Abstract
Synaptotagmins (Syts) are a family of vesicle proteins that have been implicated in both regulated neurosecretion and general membrane trafficking. Calcium-dependent interactions mediated through their C2 domains are proposed to contribute to the mechanism by which Syts trigger calcium-dependent neurotransmitter release. Syt IV is a novel member of the Syt family that is induced by cell depolarization and has a rapid rate of synthesis and a short half-life. Moreover, the C2A domain of Syt IV does not bind calcium. We have examined the biochemical and functional properties of the C2 domains of Syt IV. Consistent with its non–calcium binding properties, the C2A domain of Syt IV binds syntaxin isoforms in a calcium-independent manner. In neuroendocrine pheochromocytoma (PC12) cells, Syt IV colocalizes with Syt I in the tips of the neurites. Microinjection of the C2A domain reveals that calcium-independent interactions mediated through this domain of Syt IV inhibit calcium-mediated neurotransmitter release from PC12 cells. Conversely, the C2B domain of Syt IV contains calcium binding properties, which permit homo-oligomerization as well as hetero-oligomerization with Syt I. Our observation that different combinatorial interactions exist between Syt and syntaxin isoforms, coupled with the calcium stimulated hetero-oligomerization of Syt isoforms, suggests that the secretory machinery contains a vast repertoire of biochemical properties for sensing calcium and regulating neurotransmitter release accordingly.
INTRODUCTION
Synaptotagmins (Syts) are a large family of vesicle proteins implicated in neurotransmitter release from neural and neuroendocrine tissues. All Syt isoforms are characterized by an amino-terminal intravesicular domain, a single transmembrane domain, and a large cytoplasmic region containing two homologous repeats termed C2 domains (C2A and C2B). A role for the C2 domains of Syt I in modulating neurotransmitter release is well established (O’Connor et al., 1994; Südhof and Rizo, 1996). The first C2 (C2A) domain of Syt I consists of an eight-stranded β-sandwich containing a C2 key (Sutton et al., 1995). Structural and mutational studies indicate that calcium binds to the top of the domain via five clustered aspartic acid residues. Calcium binding does not induce significant conformational changes in the Syt I C2A domain but rather increases its affinity for effector molecules such as negatively charged phospholipids and the plasma membrane protein syntaxin 1a (Stx 1a) (Perin et al., 1990; Bennett et al., 1993a; Davletov and Südhof, 1993; Chapman et al., 1995; Li et al., 1995; Shao et al., 1996, 1997). Microinjected recombinant Syt I C2A fragments inhibit neurotransmitter release from squid giant presynaptic terminals, adrenal chromaffin cells, and neuroendocrine PC12 cells (Elferink et al., 1993; Mikoshiba et al., 1995; Ohara-Imaizumi et al., 1997). Mutational studies with recombinant Syt I C2A fragments reveal that the inhibitory effect of these fragments on neurotransmitter release from PC12 cells involves calcium-independent events mediated through a polybasic motif, which is highly conserved in the C2A domains of eight Syt isoforms (Thomas and Elferink, 1998). Therefore, the C2A domain of Syt I contains distinct calcium-dependent and -independent activities, which may function in concert to trigger neurotransmitter release.
The second C2 domain (C2B) of Syt I mediates several calcium-dependent and -independent interactions. The Syt I C2B domain binds clathrin AP-2, high inositol polyphosphates, brain-specific soluble NSF attachment protein, N-type calcium channels, and Stx 1a in a calcium-independent manner (Niinobe et al., 1994; Zhang et al., 1994; Li et al., 1995; Kee and Scheller, 1996; Sheng et al., 1997). Conversely, calcium promotes the oligomerization of Syt I through its C2B domain (Chapman et al., 1996). Evidence supporting a role for calcium-stimulated oligomerization in Syt I function comes from genetic studies. In Drosophila, a subset of mutations in the Syt I C2B domain reduces the calcium responsiveness of neurotransmitter release (Littleton et al., 1994). These studies suggest that oligomer assembly is essential for Syt I function in synaptic transmission.
The high degree of conservation between the C2 domains of neural-specific and ubiquitously expressed Syts suggests that these proteins play a critical role in regulated neurosecretion as well as general membrane trafficking events. Syt IV expression is restricted to neural and neuroendocrine tissues (Vician et al., 1995). Syt IV is an immediate-early gene, whose expression is induced after kainic acid–induced seizures in rat brain and depolarization of neuroendocrine PC12 cells (Vician et al., 1995). The C2A domain of Syt IV does not bind calcium because of the presence of a serine residue at position 244 in the putative calcium coordination site. Substitution of this serine residue with an aspartic acid residue endows the Syt IV C2A domain with calcium binding properties (von Poser et al., 1997).
In this study, we examine the biochemical and functional properties of the C2 domains of Syt IV. Our studies demonstrate that the C2A domain of Syt IV contains distinct calcium-independent Stx binding properties, which may modulate neurotransmitter release. Microinjection of a recombinant Syt IV C2A fragment perturbs depolarization-induced secretion from PC12 cells. Furthermore, the inhibitory effect of this fragment involves calcium-independent interactions. Conversely, the C2B domain of Syt IV possesses calcium binding properties, which promote both the homo-oligomerization of Syt IV and hetero-oligomerization with Syt I. Together, our results suggest that Syt IV may function in calcium regulated secretion.
MATERIALS AND METHODS
Materials
All chemicals and reagents were purchased from Sigma (St. Louis, MO) and Fisher Scientific (Houston, TX) unless stated otherwise. Recombinant Stxs 2–4 in pGEX-KG and anti-HPC-1 antibody were gifts from Mark Bennett (University of California, Berkeley, CA). Anti–dopamine-β-hydroxylase (DβH) antibody was a gift from Ruth Angeletti (Albert Einstein College of Medicine, Bronx, NY). Plasmids encoding Syt I–VIII-GST fusion proteins, encompassing the C2A and C2B domains, were a gift from Dr. Tom Südhof (University of Texas Southwestern Medical Center, Dallas, TX), and a plasmid encoding a GST fusion protein of the C2 domain of neuronal precursor cell-expressed developmentally down-regulated 4 (Nedd4) was a gift from Dr. Daniela Rotin (The Hospital for Sick Children, Toronto, Ontario, Canada). α-Latrotoxin was purchased from Alomone Laboratories (Jerusalem, Israel). The anti-hemagglutinin (HA) antibody 12CA5 was purchased from Boehringer Mannheim (Indianapolis, IN).
Plasmid Construction and GST Fusion Proteins
For Stx binding and microinjection studies, plasmids encoding recombinant GST-Syt IV fusion proteins were constructed as follows: a cDNA fragment encoding the C2A domain of Syt IV (Syt IV C2A; amino acids 152–282) was generated by PCR using the following primers: 5′-GCG GAA TTC CGG AGA AGC TGG GCA CTC TC-3′ and 5′-CCC AAG CTT TCA AGC ATT TCT CTT GAT G-3′. A mutant Syt IV C2A fragment (Syt IV C2A/AAAA) was generated using outside primers listed above and the following internal mutagenic primers: 5′-GAC AAT CTT ACC AGA GGC AGC AGC AGC AGT GAA AAC CAG AGT GCT GAG G-3′ and 5′-CCT CAG CAC TCT GGT TTT CAC TGC TGC TGC TGC CTC TGG TAA GAT TGT C-3′. The PCR products were directionally cloned into the unique EcoRI and HindIII (New England Biolabs, Beverly, MA) sites of pGEX-KG (Guan and Dixon, 1991). The plasmid encoding a GST fusion protein with the C2A domain of Syt I (residues 140–267) has been described elsewhere (Thomas and Elferink, 1998). Plasmids encoding epitope-tagged rat Stxs 2–4-GST fusion proteins were constructed in a derivative of the expression vector pGEX-KG, in which oligonucleotides encoding the HA epitope (MYPYDVPDYA) were directionally cloned into the polylinker of pGEX-KG in frame with GST, generating pGEX-KG:HA. cDNAs encoding the cytoplasmic domains of Stxs 2–4 were inserted into the EcoRI and HindIII sites of pGEX-KG:HA so that the HA epitope extended from the amino terminus of each Stx isoform. To create a GST fusion protein for Syt IV antibody affinity purification, a cDNA fragment encoding the C2A domain and adjacent spacer region of Syt IV (amino acids 54–262) was generated by PCR from rat Syt IV cDNA (Vician et al., 1995) using primers 5′-GCG GGA TCC GTG CAC GTG CTT AAA GGA G-3′ and 5′-GCG GAA TTC CTC TTG ATG ATC TCT CTG G-3′, and cloned into the EcoRI and HindIII sites of the expression vector pGEX-2T (Pharmacia, Piscataway, NJ). For oligomerization studies, Syt I (amino acids 95–421) was amplified by reverse transcription PCR as previously described (Vician et al., 1995). Syt IV (amino acids 54–425) was amplified by PCR from rat Syt IV cDNA (Vician et al., 1995) using primers 5′-GCG GTC GAC GGT CAC GTG CTT AAA GGA G-3′ and 5′-GCG GCG GCC GCC CAC AAG CTT ACA ATC GCG-3′. Syt I and Syt IV PCR products were cloned into a modified version of the in vitro translation vector pSP64 Poly(A). Plasmids encoding a GST-Syt I C2B fragment (amino acids 248–421) and a Nedd4 C2 fragment (amino acids 78–218) have been previously described (Bennett et al., 1993b; Plant et al., 1997). Syt IV C2B (amino acids 258–425) domains were amplified by PCR from rat Syt IV cDNA using the primer pair 5′-GCG GTC GAC GCT CGT TCC TCT TTC AGG G-3′ and 5′-GCG GCG GCC GCC CAC AAG CTT ACA ATC GCG-3′. These PCR products were cloned into the expression vector pGEX-2T. After expression in Escherichia coli DH5α or JM109, all fusion proteins were purified from bacterial lysates by glutathione–agarose chromatography (Guan and Dixon, 1991).
Oligomerization of Syt I and IV
Syt I (amino acids 95–421) and Syt IV (amino acids 54–425) were in vitro translated using the TnT-coupled reticulocyte lysate system (Promega, Madison, WI) according to the manufacturer’s instructions. Five microliters of in vitro–translated Syt I or Syt IV were incubated with 10 μg of GST-Syt IV C2A, GST-Syt IV C2B, GST-Syt I C2B, or GST alone in binding buffer (50 mM Tris-HCl, pH 8.0, 100 mM KCl, 1% nonfat dry-milk, and 0.05% Tween 20) supplemented with 1 mM CaCl2 or 2 mM EGTA for 1 h at 4°C. The samples were washed three times in binding buffer, solubilized in 1% SDS, passed over a Chroma Spin-30 column (Clontech, Palo Alto, CA) previously equilibrated in PBS (137 mM NaCl, 2.68 mM KCl, 8.1 mM Na2HPO4, and 1.47 mM KH2PO4) to remove high concentrations of calcium, resuspended in SDS loading buffer, and fractionated by 12% SDS-PAGE. The gels were dried and exposed to autoradiographic film overnight.
Antibody Preparation and Purification
A polyclonal antisera against a soluble recombinant Syt IV fragment encompassing residues 54–262 was generated as described elsewhere (Ferguson et al., 1999). Anti-Syt IV antibodies were affinity purified using the soluble recombinant Syt IV fragment coupled to Affi-Gel 15 (Bio-Rad, Hercules, CA) according to the manufacturer’s specifications. After elution from the Syt IV affinity column, IgG fractions were pooled, supplemented with 150 mM NaCl, 5 mg/ml BSA, and 25% glycerol, and anti-Syt IV antibody aliquots were stored at −80°C.
Western Blot Analysis
For Western analysis, protein complexes were fractionated by SDS-PAGE and transferred to nitrocellulose, and the filters were blocked overnight at 4°C in buffer A (150.4 mM NaCl, 10 mM Tris-Cl, pH 7.4, 0.1% Tween 20 [TBST], and 5% dry milk). After blocking, the filters were incubated in buffer A for 1 h at room temperature with primary antibodies: anti-Stx 1a mAb (HPC-1) diluted 1:10,000, anti-HA antibody (12CA5) diluted 1:3000, affinity-purified anti-Syt IV antibody (1:1000), and anti-GST mAb (1:1000) (Zymed, San Francisco, CA). The blots were washed five times with TBST before incubation in buffer A with the appropriate HRP-conjugated 2° antibody diluted 1:5000 for 1 h at room temperature. Blots were washed five times with TBST followed by a single wash in 0.1 M Tris-Cl, pH 8.6, for 10 min. Immunoreactivity was visualized by enhanced chemiluminescence and autoradiography (Thomas and Elferink, 1998).
Stx Binding Studies
Binding studies using recombinant Syt and Stx isoforms were performed as described previously (Thomas and Elferink, 1998) with the following modifications. Briefly, 10 μg of GST-Syt I and GST-Syt IV C2A fragments immobilized on glutathione agarose beads were incubated with a 100 nM concentration of the indicated Stx isoform in the presence or absence of 3 mM CaCl2. For studies involving native Stx 1a, rat brain synaptosomes were prepared using established conditions with the following changes (Chapman et al., 1995). Rat brain detergent extracts were prepared by solubilizing synaptosomes in buffer B (50 mM HEPES-HCl, pH 7.6, 100 mM NaCl, and 1% Triton X-100) at a detergent-to-protein ratio of 10:1, with 10 strokes in a tight fitting Teflon glass homogenizer, followed by mixing end-over-end for 2 h at 4°C. Eight hundred micrograms of the solubilized rat brain synaptosomes were incubated in the presence or absence of 3 mM CaCl2 with 15 μg of recombinant GST-Syt I and IV C2A domains, GST only, or the Nedd4 C2 domain immobilized on glutathione–agarose beads in buffer C (10 mM HEPES-NaOH, pH 7.4, 0.15 M NaCl, 2 mM MgCl2, 0.2% Triton X-100, and 0.5 mM EGTA). Protein complexes were recovered by brief centrifugation, washed four times with buffer C, and fractionated by SDS-PAGE. After transfer to nitrocellulose, blots were probed with HPC-1 as outlined above.
Cell Culture Conditions and Transfections
COS-7 cells were grown to 50% confluence in 60-mm tissue culture plates containing Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS. For LipofectAMINE transfections (Life Technologies, Grand Island, NY), full-length Syt IV was cloned into the eukaryotic expression vector pcDNA3.1 zeo+ (Invitrogen, San Diego, CA) in both the forward and reverse orientations. After transfection, cells were harvested with 1% SDS in PBS and homogenized in a Teflon glass homogenizer, and cell lysates were analyzed by Western analysis. For Western analysis of Syt IV expression, PC12 cells were first grown to 70% confluence in 60-mm tissue culture plates containing DMEM supplemented with 10% FBS and 5% heat-inactivated horse serum. To prepare differentiated cells, dishes were treated with 50 ng/ml nerve growth factor (NGF) in DMEM containing 2% FBS and 1% horse serum for 48 h. PC12 lysates were prepared by homogenization, and 10 μg of each lysate were fractionated by SDS-PAGE and analyzed by Western blotting.
PC12 Cell Microinjections and Confocal Microscopy
PC12 cell culture, microinjection, detection, and quantitation of DβH surface immunoreactivity were performed as previously described (Elferink et al., 1993; Thomas and Elferink, 1998). For microinjection studies, soluble recombinant C2A and C2 fragments from Syt IV and Nedd4, respectively, were expressed as GST fusion proteins, purified by affinity chromatography, isolated by thrombin cleavage, and dialyzed in injection buffer as previously described (Thomas and Elferink, 1998). Before microinjection, soluble fragments were diluted to a working concentration of 0.75 μg/μl in microinjection buffer containing 3 mg/ml Texas Red–conjugated dextran 10,000. α-Latrotoxin–evoked secretion was performed using a stimulation buffer devoid of CaCl2 and supplemented with 2.2 mM MgCl2 and 5 mM EGTA. Cells were stimulated at 37°C for 10 min with 9.6 μM α-latrotoxin, fixed, and processed for DβH cell surface staining. For immunofluorescence confocal microscopy, NGF-differentiated PC12 cells were depolarized in a stimulation buffer (10 mM HEPES, pH 7.4, 77 mM NaCl, 55 mM KCl, 2.2 mM CaCl2, 0.33 M Na2HPO4, 0.44 M KH2PO4, NaHCO3, and 5.6 mM glucose) for 10 min to induce Syt IV production, followed by a 2-h recovery in DMEM containing 2% NGF. Cells were then fixed and stained with affinity-purified anti-Syt IV (1:100) and anti-Syt I (1:100) antibodies, using previously described conditions (Elferink et al., 1993; Thomas and Elferink, 1998). Immunofluorescence confocal microscopy was performed using a Zeiss 310 laser scanning microscope (Wayne State University, School of Medicine).
RESULTS
The Specificity of Syt–Stx Interactions Differs for Syt I and Syt IV
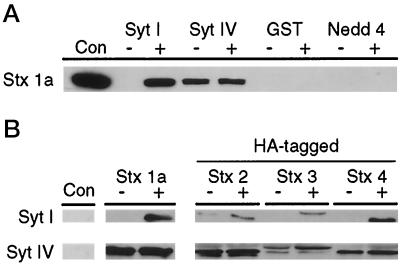
Syt I has been postulated to function in exocytosis through a mechanism involving interactions with the plasma membrane protein Stx 1a through the C2A domain (Südhof and Rizo, 1996). To determine whether Syt IV is also capable of interacting with native Stx 1a, recombinant Syt IV C2A was immobilized on glutathione–agarose and incubated with detergent-solubilized rat brain synaptosomes in the absence and presence of calcium. Recombinant protein–synaptosome complexes were detected by Western blot analysis using the anti-Stx 1a antibody HPC-1. As shown in Figure 1A, the Syt IV C2A domain binds Stx 1a in the absence and presence of calcium. In contrast, the Syt I C2A domain bound Stx 1a in a calcium-dependent manner, consistent with previous studies (Chapman et al., 1995; Li et al., 1995). As expected, GST alone fails to bind Stx 1a. To confirm the specificity of the Syt IV–Stx 1a interactions, we examined the Stx binding properties of the C2 domain from Nedd4. Nedd4 is a ubiquitin protein ligase, which interacts directly with Na+ channels in apical membranes of epithelial cells. The C2 domain of Nedd4 binds phospholipids and membranes in a calcium-dependent manner and is involved in localizing the protein to the apical membranes in epithelial cells (Staub et al., 1996; Plant et al., 1997). Given the calcium binding properties of the Nedd4 C2 domain, we examined its Stx 1a binding properties. No Stx 1a binding was observed with the Nedd4 C2 domain in the presence or absence of calcium (Figure 1A) using a wide concentration range of native Stx 1a (our unpublished results). Together, these data indicate that the C2A domain of Syt IV interacts specifically with Stx 1a, and that this interaction is not enhanced by calcium.
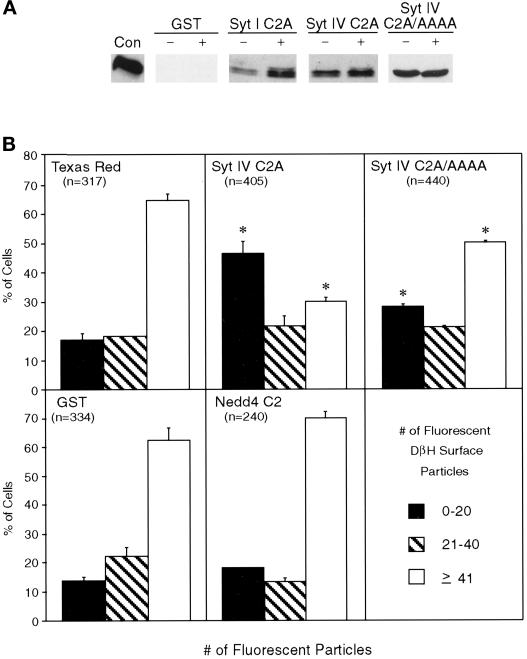
Figure 1.
Distinct Syt–Stx interactions exist for Syt I and Syt IV isoforms. (A) The indicated recombinant GST fusion proteins were immobilized on glutathione–agarose and incubated with 800 μg of rat brain synaptosomes in the absence (−) or presence (+) of 3 mM calcium. Protein complexes were isolated, fractionated by SDS-PAGE, and examined by Western analysis. Stx 1a binding was detected with HPC-1, followed by enhanced chemiluminescence. One hundred nanograms of soluble Stx 1a (Con) were used as a Western control. (B) Ten micrograms of immobilized recombinant Syt I and IV C2A domains were incubated with 100 nM soluble recombinant Stx 1a (amino acids 4–266) or HA-tagged Stxs 2 (amino acids 4–264), 3 (amino acids 4–264), or 4 (amino acids 1–268), and the protein complexes were examined by Western analysis. Stx 1a binding was detected with the anti-Stx 1a antibody HPC-1. Binding of HA-tagged Stxs 2–4 was detected with the anti-HA antibody 12CA5. No binding was detected with GST alone (Con).
The identification of multiple Syt and Stx isoforms in mammals suggests that isoform-specific Syt–Stx interactions may confer specificity to regulated secretion. To examine the specificity of Syt–Stx interactions, we compared the binding of recombinant Stxs 1a, 2, 3, and 4 with the C2A domains of Syts I and IV. The C2A domains of Syts I and IV were expressed as GST fusion proteins, immobilized on glutathione–agarose, and incubated with soluble recombinant Stxs. For detection purposes, Stxs 2–4 were HA tagged on their amino termini (Bennett et al., 1993a). Binding was detected by Western blot analysis using anti-HPC-1 (Stx 1a) or anti-HA epitope (Stxs 2–4) (Figure 1B). In the presence of calcium, strong binding to the C2A domain of Syt I is observed with Stx 1a and Stx 4. In contrast, lower levels of calcium-dependent binding are observed with Stx 2 and Stx 3. The C2A domain of Syt IV binds to each of the four Stxs tested; however, the strongest binding is observed with Stx 1a and Stx 2. In contrast to the calcium-dependent interactions of the Syt I C2A domain with the recombinant Stx proteins, Syt IV–Stx interactions are calcium independent, consistent with the non–calcium binding nature of the Syt IV C2A domain. No binding was observed with GST alone (Con) or with the recombinant Nedd4 C2 fragment (our unpublished results). These data indicate that both the specificity and calcium dependency of Syt–Stx interactions differ significantly between the C2A domains of Syt I and Syt IV.
The C2B Domain of Syt IV Mediates Homo- and Hetero-Oligomerization
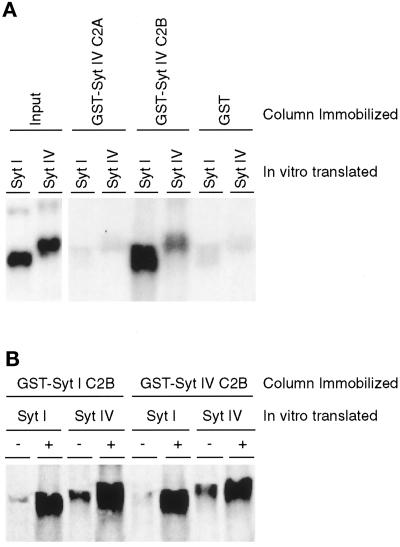
Biochemical and genetic studies suggest that Syt I acts as a multimeric complex to regulate neurotransmitter release (Littleton et al., 1994; Chapman et al., 1996). In detergent-solubilized rat brain extracts, native Syt I exists as an oligomeric structure, the basic unit of which is a homodimer (Geppert et al., 1994; Chapman et al., 1996). Dimerization of Syt I occurs through the C2B domain and is enhanced by calcium (Chapman et al., 1996). In Drosophila, mutations in the C2B domain of Syt I lower the calcium responsiveness of the dimeric complex, suggesting that Syt I functions as a homo-oligomer during synaptic transmission (Littleton et al., 1994). Comparison of the rat Syt I and Syt IV amino acid sequences suggests that, akin to the Syt I C2 domains, the C2B domain of Syt IV may also form a C2 key, which is capable of binding calcium. To examine the calcium binding properties of the Syt IV C2B domain, we compared the oligomerization properties of Syt IV with Syt I. Immobilized recombinant C2A and C2B Syt IV fragments were incubated with soluble, in vitro–translated, radiolabeled Syt I or Syt IV. Binding was detected by autoradiography (Figure 2). GST alone or the C2A domain of Syt IV shows essentially no binding (Figure 2A). In contrast, strong Syt I and Syt IV binding is observed with the C2B domain of Syt IV. These results indicate that the C2B domain of Syt IV is capable of both homo- and hetero-oligomerization with Syt I. To determine the calcium dependence of these interactions, these studies were performed in the presence or absence of calcium (Figure 2B). In the presence of calcium, both immobilized recombinant Syt I and Syt IV C2B domains interact with in vitro–translated Syt I and Syt IV; both Syt I C2B and Syt IV C2B form homo- and hetero-oligomers. Furthermore, calcium-dependent homo- and hetero-oligomer interactions were detected with an in vitro–translated, radiolabeled Syt IV C2B fragment and not a Syt IV C2A fragment (our unpublished results). Together, these data indicate that the C2B domain of Syt IV exhibits calcium binding properties, which promote both the formation of Syt IV homo-oligomers as well as the formation of hetero-oligomers with the C2B domain of Syt I.
Figure 2.
Calcium-dependent, C2B domain–mediated homo- and hetero-oligomerization of Syt I and Syt IV. (A) Five microliters of in vitro–translated, [35S]methionine-labeled cytoplasmic domain of Syt I and Syt IV were incubated with 10 μg of immobilized GST-Syt IV C2A, GST-Syt IV C2B, or GST alone in the presence of calcium. Protein complexes were isolated and analyzed by SDS-PAGE. Gels were dried and exposed to autoradiographic film overnight. In vitro–translated, [35S]methionine-labeled Syt I and Syt IV products are shown in the left two lanes (Input). (B) Five microliters of in vitro–translated, [35S]methionine-labeled cytoplasmic domain of Syt I and Syt IV were incubated with 10 μg of immobilized GST-Syt I C2B or GST-Syt IV C2B in the absence (−) or presence (+) of calcium. Protein complexes were analyzed as described above.
Syt IV and Syt I Colocalize in the Tips of Neurites in NGF-differentiated PC12 Cells
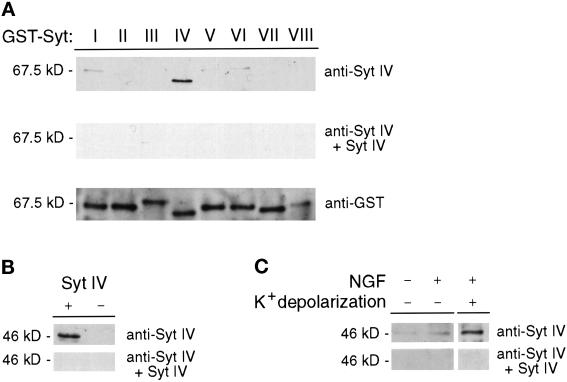
We have recently used biochemical approaches to demonstrate that Syt IV colocalizes with Syt I to the large dense core vesicles (LDCVs) and synaptic-like microvesicles (SLMVs) of neuroendocrine PC12 cells (Ferguson et al., 1999). To examine the subcellular localization of these Syt isoforms in vivo, we prepared affinity-purified anti-Syt IV antibodies. Before staining PC12 cells, we examined the specificity of the affinity-purified antibody for Syt IV by Western blot analysis against recombinant Syt I–VIII isoforms expressed as GST fusion proteins. As shown in Figure 3A (top panel), the anti-Syt IV antibody reacts only with recombinant Syt IV and not with the other Syt isoforms tested. Additionally, Syt IV immunoreactivity is blocked by preincubating the antibody with soluble Syt IV protein (Figure 3A, middle panel). Probing a second filter with anti-GST antibody confirms the presence of all GST-Syt proteins (Figure 3A, bottom panel). To further confirm the specificity of the affinity-purified antibody, we transiently expressed the Syt IV cDNA in COS-7 cells. Western analysis of COS-7 cell lysates detects an immunoreactive 46-kDa product present in cells transfected with Syt IV (consistent with its predicted molecular mass) but not in cells transfected with a plasmid containing the Syt IV cDNA in the reverse orientation (Figure 3B, top panel) or parental vector only (our unpublished results). Furthermore, preincubating the antibody with recombinant Syt IV C2A domain abolishes Syt IV immunoreactivity (Figure 3B, bottom panel). We next examined the ability of our affinity-purified antisera to detect Syt IV in NGF-differentiated and undifferentiated PC12 cells (Figure 3C, top panel). Although Syt IV immunoreactivity is not detected in undifferentiated PC12 cells, low levels are detected in cells differentiated with NGF for 48 h. In a recent study, we demonstrated that Syt IV is a relatively labile protein in PC12 cells, with a half-life of 2 h (Ferguson et al., 1999). However, expression of Syt IV in PC12 cells can be induced by chemical depolarization. Therefore, we examined the effect of chemical depolarization on expression of the Syt IV protein in NGF-differentiated PC12 cells. As shown in Figure 3C, chemical depolarization of NGF-differentiated PC12 cells with 55 mM KCl for 10 min induces significant expression of Syt IV protein; Syt IV immunoreactivity is blocked by preincubating the antibody with soluble recombinant Syt IV protein.
Figure 3.
Affinity-purified anti-Syt IV antibody does not react with other Syt isoforms. (A) Five hundred nanograms of each soluble Syt I–VIII-GST fusion protein were subjected to SDS-PAGE and analyzed by Western blotting. Filters were probed with the affinity-purified Syt IV antibody in the absence (A, top panel) or presence (A, middle panel) of 500 μg of soluble Syt IV. The presence of GST-Syt fusion proteins was confirmed by probing a filter with an anti-GST mAb (A, bottom panel). (B) Cell lysates from COS-7 cells transiently transfected with full-length Syt IV cDNA cloned into the eukaryotic expression vector pcDNA3.1 zeo+, in the forward (+) and reverse (−) orientations, were examined for expression of Syt IV by Western analysis. Filters were probed in the absence (B, top panel) or presence (B, bottom panel) of 500 μg of soluble Syt IV. (C) PC12 cells treated as indicated were examined for Syt IV expression by Western analysis. Filters were probed with anti-Syt IV antibody in the absence (C, top panel) or presence (C, bottom panel) of 500 μg of soluble Syt IV.
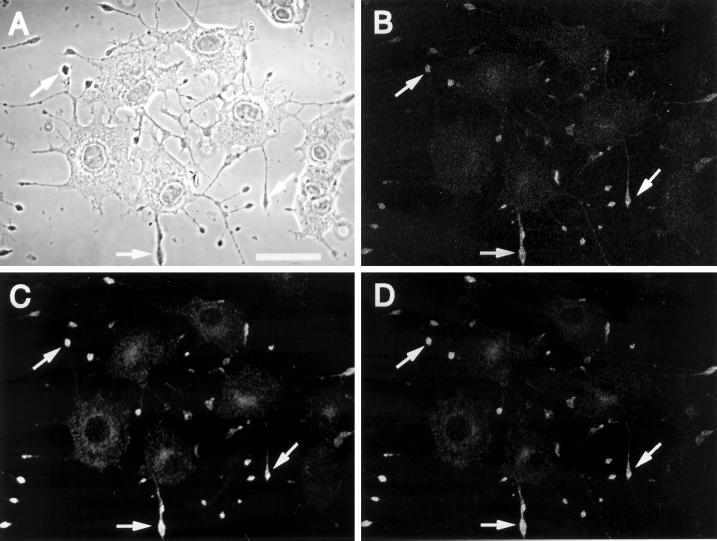
To compare the subcellular localization of Syt IV with Syt I in vivo, we performed double immunofluorescent labeling and confocal microscopy on NGF-differentiated PC12 cells. Although Syt IV is expressed at low levels in PC12 cells (Ferguson et al., 1999), native Syt IV is not readily detected in the tips of these cells by immunofluorescent microscopy (our unpublished results). Chemical depolarization induces the rapid synthesis of Syt IV in the cell body, reaching maximal levels in the tips within 1–2 h after depolarization (our unpublished results). Therefore, we performed our subcellular localization studies on depolarized PC12 cells. As shown in Figure 4, Syt I and Syt IV immunoreactivities concentrate in the tips of the neurites (compare A–C). Furthermore, Syt IV immunoreactivity in the tips of extending neurites overlaps with Syt I (Figure 4D), consistent with the distribution of LDCVs and SLMVs in PC12 cells. Coupled with our recent studies demonstrating that Syt I and Syt IV coprecipitate from a PC12 cell fraction enriched in LDCVs and SLMVs (Ferguson et al., 1999), these data indicate the vesicular colocalization of these Syt isoforms.
Figure 4.
Syt I and Syt IV colocalize to the tips of neurites in PC12 cells in vivo. NGF-differentiated PC12 cells were K+ depolarized and 2 h after depolarization analyzed by double immunofluorescence confocal microscopy. A phase-contrast image of representative cells is shown in A. Cells were costained for endogenous Syt I (B) and Syt IV (C) and detected using secondary antibodies coupled to RITC and FITC, respectively. Representative areas of overlap between Syt I and Syt IV in these panels and the merged image (D) are indicated by the arrows in the tips of PC12 cells. Bar, 20 μm.
The C2A Domain of Syt IV Inhibits Calcium-regulated Secretion from Microinjected PC12 Cells
Although genetic and microinjection studies support a role for Syt I in calcium-regulated secretion, the contribution of other Syt isoforms to this process remains to be determined. We previously demonstrated that a microinjected recombinant Syt I fragment encompassing the C2A domain inhibits calcium-regulated secretion from PC12 cells (Elferink et al., 1993). Interestingly, the inhibitory effect on secretion by recombinant Syt I C2A fragments occurs independently of calcium-mediated interactions with Stx 1a and is conferred by a novel polybasic motif unique to the C2A domains of several Syt isoforms (Thomas and Elferink, 1998). Given the enriched expression of Syt IV in PC12 cells after depolarization, Syt IV’s vesicular colocalization with Syt I, and the presence of a C2A polybasic motif in Syt IV, the Syt IV C2A domain may inhibit neurotransmitter release when injected into PC12 cells. Additionally, we prepared and assayed a new recombinant Syt IV C2A fragment in which the polybasic motif KKHK at residues 202–205 was replaced with alanines (Syt IV C2A/AAAA). We examined the functional consequences of these mutations on 1) Stx 1a interactions and 2) calcium-regulated secretion when microinjected into PC12 cells. For Stx 1a binding studies, immobilized GST-Syt IV C2A wild-type and mutant fragments were incubated with detergent-solubilized rat brain synaptosomes in the presence or absence of calcium. Binding of native Stx 1a from the synaptosome extract was determined by Western blot analysis (Figure 5A). Recombinant wild-type and mutant Syt IV C2A fragments both bind native Stx 1a; however, binding is calcium independent, consistent with the non–calcium binding properties of the Syt IV C2A domain. As expected, calcium stimulates the binding of native Stx 1a to the recombinant Syt I C2A fragment, whereas no binding is observed with GST only (Figure 5A) or Nedd4 (our unpublished results), demonstrating the specificity of these Syt IV–Stx 1a interactions. These data suggest that like the wild-type recombinant Syt IV C2A fragment, the mutant Syt IV C2A domain is a well-folded protein.
Figure 5.
The polybasic motif in the C2A domain of Syt IV functions in calcium-regulated secretion. (A) The indicated recombinant GST fusion proteins were immobilized on glutathione–agarose and incubated with 800 μg of rat brain synaptosomes in the presence (+) or absence (−) of 3 mM calcium. Protein complexes were isolated, fractionated by SDS-PAGE, and examined by Western analysis. Stx 1a binding was detected with HPC-1, followed by enhanced chemiluminescence. One hundred nanograms of soluble Stx 1a (Con) were used as a Western control. (B) NGF-differentiated PC12 cells were coinjected with Texas Red–conjugated dextran and the indicated soluble recombinant Syt IV or Nedd4 fragments. Control cells were injected with Texas Red–conjugated dextran only (Texas Red) or coinjected with a control GST extract (GST). One hour after microinjection, the cells were K+ depolarized in the presence of calcium, and DβH surface immunoreactivity was detected with a fluorescein-labeled secondary antibody. The numbers of individual fluorescent particles were counted, regardless of their size, and are presented as a percent of the total number of cells injected. Data are shown as the mean of two independent experiments ± SD with the total number of injected cells (n). Significant differences (p ≤ 0.01, χ2 analysis) between experimental and control treatments are indicated with an asterisk.
We previously demonstrated that depolarization of PC12 cells promotes the fusion of LDCVs with the plasma membrane, exposing an intravesicular form of the enzyme DβH on the cell surface that can be quantitated by immunofluorescent microscopy (Bennett et al., 1993a; Elferink et al., 1993; Thomas and Elferink, 1998). Using this in vivo secretion assay, we compared the effects of microinjecting wild-type and mutant Syt IV C2A fragments on calcium-regulated secretion from NGF-differentiated PC12 cells (Figure 5B). After depolarization, high levels of DβH surface staining (≥41 fluorescent particles) is observed on 60–65% of control cells injected with Texas Red–conjugated dextran only or coinjected with a control GST extract or the soluble C2 domain of Nedd4. In contrast, microinjection of the wild-type Syt IV C2A domain produces a 50% reduction in maximal calcium-regulated secretion from PC12 cells (≥41 fluorescent particles). Microinjection of the soluble recombinant Syt IV C2A fragment containing mutations in the polybasic motif (Syt IV C2A/AAAA) results in a 20% reduction in maximal calcium-regulated secretion. The differences between the relative blocking effects of the wild-type and mutant Syt IV C2A domains are statistically significant (p < 0.0001, χ2 analysis), suggesting that the polybasic motif is important for the inhibitory effect of the microinjected Syt IV C2A domains. Therefore, these data indicate that, akin to studies with Syt I, the C2A domain of Syt IV blocks secretion when microinjected into PC12 cells. Furthermore, the blocking effect of the Syt IV C2A domain on calcium-regulated secretion is mediated, at least partially, through the polybasic motif.
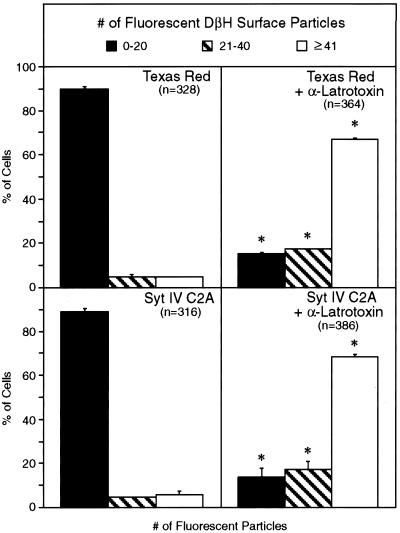
To ensure that the injected recombinant Syt IV C2A fragment interferes with the normal calcium-mediated cellular secretory machinery, we performed a series of microinjection experiments using α-latrotoxin. α-Latrotoxin is a potent neurotoxin from black widow spider venom, which is proposed to bypass the calcium requirement for vesicle fusion and trigger neurotransmitter release from neural and neuroendocrine cells (Rosenthal et al., 1990). We hypothesized that, if the recombinant Syt IV C2A fragment impaired the functional secretory machinery, then α-latrotoxin would overcome the inhibitory effect of this fragment in our in vivo secretion assay. In the absence of calcium and α-latrotoxin, minimal DβH cell surface staining is observed on 90% of control cells (0–20 fluorescent particles) injected with control GST extracts (our unpublished results), Texas Red alone, or the wild-type Syt IV C2A fragment (Syt IV C2A) (Figure 6). α-Latrotoxin treatment of cells injected with either Texas Red alone or Syt IV C2A triggers calcium-independent secretion, as indicated by an increase to 70% of cells with maximal DβH cell surface staining (≥41 fluorescent particles). Together, these data indicate that microinjection of a recombinant Syt IV C2A fragment specifically impairs neurotransmitter release from neuroendocrine PC12 cells by interfering with the endogenous regulated secretory machinery.
Figure 6.
α-Latrotoxin reverses the inhibitory effect of the Syt IV C2A domain on calcium-regulated secretion from PC12 cells. NGF-differentiated PC12 cells were injected with Texas Red–conjugated dextran alone (Texas Red) or a soluble recombinant fragment encompassing the C2A domain of Syt IV (Syt IV C2A). One hour after injection, cells were K+ depolarized in a calcium-free buffer supplemented with 2.2 mM MgCl2, 5 mM EGTA, and α-latrotoxin at a final concentration of 9.6 μM. DβH surface immunoreactivity was quantified and is presented as described in the legend to Figure 5. Data are shown as the mean of two independent experiments ± SD with the total number of injected cells (n). Significant differences (p ≤ 0.01, χ2 analysis) between experimental and control treatments are indicated with an asterisk.
DISCUSSION
Syts are a family of vesicle proteins that are proposed to function as regulators of exocytosis (O’Connor et al., 1994; Südhof and Rizo, 1996). The high level of conservation between the C2 domains of 11 isoforms characterized to date suggests an important role for these domains in Syt function. Syt IV is the product of an immediate early gene whose expression is up-regulated in rat brain during seizure induction and in response to depolarization in PC12 cells (Vician et al., 1995). We recently demonstrated that Syt IV coprecipitates with Syt I in fractions enriched in SLMVs and LDCVs of neuroendocrine PC12 cells (Ferguson et al., 1999). Furthermore, Syt IV is a labile protein with a half-life of ∼2 h in PC12 cells. Therefore, Syt IV may have a unique role in regulating neurotransmitter release from neurons and neuroendocrine cells. In this paper, we extend these findings by examining the biochemical and functional properties of the C2 domains of Syt IV. Although the C2A domain of Syt IV binds Stxs 1a, 2, 3, and 4, binding is calcium independent, consistent with the inability of the Syt IV C2A domain to bind calcium (Li et al., 1995). In neuroendocrine PC12 cells, Syt IV colocalizes with Syt I in the tips of the neurites. Furthermore, calcium-independent interactions mediated through the C2A domain of Syt IV are functionally important for neurotransmitter release from PC12 cells.
Unlike the C2A domain, the C2B domain of Syt IV contains calcium binding properties that enable the assembly of both Syt IV homo- and hetero-oligomers with Syt I, consistent with the vesicular colocalization of these Syt isoforms. Consistent with our studies, we have observed calcium-dependent binding of recombinant GST-Syt IV C2B domain with native Syt I overexpressed in COS-7 cells (Thomas and Elferink, unpublished observation). While this report was under review, a separate study demonstrated calcium-dependent hetero-oligomerization between recombinant Syt IV and native Syt I derived from detergent-solubilized brain extracts (Chapman et al., 1998), confirming our observations. Together these studies demonstrate that the C2B domain of Syt IV is capable of calcium-dependent oligomerization.
During synaptic transmission, calcium influx into the nerve terminal promotes the fusion of docked vesicles with the presynaptic plasma membrane (DeBello et al., 1993; Südhof and Rizo, 1996). Biochemical and genetic studies reveal that the C2 domains of Syt I contain distinct calcium binding properties, which may function to sense calcium influx and trigger vesicle fusion with the plasma membrane (Davletov and Südhof, 1993; Broadie et al., 1994; Geppert et al., 1994; Littleton et al., 1994; Li et al., 1995; Damer and Creutz, 1996; Sugita et al., 1996). The dimerization of Syt I via its C2B domain occurs at low calcium concentrations (3–10 μM), whereas calcium concentrations comparable with those triggering exocytosis (20–200 μM) promote the binding of Syt I dimers to Stx 1a (Chapman et al., 1996). The ability of increasing calcium levels to sequentially trigger Syt I dimerization followed by Stx 1a binding may enable Syt I to both sense calcium and trigger neurotransmitter release at sites of calcium influx (Heidelberger et al., 1994; Chapman et al., 1996). Consistent with this model, discrete mutations in the Drosophila Syt I C2B domain abolish the formation of Syt I dimers, thereby decreasing the calcium responsiveness of the secretory machinery (Littleton et al., 1994). Furthermore, half-maximal binding of Stx 1a to the C2A domain of Syt I occurs at ≥200 μM calcium, consistent with the levels required for vesicle fusion at synaptic sites (Heidelberger et al., 1994; Li et al., 1995). Therefore, our observations that different combinatorial interactions exist between Syt and Stx isoforms, coupled with calcium-stimulated hetero-oligomerization of Syt isoforms, suggest that the secretory machinery contains a vast repertoire of biochemical properties for sensing calcium and regulating neurotransmitter release accordingly.
The tight coupling between a rise in intracellular calcium and the subsequent fusion event is a unique feature of neurotransmitter release. The calcium-dependent properties of the Syt I C2A domain are consistent with Syt I’s proposed role as a low-affinity calcium sensor for fast calcium-dependent exocytosis (Brose et al., 1992; Davletov and Südhof, 1993). The C2A domain of Syt IV contains a serine at residue 244, which prevents calcium binding to the C2A metal coordination site. Our functional studies on the Syt IV C2A domain suggest that this non–calcium binding domain might also function in calcium-regulated secretion from PC12 cells. In light of its non–calcium binding properties, how can Syt IV modulate neurotransmitter release in response to calcium? It is tempting to speculate that Syt IV may alter the fusogenic properties of synaptic and secretory vesicles by interacting directly with bona fide components of the calcium-sensing machinery. Therefore, the calcium-stimulated hetero-oligomerization of Syt I and Syt IV may provide the regulated secretory machinery with a mechanism to temporally regulate Syt I-mediated vesicle fusion. The inducibility and short half-life of Syt IV may offer an additional level of control on the secretory machinery. The formation of Syt IV–Syt I hetero-oligomers is contingent on Syt IV levels, which are mediated by depolarization-induced changes in Syt IV gene expression. Therefore, neurotransmitter release may be modulated directly by rapid signaling events involving calcium and indirectly by calcium- and cAMP-mediated regulation of Syt IV availability for incorporation into the cellular secretory machinery.
Microinjection of non–calcium binding recombinant Syt IV C2A fragments inhibits calcium-regulated secretion from PC12 cells. The ability of α-latrotoxin to reverse the inhibitory effect of injected Syt IV C2A fragments suggests that these fragments function to specifically perturb the regulated cellular secretory machinery. Mutational analysis of the Syt IV C2A domain reveals that the polybasic motif KKHK confers an inhibitory effect of these fragments on Syt function in vivo. The presence of a polybasic motif in the C2A domains of eight Syt isoforms supports the hypothesis that calcium-independent events mediated through this motif are important for neurotransmitter release in general (Thomas and Elferink, 1998). Nedd4 fragments, fully capable of binding phospholipids in a calcium-dependent manner, lack the polybasic motif and fail to inhibit calcium-regulated secretion from PC12 cells. These results suggest that the inhibitory effect observed with Syt I and Syt IV C2A fragments is not due to nonspecific interactions but rather an interaction with a common effector molecule. In support of our hypothesis, microinjection of a 15-residue peptide containing the Syt I polybasic motif reversibly inhibits neurotransmitter release from giant squid presynaptic terminals (Bommert et al., 1993). Similarly, mutation of the C2A polybasic motif in recombinant Syt I C2A fragments reverses their inhibitory effect on neurotransmitter release from microinjected PC12 cells (Thomas and Elferink, 1998), independent of the calcium binding properties of the Syt I C2A fragments. Whether these calcium-independent events function directly to modulate vesicle fusion or regulate specific sequential steps leading to neurotransmitter release remains to be determined. Thus, given the unusual binding properties of its C2 domains and its inducibility after neuronal excitation, Syt IV may play a unique role in modulating neurotransmitter release.
ACKNOWLEDGMENTS
We thank Dina M. Francescutti-Verbeem for technical assistance and members of the Elferink and Herschman laboratories for helpful discussion. This work was supported by grants GM-53189 (to L.A.E.) and NS-28660 (to H.R.H.) and training grant NS-07107 (to G.D.F) from the National Institutes of Health.
Abbreviations used:
- DβH
dopamine-β-hydroxylase
- DMEM
Dulbecco’s modified Eagle’s medium
- HA
hemagglutinin
- LDCV
large dense core vesicle
- Nedd4
neuronal precursor cell-expressed developmentally down-regulated 4
- NGF
nerve growth factor
- SLMV
synaptic-like microvesicle
- Stx
syntaxin
- Syt
synaptotagmin
REFERENCES
- Bennett MK, García-Arrarás JE, Elferink LA, Peterson K, Fleming AM, Hazuka CD, Scheller RH. The syntaxin family of vesicular transport receptors. Cell. 1993a;74:863–873. doi: 10.1016/0092-8674(93)90466-4. [DOI] [PubMed] [Google Scholar]
- Bennett MK, Miller KG, Scheller RH. Casein kinase II phosphorylates the synaptic vesicle protein p65. J Neurosci. 1993b;13:1701–1707. doi: 10.1523/JNEUROSCI.13-04-01701.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bommert K, Charlton MP, DeBello WM, Chin GJ, Betz H, Augustine GJ. Inhibition of neurotransmitter release by C2-domain peptides implicates synaptotagmin in exocytosis. Nature. 1993;363:163–165. doi: 10.1038/363163a0. [DOI] [PubMed] [Google Scholar]
- Broadie K, Bellen HJ, DiAntonio A, Littleton JT, Schwarz TL. Absence of synaptotagmin disrupts excitation-secretion coupling during synaptic transmission. Proc Natl Acad Sci USA. 1994;91:10727–10731. doi: 10.1073/pnas.91.22.10727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose N, Petrenko AG, Südhof TC, Jahn R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 1992;256:1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- Chapman ER, An S, Edwardson JM, Jahn R. A novel function for the second C2 domain of synaptotagmin. J Biol Chem. 1996;271:5844–5849. doi: 10.1074/jbc.271.10.5844. [DOI] [PubMed] [Google Scholar]
- Chapman ER, Desai RC, Davis AF, Tornehl CK. Delineation of the oligomerization, AP-2 binding, and synprint binding region of the C2B domain of synaptotagmin. J Biol Chem. 1998;273:32966–32972. doi: 10.1074/jbc.273.49.32966. [DOI] [PubMed] [Google Scholar]
- Chapman ER, Hanson PI, An S, Jahn R. Ca2+ regulates the interaction between synaptotagmin and syntaxin 1. J Biol Chem. 1995;270:23667–23671. doi: 10.1074/jbc.270.40.23667. [DOI] [PubMed] [Google Scholar]
- Damer CK, Creutz CE. Calcium-dependent self-association of synaptotagmin I. J Neurochem. 1996;67:1661–1668. doi: 10.1046/j.1471-4159.1996.67041661.x. [DOI] [PubMed] [Google Scholar]
- Davletov BA, Südhof TC. A single C2 domain from synaptotagmin I is sufficient for high affinity Ca2+/phospholipid binding. J Biol Chem. 1993;268:26386–26390. [PubMed] [Google Scholar]
- DeBello WM, Betz H, Augustine GJ. Synaptotagmin and neurotransmitter release. Cell. 1993;74:947–950. doi: 10.1016/0092-8674(93)90716-4. [DOI] [PubMed] [Google Scholar]
- Elferink LA, Peterson MR, Scheller RH. A role for synaptotagmin (p65) in regulated exocytosis. Cell. 1993;72:153–159. doi: 10.1016/0092-8674(93)90059-y. [DOI] [PubMed] [Google Scholar]
- Ferguson GD, Thomas DM, Elferink LA, Herschman HR. Synthesis, degradation, and subcellular localization of synaptotagmin IV, a neuronal immediate early gene product. J Neurochem. 1999;72:1821–1831. doi: 10.1046/j.1471-4159.1999.0721821.x. [DOI] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Südhof TC. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- Guan K, Dixon JE. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Heidelberger R, Heinemann C, Neher E, Matthews G. Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature. 1994;371:513–515. doi: 10.1038/371513a0. [DOI] [PubMed] [Google Scholar]
- Kee Y, Scheller RH. Localization of synaptotagmin-binding domains on syntaxin. J Neurosci. 1996;16:1975–1981. doi: 10.1523/JNEUROSCI.16-06-01975.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Ullrich B, Zhang JZ, Anderson RGW, Brose N, Südhof TC. Ca2+-dependent and -independent activities of neural and nonneural synaptotagmins. Nature. 1995;375:594–599. doi: 10.1038/375594a0. [DOI] [PubMed] [Google Scholar]
- Littleton JT, Stern M, Perin M, Bellen HJ. Calcium dependence of neurotransmitter release and rate of spontaneous vesicle fusions are altered in Drosophila synaptotagmin mutants. Proc Natl Acad Sci USA. 1994;91:10888–10892. doi: 10.1073/pnas.91.23.10888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikoshiba K, Fukuda M, Moreira JE, Lewis FMT, Sugimora M, Niinobe M, Llinás R. Role of the C2A domain of synaptotagmin in transmitter release as determined by specific antibody injection into the squid giant synapse preterminal. Proc Natl Acad Sci USA. 1995;92:10703–10707. doi: 10.1073/pnas.92.23.10703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinobe M, Yamaguchi Y, Fukuda M, Mikoshiba K. Synaptotagmin is an inositol polyphosphate binding protein: isolation and characterization as an Ins 1,3,4,5-P4 binding protein. Biochem Biophys Res Commun. 1994;205:1036–1042. doi: 10.1006/bbrc.1994.2770. [DOI] [PubMed] [Google Scholar]
- O’Connor V, Augustine GJ, Betz H. Synaptic vesicle exocytosis: molecules and models. Cell. 1994;76:785–787. doi: 10.1016/0092-8674(94)90352-2. [DOI] [PubMed] [Google Scholar]
- Ohara-Imaizumi M, Fukuda M, Niinobe M, Misonou H, Ikeda K, Murakami T, Kawasaki M, Mikoshiba K, Kumakura K. Distinct roles of C2A and C2B domains of synaptotagmin in the regulation of exocytosis in adrenal chromaffin cells. Proc Natl Acad Sci USA. 1997;94:287–291. doi: 10.1073/pnas.94.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin MS, Fried VA, Mignery GA, Jahn R, Südhof TC. Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature. 1990;345:260–263. doi: 10.1038/345260a0. [DOI] [PubMed] [Google Scholar]
- Plant PJ, Yeger H, Staub O, Howard P, Rotin D. The C2 domain of the ubiquitin protein ligase Nedd4 mediates Ca2+-dependent plasma membrane localization. J Biol Chem. 1997;272:32329–32336. doi: 10.1074/jbc.272.51.32329. [DOI] [PubMed] [Google Scholar]
- Rosenthal L, Zacchetti D, Madeddu L, Meldolesi J. Mode of action of α-latrotoxin: role of divalent cations in Ca2+-dependent and Ca2+-independent effects mediated by the toxin. Mol Pharmacol. 1990;38:917–923. [PubMed] [Google Scholar]
- Shao X, Davletov BA, Sutton RB, Südhof TC, Rizo J. Bipartite Ca2+-binding motif in C2 domains of synaptotagmin and protein kinase C. Science. 1996;273:248–251. doi: 10.1126/science.273.5272.248. [DOI] [PubMed] [Google Scholar]
- Shao X, Li C, Fernandez I, Zhang X, Südhof TC, Rizo J. Synaptotagmin-syntaxin interaction: the C2 domain as a Ca2+-dependent electrostatic switch. Neuron. 1997;18:133–142. doi: 10.1016/s0896-6273(01)80052-0. [DOI] [PubMed] [Google Scholar]
- Sheng Z-H, Yokoyama CT, Catterall WA. Interaction of the synprint site of N-type Ca2+ channels with the C2B domain of synaptotagmin I. Proc Natl Acad Sci USA. 1997;94:5405–5410. doi: 10.1073/pnas.94.10.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub O, Dho S, Henry PC, Correa J, Ishikawa T, McGlade J, Rotin D. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle’s syndrome. EMBO J. 1996;15:2371–2380. [PMC free article] [PubMed] [Google Scholar]
- Südhof TC, Rizo J. Synaptotagmins: C2-domain proteins that regulate membrane traffic. Neuron. 1996;17:379–388. doi: 10.1016/s0896-6273(00)80171-3. [DOI] [PubMed] [Google Scholar]
- Sugita S, Hata Y, Südhof TC. Distinct Ca2+-dependent properties of the first and second C2-domains of synaptotagmin I. J Biol Chem. 1996;271:1262–1265. doi: 10.1074/jbc.271.3.1262. [DOI] [PubMed] [Google Scholar]
- Sutton RB, Davletov BA, Berghuis AM, Südhof TC, Sprang SR. Structure of the first C2 domain of synaptotagmin I: a novel Ca2+/phospholipid-binding fold. Cell. 1995;80:929–938. doi: 10.1016/0092-8674(95)90296-1. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Elferink LA. Functional analysis of the C2A domain of synaptotagmin 1: implications for calcium-regulated secretion. J Neurosci. 1998;18:3511–3520. doi: 10.1523/JNEUROSCI.18-10-03511.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vician L, Lim IK, Ferguson G, Tocco G, Baudry M, Herschman HR. Synaptotagmin IV is an immediate early gene induced by depolarization in PC12 cells and in brain. Proc Natl Acad Sci USA. 1995;92:2164–2168. doi: 10.1073/pnas.92.6.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Poser C, Ichtchenko K, Shao X, Rizo J, Südhof TC. The evolutionary pressure to inactivate. J Biol Chem. 1997;272:14314–14319. doi: 10.1074/jbc.272.22.14314. [DOI] [PubMed] [Google Scholar]
- Zhang JZ, Davletov BA, Südhof TC, Anderson RGW. Synaptotagmin I is a high affinity receptor for clathrin AP2: implications for membrane recycling. Cell. 1994;78:751–760. doi: 10.1016/s0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]