Abstract
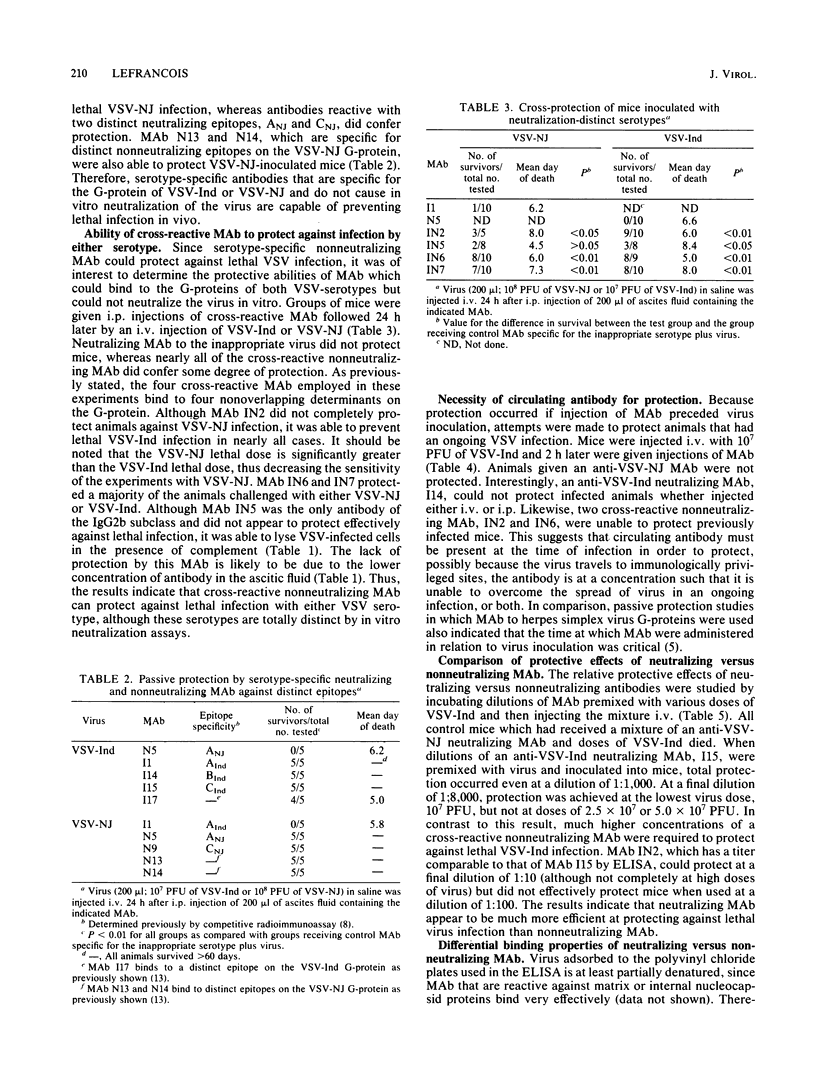
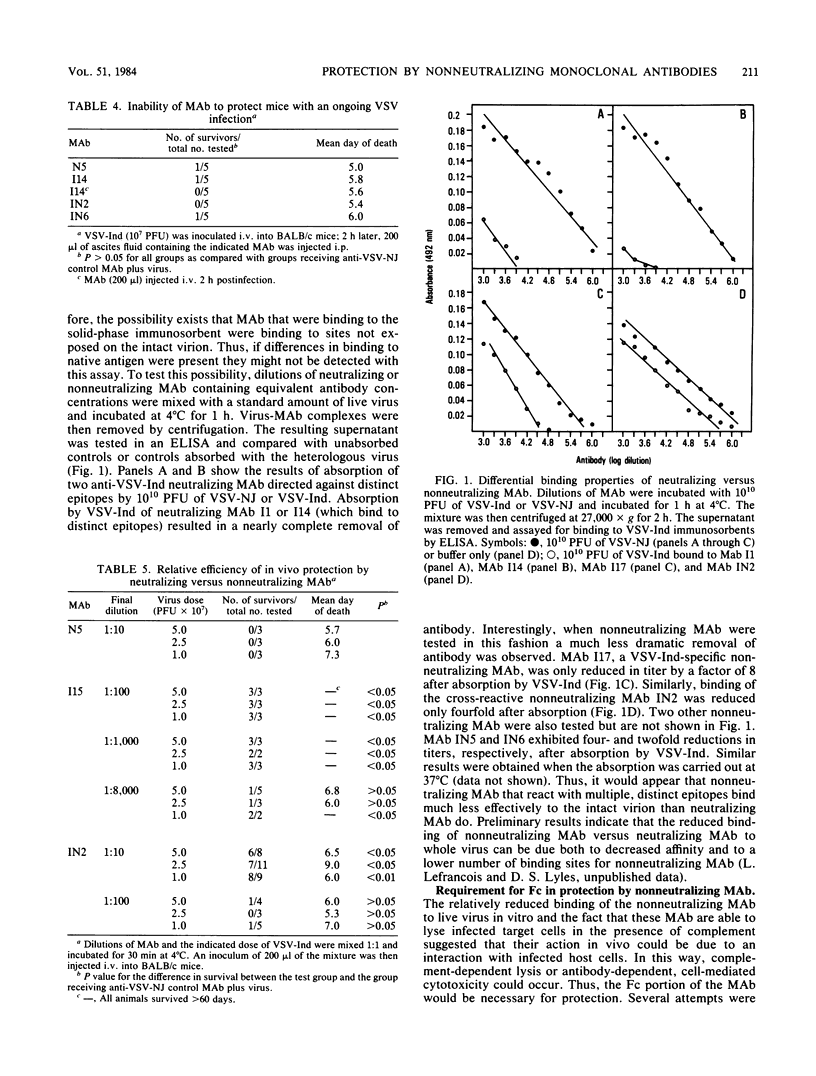
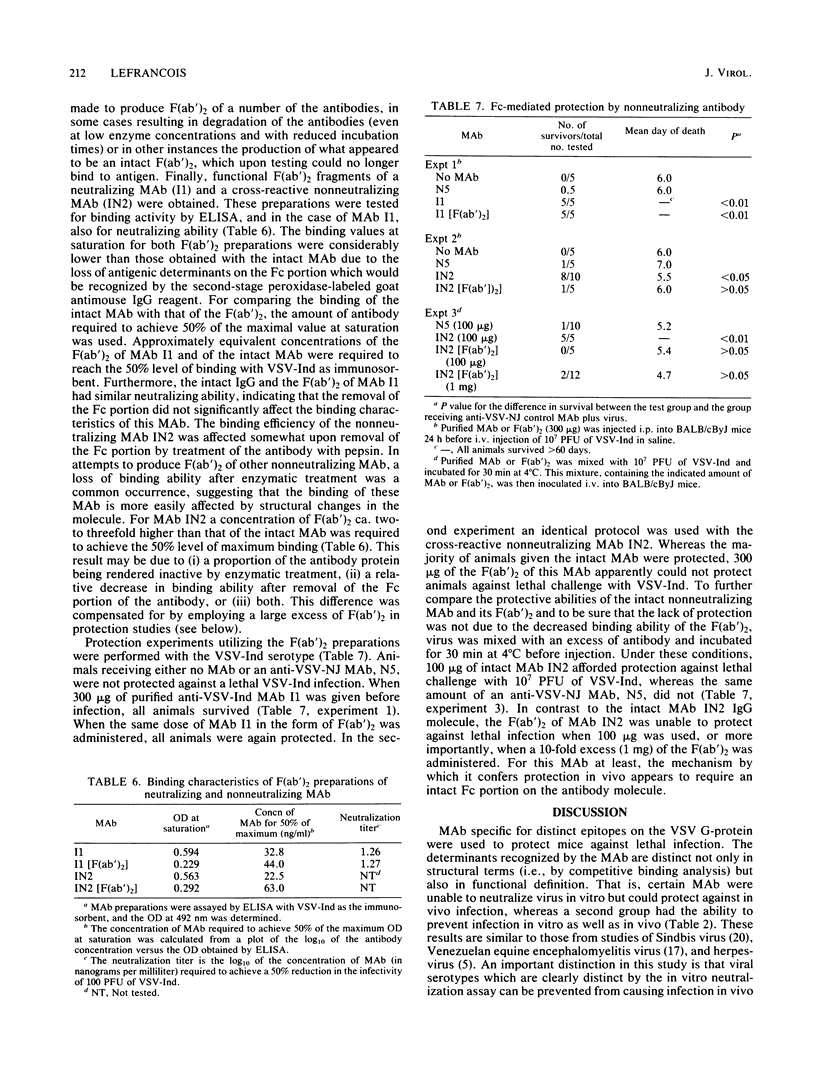
Monoclonal antibodies (MAb) reactive with the glycoprotein of vesicular stomatitis virus (VSV) serotypes Indiana (VSV-Ind) and New Jersey (VSV-NJ) were used to protect mice against lethal infection. MAb which reacted with a number of distinct epitopes and which could neutralize the virus in vitro could also protect against infection in vivo. MAb which could not neutralize the virus in vitro but which were specific for the glycoprotein of a single serotype were also able to protect mice against lethal VSV challenge. Interestingly, a group of MAb which cross-reacted with the glycoproteins of VSV-Ind and VSV-NJ could passively protect against challenge with either serotype. It was shown that as early as 2 h after infection, neither neutralizing nor nonneutralizing MAb could protect. Nonneutralizing MAb were found to be less effective at in vivo protection than neutralizing MAb. Furthermore, nonneutralizing MAb demonstrated a much lower binding efficiency to intact virions than did neutralizing MAb. These observations, plus the fact that the nonneutralizing MAb could lyse virus-infected cells in the presence of complement, suggested that in vivo protection by these antibodies may involve cell-associated viral determinants. To compare the mechanisms by which neutralizing and nonneutralizing MAb protected in vivo, F(ab')2 fragments were used in protection experiments. Although the F(ab')2 of a neutralizing MAb was still able to protect animals lethal virus challenge, the F(ab')2 of a cross-reactive nonneutralizing MAb was unable to do so. The reactivity of nonneutralizing MAb with virions and the apparent necessity of an intact Fc portion for protection further distinguish these antibodies from those MAb that are able to neutralize VSV solely by binding to the glycoprotein.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balachandran N., Bacchetti S., Rawls W. E. Protection against lethal challenge of BALB/c mice by passive transfer of monoclonal antibodies to five glycoproteins of herpes simplex virus type 2. Infect Immun. 1982 Sep;37(3):1132–1137. doi: 10.1128/iai.37.3.1132-1137.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanas A. C., Gould E. A., Clegg J. C., Varma M. G. Monoclonal antibodies to Sindbis virus glycoprotein E1 can neutralize, enhance infectivity, and independently inhibit haemagglutination or haemolysis. J Gen Virol. 1982 Jan;58(Pt 1):37–46. doi: 10.1099/0022-1317-58-1-37. [DOI] [PubMed] [Google Scholar]
- Collins J. J., Sackie D. M., Johnson G. R. Immunotherapy of murine leukemia. IX. The requirement for the Fc portion of antibody for successful passive serum therapy of Friend leukemia virus-induced disease. Virology. 1983 Apr 15;126(1):259–266. doi: 10.1016/0042-6822(83)90477-4. [DOI] [PubMed] [Google Scholar]
- Dix R. D., Pereira L., Baringer J. R. Use of monoclonal antibody directed against herpes simplex virus glycoproteins to protect mice against acute virus-induced neurological disease. Infect Immun. 1981 Oct;34(1):192–199. doi: 10.1128/iai.34.1.192-199.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields B. N., Hawkins K. Human infection with the virus of vesicular stomatitis during an epizootic. N Engl J Med. 1967 Nov 9;277(19):989–994. doi: 10.1056/NEJM196711092771901. [DOI] [PubMed] [Google Scholar]
- Flamand A., Wiktor T. J., Koprowski H. Use of hybridoma monoclonal antibodies in the detection of antigenic differences between rabies and rabies-related virus proteins. II. The glycoprotein. J Gen Virol. 1980 May;48(1):105–109. doi: 10.1099/0022-1317-48-1-105. [DOI] [PubMed] [Google Scholar]
- Gerhard W., Webster R. G. Antigenic drift in influenza A viruses. I. Selection and characterization of antigenic variants of A/PR/8/34 (HON1) influenza virus with monoclonal antibodies. J Exp Med. 1978 Aug 1;148(2):383–392. doi: 10.1084/jem.148.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley J. M., Emerson S. U., Wagner R. R. The glycoprotein of vesicular stomatitis virus is the antigen that gives rise to and reacts with neutralizing antibody. J Virol. 1972 Dec;10(6):1231–1235. doi: 10.1128/jvi.10.6.1231-1235.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lefrancios L., Lyles D. S. The interactionof antiody with the major surface glycoprotein of vesicular stomatitis virus. I. Analysis of neutralizing epitopes with monoclonal antibodies. Virology. 1982 Aug;121(1):157–167. [PubMed] [Google Scholar]
- Lefrancois L., Lyles D. S. Antigenic determinants of vesicular stomatitis virus: analysis with antigenic variants. J Immunol. 1983 Jan;130(1):394–398. [PubMed] [Google Scholar]
- Lefrancois L., Lyles D. S. Cytotoxic T lymphocytes reactive with vesicular stomatitis virus: analysis of specificity with monoclonal antibodies directed to the viral glycoprotein. J Immunol. 1983 Mar;130(3):1408–1412. [PubMed] [Google Scholar]
- Lefrancois L., Lyles D. S. The interaction of antibody with the major surface glycoprotein of vesicular stomatitis virus. II. Monoclonal antibodies of nonneutralizing and cross-reactive epitopes of Indiana and New Jersey serotypes. Virology. 1982 Aug;121(1):168–174. doi: 10.1016/0042-6822(82)90126-x. [DOI] [PubMed] [Google Scholar]
- Lostrom M. E., Stone M. R., Tam M., Burnette W. N., Pinter A., Nowinski R. C. Monoclonal antibodies against murine leukemia viruses: identification of six antigenic determinants on the p 15(E) and gp70 envelope proteins. Virology. 1979 Oct 30;98(2):336–350. doi: 10.1016/0042-6822(79)90557-9. [DOI] [PubMed] [Google Scholar]
- Mathews J. H., Roehrig J. T. Determination of the protective epitopes on the glycoproteins of Venezuelan equine encephalomyelitis virus by passive transfer of monoclonal antibodies. J Immunol. 1982 Dec;129(6):2763–2767. [PubMed] [Google Scholar]
- Miyoshi K., Harter D. H., Hsu K. C. Neuropathological and immunofluorescence studies of experimental vesicular stomatitis virus encephalitis in mice. J Neuropathol Exp Neurol. 1971 Apr;30(2):266–277. doi: 10.1097/00005072-197104000-00008. [DOI] [PubMed] [Google Scholar]
- Oakes J. E., Lausch R. N. Role of Fc fragments in antibody-mediated recovery from ocular and subcutaneous herpes simplex virus infections. Infect Immun. 1981 Jul;33(1):109–114. doi: 10.1128/iai.33.1.109-114.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaljohn A. L., Johnson E. D., Dalrymple J. M., Cole G. A. Non-neutralizing monoclonal antibodies can prevent lethal alphavirus encephalitis. Nature. 1982 May 6;297(5861):70–72. doi: 10.1038/297070a0. [DOI] [PubMed] [Google Scholar]
- Stampfer M., Baltimore D., Huang A. S. Ribonucleic acid synthesis of vesicular stomatitis virus. I. Species of ribonucleic acid found in Chinese hamster ovary cells infected with plaque-forming and defective particles. J Virol. 1969 Aug;4(2):154–161. doi: 10.1128/jvi.4.2.154-161.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley D. C., Wilson I. A., Skehel J. J. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981 Jan 29;289(5796):373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]



