Abstract
Objectives. To analyse consecutive patients with RA in usual rheumatology care between 1980 and 2004 at two settings for the proportion of patients taking MTX, interval from patient presentation to MTX prescription and radiographic and functional status outcomes.
Methods. Longitudinal study of all patients seen in usual care between 1980 and 2004, 1982 consecutive patients in Jyväskylä, Finland and 738 consecutive patients in Nashville, TN, USA. Clinical status was assessed as Larsen radiographic scores in Jyväskylä and modified health assessment questionnaire (MHAQ) in Nashville.
Results. The probability of initiating MTX within 5 yrs after presentation increased from <5% in Jyväskylä before 1989 to >90% in 2000–04, and from 25% in Nashville in 1980–84 to >90% since 1995. The median interval from presentation to MTX initiation in Jyväskylä was 14 yrs in 1980–84 vs 8.6 in 1985–89, 4.5 in 1990–94, 1.8 in 1995–99 and <1 yr in 2000–05; in Nashville, median intervals were 8.6 yrs in 1980–84, 4.4 years in 1985–89, and <2 months in 1990–95, 1995–2000 and 2000–05. Patient outcomes were substantially improved in both settings: in Jyväskylä, mean 5-yr Larsen radiographic scores (0–100) were 15.7 in 1980–84 vs 4.0 in 1995–99; in Nashville, mean MHAQ scores (0–3) for physical function were 1.13 in 1980–84 vs 0.57 in 2000–04.
Conclusion. Early MTX in usual clinical care of RA increased from <5% in 1980 to >90% in 2004. Over this period, substantially improved outcomes were seen, most of which antedated biological agents.
Keywords: Rheumatoid arthritis, Health assessment questionnaire, Modified health assessment questionnaire, Methotrexate, Outcomes
Introduction
Three major advances in treatment of RA over the last two decades include: (i) early intervention toward ‘tight control’ of inflammation to prevent joint damage [1–3]; (ii) emergence of weekly low-dose MTX as the ‘anchor drug’ for RA [4–6]; and (iii) targeted therapies with biological agents [7–9]. MTX provides substantially greater efficacy [4, 5, 10–12], effectiveness [13–17], tolerability [18, 19] and lower toxicity [13, 14, 20] than previously available DMARDs. Significantly better outcomes are seen at this time compared with earlier periods [16, 17, 21–25].
Recent medical literature concerning RA has been dominated by targeted biological agents. The ascendancy of early treatment with weekly low-dose MTX for RA over the past 25 yrs remains relatively under-recognized [26]. Biological agents are a major advance, taken by 20–50% of the patients with RA at aggressive treatment centres, and fewer at other sites [27]. Nonetheless, MTX remains the most widely prescribed medication for RA at this time. However, many non-rheumatologists continue to regard potential risks of weekly low-dose MTX for rheumatic disease as similar to those of high-dose MTX (as an anti-neoplastic agent), and may discontinue its use for relatively minor liver function abnormalities or for elective surgery, with frequent deleterious effects.
This report examines three phenomena concerning MTX use over 25 yrs from 1980 to 2004 in 1982 RA patients in Jyväskylä, Finland and 738 RA patients seen by T.P. in Nashville, TN, USA: (i) the proportion of patients taking MTX; (ii) the interval from patient presentation to prescription; and (iii) radiographic and functional status outcomes.
Patients and methods
A database has been maintained on all patients with RA seen at Jyväskylä Central Hospital, Jyväskylä, Finland [28], and all patients seen by TP at Vanderbilt University in Nashville, TN, USA [20] since 1980.
Jyväskylä Central Hospital is the only rheumatology centre in the Central Finland Health Care District and serves a population of 265 000. All new patients with suspected RA are referred to this clinic for diagnosis and therapy. Most patients with persistent RA continue their care at this hospital with regular visits to outpatient and day-care clinics. The Central Finland RA Database includes demographic and clinical data on patients with RA who have been seen in Jyväskylä Central Hospital since 1980. This database was begun in 1995 with retrospective entry of data collected at all clinic visits of all RA patients seen from 1980 to 95 and ongoing entry of data collected at all visits of RA patients since 1995 [29]. The Jyväskylä patients provide a true inception cohort, as all patients are seen for their first visit with RA prior to any long-term treatment.
The Vanderbilt weekly academic rheumatology clinic of TP in Nashville is a US university site. This database was begun in 1989 with retrospective entry of data collected at all clinic visits of all patients seen from 1980 to 1989, and ongoing entry of data collected at all clinic visits since 1989. Most of the Nashville patients were seen prior to long-term treatment of RA, but some patients were seen for their first visit after having been treated by other physicians, including other rheumatologists in other areas. Fewer than 20 patients had prior treatment with MTX, none prior to 1995.
The databases contain demographic data, standard rheumatology measures including joint counts, radiographs, laboratory measures and patient questionnaires. In Jyväskylä, the outcome measure available in most patients since 1980 is a radiograph, which is taken at baseline and during follow-up 5 yrs after the diagnosis. In Nashville, the outcome measure available in most patients since 1980 is a modified health assessment questionnaire (MHAQ) [30] and successor multidimensional HAQ (MDHAQ) [31], which includes all items in the MHAQ. All patients since 1980 have completed a HAQ, MHAQ or MDHAQ at all visits; therefore, scores for physical function and a visual analogue scale (VAS) for pain are available to compare status over time in all patients.
Previous reports from these two sites have indicated improved outcomes in some of these patients [24, 25]. This report includes new analyses concerning therapies with MTX and other DMARDs, and extends previous analyses to the entire databases from 1980 to 2004. The interval between patient presentation and initiation of MTX, as monotherapy or in combination with other medications, was calculated and presented separately for each site for five different 5-yr periods between1980 and 2004, including 1980–84, 1985–90, 1990–94, 1995–99 and 2000–04, using Kaplan–Meier statistics.
The percentage of patients who were taking MTX as the first DMARD was computed. In the Jyväskylä cohort, radiographs taken 5 (3.5–6.5) yrs after patient presentation were scored according to the Larsen method (0–100) [32, 33]. In the Nashville cohort, the last MHAQ (0–3) and pain VAS (0–10) values of each patient were scored in the period in which the patient was first seen. Data concerning each patient are included in only one of the 5-yr periods reported.
Data were computed as mean, s.d., median and interquartile range for continuous variables, and as percentages for dichotomous variables. The mean and 95% CI values for Larsen scores, MHAQ and pain were calculated.
Results
Patient characteristics
Patients from Jyväskylä had a mean age of 55 yrs; 67% were females and 62% were positive for RF (Table 1). Patients in Nashville were quite similar, with a mean age of 54 yrs, while 61% were females and 63% RF-positive. Patients in the two cohorts differed in median duration of symptoms at first visit, which was 6 months in Jyväskylä and 4 yrs in Nashville, reflecting different referral patterns at the two sites.
Table 1.
Characteristics of patients at two sites, one in Finland and one in the United States, in 5-yr periods from 1980 to 2004
| Period of the first visit | 1980–1984 | 1985–1989 | 1990–1994 | 1995–1999 | 2000–2004 | Total |
|---|---|---|---|---|---|---|
| Jyväskylä, Finland | ||||||
| Number of patients | 219 | 305 | 363 | 508 | 497 | 1892 |
| Age, mean (s.d.), yrs | 50 (15) | 53 (15) | 55 (16) | 57 (15) | 58 (16) | 55 (16) |
| Female (%) | 69 | 64 | 67 | 66 | 69 | 67 |
| RF-positive (%) | 74 | 76 | 66 | 57 | 51 | 62 |
| Duration of symptoms, median (IQR) at presentation, months | 9 (5, 23) | 6 (4, 12) | 6 (3, 14) | 5 (3, 12) | 5 (3, 11) | 6 (3, 12) |
| Total follow-up, median (IQR), yrs | 16 (12, 20) | 12 (6, 15) | 7 (3, 11) | 5 (4, 7) | 2 (1, 2) | 5 (2, 10) |
| Median number of years from presentation to initiation of MTX, cumulative percentage (95% CI) | 14 (12, 16) | 8.6 (7.6, 9.6) | 4.5 (3.7, 5.3) | 1.8 (1.5, 2.2) | 0.5 (0.3, 0.7) | 4.4 (3.9, 5.0) |
| Nashville, TN, USA | ||||||
| Number of patients | 216 | 185 | 141 | 93 | 103 | 738 |
| Age, mean (s.d.), yrs | 55 (15) | 54 (14) | 53 (15) | 54 (12) | 54 (15) | 54 (14) |
| Female (%) | 69 | 73 | 70 | 68 | 72 | 71 |
| RF-positive (%) | 69 | 58 | 59 | 60 | 70 | 63 |
| Duration of disease, median (IQR) at presentation, yrs | 5 (1, 12) | 4 (1, 13) | 3 (1, 8) | 3 (1, 11) | 6 (1, 12) | 4 (1, 12) |
| Total follow-up, median (IQR), yrs | 4.2 (0.1, 10) | 3.8 (0.3, 7.9) | 1.3 (0.2, 7.6) | 3.1 (0.1, 5.8) | 0.7 (0.2, 2.0) | 2.2 (0.1, 6.9) |
| Median number of years from presentation to initiation of MTX, cumulative percentage (95% CI) | 8.6 (7.5, 9.6) | 4.4 (2.9, 5.9) | 0.2 (0, 0.3) | 0.1 (0.1, 0.2) | 0 (0, 0) | 1.6 (1.0, 2.2) |
Interval after presentation until treatment with MTX
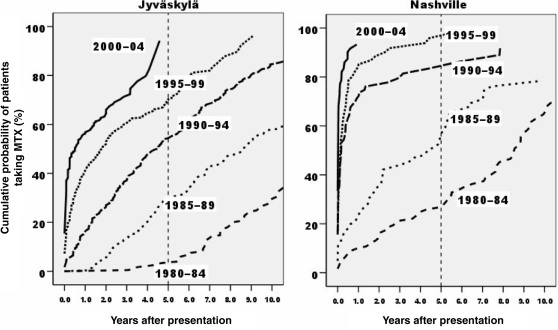
In Jyväskylä, the median interval from presentation to initiation of MTX fell from 14 yrs in 1980–84 to 8.6 yrs in 1985–89, 4.5 yrs in 1990–94, 1.8 yrs in 1995–99 and <1 yr in 2000 (Table 1, Fig. 1). In Nashville, the median interval fell from 8.6 yrs in 1980–84 to 4.4 yrs in 1985–89 to <2 months in the three 5-yr periods beginning in 1990 (Table 1, Fig. 1). The probability of starting MTX by 5 yrs after presentation increased from <5% in Jyväskylä in 1984–89 to 50% in 1990–94 and >90% in 2000–04, and from 25% in Nashville in 1984–89 to >50% in 1985–89, >80% in 1990–94 and >90% since 1995 (Fig. 1).
Fig. 1.
Interval between patient presentation and initiation of MTX in Jyväskylä, Finland and Nashville, TN, according to the period of patient presentation.
The first DMARD used in the highest number of patients in 1980–84 at both sites was intramuscular gold (Table 2). However, use of intramuscular gold as the first DMARD declined from 63.5% to 0.2% in Jyväskylä, and from 27.3% to 1.5% in Nashville, between 1980–84 and 2000–04. The proportion of patients who took MTX as the first DMARD increased from 0% to 31% in Jyväskylä and from 10% to 78% in Nashville between 1980 and 2004 (Table 2).
Table 2.
The first DMARD for RA in Jyväskylä, Finland and the first DMARD at presentation in Nashville, TN, USA, per 5-yr period since 1980
| 1980–1984 | 1985–1989 | 1990–1994 | 1995–1999 | 2000–2004 | |
|---|---|---|---|---|---|
| Jyväskylä,Finland | |||||
| Number of patients | 219 | 305 | 363 | 508 | 497 |
| Intramuscular gold, n (%) | 139 (63.5) | 171 (56.1) | 51 (14.0) | 12 (2.4) | 1 (0.2) |
| HCQ, n (%) | 72 (32.9) | 35 (11.5) | 29 (8.0) | 44 (8.7) | 70 (14.1) |
| SSZ, n (%) | 2 (0.9) | 92 (30.2) | 257 (70.8) | 366 (72.0) | 257 (51.7) |
| MTX, n (%) | 0 | 0 | 1 (0.3) | 66 (13.0) | 88 (17.7) |
| MTX in combination, n (%) | 0 | 0 | 14 (3.9) | 11 (2.2) | 66 (13.3) |
| Other, n (%) | 0 | 3 (3.0) | 8 (2.2) | 4 (0.8) | 7 (1.4) |
| No medication, n (%) | 6 (2.7) | 4 (1.3) | 3 (0.8) | 5 (1.0) | 8 (1.6) |
| Nashville, TN, USA | |||||
| Number of patients | 216 | 185 | 141 | 93 | 103 |
| Intramuscular gold, n (%) | 59 (27.3) | 18 (8.9) | 5 (3.5) | 3 (2.3) | 1 (1.5) |
| HCQ, n (%) | 23 (10.6) | 12 (6.5) | 25 (17.7) | 10 (10.8) | 4 (3.9) |
| Auranofin, n (%) | 9 (4.2) | 30 (16.2) | 7 (5.0) | 0 | 0 |
| AZA, n (%) | 11 (5.1) | 6 (3.2) | 2 (1.4) | 0 | 1 (1.0) |
| MTX, n (%) | 21 (9.7) | 48 (25.9) | 77 (54.6) | 58 (62.4) | 68 (66.0) |
| MTX in combination, n (%) | 1 (0.5) | 0 | 3 (2.1) | 8 (8.6) | 12 (11.7) |
| LEF, n (%) | 0 | 0 | 0 | 1 (1.1) | 3 (2.9) |
| Biological agent, n (%) | 0 | 0 | 0 | 1 (1.1) | 6 (5.8) |
| Other, n (%) | 7 (3.2) | 8 (4.3) | 2 (1.4) | 0 | 1 (1.0) |
| Prednisone only, n (%) | 29 (13.4) | 29 (15.7) | 16 (11.3) | 10 (10.8) | 6 (5.8) |
| No medication, n (%) | 56 (25.9) | 34 (18.4) | 4 (2.8) | 2 (2.2) | 1 (1.0) |
Clinical status in patients seen at different periods
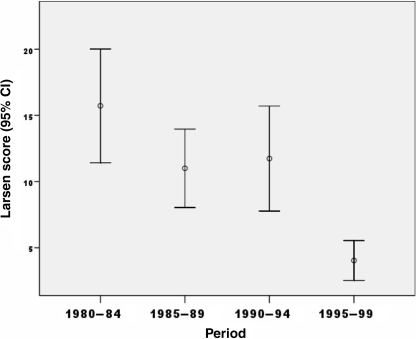
In Jyväskylä, clinical outcome over the years was analysed according to Larsen radiographic score 5 yrs after onset of disease. The mean score fell from 15.7 in 1980–84 to 11.0 in 1985–89, was level at 11.7 from 1990–94, but with further decline in 1995–99 to 4.0 (5-yr radiographs were not available after 2000) (Fig. 2).
Fig. 2.
Larsen radiographic scores in 295 patients in Jyväskylä, Finland, 5 yrs after presentation (diagnosis), according to the period of the presentation.
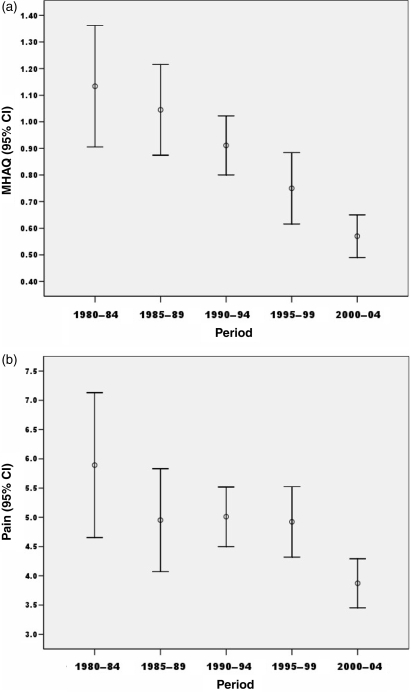
In Nashville, clinical outcomes were assessed according to the MHAQ scores for physical function and pain. Mean scores for physical function (0–3) declined from 1.13 in 1980–84 to 1.04 in 1985–89, 0.91 in 1990–94, 0.75 in 1995–99 and 0.57 in 2000–04 (Fig. 3a). A similar decline in last mean pain (0–10) score was also seen, from 5.9 in 1980–84 to 4.9 in 1985–89, 5.0 in 1990–94, 4.9 in 1995–99 and 3.8 in 2000–04 (Fig. 3b). These results were seen although the disease duration at final visit was increased from 11.5 yrs in 1980–84 to 14.5 yrs in 2000–04 (data not shown).
Fig. 3.
Patient functional status according to (a) MHAQ and (b) pain, in 596 patients in Nashville, TN at final visit, according the period when last visit occurred.
Discussion
This report presents five observations in patients with RA over 25 yrs from 1980 to 2005 at two sites in Finland and Tennessee: (i) weekly, low-dose MTX has replaced intramuscular gold injections as the primary therapy for RA, taken by >90% of the patients; (ii) MTX has been used at progressively shorter intervals after presentation, to the point where it is prescribed within a month of presentation at these sites; (iii) shortening of the interval from diagnosis to initiation of MTX at the US site antedated the Finnish site by about a decade; (iv) these changes have coincided with substantially improved radiographic outcomes in Finland and functional outcomes in Nashville; and (v) the improved outcomes seen in most of these patients antedated availability of biological agents for RA.
Several limitations are seen in this study. The most important limitation is that improved patient outcomes over the 25-yr period cannot be attributed definitively to early treatment with MTX, as improved outcomes could result in part or entirely from other developments including a secular trend towards milder disease [34, 35], a higher proportion of patients referred for specialist care (so that the relative proportion of severe patients is falling) and improved general health and care in the community beyond specific treatment for RA. Nonetheless, data in patients with RA from 22 countries in 2005–06 indicate high disease activity levels in many countries at this time, associated with lesser use of MTX and other DMARDs [27]. Therefore, it appears likely that early treatment with MTX may play at least some role in improved outcomes.
A second limitation is that it might appear ideal to document long-term effectiveness of weekly low-dose MTX in randomized controlled clinical trials. However, long-term clinical trials over 5–10 yrs in a symptomatic disease such as RA cannot be performed for logistic and ethical reasons [36], particularly with documentation of the efficacy of aggressive vs traditional therapy of RA in randomized controlled clinical trials [37–39].
Third, it would be ideal if the entire core data set were available from each patient at each visit. However, data concerning radiographs and MHAQ appear representative of two major indicators of RA progression and these may be the ‘best available’ data over the 25-yr period.
Fourth, the study includes only two sites. However, few settings have available clinical data concerning therapies taken by consecutive patients over 25 yrs. The unusual databases from Jyväskylä, Finland and by T.P. in Nashville, TN, USA, provided an opportunity to document changes in MTX therapy over the years. The similarity of results from the two sites and similarity at this time to many sites in many countries [40] suggest that the observations may be at least in part generalizable, although there are sites with lesser use of MTX at this time.
MTX courses were reported in 1992 to be continued over at least 5 yrs in >50% of the patients, in marked contrast to 10–20% of courses of parenteral gold, penicillamine, HCQ and AZA, which were continued at 5 yrs [14]. These data were interpreted to suggest that patients found that MTX provided greater long-term effectiveness, lesser long-term toxicity and the absence of loss of efficacy seen with traditional DMARDs [14]. More recently, MTX was found to be continued by 80% of the patients at 5 yrs in the Nashville clinic of T.P. between 1990 and 2003 [20].
MTX was introduced in 1951 as an anti-neoplastic agent [41, 42]. In high doses, it is a cytotoxic agent with substantial potential toxicity and low tolerability. However, weekly low-dose MTX, as used in treatment of RA, appears to act primarily as an effective and very well-tolerated anti-inflammatory agent [43]. Weekly low-dose MTX in RA may be one of the best available medications for any chronic disease at this time, rivalling insulin and proton pump inhibitors in being continued by most patients over the years as a result of effectiveness, tolerability and safety. Nonetheless, clinicians and patients often do not distinguish between high-dose MTX used to treat neoplastic disease and weekly low-dose MTX, and often discontinue its use unnecessarily, e.g. around elective surgery, with deleterious effects for the patient.
The effectiveness and low toxicity of weekly, low-dose MTX remain under-recognized. Indeed, with the use of MTX, many patients with RA do not develop significant joint damage at this time, as indicated in low radiographic scores in Finland [24] and Nashville [25]. Work disability rates [44] and mortality rates appear to be falling, associated with good response to therapies including MTX [16, 17]. These data indicate a major change in outcomes of RA associated with widespread use of MTX.
In conclusion, we have documented a substantial increase in proportion of patients treated and time to treatment of patients with RA with MTX over a 25-yr period at two sites, one in Finland and one in the United States. At this time, most patients are treated with MTX within the first year of disease, if not within the first few months, as the ‘anchor drug’ for treatment of RA, at least at these two sites, and many other sites. Evidence of substantially improved patient status was seen over the 25 yrs, although this clinical improvement may have resulted from other causes than early treatment with MTX.

Acknowledgements
The authors thank Melissa Gibson, Christopher Swearingen, and Vicky Goodin for careful assistance in management of the Nashville database.
Funding: This work has been supported in part by grants from the Arthritis Foundation, and the Jack C. Massey Foundation (to T.P.) and EVO-funds at Central Finland Health Care District, 40620 Jyväskylä, Finland (to T.S.).
Disclosure statement: T.P. has received research support from Health Report Services Inc., which also receives funds from Abbott Immunology, Amgen, Aventis, Bristol-Myers-Squibb, Centocor, Pfizer, Novartis, UCB Pharma and Wyeth. T.S. is a consultant for Abbott Laboratories, Sanofi-Aventis, UCB Pharma and Wyeth.
References
- 1.Pincus T. The case for early intervention in rheumatoid arthritis. J Autoimmun. 1992;5(Suppl A):209–26. doi: 10.1016/0896-8411(92)90036-p. [DOI] [PubMed] [Google Scholar]
- 2.Emery P, Salmon M. Early rheumatoid arthritis: time to aim for remission? Ann Rheum Dis. 1995;54:944–7. doi: 10.1136/ard.54.12.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinblatt ME. Rheumatoid arthritis: treat now, not later! [Editorial] Ann Intern Med. 1996;124:773–4. doi: 10.7326/0003-4819-124-8-199604150-00012. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmeister RT. Methotrexate therapy in rheumatoid arthritis: 15 years experience. Am J Med. 1983;75(Suppl 6A):69–73. doi: 10.1016/0002-9343(83)90477-1. [DOI] [PubMed] [Google Scholar]
- 5.Weinblatt ME, Glass DN. Trentham DE. Methotrexate for rheumatoid arthritis. N Engl J Med. 1985;313:639–40. doi: 10.1056/NEJM198509053131014. [DOI] [PubMed] [Google Scholar]
- 6.Pincus T, Yazici Y, Sokka T, Aletaha D, Smolen JS. Methotrexate as the ‘anchor drug’ for the treatment of early rheumatoid arthritis. Clin Exp Rheumatol. 2003;21:S179–85. [PubMed] [Google Scholar]
- 7.Maini RN, Elliot MJ, Brennan FM, Feldman M. Beneficial effects of tumour necrosis factor alpha (TNF-α) blockade in rheumatoid arthritis (RA) Clin Exp Immunol. 1995;101:207–12. doi: 10.1111/j.1365-2249.1995.tb08340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Vollenhoven RF, Askling J. Rheumatoid arthritis registries in Sweden. Clin Exp Rheumatol. 2005;23:S195–200. [PubMed] [Google Scholar]
- 9.Furst DE, Breedveld FC, Smolen JS, et al. Updated consensus statement on biological agents, specifically tumour necrosis factor {alpha} (TNF{alpha}) blocking agents and interleukin-1 receptor antagonist (IL-1ra), for the treatment of rheumatic diseases, 2005. Ann Rheum Dis. 2005;64:iv2–14. doi: 10.1136/ard.2005.044941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willkens RF, Watson MA. Methotrexate: a perspective of its use in the treatment of rheumatic diseases. J Lab Clin Med. 1982;100:314–21. [PubMed] [Google Scholar]
- 11.Jeurissen MEC, Boerbooms AMT, van de Putte LBA, Doesburg WH, Lemmens AM. Influence of methotrexate and azathioprine on radiologic progression in rheumatoid arthritis: a randomized, double-blind study. Ann Intern Med. 1991;114:999–1004. doi: 10.7326/0003-4819-114-12-999. [DOI] [PubMed] [Google Scholar]
- 12.Drosos AA, Tsifetaki N, Tsiakou EK, et al. Influence of methotrexate on radiographic progression in rheumatoid arthritis: a sixty-month prospective study. Clin Exp Rheumatol. 1997;15:263–7. [PubMed] [Google Scholar]
- 13.Kremer JM, Lee JK. A long-term prospective study of the use of methotrexate in rheumatoid arthritis: update after a mean of fifty-three months. Arthritis Rheum. 1988;31:577–84. doi: 10.1002/art.1780310501. [DOI] [PubMed] [Google Scholar]
- 14.Pincus T, Marcum SB, Callahan LF. Long-term drug therapy for rheumatoid arthritis in seven rheumatology private practices: II. Second-line drugs and prednisone. J Rheumatol. 1992;19:1885–94. [PubMed] [Google Scholar]
- 15.Weinblatt ME, Maier AL, Fraser PA, Coblyn JS. Longterm prospective study of methotrexate in rheumatoid arthritis: conclusion after 132 months of therapy. J Rheumatol. 1998;25:238–42. [PubMed] [Google Scholar]
- 16.Krause D, Schleusser B, Herborn G, Rau R. Response to methotrexate treatment is associated with reduced mortality in patients with severe rheumatoid arthritis. Arthritis Rheum. 2000;43:14–21. doi: 10.1002/1529-0131(200001)43:1<14::AID-ANR3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Choi HK, Hernán MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359:1173–7. doi: 10.1016/S0140-6736(02)08213-2. [DOI] [PubMed] [Google Scholar]
- 18.Aletaha D, Smolen JS. The rheumatoid arthritis patient in the clinic: comparing more than 1300 consecutive DMARD courses. Rheumatology. 2002;41:1367–74. doi: 10.1093/rheumatology/41.12.1367. [DOI] [PubMed] [Google Scholar]
- 19.Kapral T, Stamm T, Machold KP, Montag K, Smolen JS. Methotrexate in rheumatoid arthritis is frequently effective, even if re-employed after a previous failure. Arthritis Res Ther. 2006;8:R46. doi: 10.1186/ar1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yazici Y, Sokka T, Kautiainen H, Swearingen C, Kulman I, Pincus T. Long term safety of methotrexate in routine clinical care: discontinuation is unusual and rarely the result of laboratory abnormalities. Ann Rheum Dis. 2005;64:207–11. doi: 10.1136/ard.2004.023408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergstrom U, Book C, Lindroth Y, Marsal L, Saxne T, Jacobsson L. Lower disease activity and disability in Swedish patients with rheumatoid arthritis in 1995 compared with 1978. Scand J Rheumatol. 1999;28:160–5. doi: 10.1080/03009749950154239. [DOI] [PubMed] [Google Scholar]
- 22.Sokka TM, Kaarela K, Möttönen TT, Hannonen PJ. Conventional monotherapy compared to a ‘sawtooth’ treatment strategy in the radiographic procession of rheumatoid arthritis over the first eight years. Clin Exp Rheumatol. 1999;17:527–32. [PubMed] [Google Scholar]
- 23.Krishnan E, Fries JF. Reduction in long-term functional disability in rheumatoid arthritis from 1977 to 1998: a longitudinal study of 3035 patients. Am J Med. 2003;115:371–6. doi: 10.1016/s0002-9343(03)00397-8. [DOI] [PubMed] [Google Scholar]
- 24.Sokka T, Kautiainen H, Häkkinen K, Hannonen P. Radiographic progression is getting milder in patients with early rheumatoid arthritis. Results of 3 cohorts over 5 years. J Rheumatol. 2004;31:1073–82. [PubMed] [Google Scholar]
- 25.Pincus T, Sokka T, Kautiainen H. Patients seen for standard rheumatoid arthritis care have significantly better articular, radiographic, laboratory, and functional status in 2000 than in 1985. Arthritis Rheum. 2005;52:1009–19. doi: 10.1002/art.20941. [DOI] [PubMed] [Google Scholar]
- 26.Klaukka T, Kaarela K. Methotrexate is the leading DMARD in Finland. Ann Rheum Dis. 2003;62:494–6. doi: 10.1136/ard.62.5.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sokka T, Kautiainen H, Toloza S, et al. QUEST-RA: quantitative clinical assessment of patients with rheumatoid arthritis seen in standard rheumatology care in 15 countries. Ann Rheum Dis. 2007;66:1491–6. doi: 10.1136/ard.2006.069252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sokka T, Krishnan E, Häkkinen A, Hannonen P. Functional disability in rheumatoid arthritis patients compared with a community population in Finland. Arthritis Rheum. 2003;48:59–63. doi: 10.1002/art.10731. [DOI] [PubMed] [Google Scholar]
- 29.Sokka T, Kautiainen H, Hannonen P, Pincus T. Changes in health assessment questionnaire disability scores over five years in patients with rheumatoid arthritis compared with the general population. Arthritis Rheum. 2006;54:3113–8. doi: 10.1002/art.22130. [DOI] [PubMed] [Google Scholar]
- 30.Pincus T, Summey JA, Soraci SA, Jr, Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26:1346–53. doi: 10.1002/art.1780261107. [DOI] [PubMed] [Google Scholar]
- 31.Pincus T, Sokka T, Kautiainen H. Further development of a physical function scale on a multidimensional health assessment questionnaire for standard care of patients with rheumatic diseases. J Rheumatol. 2005;32:1432–9. [PubMed] [Google Scholar]
- 32.Larsen A. A radiological method for grading the severity of rheumatoid arthritis. Espoo: Meder-Offset. 1974:1–125. doi: 10.3109/03009747509165261. [DOI] [PubMed] [Google Scholar]
- 33.Kaarela K, Kautiainen H. Continuous progression of radiological destruction in seropositive rheumatoid arthritis. J Rheumatol. 1997;24:1285–7. [PubMed] [Google Scholar]
- 34.Silman A, Davies P, Currey HLF, Evans SJW. Is rheumatoid arthritis becoming less severe? J Chronic Dis. 1983;36:891–7. doi: 10.1016/0021-9681(83)90011-5. [DOI] [PubMed] [Google Scholar]
- 35.Spector TD, Hart DJ, Powell RJ. Prevalence of rheumatoid arthritis and rheumatoid factor in women: evidence for a secular decline. Ann Rheum Dis. 1993;52:254–7. doi: 10.1136/ard.52.4.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piper JM, Ray WA, Griffin MR, Fought R, Daugherty JR, Mitchel E., Jr Methodological issues in evaluating expanded Medicaid coverage for pregnant women. Am J Epidemiol. 1989;129:837–49. doi: 10.1093/oxfordjournals.aje.a115692. [DOI] [PubMed] [Google Scholar]
- 37.Grigor C, Capell H, Stirling A, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet. 2004;364:263–9. doi: 10.1016/S0140-6736(04)16676-2. [DOI] [PubMed] [Google Scholar]
- 38.Goekoop-Ruiterman YPM, de Vries-Bouwstra JK, Allaart CF, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum. 2005;52:3381–90. doi: 10.1002/art.21405. [DOI] [PubMed] [Google Scholar]
- 39.Fransen J, Moens HB, Speyer I, van Riel PLCM. Effectiveness of systematic monitoring of rheumatoid arthritis disease activity in daily practice: a multicentre, cluster randomised contolled trial. Ann Rheum Dis. 2005;64:1294–8. doi: 10.1136/ard.2004.030924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sokka T, Envalds M, Pincus T. Treatment of rheumatoid arthritis: a global perspective on the use of antirheumatic drugs. Mod Rheumatol. 2008;18:228–39. doi: 10.1007/s10165-008-0056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elion GB, Hitchings GH, Sherwood MB, Vanderwerff H. Folic acid-like activity from xanthopterin. Arch Biochem. 1950;26:337–42. [PubMed] [Google Scholar]
- 42.Elion GB Nobel lecture in physiology or medicine—1988. The purine path to chemotherapy. In Vitro Cell Dev Biol. 1989;25:321–30. doi: 10.1007/BF02624593. [DOI] [PubMed] [Google Scholar]
- 43.Cronstein BN. Molecular therapeutics: methotrexate and its mechanism of action. Arthritis Rheum. 1996;39:1951–60. doi: 10.1002/art.1780391203. [DOI] [PubMed] [Google Scholar]
- 44.Puolakka K, Kautiainen H, Mottonen T, et al. Impact of initial aggressive drug treatment with a combination of disease-modifying antirheumatic drugs on the development of work disability in early rheumatoid arthritis. A five-year randomized followup trial. Arthritis Rheum. 2004;50:55–62. doi: 10.1002/art.11436. [DOI] [PubMed] [Google Scholar]