Abstract
Radiesse® (Bioform Inc, USA) is a sterile, latex-free, non-pyrogenic, semi-solid, cohesive subdermal, injectable implant, whose principal component is synthetic calcium hydroxylapatite, a biocompatible material with over 20 years of use in medicine. The semi-solid nature of the product is created by suspending calcium hydroxylapatite microspheres of 25–45 microns diameter in a gel carrier of carboxymethylcellulose. The product has FDA approval for esthetic facial augmentation in the US. Such approval includes the long-lasting correction of moderate to severe facial wrinkles and folds and the treatment of facial fat loss due to immunodeficiency virus infection. Diverse facial regions can be injected in order to ameliorate or enhance some features: glabellar lines, subdermal support of the brows, malar and buccal fat pads, tear troughs, nasolabial folds, nose, lips, perioral region, marionette lines, oral commisures and chin among others, as well as saucerized acne scars. Other medical indications include nipple projection for nipple areolar reconstruction, urinary incontinence, vesicoureteral reflux, vocal cord augmentation, and use as a radiographic tissue marker. The average lasting result is from 12 to 18 months. Radiesse can be considered an effective soft-tissue filler for overall longevity, biocompatibility, and low rate of side effects.
Keywords: hydroxylapatite, facial augmentation, Radiesse
Facial fillers
Throughout recorded history, women and men have been trying to achieve and preserve a youthful appearance, and many techniques have been designed in order to rejuvenate the face. The perception of beauty has been influenced all over the world and throughout history by many factors, including ethnic conditions, geographic location, socioeconomic situation, and life styles, among others.
Over time, with exposure to sunlight, nutritional habits, and genetic patterns in addition to other factors, the skin starts lose its youthful appearance, especially in the face (Glogau 1996; Jacovella 2006). The most common esthetic signs of facial aging include visibility of bony landmarks, skin wrinkles, deep furrows, prominence of nasolabial folds, perioral vertical rhytids, ptosis of the oral commisures, and thinning of the lips. These changes arise from the loss of subcutaneous tissue, associated with thinning of the dermis (Glogau 1996; Jacovella 2006).
Facial rejuvenation treatments, both surgicaland non-surgical, try to meet patients’ needs according to their esthetic standards. A lot of procedures have been described to reverse some aging changes and attempt to achieve satisfactory facial rejuvenation.
To achieve success in esthetic medical procedures, it is necessary to have a well-defined notion of beauty and be able to analyze and match the main patient features with his or her desires. Every year, more aging patients present themselves to the plastic surgeon or dermatologist requesting facial rejuvenation.
Conventional rejuvenation surgery likely remains the treatment of choice for patients requiring extensive esthetic facial changes (Jansen and Graivier 2003; Rohrich et al 2003). However, injectable facial fillers are increasingly being seen as a very good option to ameliorate certain aging signs in patients who are not looking for a remarkable change in physical distinctiveness but to appear more rested and confident. These aging signs include glabellar lines, deep nasolabial folds, marionette lines, buccal commissures, peri-lips wrinkles, and other cases that need minor enhancement of some facial features (Jacovella 2007). In such cases, fillers appear to provide more “real-time” results, not characterized by extensive swelling and prolonged recuperative periods sometimes found in face lifts and other surgical procedures (Jacovella 2006; Jacovella et al 2006).
Furthermore, plastic surgeons often find facial dermal fillers to be a good complement to surgical rejuvenation procedures such as a face lift, and to non-surgical procedures such as laser resurfacing treatments and chemical peels (Narins and Bowman 2005; Burroughs et al 2006; Roy et al 2006).
Materials were first used for filling purposes more than 100 years ago, when paraffin was injected into the scrotum, with terrible results (Klein and Elson 2000; Murray et al 2005). In 1893, autologous fat transfer was described as a very good substitute material for soft tissue agumentation. Other materials including medical silicone were developed for the same purpose, but due do many severe complications, this substance has not remained one of choice (Murray et al 2005). Complications associated with injectable liquid silicone may occur at any time in the distant future, even after an initially successful treatment. The element of most concern is that when complications do occur, they frequently produce very difficult or untreatable clinical situations (Rohrich and Potter 2004).
In the early 1970s, Stanford University investigators presented a preliminary study on injectable collagen. A decade or so later, collagen was approved by the FDA for medical use (Tzikas 2004; Kanchwala et al 2005). In some ways, the approval of collagen marked a milestone for esthetic practice. Its approval also served as a catalyst for the continuing search for better filler materials for the face. Since then, researchers have been driving both plastic surgeons and dermatologists by providing a wide range of options for facial fillers.
Classification and ideal properties of facial fillers
Different classifications can be considered. Chemically, the available products can be classified into two different categories: biologic and synthetic substances (Jacovella 2007).
Biologic materials include bovine collagen, animal hyaluronic acid, and autologous fat. Synthetic fillers include non-animal hyaluronic acid, polymethyl methacrylate (PMMA) microspheres, and hydroxylapatite micro-particles, among others. Several authors have also described similar categories (Jansen and Graivier 2003, 2006; Rohrich et al 2003; Tzikas 2004; Murray et al 2005; Narins and Bowman 2005; Broder and Cohen 2006; Klein and Elson 2006; Sengelmann et al 2007).
In terms of durability of results, fillers can be classified as follows: Short lasting – up to 6 months; long lasting or semi permanent – up to 3 years; and permanent – more than 3 years.
Independently of the product, the ideal characteristics of soft-tissue filling material have been identified. They include acceptable filler longevity, biocompatibility, non-migratory, low adverse event profile, and a reasonable cost-benefit ratio (Jansen and Graivier 2003; Rohrich et al 2003; Marmur et al 2004; Tzikas 2004; Felderman 2005; Broder and Cohen 2006; Burroughs and Anderson 2006; Dover 2006; Jacovella 2006; Sengelmann et al 2007).
With reference to the above-mentioned classifications, calcium hydroxylapatite (CaHA) may be considered a synthetic and long-lasting/semipermanent dermal filler. Radiesse® offers many of the desirable mentioned properties of a facial filler, particularly for deep dermal defects (Elson 2006) and for non-surgical contouring augmentation (Busso and Karlsberg 2006).
Product description
Radiesse® (Bioform Medical Inc. USA) is a sterile, latex-free, non-pyrogenic, semi-solid, cohesive subdermal, injectable implant, whose principal component is synthetic CaHA, a biocompatible material with over 20 years of use in medicine (Hobar et al 2000; Havlik et al 2002). The product is supplied in 1.3 mL disposable syringes with Luer-lock fittings. (Bioform Medical Inc 2007). The semi-solid nature of the product is created by suspending CaHA microspheres of 25–45 micron diameter in a gel carrier that consists primarily of sterile water and glycerin. The gel structure is formed by the addition of a small amount of carboxymethylcellulose (USP). The gel is dissipated in vivo and replaced with soft tissue growth, while the CaHA remains at the site of injection (Bioform Medical Inc. 2007).
CaHA is biodegradable, following the same metabolic pathway as bone debris resulting from common bone fractures. After 2–3 months, the carboxymethylcellulose is resorbed and replaced by collagen. Finally a gradual breakdown of the particles occurs, until complete phagocytosis is achieved (Misiek and Kent 1984; Probeck and Rothstein 1989; Pettis et al 1990; Flaharty 2000; Sklar and White 2004). The CaHA microspheres present in Radiesse have the same chemical composition as the inorganic constituent of teeth and bone, and exhibit an extensive safety profile (Havlik et al 2002). As a bioceramic, CaHA is native to the body with no antigencity. Moreover, the biocompatibility of CaHA has been extensively tested in preclinical studies, and it has been shown to be non-toxic and non-mutagenic (Flaharty 2000).
Clinical, histologic, and electron microscopic findings after in vivo injections have demonstrated excellent tolerance. Biocompatibility studies on CaHA implants have all been characterized by minimal, if any, inflammatory response, with no foreign body reaction and without evidence of local or systemic toxicity (Flaharty 2000; Havlik et al 2002; Marmur et al 2004).
When injected as small microspheres, CaHA acts as a scaffold that promotes new tissue formation similar to its surrounding environment. Inside soft tissues such as the dermis, deposited particles support fibroblastic ingrowth and new collagen formation, without calcification. In other words, the properties of CaHA mimic the environment into which it is placed. No osseous infiltration has been found. No migration from the sites of injection has been observed (Felderman 2005).
After opening the syringe, the unused material after first treatment can be recapped and kept for the same patient until 3 months, for future administration. It is important that no visible air bubbles are present inside the syringe to avoid future hardening of the material.
Regulatory status
With many injectable dermal fillers on the market, the American Society of Plastic Surgeons and the American Society for Aesthetic Plastic Surgery clarified legal and regulatory issues (American Society of Plastic Surgeons and American Society of Aesthetic Plastic Surgery 2006).
Radiesse is considered a “medical device”. It has already received FDA approval for esthetic facial augmentation in the US. Such approval includes the long-lasting correction of moderate to severe facial wrinkles and folds, and the treatment of facial fat loss due to immunodeficiency virus infection.
The product also has approval for plastic and reconstructive surgery, including soft tissue augmentation for the facial area in the following countries: Argentina, Canada, Israel, Mexico, Romania, Turkey, and all countries within the EU (Bioform Medical Inc. 2007). Radiesse is not appoved in Australia for cosmetic indications. It is advisable to determine the regulatory status of the country in which the clinician is practicing.
Radiesse is provided with 3 self-adhesive identification labels with the following data: lot number, quality control number, and last date allowed to be used.
Indications
Esthetic facial main indications and contraindications
What results are to be expected – subtle or quite spectacular? It depends on the patient’s look before treatment with fillers and on their specific expectations. Indications have to be carefully discussed in advance with the physician, remembering that some signs of aging will be attenuated but will not disappear. According to specific facial conditions, treatments have to be selected in order to better treat the cases.
Sagging skin almost always suggests a surgical solution. Intracutaneous rhytides due to sun exposure are better treated with laser resurfacing or chemical peels. Patients with deep furrows, vertical glabellar lines, and nasolabial folds are very good candidates for facial fillers such as Radiesse (Roy et al 2006). Several facial regions can be injected in order to ameliorate or enhance some features: glabellar lines, subdermal support of the brows, malar and buccal fat pads, tear troughs, nasolabial folds, nose, lips, perioral region, marionette lines, oral commisures, and chin, among others.
Radiesse is also a good solution to correct some lesions such as acne scars. In a preliminary study of 10 patients, Goldberg and colleagues (2006) noted that saucerized acne scars responded well to treatment but ice-pick scars did not.
Other aging signs such as crow’s feet are partly a consequence of dynamic expression of the orbicularis oculi muscles. Due to the possibility of “speed bump-like nodules”, Radiesse is not an ideal product for this region. Moreover, patients with one or more of the following conditions should not receive Radiesse implants: acute or chronic skin infection that involves the site to be treated, existing keloidal scars, systemic collagen diseases, severe bleeding disorders, and presence of foreign bodies such as silicone (Jacovella 2006). Individuals with body dysmorphic disorders and unrealistic expectations should no be injected with Radiesse either.
Other medical indications
Evans and co-workers presented an interesting article on the use of CaHA for nipple projection after failed nipple-areolar reconstruction (Evans et al 2005). Although the study described early results with only 6 patients, the technique appears to be promising. This initial pilot study suggests that Radiesse is an effective, safe, and reliable method for immediate results, according to subjective data and patient satisfaction.
In addition to esthetic use, Radiesse has been successfully used in the following conditions: stress urinary incontinence, vesicoureteral reflux, vocal cord augmentation, and as a radiographic tissue marker (Mayer et al 2001; Marmur et al 2004).
Techniques
Preparation
The procedures can be performed in an office setting on an outpatient basis. Patients must provide specific informed consent for the treatment with Radiesse for soft tissue augmentation before initiation of the procedures (Reisman 2006). It is advisable to keep identification stickers with the product data attached to the medical record.
The areas to be injected must be appropriately marked with the patient in an upright position. As with all esthetic surgical and dermatological procedures, pretreatment pictures are mandatory.
Patients must be warned to avoid agents that can cause bleeding, for example, aspirin and some anti-inflammatory drugs, for 7 days prior to injection, which can help reduce hematoma formation (Narins and Bowman 2005).
Patient position
Treatments are usually performed with the patient in a sitting or semi-sitting position, which allows the physician to inject the desired sites while effects of gravity influence the skin.
Anesthesia
Depending on the anatomical area, patient’s sensitivity, and personal medical preferences, regional infiltrative or nerve block anesthesia may be used. In a very interesting and instructive article on local and regional block anesthesia, Gmyrek and Dahdah (2006) present many interesting guidelines about this important subject. The following techniques can be applied before Radiesse injections.
Topical anesthesia
The suggested drug is betacaine 10% as a gel applied to the treatment area for at least 15–20 minutes before Radiesse injection.
Infiltrative anesthesia
Infiltrative anesthesia has the advantage of a rapid onset of action after injection. Because infiltration of the substance frequently distorts the landmarks of the site, the area to be anesthetized must be identified and marked preceding the injection.
Nerve blocks
Nerve blocks are helpful mainly when the infiltration of the anesthetic solution may cause undesirable distortion of the surgical site or require an amount of anesthetic that exceeds the maximum recommended dose. Because most nerves are subdermal, it is recommended that the local anesthetic is placed in a subcutaneous location using a 30-gauge needle. According to the regions to be treated, the most common nerve territories can be blocked as follows.
Supraorbital and supratrochlear block: The supraorbital and supratrochlear nerves innervate the frontal part of scalp and forehead. Both nerves are branches of the first division or ophthalmic branch of the trigeminal nerve (Gmyrek and Dahdah 2006).
Supraorbital and supratrochlear nerve blocks can be approached from either the area of the supraorbital foramen or the area of the supratrochlear notch. For this region, 1 mL per side is adequate.
Infraorbital block: The infraorbital nerve innervates the lower eyelid, medial aspect of the cheek, upper lip, and lateral portion of the nose. It is a branch of the second division or maxillary branch of the trigeminal nerve. The infraorbital nerve leaves the skull through the infraorbital foramen, which is 1 cm inferior to the infraorbital ridge.
There are two approaches for infraorbital block: through direct trans-cutaneous injection or via intra-oral injection. In both cases, the infraorbital foramen should be palpated and the drug injected near but not into the canal that surrounds the nerve, in order to avoid nerve injury. An average dose of 2 mL is suggested.
Mental nerve block: The mental nerve innervates the lower lip and chin. It is a branch of the third division or mandibular portion of the trigeminal nerve. The mental nerve exits the skull through the mental foramen. The intraoral approach is preferred. The needle has to be inserted in the inferior labial sulcus at the apex of the first bicuspid. An average dose of 1.5 mL is sufficient to block the inferior lip.
Combination techniques
Some techniques can be combined for minimizing discomfort during anesthetic injection. They include the use of topical anesthetic on the skin or into the mucosa and the use of ice cubes, before performing infiltrative or nerve block anesthesia. Generally topical anesthetic may be used prior to applying the ice (Comite et al 2007). Since topical anesthesia itself is not often enough to avoid pain, combination techniques are strongly recommended.
Injection techniques: general guidelines
Some considerations must be taken into account to select a technique: area to be treated, depth of the injection, and desired injecting volume.
Needle selection is the next step. As a general rule, the smallest needle-gauge should be used that can deliver the product appropriately (Murray et al 2005). The suggested needles are 27-gauge 0.25 and 25-gauge 0.25 inch (6 mm) long. The product must be injected in the deep dermis or subdermally, placing the needle with an angle of 30–45 degrees (Jacovella 2006; Jansen and Graivier 2006).
Intradermal or superficial dermal injections are not recommended because they can cause visible white nodules in the superficial dermis (Flaharty 2000; Fagien 2006). After the needle is introduced in the proper plane, the product is injected in the created tract during slow withdrawal of the syringe. Needle adaptations may include bending to access particular contours of the face.
As a practical rule it is advisable to gradually achieve the final results over several sessions (Narins and Bowman 2005).
Different specific maneuvers can be used depending on the above-mentioned factors: linear threading, serial puncture, fanning, and cross hatching.
In linear threading the needle is introduced along the entire fold or wrinkle, longitudinally in a subcutaneous plane. Depending on the anatomical structures to be filled, one, two, or three bands of the product are delivered in parallel rows, as the needle is withdrawn. In the serial puncture technique small amount amounts of the product are injected one beside the other, perpendicularly to the fold. The fanning technique involves a radial trajectory of the needle from the puncture site with a delicate back and forth movement. The product is delivered as the needle is withdrawn. In the cross hatching technique the product is injected from two different perpendicular approaches. Linear and parallel threading deposits can be made from different sites in one direction and some deposits can be are made perpendicularly to the first.
Specific techniques according to different regions
Glabellar vertical lines
The glabellar wrinkles and furrows develop during progression of aging progression due both to static and dynamic factors. Such lines usually pose little problem for implants because of the thickness of the dermis (Figure 1).
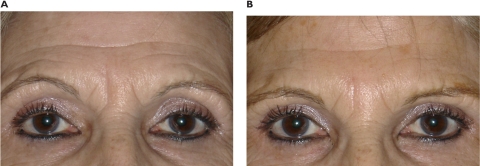
Figure 1.
Pre-treatment (A) and post-treatment (B) at 9 months after 0.5 mL of Radiesse, in a 65-year-old woman with two static vertical glabellar lines.
Although some concerns about tissue necrosis and serious complications such as embolization have been noted in the literature, no adverse effects have been observed by the author (Jacovella 2006; Jacovella et al 2006). Nevertheless, caution should be the rule and treatment of this area by the trainee injector is not advised.
To anesthetize the region, a supratrochlear block with 0.5 mL of lidocaine per side is sufficient.
To correct the vertical lines, a 27-gauge, 0.5-inch (12-mm)-long needle is used in a linear threading deposition. The needle is introduced longitudinally into the deep dermis to create a tunnel; CaHA is then injected into the created space by simultaneously slowly withdrawing the syringe and pressing the compound from the syringe into the dermis.
To reduce muscle movement, better results are obtained by previous treatment with botulinum toxin. This procedure allows for weakening of the brow depressors (Flaharty 2000; Kanchwalla et al 2005; Jacovella 2006).
The suggested amount of Radiesse is 0.3–0.5 mL.
Cheek and infraorbital region
Small or not very noticeable cheeks and lack of infraorbital projection as individual or ethnic features are frequent indications for fillers in patients looking for a simple youthful look (Figure 2). Some patients are aware of a sunken upper maxillary facial region as a sign of illness or tiredness. Radiesse can provide volume augmentation to ameliorate this aspect. By restoring structural support and fullness of the malar and submalar areas, the shadowing effect associated with aging of the region can be diminished, resulting in a better appearance.
Figure 2.
Pre-treatment (A) and post-treatment (B) at 18-month follow up of a 44-year-old woman with sunken upper maxillary anatomical region. The implant of 1.5 mL of Radiesse provided an enhanced infra-orbital area. The patient was injected by Dr. Miles Graivier.
It is advisable to plan augmentation of cheek and adjacent areas in the context of the whole face, not only just the overlying malar eminence (Busso and Karslberg 2006). The treatment should be planned in consultation with the patient (Jansen and Graivier 2006). Infiltrative or nerve block anesthesia can be used in such areas.
A 25-gauge, 1-inch (25-mm)-long needle is recommended. The puncture site must be distal to the desired area to be injected and the combination of cross-hatching and fanning techniques is preferred to allow adequate spread of the product. Depth of injection is of paramount importance in this area. Jansen and Graivier (2006) propose beginning injecting in the more superior aspect of the zone, in the direct subermal subcutaneous plane. Better results are achieved when multiple fine threads are delivered in multiple planes, allowing the scaffolding effects of tissue in-growing among the product (Busso and Karlsberg 2006).
After product placement, immediate molding is advisable to smooth the surface and achieve the desired shape. Overcorrection is hardly ever necessary. However, some clinicians prefer to overcorrect in anticipation of initial partial resorption. The suggested volumes for this area are 2–4 mL, even though 1 mL per side can provide a subtle noticeable result.
Nose
Even though conventional esthetic rhinoplasty remains the treatment of preference for the most important nasal deformities, injectable facial fillers may provide an attractive alternative in minor defects (Figures 3 and 4). Moreover some internal nasal valve collapse after previous surgery or trauma can be find repaired by injecting HA filler.
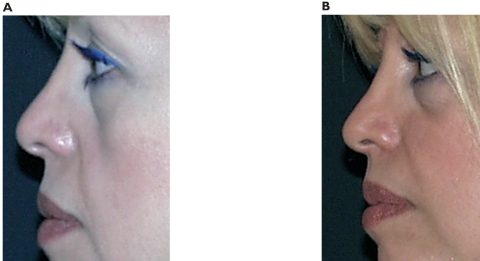
Figure 3.
Pre-treatment (left) and post-treatment (right) at a 12-month follow up, in a profile view of a 42-year-old woman with a primary aesthetic rhinoplasty. Enhancement of tip projection was achieved with 0.5 mL. No touch-up was necessary after first implant.
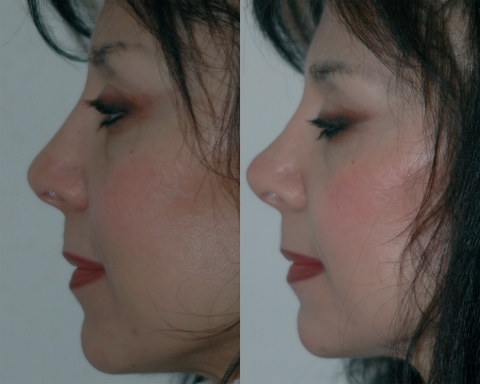
Figure 4.
Pre-treatment (left) and post-treatment (right) at a 12-month follow up, in ¾ view of the same patient as in Figure 3.
Mastery of both nasal anatomy and precise surgical technique is mandatory before performing nasal corrections with fillers (Fagien 2006).
The following anatomical regions can be implanted in order to improve esthetic appearance: fronto-nasal angle, dorsum, nasal tip, and naso-labial angle.
Patients can be injected once, twice, or thrice, starting always with a low volume. As is the case with other areas of treatment with Radiesse, but especially in this one, overcorrection is to be avoided.
Patients with and without previous surgery are suitable for CaHA implants. In order to avoid distortions, supratrochlear and infraorbital nerve block anesthesia is recommended. A waiting time of 10–15 minutes diminishes bleeding.
Proceeding with back and forth multidirectional technique, and by using delicate and limited fanning soft movements with a 27- or 25-gauge needle connected to the syringe, the physician can create a subcutaneous space to hold the filler. The product is injected in the previously created space during slow withdrawal of the needle. Placing the puncture site distal to the regions to be corrected is strongly recommended.
Because of the many sebaceous glands in the nasal tip, approaching this area from the dorsum with a 25-gauge, 1-inch (25-mm)-long needle is desirable.
Micropore-like tapes can be applied around the corrected areas in order to provide desired shape and diminish swelling deformities.
The suggested maximum volumes for each of the nasal regions are: fronto-nasal angle, 1.5 mL; dorsum, 0.5 mL; tip, 0.5 mL; and nasolabial angle, 1.5 mL.
Besides esthetic indications, Radiesse can provide improvement in some cases of nasal flow impairment.
In a very interesting paper, Nyte (2006) describes spreader graft injection with Radiesse for a non-surgical solution in internal nasal valve collapse in 23 patients.
Some causes such as prior rhinoplasty, nasal trauma, or weakness of the upper lateral cartilages can result in an internal valve collapse.
Before proceeding with the implantation, a Q-tip can be used to displace the involved upper lateral cartilages and therefore simulate the spreader graft effect.
With the aid of a head lamp and a nasal speculum, small drops (0.05 mL) of CaHA are injected into the submucoperichondrial and submucosal planes at the junction points of the upper lateral cartilages and the dorsal cartilaginous septum. A 23-gauge, 1.5-inch (37-mm) needle and 0.10–0.15 mL are proposed (Nyte 2006). The area must be carefully inspected after each injection.
The CaHA provides support to this area and allows air flow improvement, due to the corresponding increase of the internal nasal valve angle. Some middle external vault fullness due to temporary edema can be expected as adverse effect.
Although this indication appears to be safe, convenient, and cost-effective alternative to conventional graft surgery, closed or open rhinoplasty, with and without autologous cartilages, are still the first preference in most of nasal deformities, both esthetic and functional.
Mid face lipoatrophy associated with HIV
First described in 1998, lipodystrophy syndrome is characterized by peripheral lipoatrophy, central lipohypertrophy and other signs and symptoms (Silvers et al 2006). Lipoatrophy refers to the loss of subcutaneous fat in the face, arms, legs, and buttocks. Although the pathogenesis of this syndrome is not fully understood, recent hypotheses suggest that impairment of adipocyte differentiation may play a role in this disease.
Facial lipoatrophy likely results in part from infection with HIV and in part from highly active antiretroviral treatment as a consequence of the infection. Fat loss is most notably in the cheeks and can cause severe psychological effects because it can be an identifying stigma of HIV infection (Comite et al 2006; Silvers et al 2006).
Infiltrative anesthesia is suggested. The product must be injected in the deep subcutaneous plane by a cross-hatching technique. Depending of the degree of hollowness caused by the absence of fat tissue, volumes may vary from patient to patient. Silvers et al (2006) proposed amounts from 2 to 20 mL, considering several injections in the same areas.
Considering the lack of fat in these patients, Radiesse appears to be a very good option as volume filler.
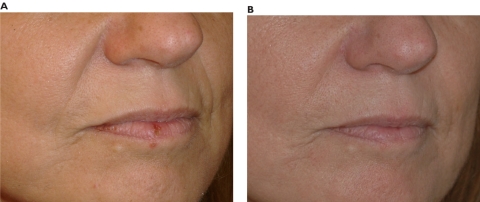
Nasolabial folds
The nasolabial folds are one of the most frequently treated facial areas (Figures 5 and 6). The physician should begin by determining the amount of skin redundancy with a pinch test. Depending on the surgeon’s experience, if the redundancy is too large, a surgical face lift could be considered (Jacovella 2006).
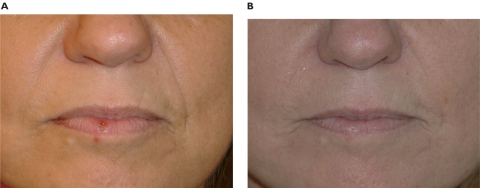
Figure 5.
Pre-treatment (A) and post-treatment (B) of a 52-year-old woman with noticeable nasolabial folds. After 0.5 mL in each side and a touch-up at 3 months, the results are shown after a 12-month follow up period. The total amount in each side was 0.8 mL.
Figure 6.
Pre-treatment (A) and post-treatment (B) of the same patient as in Figure 5, in ¾ view.
Some degree of natural asymmetry is common and part of an individual’s beauty. For this reason, time to observe and identify subtle asymmetries must be taken in account, and discussed with the patient according to their expectations (Jansen and Graivier 2006).
After marking the area to be treated, 1 mL of infiltrative anesthesia per side is injected and a 10-minute waiting time is recommended. Some authors such Alam and Yoo (2007) suggest miniblocks of 0.2 mL of 0.5% lidocaine, injected into the sulcus superior to the third incisor bilaterally.
The product may be injected the first time to achieve a moderate correction and proceed with a touch-up, 4 weeks later. In the folds, a 25-gauge, 1-inch (25-mm)-long needle or a 27-gauge, 1.25-inch (31-mm) needle can be chosen. The smaller one is sufficient to deliver the material smoothly without clogging. Nonetheless the 25-gauge allows a thicker layer in one delivery.
Two techniques or the combination of both can be performed: linear and serial puncture (Jacovella 2006; Alam and Yoo 2007).
To perform the first, the needle is introduced along the entire fold longitudinally in a subcutaneous plane, starting at the inferior end and advancing up to the nose. Depending on the depth of the folds, one, two, or three bands of Radiesse must be implanted in parallel rows, through the same starting puncture. There is no need to remove the needle between threads. As the needle is withdrawn, the product is injected while the passive hand of the operator palpates the area and feels the deliver of the material.
The serial puncture technique, perpendicular to the fold, is suggested as a structural support. In this case, the product should be delivered in a deeper plane first and completed with the linear mentioned maneuver.
Suggested volumes are 0.5–1.5 mL per side.
The filler material can be massaged and modeled to ensure even placement and to avoid an irregular surface appearance. With the operator’s index finger inside the mouth and the thumb outside, the whole length of the nasolabial fold is compressed to create a flat shaped material deposit (Alam and Yoo 2007).
Lips
The lips are considered one of the key features of facial beauty. The ideal lip augmentation procedure should grant predictable results without visible scars. The high number of techniques available for lip enhancement reflects the lack of an ideal and completely reliable method with predictable and reproducible results. Some of them include complex surgical techniques and the use of autologous or synthetic materials.
Dermal flaps and superficial musculoaponeurotic flaps, for example, may present serious complications such as asymmetry, noticeable scars, and bridge restriction scars. These scars, if not hypertrophic, can be quite apparent and may necessitate permanent application of camouflage cosmetics. Despite corrections remaining for 5 years, such procedures imply prolonged surgical techniques (Jacovella 2006).
Other materials such as polytetrafluoroethylene semi-solid implants offer permanent augmentation results. Segments of the product have to be cut to shape and size and introduced through an incision in the posterior labial mucosa. Despite achieving permanent results, the need for temporary sutures, a mandatory 2 days of liquid diet, and the possibility of malposition, are considered disadvantages compared with fillers.
Many indications are taken into account in the aging mouth: vertical wrinkles, reduction in vermillion height, disappearance of Cupid’s bow, and low volume of the lips.
According to these signs it is very important to help the patient define the specific goals of treatment.
The biggest disadvantage of permanent fillers is their permanence. If the desired results are not achieved, removal of these permanent fillers is difficult at best and even sometimes impossible (Jacovella et al 2006).
Because of the greater degree of motion, lip augmentation with fillers is not as forgiving as in the nasolabial region. For that reason previous training and delicate technique are mandatory (Kanchwala et al 2005).
Radiesse can provide long-lasting results without the above-mentioned drawbacks of both surgical and non-surgical techniques.
Troncular anesthesia is strongly recommended. Infraorbital nerve and mental nerve blocks provide effective anesthesia and reduce the chance of unacceptable distortion sometimes seen in direct infiltration (Gymrek and Dahdah 2006).
A 27-gauge or 25-gauge, 1-inch (25-mm)-long needle can be selected for this area, to limit the number of punctures and to diminish both the incidence of bleeding and hematomas. The preferred plane is between the mucosal border and the orbicular oris muscle. The specific technique consists of creating two puncture wounds for each side, since each half of the length of the upper lip and lower lip has to be approached from the two corners of the mouth (Jacovella et al 2006).
To avoid involuntary spreading, special care should be to hold the lip firmly with the thumb and the forefinger of the passive hand, while introducing the needle with the other hand. Only one-layer injections of ≤0.5 mL per site are suggested, while softly withdrawing the needle.
When lip fullness is desired in addition to vermillion enhancement, a deeper second line can be injected. The philtral columns have to be treated separately with a 27-gauge 0.25-inch (6-mm) needle (Jacovella 2006).
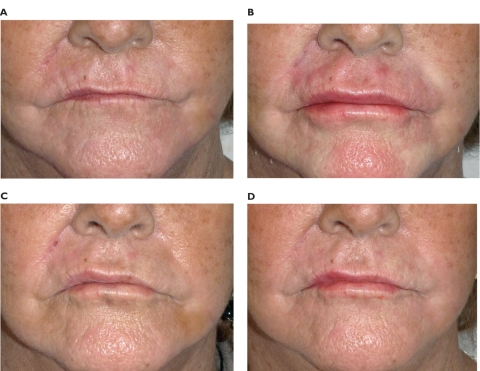
The total ideal amount is no more than 1 mL for each lip, at least during the first treatment. Beginning with low volumes is always advisable, especially in the treatment of lips (Figures 7 and 8).
Figure 7.
Pre-treatment (A), immediate at 24 hours post-treatment (B), after 15 days (C), and at 12 months post-treatment (D) of a 68-year-old woman. The total amounts were 0.7 mL for the upper lip and 0.5 mL for the lower lip. No touch-ups were performed after first injection.
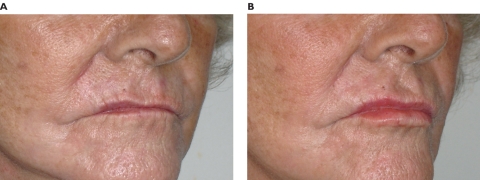
Figure 8.
Pre-treatment (A) and post-treatment (B) of the previous patient in a ¾ view. Despite a previous lip asymmetry, a total amount of 1.2 mL of Radiesse in both lips provided enhancement and projection of vermilion border and filtrum. Radial lip lines were lessened.
To avoid speed bump-like deformities, vertical lines should not be directly filled. The linear threading technique itself can help remove fine radial rhytids, as the overlying skin stretches out. Nevertheless, some practitioners prefer to fill such lines directly (Jansen and Graivier 2003, 2006).
Other areas
Chin and jawlines can be treated very similarly to cheeks. Because the patient’s physiognomy can significantly change from supine to upright positions, the patient must be positioned in the Frankfurt plane to determine some important areas such as central dimple creation and prejowl sulcus defects (Jansen and Graivier 2006).
The subcutaneous plane allows easy molding and shaping around the inferior border of the mandible. Special care must be taken to avoid product displacement and the appearance of “witch chin” deformity.
Facial asymmetries can be also corrected depending on the required volume.
Treatment combinations
Radiesse can be combined with some other fillers, such as hyaluronic acid, and with botulinum toxin, laser resurfacing, different dermal peels, and radiofrequency treatments.
Godin and colleagues presented an interesting study in which Radiesse and non-animal hyaluronic acid are combined to improve facial enhancement (Godin et al 2006). Many patients have received semipermanent fillers after temporary fillers without interference (Lemperle et al 2006).
Botulinum toxin can decrease dynamic wrinkles prior to Radiesse implant. In this way, especially in glabellar furrows and marionette lines, filling effects can be improved. It is recommended to start with botulinum toxin and wait for at least a week before proceeding with CaHA.
Combination treatments such as laser, chemical peels, and dermabrasion should be performed before facial filler injection. Because long-lasting implants usually lie in a dermal deep plane, they are not affected by laser (Lemperle et al 2006).
In an experimental preclinical study, Shumaker et al (2006), presented the effects of monopolar radiofrequency over soft tissue fillers. The preliminary results showed an increase in fibroplasia and collagen deposition surrounding Radiesse.
Every patient’s circumstances and treatment needs are different. Each case must be considered as unique according to specific signs, patients’ desires, and real possibilities.
Results
Since beauty is in the eye of the beholder, discussing results is difficult. No filler can stop time. Longevity, patients’ satisfaction, adverse events, and complications all influence how results are interpreted.
Longevity
How long will the effects last? How long depends on many factors and cannot exactly be predicted. Longevity depends on many factors, such as the patient’s age and metabolism, and the relative dynamic motion of the area in which the implant is placed (Felderman 2005).
Real “persistence of the product” in the body versus “clinical visible effect” concepts must be clearly explained, discussed, and understood by the patient before every treatment.
Many published reports state that results achieved during the first post-procedural month or after the last touch-up when needed remain almost unchanged until 12 months. After 12–18 months, the achieved volumes begin to diminish, though some results can be noted 24 months post injection. The average longevity therefore could be considered to be 12–18 months (Tzikas 2004; Kanchwala et al 2005; Broder and Cohen 2006; Jacovella 2006; Jacovella et al 2006; Jansen and Graivier 2006; Silvers et al 2006). Longer duration of effect, up to 24 months, can be seen in some patients, with a touch-up at the 12th month of half the amount injected the first time (Felderman 2005; Jacovella 2006).
Patient satisfaction
Patient satisfaction results for Radiesse have been assessed in several studies. In a possible self-reporting options range very good, good, acceptable, and non-acceptable, Jacovella et al reported 87% of very good results in a preliminary study (Jacovella et al 2006). Tzikas (2004) presented a patient satisfaction response of 47% excellent and 41% good. Jansen and Graivier (2006) concluded that 89% of 609 patients would choose Radiesse again. Roy and colleagues presented an overwhelming positive response to the look and feel of the implant, averaging 4.6 on a grading scale from 1 to 5 (Roy et al 2006).
Unpleasant effects and complications
Most of the unpleasant effects and complications associated with Radiesse injection are technique-related (Jacovella 2006).
Unpleasant effects
As temporary adverse effects, ecchymosis and hematoma may be expected in approximately 5% of patients. Redness and swelling immediately after treatment is very frequent. Both signs can persist for 5 days. Unpleasant effects usually resolve spontaneously.
Main complications
Nodules and granulomas
Nodules and granulomas have been described as complications. Lip nodules are relatively frequent because of product crowding, and are technique-associated. Granulomas are due to excessive immunological reaction and are not that frequent.
According to Kanchwala et al (2005), Radiesse should not be injected in lips because of a high incidence of mucosal nodules, but another study has shown a low incidence of lip nodules (Jacovella et al 2006).
Both nodules and granulomas can be treated with triamcinolone local injection at first time or through a small incision in persistent cases (Zide 2003; Jacovella 2006; Jansen and Graivier 2006).
Asymmetries
Asymmetries can occur immediately after injections. It is wise to refrain from additional treatment for at least 2 or 3 weeks, until the acute inflammatory effects disappear. After that time, persistent asymmetries can be corrected, starting always with small amounts of the product.
Comments and conclusions
The high number of available injectable facial fillers is increasing steadily. The election of the most appropriate filling material is based on multiple factors, including, most importantly, patient safety first, longevity, cost-effectiveness, precise indications, and regulatory status, among others. Consequently it is advisable to make the best decision, considering the right products, at the right place, at the right time (Werschler 2006). Any procedure performed on an elective basis must have a predictable result.
Several other types of facial filler compounds can be mentioned. Autologous fat transfer by injection has been proposed for large volume bulking purposes. Despite the advantage of being the own patient’s tissue, two other disadvantages can be considered. First, it implies two surgical procedures: taking the fat and injecting the fat. Second, lasting results are unpredictable, even though permanence can be improved by adding endothelial growth factor.
Taking into account that aging is a continuous process, persistence of permanent fillers can be disproportional to the loss of connective tissue, and visible bumps in some regions could result in an unesthetic appearance.
Because of many complications such as big granulomas, migration of product, serious deformities, and systemic reactions, silicone injections should not be used. The significant drawback of injectable liquid silicone is that the product and the respective reactive tissue cannot be removed without consequent deformity.
Short-lasting fillers such as hyaluronic acid could be used to demonstrate expected results when these are not well understood by the patient, and also for training purposes. Frequent touch-ups, every 6 months, should be taken into account when making a decision on such temporary products.
Finally, Radiesse can be considered an effective soft-tissue filler in view of its overall advantages. Much published research shows that calcium hydroxylapatite is safe and well tolerated when used properly. Most of the desirable properties of a dermal filler can be achieved with Radiesse, including acceptable longevity, biocompatibility, low rate of side effects, and a reasonable cost-benefit ratio.
Footnotes
Disclosures The author has no commercial interest in Radiesse.
References
- Alam M, Yoo SS. Technique for calcium hydroxylapatite injection for correction of nasolabial fold depressions. J Am Acad Dermatol. 2007;56:285–9. doi: 10.1016/j.jaad.2006.09.014. [DOI] [PubMed] [Google Scholar]
- American Society of Plastic Surgeons and American Society of Aesthetic Plastic Surgery. Injectable fillers: legal and regulatory risk management issues. Plast Reconstr Surg. 2006;118(Suppl):129S–32S. doi: 10.1097/01.prs.0000214727.21086.0c. [DOI] [PubMed] [Google Scholar]
- Bioform Medical Inc. Regulatory issues. 2007 [online]. Accessed October 25, 2007. URL: http: //www.radiesse.com.
- Broder KW, Cohen SR. An overview of permanent and semipermanent Fillers. Plast Reconstr Surg. 2006;118(Suppl):7S–14S. doi: 10.1097/01.prs.0000234900.26676.0b. [DOI] [PubMed] [Google Scholar]
- Burroughs JR, Anderson RL, McCann JD. Expanding roles for dermal fillers lead to better outcomes, satisfied patients. Cosmetic Surgery Times. 2006;6:10–17. [Google Scholar]
- Busso M, Karlsberg PL. Cheek augmentation and rejuvenation using Radiesse. Cosmetic Dermatology. 2006;19:583–8. [Google Scholar]
- Comite SL, Greene A, Cieszynski SA, et al. Minimizing discomfort during the injection of Radiesse™ with the use of either local anesthetic or ice. Dermatology Online J. 2007;13:5. Accesed, October 10, 2007. URL: http://dermatology.cdlib.org. [PubMed]
- Comite SL, Liu JF, Balasubramanian S, et al. Treatment of HIV-associated facial lipoatrophy with Radiance FN™ (Radiesse™) Dermatology Online J. 2006;10:2. Accessed May 25, 2007. URL: http://dermatology.cdlib.org. [PubMed]
- Dover JS. The filler revolution has just begun. Plast Reconstr Surg. 2006;117(Suppl):38S–40S. [Google Scholar]
- Elson ML. Radiesse: A fresh look at fillers. Cosmet Dermatol. 2006;19:111–12. [Google Scholar]
- Evans KK, Rasko Y, Lenert J, et al. The use of calcium hydroxylapatite for nipple projection after failed nipple-areolar reconstruction. Ann Plast Surg. 2005;55:25–29. doi: 10.1097/01.sap.0000168370.81333.97. [DOI] [PubMed] [Google Scholar]
- Fagien S. Evalution of a calcium hydroxylapatite-based implant (Radiesse) for facial soft-tissue augmentation. Plast Reconstr Surg. 2006;118(Suppl):31S–3S. doi: 10.1097/01.prs.0000234903.55310.6a. Discussion. [DOI] [PubMed] [Google Scholar]
- Felderman LI. Radiesse for facial rejuvenation. Cosmet Dermatol. 2005;18:823–6. [Google Scholar]
- Flaharty P. Radiance. Facial Plast Surg. 2000;20:165–9. doi: 10.1055/s-2004-861759. [DOI] [PubMed] [Google Scholar]
- Glogau RG. Aesthetic and anatomic analysis of the aging skin. Semin Cutan Med Surg. 1996;15:134–8. doi: 10.1016/s1085-5629(96)80003-4. [DOI] [PubMed] [Google Scholar]
- Gmyrek R, Dahdah M. Local anesthesia and regional block anesthesia. eMedicine.com. 2006 online. Accessed may 25, 2006. URL: http://www.emediciene.com/derm.
- Godin MS, Majmundar MV, Chrzanowski DS, et al. Use of Radiesse in combination with Restylane for facial augmentation. Arch Facial Plast Surg. 2006;8:92–7. doi: 10.1001/archfaci.8.2.92. [DOI] [PubMed] [Google Scholar]
- Goldberg DJ, Amin S, Hussain M. Acne scar correction using Radiesse, injectable calcium hydroxylapatite in a carrier-based gel. J Cosmetic Laser Ther. 2006;8:134–6. doi: 10.1080/14764170600891632. [DOI] [PubMed] [Google Scholar]
- Havlik RJ the PSEF Data Committee. Hydroxyapatite. Plast Reconstr Surg. 2002;15:1176–9. doi: 10.1097/01.PRS.0000020998.55126.10. [DOI] [PubMed] [Google Scholar]
- Hobar PC, Pantaloni M, Byrd HS. Porous hydroxyapatite granules for alloplastic enhancement of the facial region. Clin Plast Surg. 2000;27:557–69. [PubMed] [Google Scholar]
- Jacovella PF. Calcium hydroxylapatite (Radiesse): indications, technique and results. Clin Plast Surg. 2006;33:511. doi: 10.1016/j.cps.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Jacovella PF. Correcciones faciales estéticas con rellenos inyectables. Buenos Aires: Akadia Editorial; 2007. Spanish. [Google Scholar]
- Jacovella PF, Peiretti CB, Cunille D, et al. Long lasting results with hydroxylapatite (Radiesse) facial filler. Plast Reconstr Surg. 2006;118(Suppl):15S–21S. doi: 10.1097/01.prs.0000234902.61284.c9. [DOI] [PubMed] [Google Scholar]
- Jansen DA, Graivier MH. Evalution of a calcium hydroxylapatite-based implant (Radiesse) for facial soft-tissue augmentation. Plast Reconstr Surg. 2006;118(Suppl):22S–30S. doi: 10.1097/01.prs.0000234903.55310.6a. [DOI] [PubMed] [Google Scholar]
- Jansen DA, Graivier MH. Soft tissue substitutes in perioral augmentation. Sem in Plast Surg. 2003;17:181–98. [Google Scholar]
- Kanchwala SL, Holloway L, Bucky LP. Reliable soft tissue augmentation. Clinican comparison of injectable soft-tissue filler for facial-volumen augmentation. Ann Plast Surg. 2005;55:30–5. doi: 10.1097/01.sap.0000168292.69753.73. [DOI] [PubMed] [Google Scholar]
- Klein AW, Elson ML. The history of substances for soft tissue augmentation. Dermatol Surg. 2006;26:1096–195. [PubMed] [Google Scholar]
- Lemperle G, Rullan PP, Hazan Gauthier N. Avoiding and treating dermal filler complications. Plast Reconstr Surg. 2006;118(Suppl):92S–107S. doi: 10.1097/01.prs.0000234672.69287.77. [DOI] [PubMed] [Google Scholar]
- Marmur AS, Phelps R, Goldbeg DJ. Clinical, histologic and electron microscopic findings after injection of a calcium hydroxylapatite filler. J Cosmet Laser Ther. 2004;6:223–6. doi: 10.1080/147641704100003048. [DOI] [PubMed] [Google Scholar]
- Mayer R, Lightfoot M, Jung T. Preliminary evaluation of calcium hydroxylapatite as a transurethral bulking agent for stress urinary incontinence. Urology. 2001;57:434–8. doi: 10.1016/s0090-4295(00)01098-0. [DOI] [PubMed] [Google Scholar]
- Misiek D, Kent J. Soft tissue response to hydroxylapatite particle of different shapes. J Oral Maxillofacial Surg. 1984;42:150–60. doi: 10.1016/s0278-2391(84)80025-7. [DOI] [PubMed] [Google Scholar]
- Murray CA, Zloty D, Warshawski L. The evolution of soft tissue fillers in clinical practice. Dematol Clin. 2005;23:343–63. doi: 10.1016/j.det.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Narins RS, Bowman PH. Injectable skin fillers. Clin Plast Surg. 2005;32:151–62. doi: 10.1016/j.cps.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Nyte CP. Spreader graft Injection with calcium hydroxylapatite: a nonsurgical technique for internal nasal valve collapse. Laryngoscope. 2006;116:1291–2. doi: 10.1097/01.mlg.0000218047.06639.eb. [DOI] [PubMed] [Google Scholar]
- Pettis G, Kaban L, Glowacki J. Tissue response to composite ceramic hydroxylapatite-demineralized bone implant. J Oral Maxillofacial Surg. 1990;48:1068–74. doi: 10.1016/0278-2391(90)90291-9. [DOI] [PubMed] [Google Scholar]
- Probeck HP, Rothstein SS. Histologic observation of soft tissue responses to imported multifaceted particles and discs of hydroxylapatite. J Oral Maxillofacial Surg. 1989;42:143–9. doi: 10.1016/s0278-2391(84)80024-5. [DOI] [PubMed] [Google Scholar]
- Reisman NR. Ethics, legal issues and consent for fillers. Clin Plast Surg. 2006;3:505–10. doi: 10.1016/j.cps.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Rohrich RJ, Potter JK. Liquid injectable silicone: is there a role as a cosmetic soft-tissue filler? Plast Reconstr Surg. 2004;113:1239–41. doi: 10.1097/01.prs.0000118022.71802.78. [DOI] [PubMed] [Google Scholar]
- Rohrich RJ, Rios JL, Fagien S. Role of new fillers in facial augmentation: a cautious outlook. Plast Reconstr Surg. 2003;112:1899–902. doi: 10.1097/01.PRS.0000097307.62862.27. [DOI] [PubMed] [Google Scholar]
- Roy D, Sadick N, Mangat D. Clinical trial of a novel filler material for soft tissue augmentation of the face containing synthetic calcium hydroxylapatite microspheres. Dermatol Surg. 2006;32:1134–9. doi: 10.1111/j.1524-4725.2006.32256.x. [DOI] [PubMed] [Google Scholar]
- Sengelmann RD, Tull S, et al. Dermal fillers. eMedicine.com. 2004 online. Accessed 20 May 2007. URL: www.emedicine.com/derm/topic515.htm.
- Shumaker PR, et al. Effect of monopolar RF treatment over soft-tissue fillers on an animal model. Laser Surg Med. 2006;38:211–17. doi: 10.1002/lsm.20292. [DOI] [PubMed] [Google Scholar]
- Silvers SL, Eviatar JA, Echavez MI, et al. Prospective, open-label, 18-month trial of calcium hydroxylapatite (Radiesse) for facial soft-tissue augmentation in patients with human immunodeficiency virus-associated lipoatrophy: one year durabilitay. Plast Reconstr Surg. 2006;118(Suppl):34S–45S. doi: 10.1097/01.prs.0000234847.36020.52. [DOI] [PubMed] [Google Scholar]
- Sklar JA, White SM. Radiance FN: A new soft tissue filler. Dermatol Surg. 2004;30:764–8. doi: 10.1111/j.1524-4725.2004.30214.x. [DOI] [PubMed] [Google Scholar]
- Tzikas TL. Evaluation of the Radiance FN soft tissue filler for facial soft tissue augmentation. Arch Facial Plast Surg. 2004;6:234–39. doi: 10.1001/archfaci.6.4.234. [DOI] [PubMed] [Google Scholar]
- Werschler WP. Radiesse and other soft tissue fillers: Right products, right place, right time. Aesthetics Trends Tech. 2006 summer;5(4) [Google Scholar]
- Zide BM. Radiance: short-tern experience. Aesthetic Surg J. 2003;23:495–9. doi: 10.1016/j.asj.2003.08.004. [DOI] [PubMed] [Google Scholar]