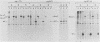Abstract
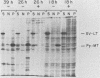
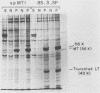
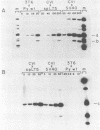
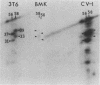
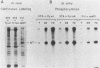
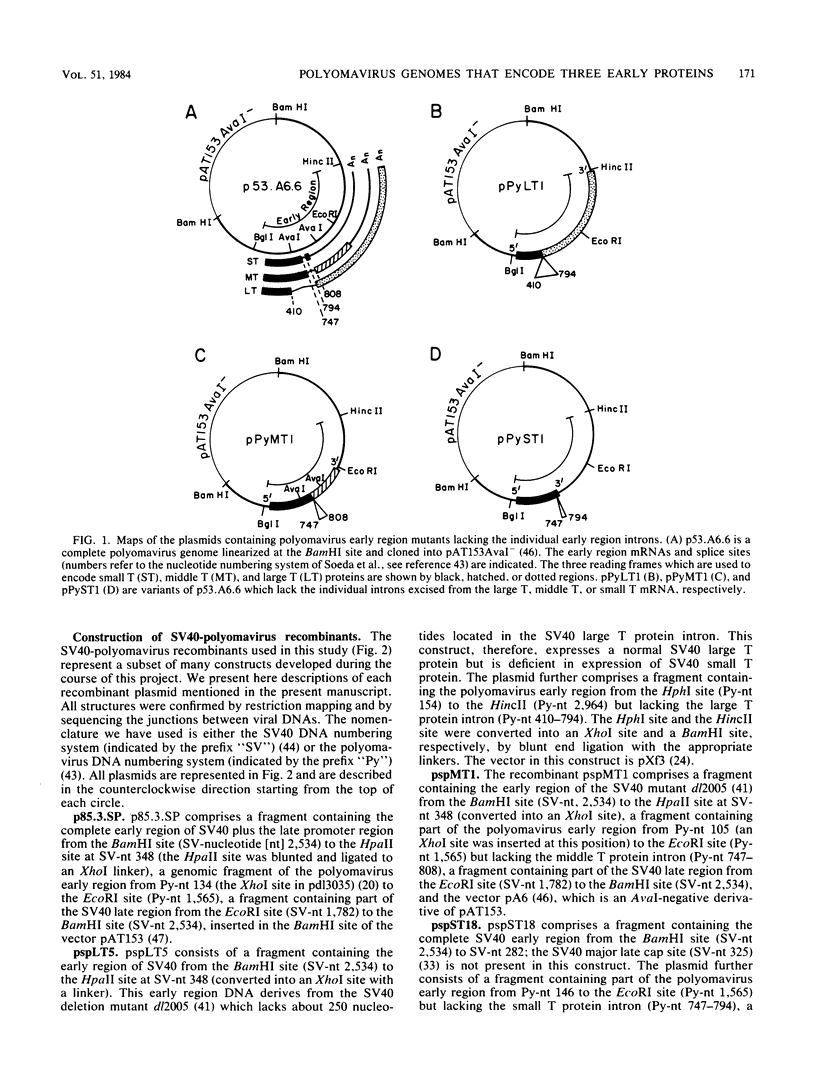
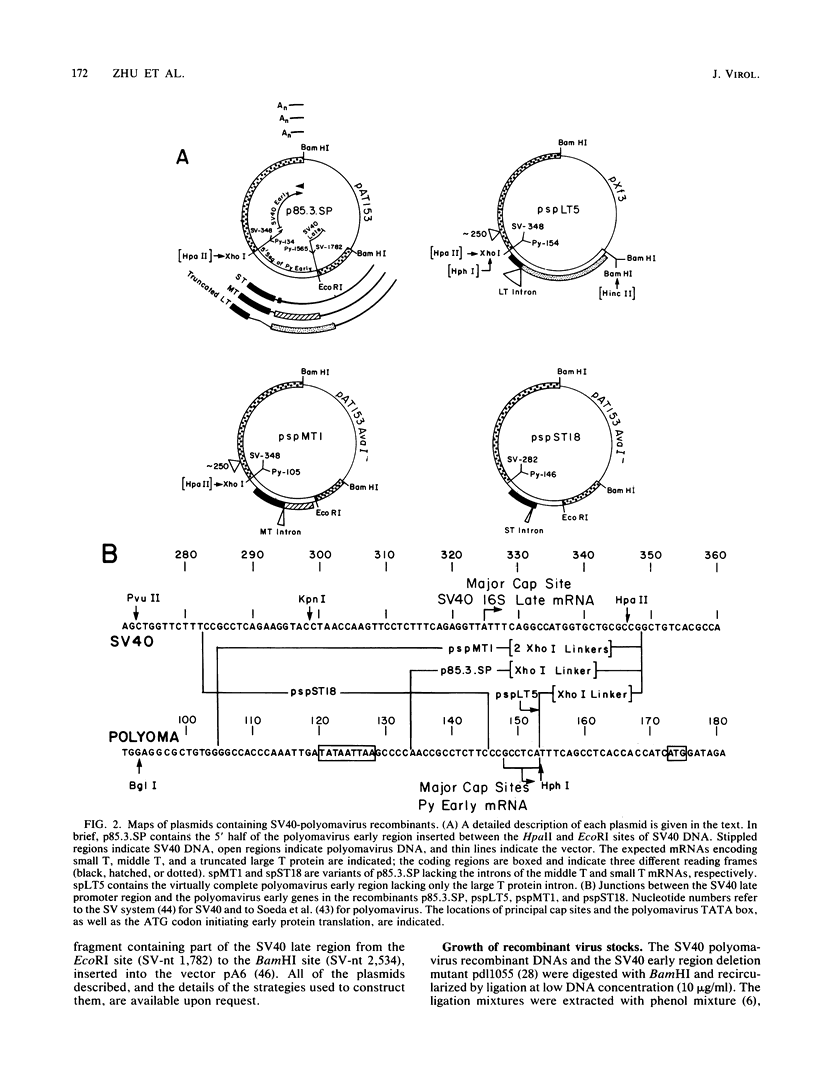
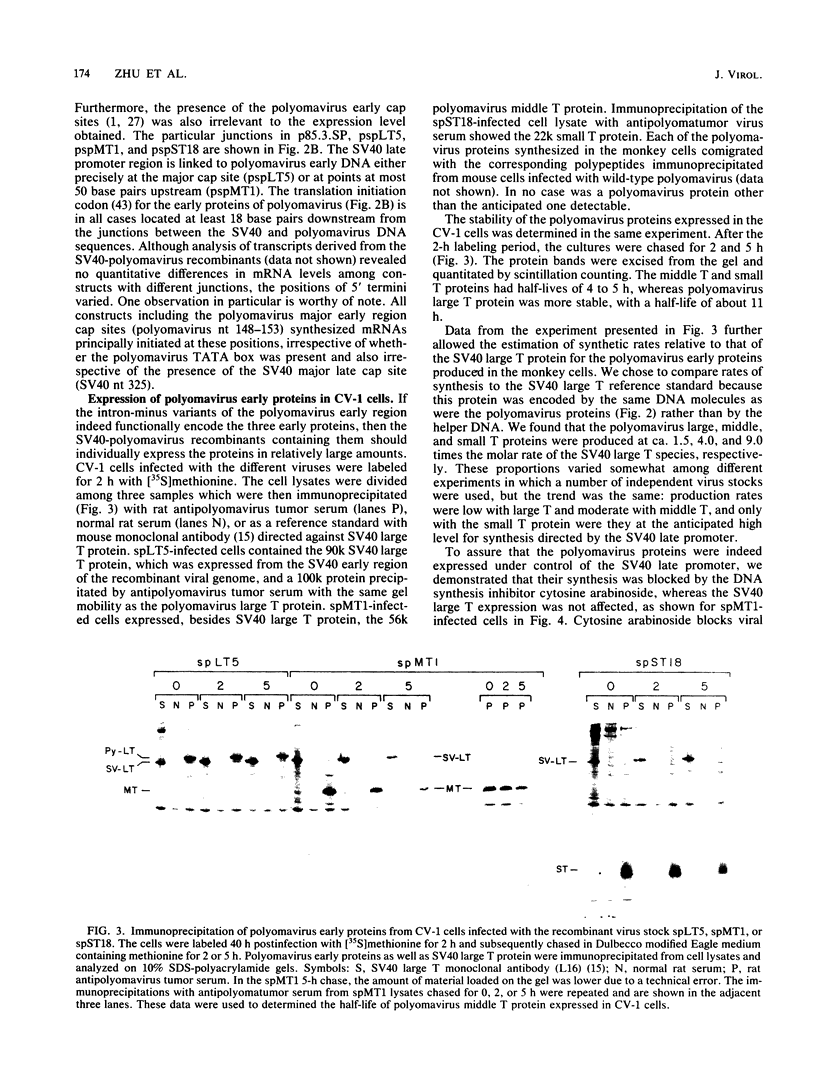
Modified polyomavirus genomes that individually encode the large and small T proteins were constructed by exchanging restriction endonuclease fragments between cDNA copies of the respective mRNAs and cloned genomic DNA. The efficacies of the new constructs, and that of the middle T protein gene described previously (R. Treisman , U. Novak, J. Favaloro , and R. Kamen , Nature [London] 292:595-600, 1981), were demonstrated with simian virus 40 (SV40)-polyomavirus recombinants in which part or all of the SV40 late region was replaced with the modified polyomavirus early genes. Each of the three recombinant viruses induced the synthesis of only the expected polyomavirus early protein in infected CV-1 cells. The rates of synthesis of large, middle, and small T proteins were ca. 1.5, 4.0, and 9.0 times the rate of synthesis of SV40 large T protein, respectively. The deletion of introns had no detrimental effect on mRNA biogenesis. Indeed, a further polyomavirus-SV40 recombinant, containing wild-type polyomavirus early region DNA, expressed an aberrant 58,000-dalton form of the middle T protein which we believe to result from utilization of a cryptic splice site. Immunofluorescence studied with monkey cells infected by the recombinant viruses allowed us to determine the cellular locations of the polyomavirus early proteins. Overproduction of the middle T protein did not result in a corresponding overproduction of the middle T protein-associated tyrosine phosphokinase activity.
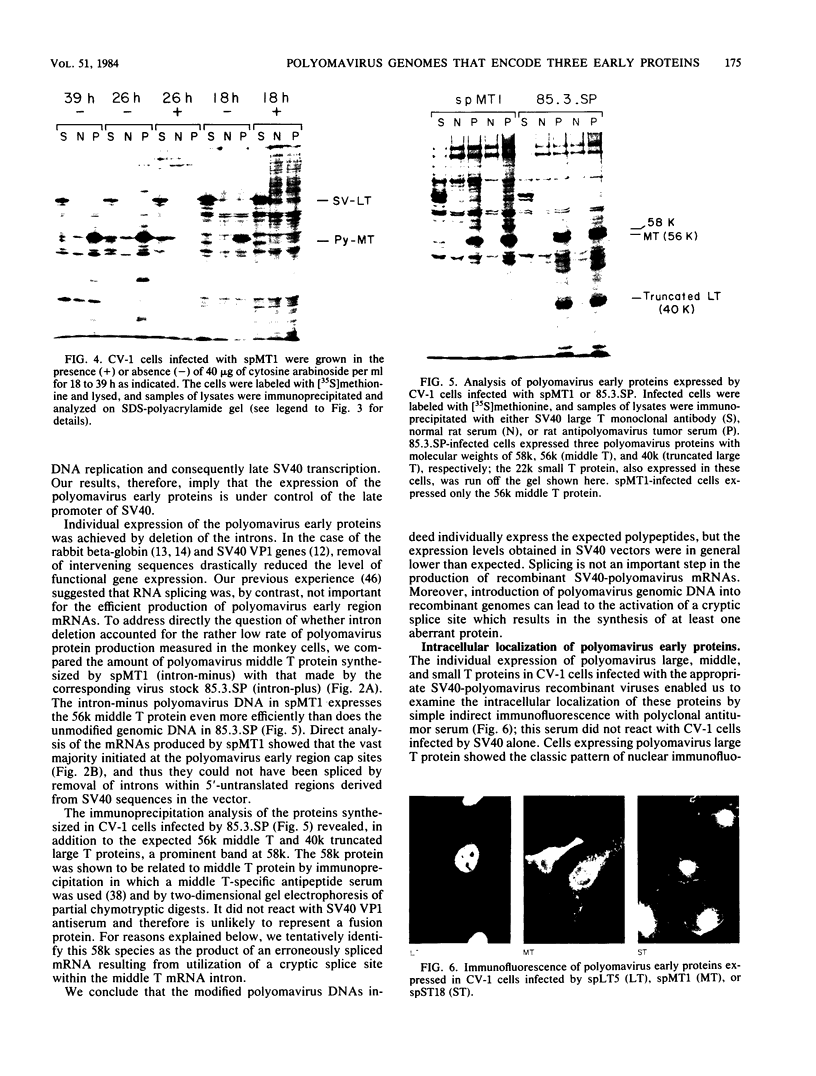
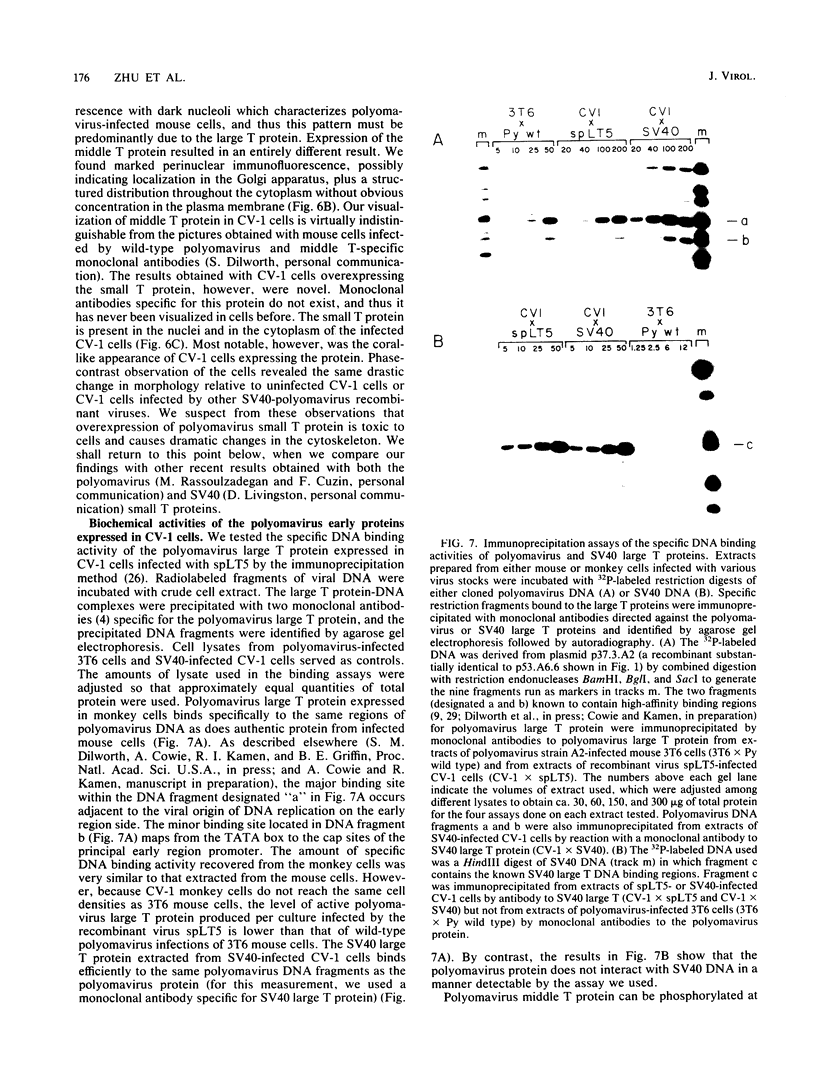
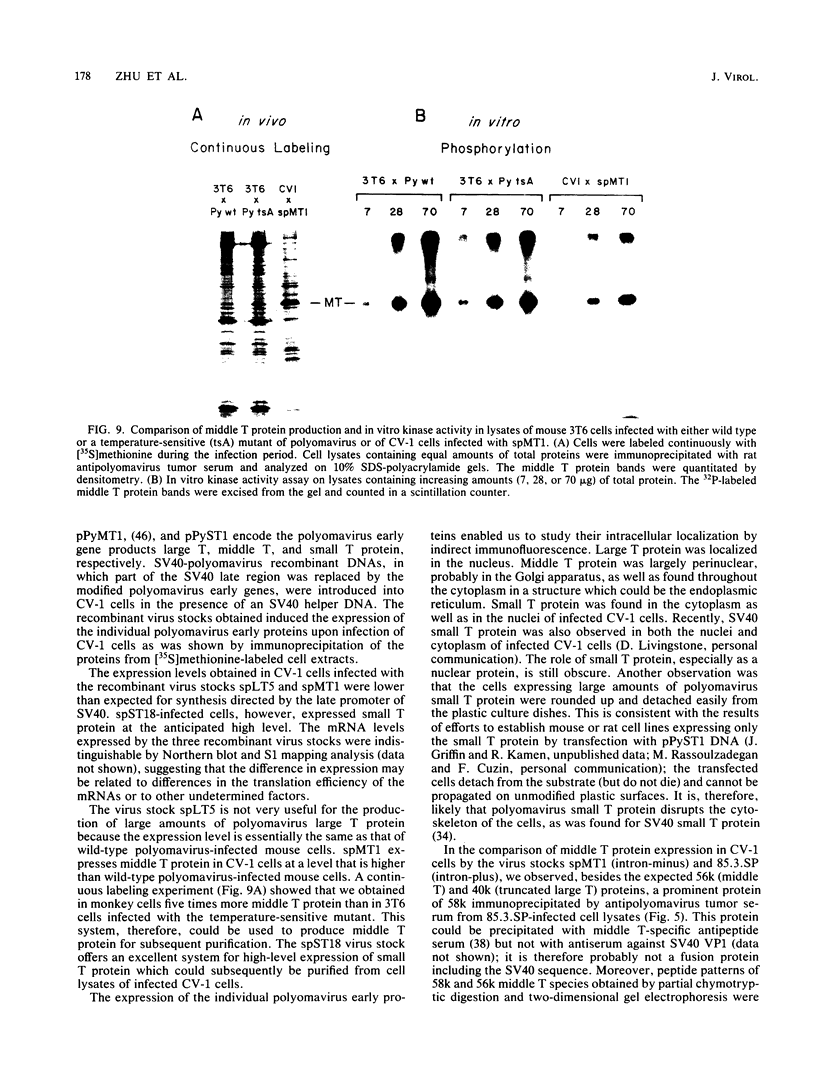
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Courtneidge S. A., Smith A. E. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature. 1983 Jun 2;303(5916):435–439. doi: 10.1038/303435a0. [DOI] [PubMed] [Google Scholar]
- Cowie A., Jat P., Kamen R. Determination of sequences at the capped 5' ends of polyoma virus early region transcripts synthesized in vivo and in vitro demonstrates an unusual microheterogeneity. J Mol Biol. 1982 Aug 5;159(2):225–255. doi: 10.1016/0022-2836(82)90494-6. [DOI] [PubMed] [Google Scholar]
- Dilworth S. M., Griffin B. E. Monoclonal antibodies against polyoma virus tumor antigens. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1059–1063. doi: 10.1073/pnas.79.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth S. M. Protein kinase activities associated with distinct antigenic forms of polyoma virus middle T-antigen. EMBO J. 1982;1(11):1319–1328. doi: 10.1002/j.1460-2075.1982.tb01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhart W., Hutchinson M. A., Hunter T. An activity phosphorylating tyrosine in polyoma T antigen immunoprecipitates. Cell. 1979 Dec;18(4):925–933. doi: 10.1016/0092-8674(79)90205-8. [DOI] [PubMed] [Google Scholar]
- FRIED M. CELL-TRANSFORMING ABILITY OF A TEMPERATURE-SENSITIVE MUTANT OF POLYOMA VIRUS. Proc Natl Acad Sci U S A. 1965 Mar;53:486–491. doi: 10.1073/pnas.53.3.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Friedmann T., Esty A., LaPorte P., Deininger P. The nucleotide sequence and genome organization of the polyoma early region: extensive nucleotide and amino acid homology with SV40. Cell. 1979 Jul;17(3):715–724. doi: 10.1016/0092-8674(79)90278-2. [DOI] [PubMed] [Google Scholar]
- Gaudray P., Tyndall C., Kamen R., Cuzin F. The high affinity binding site on polyoma virus DNA for the viral large-T protein. Nucleic Acids Res. 1981 Nov 11;9(21):5697–5710. doi: 10.1093/nar/9.21.5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Cell-surface expression of influenza haemagglutinin from a cloned DNA copy of the RNA gene. Nature. 1981 Oct 22;293(5834):620–625. doi: 10.1038/293620a0. [DOI] [PubMed] [Google Scholar]
- Gruss P., Khoury G. Gene transfer into mammalian cells: use of viral vectors to investigate regulatory signals for the expression of eukaryotic genes. Curr Top Microbiol Immunol. 1982;96:159–170. doi: 10.1007/978-3-642-68315-2_10. [DOI] [PubMed] [Google Scholar]
- Gruss P., Lai C. J., Dhar R., Khoury G. Splicing as a requirement for biogenesis of functional 16S mRNA of simian virus 40. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4317–4321. doi: 10.1073/pnas.76.9.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer D. H., Leder P. Expression of the chromosomal mouse Beta maj-globin gene cloned in SV40. Nature. 1979 Sep 6;281(5726):35–40. doi: 10.1038/281035a0. [DOI] [PubMed] [Google Scholar]
- Hamer D. H., Leder P. Splicing and the formation of stable RNA. Cell. 1979 Dec;18(4):1299–1302. doi: 10.1016/0092-8674(79)90240-x. [DOI] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayday A. C., Chaudry F., Fried M. Loss of polyoma virus infectivity as a result of a single amino acid change in a region of polyoma virus large T-antigen which has extensive amino acid homology with simian virus 40 large T-antigen. J Virol. 1983 Feb;45(2):693–699. doi: 10.1128/jvi.45.2.693-699.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Hutchinson M. A., Eckhart W. Translation of polyoma virus T antigens in vitro. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5917–5921. doi: 10.1073/pnas.75.12.5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Spurr N., Dulbecco R. Characterization of polyoma virus T antigen. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1259–1263. doi: 10.1073/pnas.74.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jat P., Novak U., Cowie A., Tyndall C., Kamen R. DNA sequences required for specific and efficient initiation of transcription at the polyoma virus early promoter. Mol Cell Biol. 1982 Jul;2(7):737–751. doi: 10.1128/mcb.2.7.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen R., Jat P., Treisman R., Favaloro J., Folk W. R. 5' termini of polyoma virus early region transcripts synthesized in vivo by wild-type virus and viable deletion mutants. J Mol Biol. 1982 Aug 5;159(2):189–224. doi: 10.1016/0022-2836(82)90493-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- McKay R. D. Binding of a simian virus 40 T antigen-related protein to DNA. J Mol Biol. 1981 Jan 25;145(3):471–488. doi: 10.1016/0022-2836(81)90540-4. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Howard B. H., Berg P. Synthesis of rabbit beta-globin in cultured monkey kidney cells following infection with a SV40 beta-globin recombinant genome. Nature. 1979 Jan 11;277(5692):108–114. doi: 10.1038/277108a0. [DOI] [PubMed] [Google Scholar]
- Pipas J. M., Adler S. P., Peden K. W., Nathans D. Deletion mutants of SV40 that affect the structure of viral tumor antigens. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):285–291. doi: 10.1101/sqb.1980.044.01.032. [DOI] [PubMed] [Google Scholar]
- Pomerantz B. J., Mueller C. R., Hassell J. A. Polyomavirus large T antigen binds independently to multiple, unique regions on the viral genome. J Virol. 1983 Sep;47(3):600–610. doi: 10.1128/jvi.47.3.600-610.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassoulzadegan M., Cowie A., Carr A., Glaichenhaus N., Kamen R., Cuzin F. The roles of individual polyoma virus early proteins in oncogenic transformation. Nature. 1982 Dec 23;300(5894):713–718. doi: 10.1038/300713a0. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M., Naghashfar Z., Cowie A., Carr A., Grisoni M., Kamen R., Cuzin F. Expression of the large T protein of polyoma virus promotes the establishment in culture of "normal" rodent fibroblast cell lines. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4354–4358. doi: 10.1073/pnas.80.14.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. B., Ghosh P. K., Lebowitz P., Weissman S. M. Gaps and duplicated sequences in the leaders of SV40 16S RNA. Nucleic Acids Res. 1978 Nov;5(11):4195–4213. doi: 10.1093/nar/5.11.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H., Figge J., Bladon M. T., Chen L. B., Ellman M., Bikel I., Farrell M., Livingston D. M. Role of small t antigen in the acute transforming activity of SV40. Cell. 1982 Sep;30(2):469–480. doi: 10.1016/0092-8674(82)90244-6. [DOI] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Schaffhausen B. S., Benjamin T. L. Phosphorylation of polyoma T antigens. Cell. 1979 Dec;18(4):935–946. doi: 10.1016/0092-8674(79)90206-x. [DOI] [PubMed] [Google Scholar]
- Schaffhausen B. S., Silver J. E., Benjamin T. L. Tumor antigen(s) in cell productively infected by wild-type polyoma virus and mutant NG-18. Proc Natl Acad Sci U S A. 1978 Jan;75(1):79–83. doi: 10.1073/pnas.75.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffhausen B., Benjamin T. L. Comparison of phosphorylation of two polyoma virus middle T antigens in vivo and in vitro. J Virol. 1981 Oct;40(1):184–196. doi: 10.1128/jvi.40.1.184-196.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffhausen B., Benjamin T. L., Pike L., Casnellie J., Krebs E. Antibody to the nonapeptide Glu-Glu-Glu-Glu-Tyr-Met-Pro-Met-Glu is specific for polyoma middle T antigen and inhibits in vitro kinase activity. J Biol Chem. 1982 Nov 10;257(21):12467–12470. [PubMed] [Google Scholar]
- Schaffhausen B. Transforming genes and gene products of polyoma and SV40. CRC Crit Rev Biochem. 1982;13(3):215–286. doi: 10.3109/10409238209114230. [DOI] [PubMed] [Google Scholar]
- Sleigh M. J., Topp W. C., Hanich R., Sambrook J. F. Mutants of SV40 with an altered small t protein are reduced in their ability to transform cells. Cell. 1978 May;14(1):79–88. doi: 10.1016/0092-8674(78)90303-3. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Smith R., Griffin B., Fried M. Protein kinase activity associated with polyoma virus middle T antigen in vitro. Cell. 1979 Dec;18(4):915–924. doi: 10.1016/0092-8674(79)90204-6. [DOI] [PubMed] [Google Scholar]
- Soeda E., Arrand J. R., Smolar N., Walsh J. E., Griffin B. E. Coding potential and regulatory signals of the polyoma virus genome. Nature. 1980 Jan 31;283(5746):445–453. doi: 10.1038/283445a0. [DOI] [PubMed] [Google Scholar]
- Treisman R., Cowie A., Favaloro J., Jat P., Kamen R. The structures of the spliced mRNAs encoding polyoma virus early region proteins. J Mol Appl Genet. 1981;1(2):83–92. [PubMed] [Google Scholar]
- Treisman R., Novak U., Favaloro J., Kamen R. Transformation of rat cells by an altered polyoma virus genome expressing only the middle-T protein. Nature. 1981 Aug 13;292(5824):595–600. doi: 10.1038/292595a0. [DOI] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Tyndall C., La Mantia G., Thacker C. M., Favaloro J., Kamen R. A region of the polyoma virus genome between the replication origin and late protein coding sequences is required in cis for both early gene expression and viral DNA replication. Nucleic Acids Res. 1981 Dec 11;9(23):6231–6250. doi: 10.1093/nar/9.23.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]