994). Possibly, a mechano-chemical motor in conjunction with the actin in the micovillous core is required for pinching off of endocytic vesicles from this membrane domain, which may explain the specific inhibition of endocytosis at this membrane domain (Gottlieb et al., 1993; Jackman et al., 1994).
Previously, we have shown that in HepG2 cells, a polarized hepatoma cell model, sphingolipids are targeted to the apical domain via transcytosis and via a direct route from the Golgi to the apical membrane (Zaal et al., 1994; Zegers and Hoekstra, 1997). Both these transport routes are regulated by protein kinase C (PKC) and protein kinase A (PKA). Moreover, the kinase-mediated changes in lipid transport correlate with changes in cell polarity. Thus, we observed that stimulation of apical lipid transport (by PKA) correlates with an enlargement of the apical membrane surface area, whereas an inhibition of apical sphingolipid transport (by PKC) correlates with a rapid loss of cell polarity, as shown by the redistribution of several apical protein markers, in particular that of actin (Zegers and Hoekstra, 1997).
Because activation of these kinases influenced both cell polarity and apical lipid transport, cytoskeletal elements are probably involved in mediating these effects. In this study, we examined the effect of compounds that are known to disrupt actin filaments or microtubules on both lipid transport and kinase-induced changes of cell polarity. We show here that the direct and indirect sphingolipid transport routes display a different sensitivity toward cytoskeletal inhibitors. Moreover, PKA-induced enlargement of the apical membrane surface area and stimulation of sphingolipid transport were inhibited by disruption of microtubules. We conclude that both actin filaments and microtubules play an important role in the biogenesis of apical membranes as reflected by a prominent interference with apically directed sphingolipid transport.
MATERIALS AND METHODS
Materials
DMEM was obtained from Life Technologies (Paisley, Scotland), and fetal calf serum (FCS) was from BioWhittaker (Verviers, Belgium). d-Sphingosine, 1β-d-glucosylsphingosine, and sphingosylphosphorylcholine were from Matreya (Pleasant Gap, PA), and 6-[N-(7-nitrobenz-2-oxa-1,3 diazol-4-yl)amino]hexanoic acid (C6-NBD) was from Molecular Probes (Eugene, OR). Latrunculin B was obtained from Calbiochem (La Jolla, CA). Dibutyryl-cAMP (dB-cAMP) was purchased from Boehringer Mannheim GmbH (Mannheim, Germany). Paraformaldehyde, high-performance TLC plates, and organic solvents were from Merck (Darmstadt, Germany). FITC-conjugated antibodies were from Nordic Immunology Laboratories (Tilburg, The Netherlands). All other chemicals were supplied by Sigma Chemical (St. Louis, MO).
Cell Culture
HepG2 cells were grown in DMEM containing 4.5 g/l glucose, supplemented with 10% FCS, 2 mM l-glutamine, penicillin (100 IU/ml), and streptomycin (100 μg/ml), at 37°C in a humidified atmosphere of 5% CO2 in air. Experiments were performed 3 d after cell plating. For biochemical experiments, cells were grown on 94-mm culture dishes. For microscopy, cells were grown on glass coverslips.
Synthesis of C6-NBD Sphingolipids
C6-NBD-ceramide (C6-NBD-Cer), C6-NBD-sphingomyelin (C6-NBD-SM), and C6-NBD-glucosylceramide (C6-NBD-GlcCer) were synthesized from C6-NBD and d-sphingosine, 1β-d-glucosylsphingosine, and sphingosylphosphorylcholine, respectively, as described elsewhere (Babia et al., 1994).
Cell Labeling and Lipid Transport Assays
Insertion and Back-Exchange of Fluorescent Lipids.
Cells were washed three times with cold PBS. C6-NBD lipids were added to cold Hanks’ buffered salt solution (HBSS) by means of ethanolic injection. Lipids from a stock solution in chloroform/methanol (2:1 vol/vol) were dried under nitrogen and solubilized in absolute ethanol. The ethanolic solution was subsequently injected into HBSS under vigorous vortexing. The final concentration of ethanol did not exceed 0.5% (vol/vol).
When required, C6-NBD lipids present in the outer leaflet of the plasma membrane were removed by a back-exchange procedure. To this end the cells were incubated for 30 min at 4°C with 5% BSA in HBSS, followed by washing with cold HBSS. This procedure was repeated once.
Transcytosis of Lipids.
Transcytosis of sphingolipid was determined by labeling the plasma membrane of HepG2 cells with C6-NBD-GlcCer or C6-NBD-SM for 30 min at 4°C. The cells were washed and subsequently incubated at 37°C for 15 min to allow for internalization and transcytosis to the bile canalicular domain. The cells were then cooled to 4°C, and the lipid remaining in the outer leaflet of the plasma membrane was removed by a back-exchange at 4°C. The apical labeling of the bile canalicular structures was determined semiquantitatively by assessing the percentage of bile canaliculi that was NBD positive (Zegers and Hoekstra, 1997). The bile canaliculi, which are easily visualized under phase contrast by their microvillar appearance, were classified as NBD positive or negative under epifluoresence illumination. In control cells, ∼70% of the total population of bile canaliculi was NBD positive after a 15-min incubation period at 37°C. Cells that were incubated with NBD lipids at 4°C only showed a background labeling of bile canaliculi of ∼10% of the total population.
Apical Delivery of De Novo-synthesized Lipids.
Cells were plated on coverslips and labeled with C6-NBD-Cer for 60 min at 4°C. To allow for synthesis of C6-NBD-GlcCer or C6-NBD-SM and their subsequent transport, an incubation was carried out at 37°C for 60 min in HBSS containing 5% BSA. After the incubation, the apical delivery of sphingolipids was determined as indicated above. In control cells, ∼50% of the total population of bile canaliculi was NBD positive after a 60-min incubation at 37°C. In cells that had been incubated at 4°C only, no NBD-positive bile canaliculi were observed (our unpublished observations).
Metabolism of C6-NBD-Cer and Basolateral Delivery of De Novo-synthesized Sphingolipids.
Cells were plated in culture dishes and labeled with C6-NBD-Cer for 60 min at 4°C. The cells were then incubated for 60 min at 37°C in HBSS, containing 5% BSA. Subsequently they were washed and scraped from the culture dish. Lipids from the incubation medium and cells were extracted and quantified as described below.
Total Endocytic Uptake of Sphingolipids.
The total endocytic uptake of sphingolipids was determined by labeling cells with C6-NBD-GlcCer or C6-NBD-SM for 30 min at 4°C. The cells were then washed and incubated at 37°C. At the end of the incubation the cells were cooled to 4°C, and the lipid remaining in the outer leaflet of the plasma membrane was removed by back-exchange at 4°C. After washing, the cells were scraped from the culture dish. Lipids from the back-exchange medium and the cells were extracted and quantified. In the time span of our experiments no degradation of C6-NBD-GlcCer or C6-NBD-SM occurred, as revealed by TLC.
Lipid Extraction and Quantification.
Lipids from cells and back-exchange media were extracted according to the method of Bligh and Dyer (1959). NBD lipids were separated by TLC and quantified fluorometrically as described previously (Kok et al., 1991)
Fluid Phase Endocytosis of HRP
The fluid phase uptake of HRP was determined as described (Nolan, 1992). Briefly, cells in 35-mm Petri dishes were washed with DMEM and then with DMEM containing 5 mg/ml BSA (DMEM/BSA). Subsequently, the cells were incubated with 2 mg/ml HRP in DMEM/BSA for 1 h at 37°C or at 4°C (control). To exclude possible uptake of HRP via the mannose receptor, 2 mg/ml yeast mannans were included during incubation. After the incubation, the cells were extensively washed with DMEM/BSA and subsequently solubilized in 1 ml 0.5% Triton X-100. The cell extracts were assayed for HRP activity by a colorimetric assay using ortho-diansidine as a substrate.
Immunofluorescence and Other Cell-staining Procedures
To stain F-actin, cells were washed with PBS and fixed for 10 s at −20°C in ethanol. F-actin was then stained using 100 ng/ml TRITC-labeled phalloidin in PBS for 30 min at room temperature. To determine the ratio of bile canaliculi per number of cells, cells were fixed with ethanol and double stained with 5 ng/ml nuclear stain Hoechst 33258 and 100 ng/ml TRITC–phalloidin for 30 min in PBS at room temperature. For staining microtubules, cells were fixed with 3% paraformaldehyde in PBS for 30 min and permeabilized with 0.1% Triton X-100 in PBS for 10 min. Cells were then washed in PBS, incubated for 30 min in PBS containing 2.5% FCS (PBS/FCS) to block nonspecific binding sites, and subsequently incubated with a primary mouse monoclonal antibody against β-tubulin, diluted in PBS/FCS (1:100). After several washings, the primary antibody was revealed with an FITC-conjugated goat anti-mouse antibody. To prevent bleaching, cells were embedded in glycerol containing 2.5% 1,4-diazobicyclo[2.2.2.]octane before microscopic examination.
Microscopy
Cells were examined using an epifluorescence microscope (Provis AX70; Olympus, New Hyde Park, NY). Photographs were taken using Ilford (Benelux, Leiden, the Netherlands) HP-5 plus film.
RESULTS
In HepG2 cells, bile canaliculi are located between adjacent cells and represent the apical membrane surface domain of these cells. Previous work has shown that fluorescent sphingolipid analogues can be transferred to the apical membrane via both basolateral to apical transcytosis and a transcytosis-independent, “direct” pathway (Zaal et al., 1994; Zegers and Hoekstra, 1997).
Transport via the direct route is demonstrated by incubating the cells with the freely diffusable, fluorescent precursor C6-NBD-Cer. This precursor specifically accumulates in the Golgi, where it is used for the synthesis of C6-NBD-GlcCer and C6-NBD-SM (Lipsky and Pagano, 1985). Besides fluorescent labeling of the Golgi, at 37°C, an additional labeling of the bile canaliculus is observed, which becomes apparent after some 20 min of incubation (Zaal et al., 1994), i.e., a time interval after which significant amounts of the C6-NBD sphingolipids can be detected intracellularly. Hence, these results indicate that metabolism of C6-NBD-Cer precedes labeling of the apical membrane and suggest that the synthesis of C6-NBD-GlcCer and C6-NBD-SM is a prerequisite for transport to and fluorescent labeling of the apical membrane in hepatocytes and HepG2 cells. To validate this hypothesis, we analyzed the effect of the sphingolipid synthesis inhibitor d-threo-1-phenyl-2-decanoyl amino-3-morpholino-1-propanol (PDMP) on the metabolism of C6-NBD-Cer and on the apical labeling by C6-NBD sphingolipids. We found that 100 μM PDMP completely inhibited the synthesis of C6-NBD-GlcCer, whereas the synthesis of C6-NBD-SM was inhibited by 85% (Table 1). Overall, the formation of fluorescent Cer metabolites was inhibited by >90%, because in control cells 44% and in PDMP-treated cells only 4% of either product was formed. The inhibition of C6-NBD-GlcCer and -SM synthesis by PDMP strongly affected the labeling of the apical membrane. When the percentage of NBD-positive bile canaliculi was determined in cells that had been incubated with C6-NBD-Cer for 60 min at 37°C, either in the absence or presence of PDMP, virtually no NBD-positive bile canaliculi could be identified in PDMP-treated cells, whereas in control cells 55% of the total population of bile canaliculi was fluorescently labeled (Table 2). This indicates that the fluorescent labeling that was observed in the bile canalicular membrane of control cells originated from either C6-NBD-SM or C6-NBD-GlcCer or both, which were transported from the Golgi to the apical membrane. In addition to their transport to the apical surface, the newly synthesized C6-NBD-GlcCer and C6-NBD-SM are also transported to the basolateral surface (Zegers and Hoekstra, 1997). However, the presence of BSA in the incubation medium extracts lipid that arrives at the outer leaflet of the basolateral surface, thereby preventing the lipids from reentering the cell. Thus trafficking to the apical domain via transcytosis, the alternative pathway that delivers sphingolipid to the apical domain, can be excluded. However, with this experimental setup, and given the need of biosynthesis for transport to occur, apical transport of newly synthesized C6-NBD sphingolipids in the direct transport route can thus be determined in a convenient and reliable manner. Transport of lipid from the basolateral to the apical domain by transcytosis is monitored after initial insertion of either C6-NBD-GlcCer or C6-NBD-SM in the basolateral membrane at low temperature. After elevating the temperature, the lipid is processed via basolateral endocytosis and subsequently transported to the apical surface. The dependence of either transport pathway on cytoskeletal elements was examined next.
Table 1.
Effect of PDMP on the metabolism of C6-NBD-Cer in HepG2 cells
| Percentage
of total C6-NBD
lipid
|
||
|---|---|---|
| Control | +PDMP | |
| C6-NBD-Cer | 56 ± 11 | 96 ± 1 |
| C6-NBD-SM | 30 ± 10 | 4 ± 1 |
| C6-NBD-GlcCer | 14 ± 2 | —a |
HepG2 cells were preincubated with or without 100 μM PDMP in HBSS for 60 min at 37°C. The cells were then cooled and labeled with 3 μM C6-NBD-Cer for 60 min at 4°C in the presence or absence of PDMP. After subsequent washing, the cells were further incubated in HBSS with or without PDMP at 37°C for 60 min. The cells were then washed and scraped from the culture dish. The lipids were extracted, and NBD lipids were analyzed and quantified as described in MATERIALS AND METHODS. Data represent values of six experiments ± SEM.
C6-NBD-GlcCer levels in PDMP-treated cells were below detection level.
Table 2.
Apical labeling in PDMP-treated cells
| Labeled bile canaliculi (%) | |
|---|---|
| Control | 55 ± 4 |
| PDMP | <1 |
HepG2 cells, plated on glass coverslips, were preincubated with or without 100 μM PDMP in HBSS for 60 min at 37°C. After cooling, the cells were labeled with 3 μM C6-NBD-Cer for 60 min at 4°C in the presence or absence of PDMP. The cells were then washed and incubated for 60 min at 37°C, in HBSS containing 5% BSA to prevent transcytosis of basolaterally arrived lipid, in the presence or absence of PDMP. Subsequently, the cells were washed, and apical labeling was determined as described in MATERIALS AND METHODS. Data represent values of four experiments ± SEM.
Direct Transport of Sphingolipids Depends on Intact Microtubules, Whereas Transcytosis Depends on Intact Actin Filaments
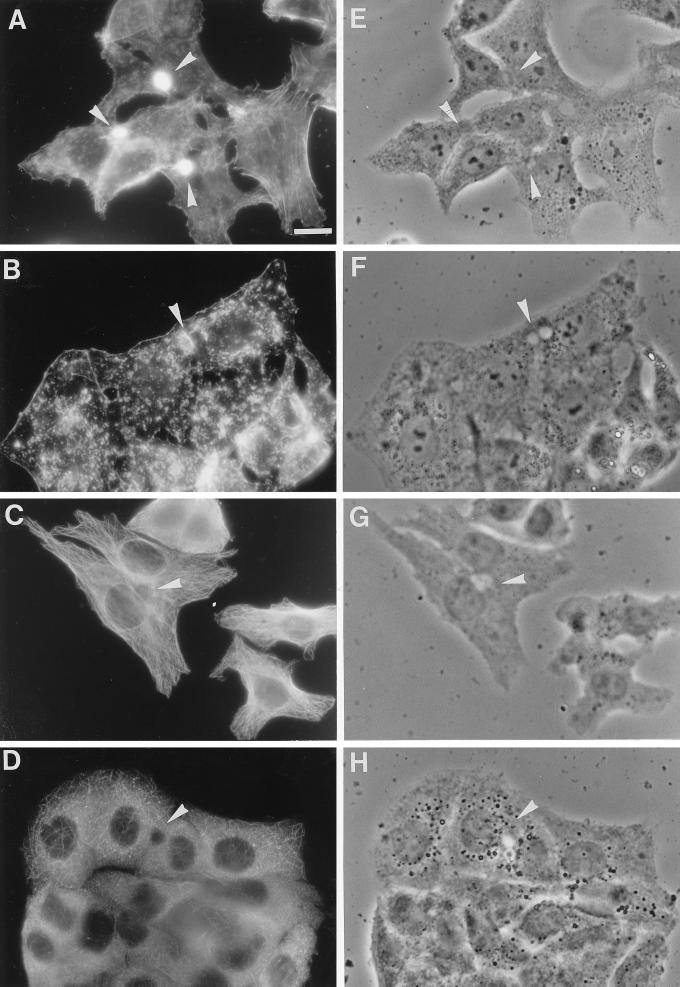
To investigate whether sphingolipid transport via both routes is dependent on cytoskeletal elements, we preincubated the cells with cytD or nocodazole, agents that are known to disrupt actin filaments and microtubules, respectively. The effects of both compounds on either the actin cytoskeleton or the microtubules in HepG2 cells are shown in Figure 1. After treatment of HepG2 cells with 10 μg/ml cytD, actin filaments were visualized by staining with TRITC-labeled phalloidin. In untreated cells (Figure 1A), a strong accumulation of actin filaments was observed underneath the apical membrane. In addition, labeling of actin filaments was found near the basolateral plasma membrane. In cells that were treated with cytD (Figure 1B), actin filaments were mainly found in small aggregates, and the accumulation at the apical plasma membrane that was seen in untreated cells was strongly reduced, although it did not disappear completely.
Figure 1.
Effect of cytoskeletal drugs on the distribution of actin filaments and microtubules in HepG2 cells. The effect of cytD on actin filaments was determined by incubating the cells with 10 μg/ml cytD in HBSS for 30 min at 37°C. After the incubation, the cells were washed and fixed in ethanol for 10 s at −20°C. Actin filaments were stained with 100 ng/ml TRITC-labeled phalloidin in PBS for 30 min at room temperature. Note the extensive staining of actin filaments around the bile canaliculus (arrowhead) and the additional staining at the basolateral membrane in control cells (A), whereas in cytD-treated cells (B) mainly small aggregates and a strongly reduced labeling around the bile canaliculus are observed. By staining cells with anti-β-tubulin, the effect of nocodazole on microtubules was evaluated. Cells were preincubated with 33 μM nocodazole in HBSS for 2 h at 37°C. The cells were then fixed with 3% paraformaldehyde for 30 min at room temperature and permeabilized with 0.1% Triton X-100. Indirect immunofluorescence staining with an antibody against β-tubulin revealed that in control cells, the microtubules formed a delicate network (C) that was severely disrupted after nocodazole treatment (D). (E–H) Phase-contrast images of A–D, respectively. Bar, 10 μm.
By staining cells for tubulin, the effect of nocodazole on the distribution of microtubules was determined. In agreement with other studies (Salas et al., 1986), we found that preincubation with 33 μM nocodazole (Figure 1D) resulted in a severe disruption of the fine microtubular network that was observed in untreated cells (Figure 1C). In contrast to the distribution of actin filaments, no strong accumulation of microtubules was observed around the bile canaliculus. In fact, although some microtubules seem to radiate from the bile canaliculus, an association with the apical membrane, as seen for actin filaments, was not present, as the immediate area near the bile canaliculus appears rather depleted of microtubules. As control experiments, we also examined the effect of nocodazole on the actin cytoskeleton and the effect of cytD on the microtubules. The results of these experiments showed neither an effect of nocodazole on the distribution of actin, nor that cytD perturbed the organization of the microtubules (our unpublished observations).
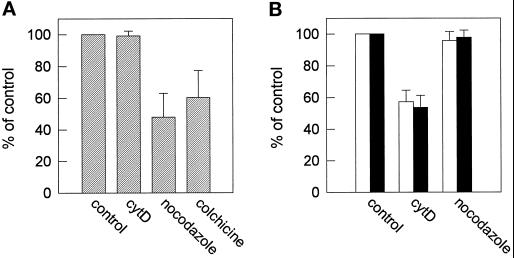
Previously, we have demonstrated that phorbol 12-myristate 13-acetate (PMA), a potent activator of PKC, induces rapid actin rearrangements and, concomitantly, inhibits apical transport of sphingolipids (Zegers and Hoekstra, 1997). We therefore hypothesized that the actin rearrangements may be responsible for the inhibition of apical lipid transport by preventing docking or exocytosis at this membrane surface, as has been described in other systems (Fath and Burgess, 1993; Fath et al., 1994; Muallem et al., 1995). To validate this hypothesis, the cells were treated with cytD to depolymerize the actin filaments, and the effect on apical sphingolipid transport via both pathways was analyzed. As shown in Figure 2, cytD treatment had no effect on the direct transport pathway (Figure 2A) but inhibited apical transport of either C6-NBD-GlcCer or C6-NBD-SM via transcytosis by ∼40% (Figure 2B). Very similar results (40–50% inhibition) were obtained when the cells were treated with latrunculin B (5 μg/ml), a drug that effectively disrupts actin filaments (Spector et al., 1983), including those in HepG2 cells (our unpublished observations). The effect of cyt D on apical sphingolipid transport was opposite to the effect of the microtubular inhibitor nocodazole. When microtubuli were disrupted with this drug, sphingolipid transport via the direct pathway was inhibited by ∼50%. Similar effects were obtained with colchicine, another microtubuli-disrupting drug (Figure 2A). However, neither nocodazole nor colchicine (our unpublished observations) treatment inhibited the apical delivery of fluorescent GlcCer or SM via transcytosis (Figure 2B).
Figure 2.
Effect of nocodazole and cytD on apical sphingolipid transport. HepG2 cells were preincubated with 10 μg/ml cytD for 30 min or 33 μM nocodazole or 1 μM colchicine for 2 h in HBSS at 37°C. The compounds were kept present during further incubations. (A) Effect of the cytoskeletal drugs on direct transport of newly synthesized sphingolipids. Cells were labeled with 3 μM C6-NBD-Cer at 4°C, as described in MATERIALS AND METHODS. After an incubation at 37°C for 60 min in back-exchange medium, the cells were washed, and apical transport was determined as described. Data represent the mean ± SEM of three or four independent experiments of cells treated with 0.1% DMSO (control), cytD, nocodazole, or colchicine. (B) Effect of cytD or nocodazole on transcytosis of exogenously inserted (i.e., in the basolateral membrane) C6-NBD-SM and C6-NBD-GlcCer. After preincubation with cytD or nocodazole, cells were labeled with 3 μM C6-NBD-GlcCer (white bars) or C6-NBD-SM (black bars) at 4°C. After warming at 37°C for 15 min in HBSS, the cells were subsequently washed, cooled, and back-exchanged. Apical transport was determined as described in MATERIALS AND METHODS. Data represent the mean ± SEM of three to five independent experiments.
Because labeling of bile canaliculi depends on the conversion of C6-NBD-Cer to its products C6-NBD-SM or C6-NBD-GlcCer as demonstrated above, an inhibition of the direct pathway may be the result of a reduced synthesis of these lipids. As a control experiment, we therefore analyzed the metabolism of C6-NBD-Cer in the presence of cytD or nocodazole, under the same experimental conditions as were used for analyzing direct lipid transport. As shown in Table 3, both compounds did not affect the synthesis of C6-NBD-SM or C6-NBD-GlcCer. However, by analyzing the incubation medium, which contained BSA to extract lipid that is transported basolaterally (see MATERIALS AND METHODS), we found that nocodazole had a small but significant inhibiting effect on basolateral transport of C6-NBD-SM but not on that of C6-NBD-GlcCer.
Table 3.
Synthesis and basolateral transport of C6NBD-GlcCer and C6-NBD-SM
| Percentage
of total NBD
lipid
|
||
|---|---|---|
| GlcCer | SM | |
| Control | 5.2 ± 0.7 (82.6 ± 5) | 24.7 ± 6 (77.2 ± 6) |
| Cytochalasin D | 4.7 ± 0.7 (82.0 ± 0.7) | 22.4 ± 4 (74.1 ± 8) |
| Nocodazole | 4.8 ± 0.9 (81.4 ± 3) | 24.1 ± 5 (62.9 ± 6) |
Cells were preincubated with or without 10 μg/ml cytD in HBSS at 37°C for 30 min or with 33 μM nocodazole for 2 h. Cells were then cooled and, in the presence or absence of the cytoskeletal drugs, incubated with 3 μM C6-NBD-Cer as described in MATERIALS AND METHODS. Subsequently the cells were incubated at 37°C for 60 min in back-exchange medium and, after washing, scraped from the culture dish. The lipids were extracted from the back-exchange medium and the cells, and the NBD lipids were quantified as described in MATERIALS AND METHODS. In parentheses, the percentage of lipid transported to the basolateral plasma membrane is indicated. Values represent the data of three experiments ± SEM.
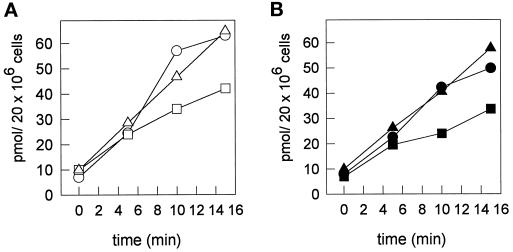
Several studies have shown that depolymerization of actin filaments by cytD inhibits endocytic uptake (Sandvig and van Deurs, 1990; Gottlieb et al., 1993; Chazaud et al., 1994; Jackman et al., 1994; Durrbach et al., 1996). We analyzed the effect of cytD on basolateral endocytosis of sphingolipids by monitoring its effect on the internalization of the fluorescent lipid analogs over the same time interval as was used in the transcytotic lipid transport assay. In cells that had been treated with either cytD (Figure 3) or latrunculin B (our unpublished observations), the internalization of C6-NBD-SM and C6-NBD-GlcCer was inhibited by ∼40–50%. By contrast, nocodazole treatment did not affect lipid internalization. Note that the extent of inhibition of basolateral endocytosis by cytD matches exactly the inhibiting effect on apical lipid transport by transcytosis (Figure 2B), as determined after the same time interval. This suggests that the inhibition of transcytotic lipid transport by actin-perturbing drugs such as cytD and latrunculin B is due to interference with an actin-dependent step during endocytic uptake from the basolateral membrane. Moreover, when we measured the uptake of HRP, we found that cytD inhibited the uptake of this fluid phase marker as well (Table 4), which would suggest cytD to act as a nonspecific inhibitor of overall endocytosis in HepG2 cells.
Figure 3.
Effect of cytD and nocodazole on the internalization of basolateral membrane-inserted C6-NBD-SM and C6-NBD-GlcCer. HepG2 cells were preincubated with or without 10 μg/ml cytD or 33 μM nocodazole in HBSS at 37°C for 30 min or 2 h, respectively. Cells were then cooled and, in the presence of the drugs, labeled with 3 μM C6-NBD-SM or C6-NBD-GlcCer at 4°C. Subsequent incubations were carried out at 37°C for the indicated time intervals. After the incubation, the cells were washed with cold PBS, back-exchanged, and scraped from the culture dish. Lipids were extracted and NBD-lipids were quantified as described in MATERIALS AND METHODS. Each value represents the mean of two independent experiments with variations between 3 and 14%. (A) Internalization of C6-NBD-GlcCer in control (○), cytD (□), and nocodazole-treated (▵) cells. (B) Internalization of C6-NBD-SM in control (•), cytD (▪), and nocodazole-treated (▴) cells.
Table 4.
Effect of cytochalasin D on fluid phase endocytosis of HRP
| HRP uptake (ng/h per 106 cells) | |
|---|---|
| Control | 8.91 ± 2.1 |
| Cytochalasin D | 4.98 ± 1.3 |
Cells were preincubated with or without 10 μg/ml cytD for 30 min in DMEM. After the preincubation, the cells were incubated with HRP for 1 h at 37°C, and the cell-associated HRP activity was determined as described in MATERIALS AND METHODS. Data represent the values of three independent experiments in duplicate ± SEM.
Nocodazole Inhibits PKA-mediated Stimulation of Lipid Transport and Expansion of the Bile Canalicular Surface Area
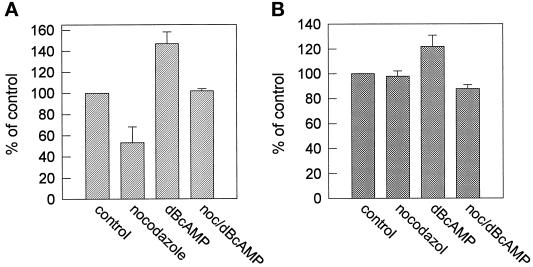
When HepG2 cells are treated with agents that stimulate the activity of the cAMP-dependent PKA, apical sphingolipid transport is enhanced by stimulation of both the direct and the transcytotic sphingolipid transport routes. Concomitantly, the number and the size of bile canaliculi increase, i.e., indicative of the system to accomplish a hyperpolarized state (Zegers and Hoekstra, 1997). To investigate whether hyperpolarization as mediated by PKA activation was dependent on the presence of intact microtubules, we preincubated HepG2 cells with 33 μM nocodazole. After the preincubation, 1 mM cell-permeable cAMP analog dB-cAMP was added to activate PKA, and the cells were subsequently incubated in the presence of dB-cAMP and nocodazole for 4 h. After fixation, the number of bile canaliculi was determined. As shown in Table 5, a 4-h treatment with dB-cAMP strongly increases the total number of bile canaliculi, thus resulting in an increase of the total apical surface area. However, in cells that were pretreated with nocodazole, the stimulating effect of dB-cAMP on the formation of bile canaliculi was completely abolished. Cells that were treated with both nocodazole and dB-cAMP showed a total number of bile canaliculi that was even slightly less than the number obtained for control cells. Apparently, the formation of bile canaliculi in both dB-cAMP-treated and nonstimulated cells is dependent on the presence of intact microtubuli. However, these observations are remarkable, because in previous work it was found that PKA activation stimulated lipid transport to the apical membrane in both the direct and indirect pathway. Yet, in the present work, a perturbation of the microtubule structure only caused an inhibition of the direct transport pathway, whereas the indirect, transcytotic pathway was unaffected (Figure 2A). We therefore examined whether the dB-cAMP-induced stimulation of apical sphingolipid transport was dependent on microtubules. Control experiments revealed that also in the presence of dB-cAMP, nocodazole effectively disrupted the organization of the microtubules (our unpublished observations; cf. Figure 1D). As shown in Figure 4B, we observed that a combined dB-cAMP–nocodazole treatment abolished the stimulatory effect of dB-cAMP per se and resulted in values of transcytotic apical transport that were similar to those in untreated cells. Thus, whereas in control cells transcytotic delivery of sphingolipid to the apical membrane is microtubule independent, the results suggest that during transcytosis in dB-cAMP-activated cells, at least part of the increment in transport may have occurred via an alternative apically directed but microtubule-dependent pathway. In the direct pathway between Golgi and plasma membrane, dB-cAMP stimulates apical sphingolipid transport by ∼40% (Zegers and Hoekstra, 1997), whereas nocodazole, as described above, inhibited transport by ∼50% (Figure 2A). After a combined dB-cAMP–nocodazole treatment, the percentage of labeled bile canaliculi was similar when compared with control values (Figure 4A). This may indicate that in contrast to the transcytotic route, the db-cAMP-induced increase of direct lipid transport does not exclusively depend on microtubules. However, because the (lack of) effect on apical lipid transport that we observed represents a combined result of a stimulator and an inhibitor of this process, it is difficult to draw conclusions concerning the relative contribution of the db-cAMP-induced stimulation and nocodazole-induced inhibition in these cells.
Table 5.
The dB-cAMP-induced hyperpolarization of HepG2 cells is microtubule dependent
| Ratio of bile canaliculi to total cells | |
|---|---|
| Control | 1:13.7 ± 0.1 |
| dB-cAMP | 1:5.8 ± 0.8 |
| Nocodazole | 1:16.2 ± 1.4 |
| dB-cAMP + nocodazole | 1:15.7 ± 4.5 |
HepG2 cells were washed and incubated with or without 1 mM dB-cAMP, 33 μM nocodazole, or both in HBSS for 4 h at 37°C. When cells were incubated with both nocodazole and dB-cAMP, the cells were preincubated with nocodazole for 30 min in HBSS before the addition of dB-cAMP. After the incubation, cells were washed and double stained for actin and nuclei, using TRITC–phalloidin and Hoechst 33258, respectively, as described in MATERIALS AND METHODS. Cells were examined by fluorescence microscopy. For each condition, five random fields were scored for the total number of cells by counting nuclei and the total number of bile canaliculi, stained with actin. Each field contained 200–300 cells. Data represent the mean ± SEM of three independent experiments.
Figure 4.
Effect of nocodazole, dB-cAMP, or both on apical sphingolipid transport. Cells were preincubated at 37°C with or without 33 μM nocodazole for 2 h and/or with 1 mM dB-cAMP for 30 min. Then, in the absence or presence of either nocodazole or dB-cAMP or both (noc/dBcAMP), direct, apical transport of newly synthesized C6-NBD-sphingolipids (A) or transcytotic transport of C6-NBD-SM (B) was determined as described in MATERIALS AND METHODS and in the legend of Figure 2. Data represent the mean ± SEM of three independent experiments.
DISCUSSION
In the present work we provide evidence that the transcytosis-dependent and -independent pathways by which apical membrane-directed sphingolipid transport in HepG2 cells occurs (Zaal et al., 1994; Zegers and Hoekstra, 1997) are regulated by different cytoskeletal elements. Thus, whereas direct transport of newly synthesized C6-NBD-GlcCer and C6-NBD-SM from the Golgi to the apical membrane is microtubule dependent, transcytosis from basolateral to apical membrane depends on actin filaments. Also, sphingolipid transport along the direct pathway to the basolateral membrane is only marginally affected by microtubule-disrupting agents. The data indicate that both pathways may, in principle, operate largely independent of each other. Interestingly, hyperpolarization, occurring when stimulating apical transport by dB-cAMP, displays an absolute dependence on microtubules.
The microtubule dependency of apical but not basolateral membrane-directed trafficking in the direct transport pathway appears to be a general feature for both sphingolipids and proteins in polarized cells, because it has also been observed in Caco-2 and MDCK cells (Eilers et al., 1989; Parczyk et al., 1989; Breitfeld et al., 1990; Matter et al., 1990; van Zeijl and Matlin, 1990; Gilbert et al., 1991; van Meer and van’t Hof, 1993). However, in contrast to what has been reported for protein transport in these cell types (Breitfeld et al., 1990; Hunziker et al., 1990; Matter et al., 1990), in HepG2 cells, basolateral to apical transcytosis is not sensitive to nocodazole. Interestingly, a microtubule-dependent step in the transcytotic pathway could be revealed in cells in which transport has been stimulated with dB-cAMP. Relative to control cells, the dB-cAMP-induced stimulation was completely abolished by nocodazole treatment. Elsewhere (van IJzendoorn et al., 1997) we have shown that dB-cAMP redirects sphingolipid trafficking in the reverse transcytotic pathway by strongly enhancing apical lipid recycling from subapical compartments. It is tempting to suggest that this step in the overall transcytotic pathway represents the dB-cAMP-facilitated increment to apical sphingolipid delivery in the indirect pathway, and that it is this particular step which is sensitive to nocodazole (Figure 4B).
In addition to the stimulating effect on transcytosis, dB-cAMP also stimulates direct sphingolipid transport. However, although nocodazole effectively inhibits this pathway, a complete inhibition was never seen, implying that also in the direct pathway, a pool of sphingolipid may be transported apically by a microtubule-independent process. Interestingly, the dB-cAMP-induced enlargement of the total apical surface area as a result of an increase in size and the total number of bile canaliculi can be completely abolished by nocodazole. Thus, hyperpolarization displays an absolute requirement for microtubules. This bears analogy to similar observations in freshly isolated hepatocytes that have retained a bile canalicular lumen between pairs of cells (Boyer and Soroka, 1995). A dB-cAMP-induced increase of the bile canalicular circumference could be largely, but not completely, blocked by nocodazole, emphasizing that the HepG2 system compares well with its natural counterpart.
The disruption of actin filaments by cytD (or latrunculin B) had opposite effects on lipid transport when compared with the effects of nocodazole. In cells that were treated with cytD, the basolateral to apical transcytosis of sphingolipid but not the direct pathway was strongly inhibited. We have previously shown that activation of PKC activity results in a rearrangement and depolymerization of actin filaments and a concomitant inhibition of sphingolipid transport via both the direct and the transcytotic pathways (Zegers and Hoekstra, 1997). We therefore hypothesized that the actin depolymerization might be responsible for the inhibition of apical sphingolipid transport by inhibiting the final delivery of vesicles to the apical membrane (Muallem et al., 1995; Fath and Burgess, 1993; Fath et al., 1994). The results presented in this study indicate, however, that the PKC-mediated inhibition of apical lipid transport is mediated via another mechanism, because disruption of actin filaments only inhibits apical sphingolipid transport via transcytosis but not via the direct pathway. In support of this conclusion is the notion that cytD inhibited basolateral endocytosis of exogenously supplied sphingolipids by ∼40%. By contrast, PMA exerts its inhibitory effect in transcytotic sphingolipid transport on a transport step subsequent to internalization; i.e., it does not affect the uptake of sphingolipid from the basolateral membrane (Zegers and Hoekstra, 1997). In this context it is also of interest to note that the internalization of GPI-linked proteins via glycolipid-enriched microdomains or caveolae is regulated by PKC and PKA activity and is tightly linked to reorganization of the actin cytoskeleton (Parton et al., 1994; Deckert et al., 1996). As we showed in a previous study (Zegers and Hoekstra, 1997), neither inhibition of transcytosis by PMA treatment nor stimulation of this transport route by dB-cAMP correlates with an inhibition or stimulation, respectively, of total uptake of the fluorescent sphingolipids. We therefore hypothesized that inhibition of transcytosis via a kinase-regulated mechanism, as mentioned above, is unlikely. However, results of a preliminary study show that treatment of HepG2 cells with 1 μg/ml filipine, a sterol-binding agent that is known to interfere with caveolae-mediated endocytosis and transcytosis (Schnitzer et al., 1994), inhibits the transcytosis of C6-NBD-SM by 15 ± 4% (n = 5), which suggests that caveolae-mediated uptake may also play a role in the transcytotic delivery of sphingolipids. However, because cytD inhibits the fluid phase uptake of HRP to an extent similar to that observed for sphingolipid internalization, we propose that the inhibition of transcytotic sphingolipid transport by cytD is primarily due to an actin-dependent step during basolateral uptake, rather than to an interference in the final apical delivery of vesicles. Indeed, although conflicting reports have appeared (see (Sandvig and van Deurs, 1990; Gottlieb et al., 1993), evidence obtained from studies of several cell types shows that the actin cytoskeleton is involved in fluid phase uptake of HRP, whereas both clathrin-dependent and clathrin-independent mechanisms were reported to be inhibited by cytD as well (Sandvig and van Deurs, 1990; Gottlieb et al., 1993; Kubler and Riezman, 1993; Chazaud et al., 1994; Jackman et al., 1994; Durrbach et al., 1996; Maples et al., 1997). In polarized cells, actin-dependent endocytosis has only been reported to take place at the apical domain. In MDCK cells, it was demonstrated that cytD inhibits both fluid phase and clathrin-dependent endocytosis at the apical membrane (Gottlieb et al., 1993), whereas in Caco-2 cells the drug inhibits the apical uptake of ricin (Jackman et al., 1994). However, in both these cell lines, endocytosis from the basolateral domain is not affected by cytD. The results of these studies are in contrast with the inhibition of basolateral endocytosis that we describe here. A possible explanation for this discrepancy might be that the HepG2 cells are not completely polarized and have retained apical membrane remnants on their basolateral membrane after trypsinization. As has been described in freshly isolated hepatocyte couplets, such remnants are retargeted to the single remaining apical pole, which depends on intact actin filaments (Gautam et al., 1987). Indeed, also in HepG2 cells, microvilli could be occasionally detected on the basolateral membrane, as revealed by electron microscopy (Zegers, unpublished observation).
In summary, the present results emphasize the complexity of apically directed membrane trafficking, the degree of which, moreover, also appears to be depend on cell type. In the case of HepG2 cells, we have shown that protein kinases (Zegers and Hoekstra, 1997) and cytoskeletal elements (this work) regulate different aspects of direct and indirect membrane flow to the apical membrane. This knowledge, in conjunction with the application of photoaffinity-labeled sphingolipid analogs (Zegers et al., 1997), will be exploited to further dissect the involvement of specific molecular markers in intracellular trafficking in HepG2 cells.
ACKNOWLEDGMENTS
This work was supported by the Netherlands Foundation for Chemical Research with financial aid from the Netherlands Foundation for Scientific Research.
REFERENCES
- Babia T, Kok JW, Hoekstra D. The use of fluorescent lipid analogues to study endocytosis of glycosphingolipids. In: Greenstein B, editor. Receptor Research Methods. London: Harwood Academic; 1994. pp. 155–174. [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Boyer JL, Soroka CJ. Vesicle targeting to the apical domain regulates bile excretory function in isolated rat hepatocyte couplets. Gastroenterology. 1995;109:1600–1611. doi: 10.1016/0016-5085(95)90649-5. [DOI] [PubMed] [Google Scholar]
- Breitfeld PP, McKinnon WC, Mostov KE. Effect of nocodazole on vesicular traffic to the apical and basolateral surfaces of polarized MDCK cells. J Cell Biol. 1990;111:2365–2373. doi: 10.1083/jcb.111.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazaud B, Muriel MP, Bauvy C, Codogno P, Aubery M, Decastel M. Requirement of either the NH4Cl-sensitive or the cytochalasin D-sensitive pathway for ricin toxicity depends upon the enterocytic state of differentiation of HT-29 cells. Eur J Cell Biol. 1994;64:15–28. [PubMed] [Google Scholar]
- Costa de Beauregard MA, Pringault E, Robine S, Louvard D. Suppression of villin expression by antisense RNA impairs brush border assembly in polarized epithelial intestinal cells. EMBO J. 1995;14:409–421. doi: 10.1002/j.1460-2075.1995.tb07017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckert M, Ticchioni M, Bernard A. Endocytosis of GPI-anchored proteins in human lymphocytes: role of glycolipid-based domains, actin cytoskeleton, and protein kinases. J Cell Biol. 1996;133:791–799. doi: 10.1083/jcb.133.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin DG, Nelson WJ. Origins of cell polarity. Cell, 1996;84:335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- Durrbach A, Louvard D, Coudrier E. Actin filaments facilitate two steps of endocytosis. J Cell Sci. 1996;109:457–465. doi: 10.1242/jcs.109.2.457. [DOI] [PubMed] [Google Scholar]
- Eilers U, Klumperman J, Hauri HP. Nocodazole, a microtubule-active drug, interferes with apical protein delivery in cultured intestinal epithelial cells (Caco-2) J Cell Biol. 1989;108:13–22. doi: 10.1083/jcb.108.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fath KR, Burgess DR. Golgi-derived vesicles from developing epithelial cells bind actin filaments and possess myosin-I as a cytoplasmically oriented peripheral membrane protein. J Cell Biol. 1993;120:117–127. doi: 10.1083/jcb.120.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fath KR, Trimbur GM, Burgess DR. Molecular motors are differentially distributed on Golgi membranes from polarized epithelial cells. J Cell Biol. 1994;126:661–675. doi: 10.1083/jcb.126.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam A, Ng OC, Boyer JL. Isolated rat hepatocyte couplets in short-term culture: structural characteristics and plasma membrane reorganization. Hepatology. 1987;7:216–223. doi: 10.1002/hep.1840070203. [DOI] [PubMed] [Google Scholar]
- Gilbert T, Le Bivic A, Quaroni A, Rodriguez Boulan E. Microtubular organization and its involvement in the biogenetic pathways of plasma membrane proteins in Caco-2 intestinal epithelial cells. J Cell Biol. 1991;113:275–288. doi: 10.1083/jcb.113.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb TA, Ivanov IE, Adesnik M, Sabatini DD. Actin microfilaments play a critical role in endocytosis at the apical but not the basolateral surface of polarized epithelial cells. J Cell Biol. 1993;120:695–710. doi: 10.1083/jcb.120.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M, et al. Role of cytoskeleton and acidification of endocytic compartment in asialoglycoprotein metabolism in isolated rat hepatocyte couplets. Hepatology. 1995;21:1413–1421. [PubMed] [Google Scholar]
- Hunziker W, Male P, Mellman I. Differential microtubule requirements for transcytosis in MDCK cells. EMBO J. 1990;9:3515–3525. doi: 10.1002/j.1460-2075.1990.tb07560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman MR, Shurety W, Ellis JA, Luzio JP. Inhibition of apical but not basolateral endocytosis of ricin and folate in Caco-2 cells by cytochalasin D. J Cell Sci. 1994;107:2547–2556. doi: 10.1242/jcs.107.9.2547. [DOI] [PubMed] [Google Scholar]
- Kok JW, Babia T, Hoekstra D. Sorting of sphingolipids in the endocytic pathway of HT29 cells. J Cell Biol. 1991;114:231–239. doi: 10.1083/jcb.114.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok JW, Hoekstra K, Eskelinen S, Hoekstra D. Recycling pathways of glucosylceramide in BHK cells: distinct involvement of early and late endosomes. J Cell Sci. 1992;103:1139–1152. doi: 10.1242/jcs.103.4.1139. [DOI] [PubMed] [Google Scholar]
- Kubler E, Riezman H. Actin and fimbrin are required for the internalization step of endocytosis in yeast. EMBO J. 1993;12:2855–2862. doi: 10.1002/j.1460-2075.1993.tb05947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky NG, Pagano RE. Intracellular translocation of fluorescent sphingolipids in cultured fibroblasts: endogenously synthesized sphingomyelin and glucocerebrosidase analogues pass through the Golgi apparatus en route to the plasma membrane. J Cell Biol. 1985;100:27–34. doi: 10.1083/jcb.100.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maples CJ, Ruiz WG, Apodaca G. Both microtubules and actin filaments are required for efficient postendocytotic traffic of the polymeric immunoglobulin receptor in polarized Madin-Darby canine kidney cells. J Biol Chem. 1997;272:6741–6751. doi: 10.1074/jbc.272.10.6741. [DOI] [PubMed] [Google Scholar]
- Matter K, Bucher K, Hauri HP. Microtubule perturbation retards both the direct and the indirect apical pathway but does not affect sorting of plasma membrane proteins in intestinal epithelial cells (Caco-2) EMBO J. 1990;9:3163–3170. doi: 10.1002/j.1460-2075.1990.tb07514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays RW, Beck KA, Nelson WJ. Organization and function of the cytoskeleton in polarized epithelial cells: a component of the protein sorting machinery. Curr Opin Cell Biol. 1994;6:16–24. doi: 10.1016/0955-0674(94)90111-2. [DOI] [PubMed] [Google Scholar]
- Muallem S, Kwiatkowska K, Xu X, Yin HL. Actin filament disassembly is a sufficient final trigger for exocytosis in nonexcitable cells. J Cell Biol. 1995;128:589–598. doi: 10.1083/jcb.128.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan CM. Receptor-mediated endocytosis and lysosomal transport. In: Magee AI, Wileman T, editors. Protein Targeting. A Practical Approach. Oxford: Oxford University Press; 1992. pp. 1–23. [Google Scholar]
- Parczyk K, Haase W, Kondor Koch C. Microtubules are involved in the secretion of proteins at the apical cell surface of the polarized epithelial cell, Madin-Darby canine kidney, J. Biol Chem. 1989;264:16837–16846. [PubMed] [Google Scholar]
- Parton RG, Joggerst B, Simons K. Regulated internalization of caveolae. J Cell Biol. 1994;127:1199–1215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakisaka S, Ng OC, Boyer JL. Tubulovesicular transcytotic pathway in isolated rat hepatocyte couplets in culture. Effect of colchicine and taurocholate. Gastroenterology. 1988;95:793–804. doi: 10.1016/s0016-5085(88)80030-1. [DOI] [PubMed] [Google Scholar]
- Salas PJI, Misek DE, Vega-Salas DE, Gundersen D, Cereijido M, Rodriguez-Boulan E. Microtubules and actin filaments are not critically involved in the biogenesis of epithelial cell surface polarity. J Cell Biol. 1986;102:1853–1867. doi: 10.1083/jcb.102.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K, van Deurs B. Selective modulation of the endocytic uptake of ricin and fluid phase markers without alteration in transferrin endocytosis. J Biol Chem. 1990;265:6382–6388. [PubMed] [Google Scholar]
- Schnitzer JE, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol. 1994;127:1217–1232. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector I, Shochet NR, Kashman Y, Groweiss A. Latrunculins: novel marine toxins that disrupt microfilament organization in cultured cells. Science. 1983;219:493–495. doi: 10.1126/science.6681676. [DOI] [PubMed] [Google Scholar]
- van IJzendoorn SCD, Zegers MMP, Kok JW, Hoekstra D. Segregation of glucosylceramide and sphingomyelin occurs in the apical to basolateral transcytotic route in HepG2 cells. J Cell Biol. 1997;137:347–357. doi: 10.1083/jcb.137.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G, van’t Hof W. Epithelial sphingolipid sorting is insensitive to reorganization of the Golgi by nocodazole, but is abolished by monensin and by brefeldin A. J Cell Sci. 1993;104:833–842. doi: 10.1242/jcs.104.3.833. [DOI] [PubMed] [Google Scholar]
- van Zeijl MJ, Matlin KS. Microtubule perturbation inhibits intracellular transport of an apical membrane glycoprotein in a substrate-dependent manner in polarized Madin-Darby canine kidney epithelial cells. Cell Regul. 1990;1:921–936. doi: 10.1091/mbc.1.12.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaal KJM, Kok JW, Sormunen R, Eskelinen S, Hoekstra D. Intracellular sites involved in the biogenesis of bile canaliculi in hepatic cells. Eur J Cell Biol. 1994;63:10–19. [PubMed] [Google Scholar]
- Zegers MMP, Hoekstra D. Sphingolipid transport to the apical plasma membrane domain in human hepatoma cells is controlled by PKC and PKA activity: a correlation with cell polarity in HepG2 cells. J Cell Biol. 1997;138:307–321. doi: 10.1083/jcb.138.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegers MMP, Kok JW, Hoekstra D. Use of photoactivatable sphingolipid analogs to monitor lipid transport in mammalian cells. Biochem J. 1997;328:489–498. doi: 10.1042/bj3280489. [DOI] [PMC free article] [PubMed] [Google Scholar]