Abstract
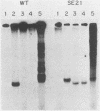
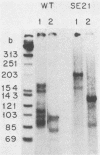
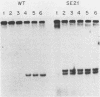
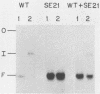
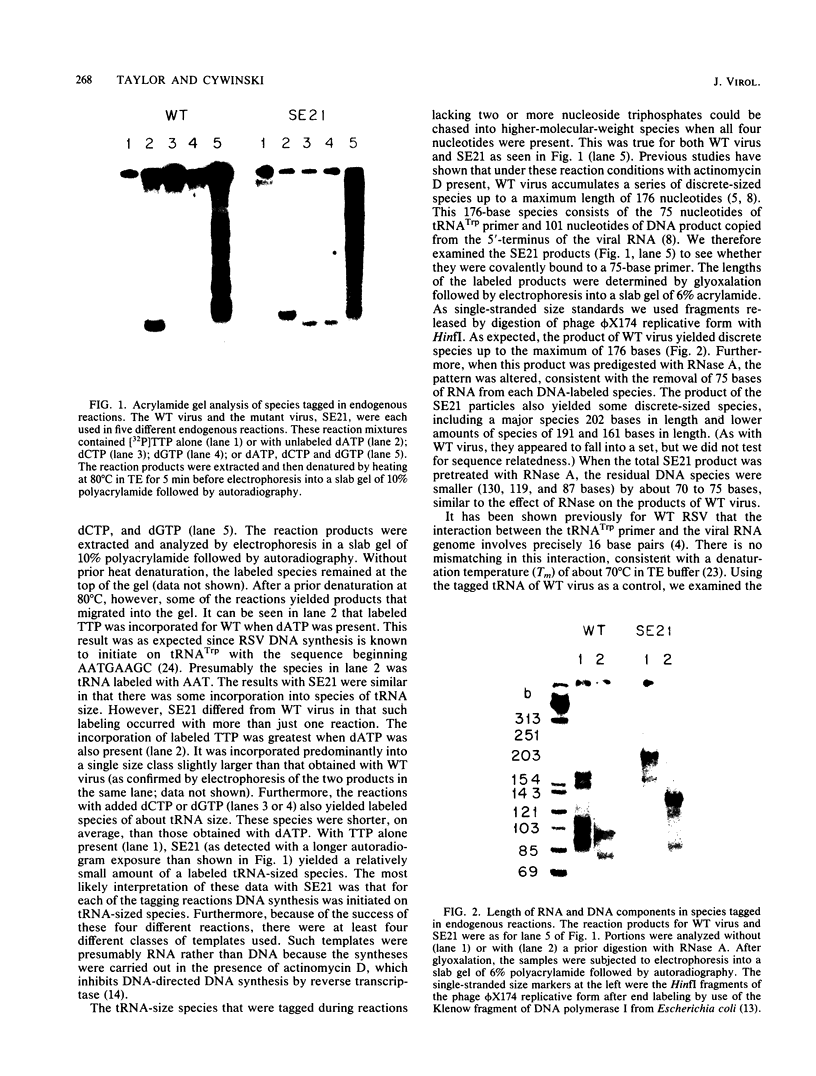
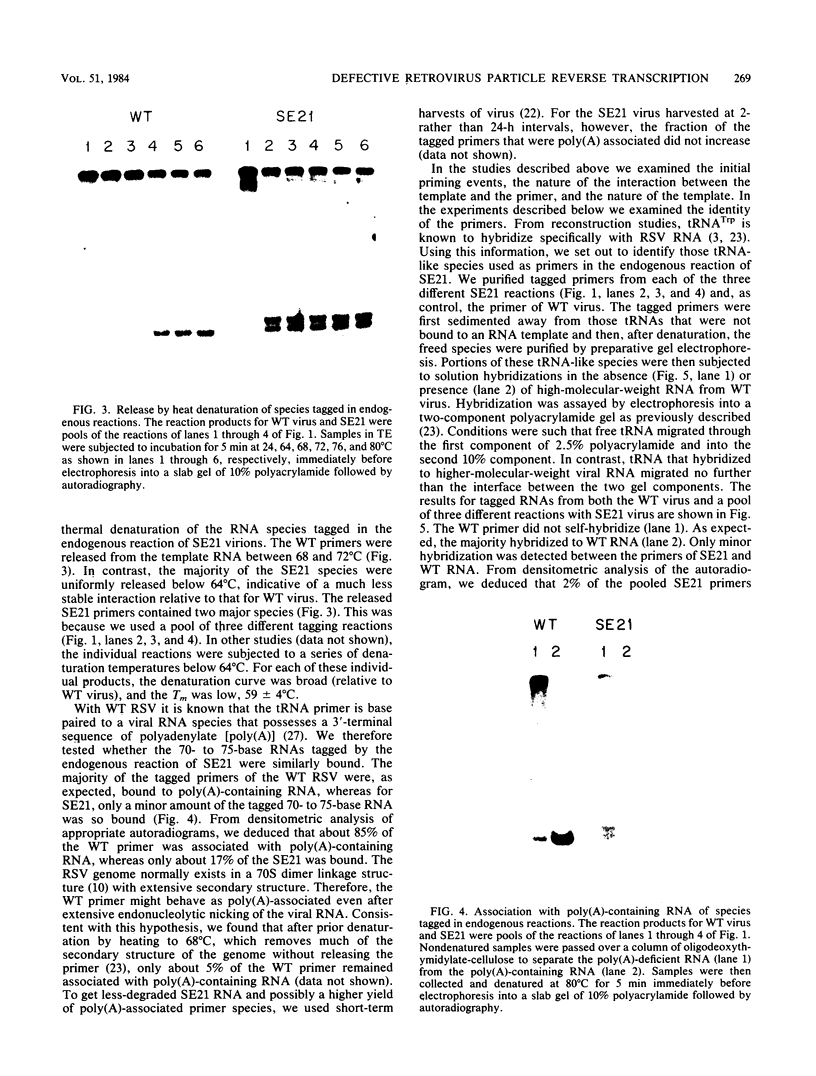
Linial and co-workers described a quail cell line, SE21Q1b, transformed by a single provirus of Rous sarcoma virus that is defective in virus assembly, in as much as the virus particles produced, SE21, contain cellular rather than viral RNA. In other respects these particles are normal, and the amount of endogenous DNA synthesis by disrupted virus particles is comparable to that of normal virus. We now report that endogenous DNA synthesis by SE21 virions uses RNA primers of the same size as tRNA species and that about 17% of these are bound to polyadenylate-containing RNA templates. Previous studies have shown that with wild-type Rous sarcoma virus, DNA synthesis is exclusively initiated on a tRNATrp species base paired to a specific location on the viral RNA. In contrast, we interpreted our data with SE21 as evidence that many different tRNA-primed initiations occurred, that predominantly species other than tRNATrp were used, and that the base pairing between template and primer RNAs included significant nucleotide mismatching. A subpopulation of the DNA synthesized by SE21 virions from tRNA-like primers was both initiated and terminated at discrete locations. These species are therefore analogous to the strong-stop DNA synthesized by wild-type virus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein L. B., Mount S. M., Weiner A. M. Pseudogenes for human small nuclear RNA U3 appear to arise by integration of self-primed reverse transcripts of the RNA into new chromosomal sites. Cell. 1983 Feb;32(2):461–472. doi: 10.1016/0092-8674(83)90466-x. [DOI] [PubMed] [Google Scholar]
- Boone L. R., Skalka A. M. Viral DNA synthesized in vitro by avian retrovirus particles permeabilized with melittin. I. Kinetics of synthesis and size of minus- and plus-strand transcripts. J Virol. 1981 Jan;37(1):109–116. doi: 10.1128/jvi.37.1.109-116.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg J. E., Sawyer R. C., Taylor J. M., Faras A. J., Levinson W. E., Goodman H. M., Bishop J. M. Transcription of DNA from the 70S RNA of Rous sarcoma virus. I. Identification of a specific 4S RNA which serves as primer. J Virol. 1974 May;13(5):1126–1133. doi: 10.1128/jvi.13.5.1126-1133.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden J. J., Quade K., Nichols J. L. Interaction of tryptophan transfer RNA with Rous sarcoma virus 35S RNA. Nature. 1976 Jan 22;259(5540):245–247. doi: 10.1038/259245a0. [DOI] [PubMed] [Google Scholar]
- Faras A. J., Taylor J. M., Levinson W. E., Goodman H. M., Bishop J. M. RNA-directed DNA polymerase of Rous sarcoma virus: initiation of synthesis with 70 S viral RNA as template. J Mol Biol. 1973 Sep 5;79(1):163–183. doi: 10.1016/0022-2836(73)90277-5. [DOI] [PubMed] [Google Scholar]
- Gallis B., Linial M., Eisenman R. An avian oncovirus mutant deficient in genomic RNA: characterization of the packaged RNA as cellular messenger RNA. Virology. 1979 Apr 15;94(1):146–161. doi: 10.1016/0042-6822(79)90445-8. [DOI] [PubMed] [Google Scholar]
- Hallick R. B., Chelm B. K., Gray P. W., Orozco E. M., Jr Use of aurintricarboxylic acid as an inhibitor of nucleases during nucleic acid isolation. Nucleic Acids Res. 1977 Sep;4(9):3055–3064. doi: 10.1093/nar/4.9.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseltine W. A., Maxam A. M., Gilbert W. Rous sarcoma virus genome is terminally redundant: the 5' sequence. Proc Natl Acad Sci U S A. 1977 Mar;74(3):989–993. doi: 10.1073/pnas.74.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagadeeswaran P., Forget B. G., Weissman S. M. Short interspersed repetitive DNA elements in eucaryotes: transposable DNA elements generated by reverse transcription of RNA pol III transcripts? Cell. 1981 Oct;26(2 Pt 2):141–142. doi: 10.1016/0092-8674(81)90296-8. [DOI] [PubMed] [Google Scholar]
- King A. M. High molecular weight RNAs from Rous sarcoma virus and Moloney murine leukemia virus contain two subunits. J Biol Chem. 1976 Jan 10;251(1):141–149. [PubMed] [Google Scholar]
- Linial M., Medeiros E., Hayward W. S. An avian oncovirus mutant (SE 21Q1b) deficient in genomic RNA: biological and biochemical characterization. Cell. 1978 Dec;15(4):1371–1381. doi: 10.1016/0092-8674(78)90062-4. [DOI] [PubMed] [Google Scholar]
- Linial M. Transfer of defective avian tumor virus genomes by a Rous sarcoma virus RNA packaging mutant. J Virol. 1981 Apr;38(1):380–382. doi: 10.1128/jvi.38.1.380-382.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell J. P., Garapin A. C., Levinson W. E., Quintrell N., Fanshier L., Bishop J. M. DNA polymerases of Rous sarcoma virus: delineation of two reactions with actinomycin. Nature. 1970 Oct 31;228(5270):433–435. doi: 10.1038/228433a0. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panet A., Haseltine W. A., Baltimore D., Peters G., Harada F., Dahlberg J. E. Specific binding of tryptophan transfer RNA to avian myeloblastosis virus RNA-dependent DNA polymerase (reverse transcriptase). Proc Natl Acad Sci U S A. 1975 Jul;72(7):2535–2539. doi: 10.1073/pnas.72.7.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G., Dahlberg J. E. RNA-directed DNA synthesis in Moloney murine leukemia virus: interaction between the primer tRNA and the genome RNA. J Virol. 1979 Aug;31(2):398–407. doi: 10.1128/jvi.31.2.398-407.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G., Glover C. tRNA's and priming of RNA-directed DNA synthesis in mouse mammary tumor virus. J Virol. 1980 Jul;35(1):31–40. doi: 10.1128/jvi.35.1.31-40.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabran J. L., Hsu T. W., Yeater C., Kaji A., Mason W. S., Taylor J. M. Analysis of integrated avian RNA tumor virus DNA in transformed chicken, duck and quail fibroblasts. J Virol. 1979 Jan;29(1):170–178. doi: 10.1128/jvi.29.1.170-178.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer R. C., Hanafusa H. Comparison of the small RNAs of polymerase-deficient and polymerase-positive Rous sarcoma virus and another species of avian retrovirus. J Virol. 1979 Mar;29(3):863–871. doi: 10.1128/jvi.29.3.863-871.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Linial M. Avian oncovirus mutant (SE21Q1b) deficient in genomic RNA: characterization of a deletion in the provirus. J Virol. 1980 Nov;36(2):450–456. doi: 10.1128/jvi.36.2.450-456.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. E., Bernstein E. H. Production and purification of large amounts of Rous sarcoma virus. Appl Microbiol. 1973 Mar;25(3):346–353. doi: 10.1128/am.25.3.346-353.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Cordell-Stewart B., Rohde W., Goodman H. M., Bishop J. M. Reassociation of 4 S and 5 S RNA's with the genome of avian sarcoma virus. Virology. 1975 May;65(1):248–259. doi: 10.1016/0042-6822(75)90025-2. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Garfin D. E., Levinson W. E., Bishop J. M., Goodman H. M. Tumor virus ribonucleic acid directed deoxyribonucleic acid synthesis: nucleotide sequence at the 5' terminus of nascent deoxyribonucleic acid. Biochemistry. 1974 Jul 16;13(15):3159–3163. doi: 10.1021/bi00712a024. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R. Site on the RNA of an avian sarcoma virus at which primer is bound. J Virol. 1975 Sep;16(3):553–558. doi: 10.1128/jvi.16.3.553-558.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Arsdell S. W., Denison R. A., Bernstein L. B., Weiner A. M., Manser T., Gesteland R. F. Direct repeats flank three small nuclear RNA pseudogenes in the human genome. Cell. 1981 Oct;26(1 Pt 1):11–17. doi: 10.1016/0092-8674(81)90028-3. [DOI] [PubMed] [Google Scholar]
- Wilde C. D., Crowther C. E., Cowan N. J. Diverse mechanisms in the generation of human beta-tubulin pseudogenes. Science. 1982 Aug 6;217(4559):549–549. doi: 10.1126/science.6178164. [DOI] [PubMed] [Google Scholar]