Abstract
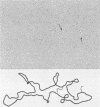
Temperature bacteriophage CP-T1 of Vibrio cholerae has a capsid that is 45 nm in diameter, a contractile tail 65 nm long and 9.5 nm wide, and a baseplate with several spikes or short tail fibers. The linear double-stranded DNA is 43.5 +/- 1.4 kilobases long, and the phage genome is both terminally redundant and partially circularly permuted. The extent of terminal redundancy is ca. 4%, and circular permutation is up to ca. 44%. Circular restriction maps have been constructed for the enzymes HindIII, EcoRI, BamHI, and PstI. By restriction endonuclease and heteroduplex analyses of phage DNA, the presence and location of a site (pac) at which packaging of phage DNA is initiated was established.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhaskaran K., Sinha V. B., Iyer S. S. Chromosome mobilization in Vibrio cholerae (biotype eltor) mediated by sex factor P. J Gen Microbiol. 1973 Sep;78(1):119–124. doi: 10.1099/00221287-78-1-119. [DOI] [PubMed] [Google Scholar]
- Bächi B., Arber W. Physical mapping of BglII, BamHI, EcoRI, HindIII and PstI restriction fragments of bacteriophage P1 DNA. Mol Gen Genet. 1977 Jun 24;153(3):311–324. doi: 10.1007/BF00431596. [DOI] [PubMed] [Google Scholar]
- Datta A., Parker C. D., Wohlhieter J. A., Baron L. S. Isolation and characterization of the fertility factor P of Vibrio cholerae. J Bacteriol. 1973 Feb;113(2):763–771. doi: 10.1128/jb.113.2.763-771.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J. C., Romig W. R. Complete and Defective Bacteriophages of Classical Vibrio cholerae: Relationship to the Kappa Type Bacteriophage. J Virol. 1975 May;15(5):1231–1238. doi: 10.1128/jvi.15.5.1231-1238.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys G. O., Trautner T. A. Maturation of bacteriophage SPPI DNA: limited precision in the sizing of mature bacteriophage genomes. J Virol. 1981 Feb;37(2):832–835. doi: 10.1128/jvi.37.2.832-835.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbesi F., Manning P. A. Biotype-specific restriction and modification of DNA in Vibrio cholerae. J Clin Microbiol. 1982 Sep;16(3):552–554. doi: 10.1128/jcm.16.3.552-554.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman R. B. Denaturation mapping of DNA. Methods Enzymol. 1974;29:451–458. doi: 10.1016/0076-6879(74)29037-2. [DOI] [PubMed] [Google Scholar]
- Jackson E. N., Jackson D. A., Deans R. J. EcoRI analysis of bacteriophage P22 DNA packaging. J Mol Biol. 1978 Jan 25;118(3):365–388. doi: 10.1016/0022-2836(78)90234-6. [DOI] [PubMed] [Google Scholar]
- Johnson S. R., Romig W. R. Transposon-facilitated recombination in Vibrio cholerae. Mol Gen Genet. 1979 Feb 16;170(1):93–101. doi: 10.1007/BF00268584. [DOI] [PubMed] [Google Scholar]
- Johnson S. R., Romig W. R. Vibrio cholerae conjugative plasmid pSJ15 contains transposable prophage dVcA1. J Bacteriol. 1981 May;146(2):632–638. doi: 10.1128/jb.146.2.632-638.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekalanos J. J., Moseley S. L., Murphy J. R., Falkow S. Isolation of enterotoxin structural gene deletion mutations in Vibrio cholerae induced by two mutagenic vibriophages. Proc Natl Acad Sci U S A. 1982 Jan;79(1):151–155. doi: 10.1073/pnas.79.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli G., Montenegro M. A., Hillenbrandt G., Scherzinger E., Trautner T. A. The genome of B. subtilis phage SPP1: assignment of 5'--3'-polarity to the complementary strands of SPP1 DNA. Mol Gen Genet. 1978 Aug 4;164(1):93–97. doi: 10.1007/BF00267603. [DOI] [PubMed] [Google Scholar]
- Morris C. F., Sinha N. K., Alberts B. M. Reconstruction of bacteriophage T4 DNA replication apparatus from purified components: rolling circle replication following de novo chain initiation on a single-stranded circular DNA template. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4800–4804. doi: 10.1073/pnas.72.12.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg J. E., Ogg B. J., Shrestha M. B., Poudayl L. Antigenic changes in Vibrio cholerae biotype eltor serotype Ogawa after bacteriophage infection. Infect Immun. 1979 Jun;24(3):974–978. doi: 10.1128/iai.24.3.974-978.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg J. E., Shrestha M. B., Poudayl L. Phage-induced changes in Vibrio cholerae: serotype and biotype conversions. Infect Immun. 1978 Jan;19(1):231–238. doi: 10.1128/iai.19.1.231-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg J. E., Timme T. L., Alemohammad M. M. General Transduction in Vibrio cholerae. Infect Immun. 1981 Feb;31(2):737–741. doi: 10.1128/iai.31.2.737-741.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C., Gauthier D., Tate A., Richardson K., Romig W. R. Expanded linkage map of Vibrio cholerae. Genetics. 1979 Feb;91(2):191–214. doi: 10.1093/genetics/91.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippsen P., Kramer R. A., Davis R. W. Cloning of the yeast ribosomal DNA repeat unit in SstI and HindIII lambda vectors using genetic and physical size selections. J Mol Biol. 1978 Aug 15;123(3):371–386. doi: 10.1016/0022-2836(78)90085-2. [DOI] [PubMed] [Google Scholar]
- Ratcliff S. W., Luh J., Ganesan A. T., Behrens B., Thompson R., Montenegro M. A., Morelli G., Trautner T. A. The genome of Bacillus subtilis phage SPP1: the arrangement of restriction endonuclease generated fragments. Mol Gen Genet. 1979 Jan 10;168(2):165–172. doi: 10.1007/BF00431442. [DOI] [PubMed] [Google Scholar]
- Schnabel H., Zillig W., Pfäffle M., Schnabel R., Michel H., Delius H. Halobacterium halobium phage øH. EMBO J. 1982;1(1):87–92. doi: 10.1002/j.1460-2075.1982.tb01129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]