Abstract
Objectives. There are no studies of fatigue levels in patients with SSc. The objective of this study was to compare fatigue in SSc to general population samples and patients with rheumatic diseases and cancer, where fatigue has been researched extensively.
Methods. SSc patients completed the General Fatigue Index (GFI) of the Multidimensional Fatigue Inventory. A systematic review was conducted to select comparison samples. Mean GFI scores from SSc patients were compared with mean scores from comparison samples with t-tests and Bonferroni corrections (family-wise P < 0.05).
Results. A total of 106 SSc patients were sampled (97 females; 28 diffuse SSc; 11.9 ± 7.9 yrs since diagnosis). Based on comparisons from the systematic review, mean GFI scores in SSc (13.3 ± 4.6) were significantly higher (greater fatigue; P < 0.05) than in two large population samples (8.7 and 9.6) and than in two samples of cancer patients in remission (9.4 and 10.0). Scores for the SSc sample were significantly lower (less fatigue) compared with two samples of cancer patients in palliative care (16.8 and 17.0). SSc GFI scores were similar to scores from patients with RA (13.4), AS (13.0) and SLE (13.1) and to scores from six studies of cancer patients in active treatment (11.1–13.5).
Conclusions. The high levels of fatigue reported in SSc were similar to patients with varying types and treatment stages of cancer and patients with other rheumatic diseases when assessed with the GFI, demonstrating that fatigue warrants greater attention in SSc.
Keywords: Systemic sclerosis, Fatigue, Systematic review, Multidimensional Fatigue Inventory
Introduction
Fatigue is defined as the experience of feeling weak, tired and lacking energy that often comes and goes in normal circumstances. Persistent fatigue from chronic illness involves ongoing exhaustion that is disproportionate to exertion and not alleviated by rest [1]. Fatigue from chronic illness decreases quality of life (QOL) by diminishing the ability to engage in meaningful personal and social activities and has important implications for employment, compliance with medical treatments and the use of healthcare services [2–8].
Patients with SSc report substantial disability and poor QOL [9–18]. Only three studies have examined frequency or impact of fatigue in SSc [19–21], and patients in one of these studies rated fatigue as more bothersome than any other symptom, including pain [20]. All three studies, however, used single-item assessments with unknown psychometric characteristics, and thus no conclusions could be drawn about fatigue levels in SSc compared with the general population or with patients in disease groups where it has received greater attention.
The objective of this study was to compare fatigue levels in SSc with fatigue levels from population samples and among patients with cancer and other rheumatic diseases. Fatigue has been more extensively researched in cancer patients than any other patient group. We used the Multidimensional Fatigue Inventory (MFI) [22] because its General Fatigue Index (GFI) provides a single indicator of overall fatigue with strong psychometric properties in multiple patient groups and because general population data are available.
Patients and methods
SSc patient data
We analysed data from SSc patients who were treated at the Johns Hopkins and University of Maryland Scleroderma Center between August 2004 and January 2006 and had a diagnosis of lcSSc or dcSSc based on ACR criteria. Detailed study procedures are documented elsewhere [9,23–25]. The study was approved by the Johns Hopkins University School of Medicine Internal Review Board. All patients provided informed consent.
Search strategy and study selection
The MEDLINE®, CINAHL® and PsycINFO® databases were searched on 12 October 2007 for studies in any language that included ≥50 subjects and reported GFI means and s.d. for general population samples, patients with rheumatic disease or patients with cancer. Studies that used abbreviated versions of the MFI or an early version with different item scaling [22] were excluded due to incomparability of scores. In the case of multiple articles published on the same cohort, the most recent article with complete data was included. Studies with mixed patient populations were included only if data for patients with cancer or a rheumatic disease were reported separately. Two investigators evaluated studies for inclusion and recorded relevant study data. Discrepancies were resolved by consensus.
Assessments
The MFI [22] is a 20-item measure designed for medically ill patients that includes five subscales of four items each: the GFI, Physical Fatigue, Reduced Motivation, Reduced Activity and Mental Fatigue. Items are worded both positively and negatively and responses are recorded on a 5-point Likert scale (1–5; ‘no, that is not true’ to ‘yes, that is true’). Scores range from 4 to 20 on each subscale/index. Higher scores indicate greater fatigue. The GFI is used when a single fatigue score is sought [26]. It is comprised of four statements (‘I feel fit’, ‘I feel tired’, ‘I am rested’, ‘I tire easily’) that assess fatigue during the previous days. Some studies linearly transformed the GFI score, to convert the 4–20 scale into a 0–100 scale. For those studies, we re-transformed the reported subscale score into the standard 4–20 scale. Data from the GFI have been published for general population samples [27, 28], for healthy non-patient groups [26,29–35], and for multiple patient groups, including RA [36], SLE [37], AS [38], cancer [22,26,39–48], Parkinson's disease [49], multiple sclerosis [50], heart failure [31, 51], chronic fatigue syndrome [26, 52] and chronic lung disease [53]. Internal consistency reliability (Cronbach's α) for the GFI ranges from 0.74 to 0.93 [22, 26, 27,29, 31, 35,39, 43, 44,52, 54]. Convergent validity is 0.77–0.79 with visual analogue fatigue scales [26, 36, 44], 0.78 with the Fatigue Severity Scale [50] and 0.84 with the Piper Fatigue Scale [55]. In addition to fatigue, depressive symptoms were measured in our SSc sample with the Center for Epidemiological Studies Depression Scale; pain with a numerical rating scale (0–10); and disability with the HAQ Disability Index.
Data analysis
In the table and text, mean ± s.d. are presented. GFI scores were compared using two-tailed t-tests based on published means and s.d. A Bonferroni correction was used to maintain the family-wise Type I error rate <0.05 within each set of comparisons (e.g. SSc vs general population studies, SSc vs studies of patients with rheumatic disease). Kendall's τ correlations were used to assess the bivariate association between demographic (age, gender, race/ethnicity), socioeconomic (education, marital status), medical (disease duration, diffuse/limited classification) and psychosocial variables (pain, depressive symptoms, disability) with fatigue.
Results
SSc sample characteristics
A total of 106 patients with lcSSc or dcSSc completed the MFI. The mean age was 55.5 ± 11.4 yrs; mean time since SSc diagnosis was 11.9 ± 7.9 yrs; 91.3% were females; 83.7% were non-Hispanic white; 67.9% were married; 67.9% completed at least some college; 73.6% had lcSSc; and 26.0% reported that they were disabled and unable to work. GFI total scores are shown in Table 1 for all patients, patients with diffuse disease and patients with limited disease. Patients with dcSSc scored higher than patients with lcSSc on the GFI, albeit not significantly so (P = 0.494). Female SSc patients had significantly higher fatigue scores than male patients (τ = 0.19, P = 0.024), but no other demographic, socioeconomic or medical variables were related to fatigue. Patient ratings of pain (τ = 0.38), depressive symptoms (τ = 0.36) and disability (τ = 0.39) were all significantly associated with fatigue (P < 0.001).
Table 1.
Summary of General Fatigue Index scores for scleroderma and comparison samples
| Reference | Population | Timing of assessment | n | Percentage of females | Mean age | General fatigue Index ± s.d. | P-values |
|---|---|---|---|---|---|---|---|
| Thombs (2008) | SSc | Mean disease duration 11.9 yrs | 106 | 91 | 56 | 13.3 ± 4.6 | |
| dcSSc | Mean disease duration 9.4 yrs | 28 | 86 | 54 | 13.8 ± 4.7 | ||
| lcSSc | Mean disease duration 12.9 yrs | 78 | 93 | 56 | 13.1 ± 4.6 | ||
| General population | |||||||
| Schwarz et al. [27] | German adults (≥14 yrs) | NA | 2037 | 56 | – | 8.7 ± 3.4* | < 0.001 |
| Watt et al. [28] | Danish adults (20–77 yrs) | NA | 1082 | 51 | – | 9.6 ± 4.5* | < 0.001 |
| Rheumatic disease | |||||||
| Da Costa et al. [37] | SLE | Mean disease duration 13.8 yrs | 130 | 100 | 45 | 13.1 ± 4.5 | 0.801 |
| Rupp et al. [36] | RA | Mean disease duration 10.7 yrs | 490 | 73 | 61 | 13.4 ± 4.9 | 0.773 |
| Van Tubergen et al. [38] | AS | Mean disease duration 12.5 yrs | 776 | 30 | 45 | 13.0 ± 3.4 | 0.212 |
| Cancer | |||||||
| Active Treatment | |||||||
| Hagelin et al. [56] | Heterogeneous cancer—radiation therapy | End of treatment | 81 | 90 | 59 | 13.5 ± 5.1 | 0.725 |
| Holzner et al. [42] | Heterogeneous cancer—chemotherapy | Beginning of 3rd cycle | 60 | 43 | 61 | 13.4 ± 5.8 | 0.870 |
| Fürst and berg [39] | Heterogeneous cancer radiation therapy | Last week of radiotherapy | 81 | 90 | 56 | 13.5 ± 5.1 | 0.726 |
| Meek et al. [44] | Heterogeneous cancer—chemo-, radiation or transplant therapies | End of treatment | 148 | NR | NR | 11.1 ± 5.0* | < 0.001 |
| Visser and Smets [46] | Heterogeneous cancer—radiation therapy | 2 weeks post-treatment | 216 | 42 | 64 | 11.7 ± 5.9 | 0.015 |
| Schneider [43] | Heterogeneous cancer—radiation/chemotherapy | Current treatment | 54 | 78 | 60 | 13.1 ± 4.0 | 0.786 |
| Palliative care | |||||||
| Hagelin et al. [56] | Heterogeneous cancer—palliative care | On admission to clinic | 229 | 60 | 68 | 16.8 ± 3.7* | < 0.001 |
| Munch et al. [40] | Heterogeneous cancer—palliative care | On referral to clinic | 130 | 42 | 62 | 17.0 ± 3.0* | < 0.001 |
| Non-active treatment | |||||||
| Holzner et al. [41] | Ovarian cancer—no active treatment | >3 months since treatment | 98 | 100 | 57 | 9.4 ± 4.4* | < 0.001 |
| Rüffer et al. [47] | Hodgkin's lymphoma —complete remission | Median 5.2 yrs since treatment | 818 | 48 | 31 | 10.0 ± 4.7* | < 0.001 |
| Mixed stages: | |||||||
| Bartsch et al. [45] | Heterogeneous cancer —mixed stages | 68% remission 8% part remission 10% progression 14% unclear | 144 | 70 | 56 | 12.1 ± 4.3* | 0.044 |
*Significantly different from mean scores for SSc patients (n = 106) at P < 0.05 using Bonferroni correction for family-wise error for each subgroup comparison.
Search results
The search process identified 114 unique studies that used the MFI. Upon review, 99 articles were excluded, including 24 reports on patients without chronic illness, 43 on patients with chronic illness other than cancer or rheumatic disease, 10 with <50 patients, 18 that did not report GFI scores, two with duplicate data, one that only reported GFI scores for patients with high fatigue scores on another fatigue measure and one that reported scores only for patients who were selected for rehabilitation based on criteria that included fatigue.
Characteristics of studies reviewed are shown in Table 1. A total of 15 studies published from 1995 to 2007 were reviewed, including 12 studies from Europe [27, 28,36,38–42,45–47,56] and three studies from North America [37, 43, 44]. There were two studies on fatigue in the general population [27, 28] and three on patients with rheumatic disease, including RA [36], SLE [37] and AS [38]. There were 10 studies (11 separate cohorts) on patients with cancer [39–47,56, 57], including six cohorts of patients in active treatment [39,42–44,46, 56], two that assessed fatigue post-treatment [41, 47], two of patients in palliative care [40, 56] and one that included patients in mixed stages, which was not classified as active, non-active or palliative care [45].
Comparison of SSc patients with selected cohorts
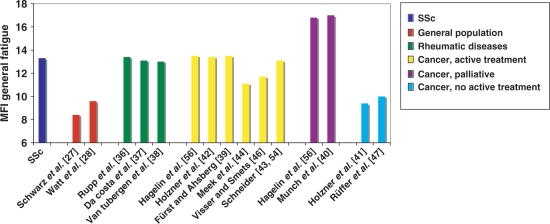
As shown in Fig. 1, there was a high level of consistency of GFI scores within patient groups. There were no significant differences (family-wise error, P < 0.05; Bonferroni adjustment) in mean GFI scores between cohorts within general population studies; rheumatic disease studies; non-active cancer treatment studies; or palliative cancer care studies. Of the six cohorts in the active cancer-treatment group, four had similar mean GFI scores (13.1–13.5) [39, 42, 43,56], which were higher than in the other two studies (11.1 and 11.7) [44, 46].
Fig. 1.
General Fatigue Index scores for SSc and comparison samples.
Based on the GFI, the SSc sample reported significantly more fatigue than both the Danish and German general population samples [27, 28]. GFI scores for the SSc sample were significantly higher than all age groups in each population sample, including the oldest age groups of Danish subjects aged 70–79 yrs and German subjects aged >75 yrs (data not shown). There were no significant differences between the SSc patients and samples of patients with other rheumatic diseases, including RA [36], SLE [37] and AS [38]. The SSc sample had significantly lower GFI scores than the two samples of cancer patients in palliative care [40, 56] and significantly higher scores than patients in remission [41, 47]. Compared with cancer patients in active treatment, the SSc patients had significantly higher scores than patients in one of the samples [44], but were not significantly different from the other five samples [39, 42,43, 46, 56].
Discussion
This is the first study to report levels of fatigue among patients with SSc using a standardized fatigue assessment instrument. The levels of fatigue reported in SSc were significantly higher than in general population samples and similar to samples of patients with other rheumatic diseases identified through systematic review. Fatigue in SSc was similar to patients in active treatment for cancer, higher than remitted cancer patients and lower than cancer patients in palliative care.
Although patients with SSc identify fatigue as a highly debilitating problem [19, 20], it has been largely ignored in the assessment and intervention literatures. The development and testing of interventions to reduce fatigue in SSc and the viability of their application in clinical settings are dependent upon fatigue assessment. Types of assessment tools that would be useful for clinical and research purposes include structured interviews based on case-definition criteria, continuous measurement scales and brief screening tools. Case-definition criteria define consensual understandings of fixed criteria to identify patients with clinically significant conditions. Measurement scales are rating scales or questionnaires that facilitate research on aetiological factors, symptom impact or change. Brief screening tools are quick, easily administered instruments to identify patients likely to meet case-definition criteria with more extensive evaluation. Research in cancer has laid the groundwork to develop fatigue case-definition criteria [58] and case-definition criteria for cancer-related fatigue appear in the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Clinical Modification (ICD-10-CM). Research in RA has identified a set of valid and reliable fatigue measurement scales based on rigorous evaluative standards [59], and recently the final plenary session of the Outcome Measures in Rheumatology Clinical Trials group (OMERACT 8) endorsed the proposal that fatigue should be measured in future studies of RA [60]. Finally, research on screening for fatigue in cancer and for major depression in primary care has shown that brief 1–3 question screening tools can facilitate effective, low-burden case detection in clinical settings [61–63]. Similar methods should be used to develop case definition, continuous assessment and screening tools that can be applied in SSc with the goal of facilitating research on intervention and subsequent implementation in clinical settings.
Limitations that should be considered in this study include the relatively small sample of SSc patients from a single centre and the large proportion of patients with lcSSc, which may have lowered fatigue estimates. Due to the small sample, the predominance of patients with lcSSc, and the lack of available data on important disease aspects, such as a measure of physician-rated disease severity, assessment of factors related to fatigue in SSc was limited. In addition, for the comparative analyses, the limited number of studies in each disease group and varied patient characteristics within and across comparison groups did not allow for assessment across samples of factors, such as age and sex, that may influence fatigue [27].
In summary, the results demonstrate that fatigue is a significant problem for patients with SSc, similar in magnitude to fatigue among patients with heterogeneous types of cancer and other rheumatic diseases. Research is needed on assessment tools to accurately screen and measure fatigue in patients with SSc, which will then lead to the creation of interventions aimed at treating fatigue in SSc.

Acknowledgements
Funding: This work was supported by the Scleroderma Foundation and the Scleroderma Research Foundation and grants from the National Institutes of Health (R21AT003250-01A1) awarded to J.A.H. B.D.T. and M.H. are supported by New Investigator Awards from the Canadian Institutes of Health Research and Établissement de Jeunes Chercheurs awards from the Fonds de la Recherche en Santé Québec.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Swain MG. Fatigue in chronic disease. Clin Sci. 2000;99:1–8. [PubMed] [Google Scholar]
- 2.Cella D, Davis K, Breitbart W, Curt G. Fatigue coalition. Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J Clin Oncol. 2001;19:3385–91. doi: 10.1200/JCO.2001.19.14.3385. [DOI] [PubMed] [Google Scholar]
- 3.Irvine D, Vincent L, Graydon JE, Bubela N, Thompson L. The prevalence and correlates of fatigue in patients receiving treatment with chemotherapy and radiotherapy. A comparison with the fatigue experienced by healthy individuals. Cancer Nurs. 1994;17:367–78. [PubMed] [Google Scholar]
- 4.Nail LM, Winningham ML. Fatigue and weakness in cancer patients: the symptoms experience. Semin Oncol Nurs. 1995;11:272–8. doi: 10.1016/s0749-2081(05)80008-7. [DOI] [PubMed] [Google Scholar]
- 5.Winningham ML, Nail LM, Burke MB, et al. Fatigue and the cancer experience: the state of the knowledge. Oncol Nurs Forum. 1994;21:23–36. [PubMed] [Google Scholar]
- 6.Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 2000;5:353–60. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 7.Vogelzang NJ, Breitbart W, Cella D, et al. Patient, caregiver, and oncologist perceptions of cancer-related fatigue: results of a tripart assessment survey. The Fatigue Coalition. Semin Hematol. 1997;34(3 Suppl. 2):4–12. [PubMed] [Google Scholar]
- 8.Ashbury FD, Findlay H, Reynolds B, McKerracher K. A Canadian survey of cancer patients’ experiences: are their needs being met? J Pain Symptom Manage. 1998;16:298–306. doi: 10.1016/s0885-3924(98)00102-x. [DOI] [PubMed] [Google Scholar]
- 9.Benrud-Larson LM, Haythornthwaite JA, Heinberg LJ, et al. The impact of pain and symptoms of depression in scleroderma. Pain. 2002;95:267–75. doi: 10.1016/S0304-3959(01)00409-2. [DOI] [PubMed] [Google Scholar]
- 10.Georges C, Chassany O, Mouthon L, et al. Validation of French version of the scleroderma health assessment questionnaire (SSc HAQ) Clin Rheumatol. 2005;24:3–10. doi: 10.1007/s10067-004-0942-3. [DOI] [PubMed] [Google Scholar]
- 11.Georges C, Chassany O, Toledano C, et al. Impact of pain in health related quality of life of patients with systemic sclerosis. Rheumatology. 2006;45:1298–302. doi: 10.1093/rheumatology/kel189. [DOI] [PubMed] [Google Scholar]
- 12.Johnson SR, Glaman DD, Schentag CT, Lee P. Quality of life and functional status in systemic sclerosis compared to other rheumatic diseases. J Rheumatol. 2006;33:1117–22. [PubMed] [Google Scholar]
- 13.Khanna D, Ahmed M, Furst DE, et al. Health values of patients with systemic sclerosis. Arthritis Rheum. 2007;57:86–93. doi: 10.1002/art.22465. [DOI] [PubMed] [Google Scholar]
- 14.Khanna D, Clements PJ, Furst DE, et al. Correlation of the degree of dyspnea with health-related quality of life, functional abilities, and diffusing capacity for carbon monoxide in patients with systemic sclerosis and active alveolitis: results from the Scleroderma Lung Study. Arthritis Rheum. 2005;52:592–600. doi: 10.1002/art.20787. [DOI] [PubMed] [Google Scholar]
- 15.Khanna D, Furst DE, Clements PJ, et al. Responsiveness of the SF-36 and the Health Assessment Questionnaire Disability Index in a systemic sclerosis clinical trial. J Rheumatol. 2005;32:832–40. [PubMed] [Google Scholar]
- 16.Poole JL, Steen VD. The use of the Health Assessment Questionnaire (HAQ) to determine physical disability in systemic sclerosis. Arthritis Care Res. 1991;4:27–31. doi: 10.1002/art.1790040106. [DOI] [PubMed] [Google Scholar]
- 17.Poole JL, Williams CA, Bloch DA, Hollak B, Spitz P. Concurrent validity of the health assessment questionnaire disability index in scleroderma. Arthritis Care Res. 1995;8:189–93. doi: 10.1002/art.1790080312. [DOI] [PubMed] [Google Scholar]
- 18.Rannou F, Poiraudeau S, Berezne A, et al. Assessing disability and quality of life in systemic sclerosis: construct validities of the Cochin Hand Function Scale, Health Assessment Questionnaire (HAQ), Systemic Sclerosis HAQ, and Medical Outcomes Study 36-Item Short Form Health Survey. Arthritis Rheum. 2007;57:94–102. doi: 10.1002/art.22468. [DOI] [PubMed] [Google Scholar]
- 19.Richards HL, Herrick AL, Griffin K, Gwilliam PD, Loukes J, Fortune DG. Systemic sclerosis: patients’ perceptions of their condition. Arthritis Rheum. 2003;49:689–96. doi: 10.1002/art.11385. [DOI] [PubMed] [Google Scholar]
- 20.van Lankveld WG, Vonk MC, Teunissen H, van den Hoogen FH. Appearance self-esteem in systemic sclerosis—subjective experience of skin deformity and its relationship with physician-assessed skin involvement, disease status and psychological variables. Rheumatology. 2007;46:872–6. doi: 10.1093/rheumatology/kem008. [DOI] [PubMed] [Google Scholar]
- 21.Suarez-Almazor ME, Kallen MA, Roundtree AK, Mayes M. Disease and symptom burden in systemic sclerosis: a patient perspective. J Rheumatol. 2007;34:1718–26. [PubMed] [Google Scholar]
- 22.Smets EM, Garssen B, Cull A, de Haes JC. Application of the multidimensional fatigue inventory (MFI-20) in cancer patients receiving radiotherapy. Br J Cancer. 1996;73:241–5. doi: 10.1038/bjc.1996.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heinberg LJ, Kudel I, White B, et al. Assessing body image in patients with systemic sclerosis (scleroderma): validation of the adapted satisfaction with appearance scale. Body Image. 2007;4:79–86. doi: 10.1016/j.bodyim.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edwards RR, Goble L, Kwan A, et al. Catastrophizing, pain, and social adjustment in scleroderma: relationships with educational level. Clin J Pain. 2006;22:639–46. doi: 10.1097/01.ajp.0000210918.26159.94. [DOI] [PubMed] [Google Scholar]
- 25.Benrud-Larson LM, Heinberg LJ, Boling C, et al. Body image dissatisfaction among women with scleroderma: extent and relationship to psychosocial function. Health Psychol. 2003;22:130–9. [PubMed] [Google Scholar]
- 26.Smets EM, Garssen B, Bonke B, De Haes JC. The multidimensional fatigue inventory (MFI): psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–25. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 27.Schwarz R, Krauss O, Hinz A. Fatigue in the general population. Onkologie. 2003;26:140–4. doi: 10.1159/000069834. [DOI] [PubMed] [Google Scholar]
- 28.Watt T, Groenvold M, Bjorner JB, Noerholm V, Rasmussen NA, Bech P. Fatigue in the Danish general population. Influence of sociodemographic factors and disease. J Epidemiol Community Health. 2000;54:827–33. doi: 10.1136/jech.54.11.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jansen AJ, Essink-Bot ML, Duvekot JJ, van Rhenen DJ. Psychometric evaluation of health-related quality of life measures in women after different types of delivery. J Psychosom Res. 2007;63:275–81. doi: 10.1016/j.jpsychores.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Weinstein AA, Deuster PA, Kop WJ. Heart rate variability as a predictor of negative mood symptoms induced by exercise withdrawal. Med Sci Sports Exerc. 2007;39:735–41. doi: 10.1249/mss.0b013e31802f590c. [DOI] [PubMed] [Google Scholar]
- 31.Hagglund L, Boman K, Olofsson M, Brulin C. Fatigue and health-related quality of life in elderly patients with and without heart failure in primary healthcare. Eur J Cardiovasc Nurs. 2007;6:208–15. doi: 10.1016/J.EJCNURSE.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Kok NF, Lind MY, Hansson BM, et al. Comparison of laparoscopic and mini incision open donor nephrectomy: single blind, randomised controlled clinical trial. Br Med J. 2006;333:221. doi: 10.1136/bmj.38886.618947.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oken BS, Zajdel D, Kishiyama S, et al. Randomized, controlled, six-month trial of yoga in healthy seniors: effects on cognition and quality of life. Altern Ther Health Med. 2006;12:40–7. [PMC free article] [PubMed] [Google Scholar]
- 34.Beutel ME, Weidner K, Schwarz R, Brahler E. Age-related complaints in women and their determinants based on a representative community study. Eur J Obstet Gynecol Reprod Biol. 2004;117:204–12. doi: 10.1016/j.ejogrb.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 35.de Rijk AE, Schreurs KM, Bensing JM. What is behind ‘I’m so tired’? Fatigue experiences and their relations to the quality and quantity of external stimulation. J Psychosom Res. 1999;47:509–23. doi: 10.1016/s0022-3999(99)00049-5. [DOI] [PubMed] [Google Scholar]
- 36.Rupp I, Boshuizen HC, Jacobi CE, Dinant HJ, van den Bos GA. Impact of fatigue on health-related quality of life in rheumatoid arthritis. Arthritis Rheum. 2004;51:578–85. doi: 10.1002/art.20539. [DOI] [PubMed] [Google Scholar]
- 37.Da Costa D, Dritsa M, Bernatsky S, et al. Dimensions of fatigue in systemic lupus erythematosus: relationship to disease status and behavioral and psychosocial factors. J Rheumatol. 2006;33:1282–8. [PubMed] [Google Scholar]
- 38.van Tubergen A, Coenen J, Landewe R, et al. Assessment of fatigue in patients with ankylosing spondylitis: a psychometric analysis. Arthritis Rheum. 2002;47:8–16. doi: 10.1002/art1.10179. [DOI] [PubMed] [Google Scholar]
- 39.Furst CJ, Ahsberg E. Dimensions of fatigue during radiotherapy. An application of the Multidimensional Fatigue Inventory. Support Care Cancer. 2001;9:355–60. doi: 10.1007/s005200100242. [DOI] [PubMed] [Google Scholar]
- 40.Munch TN, Stromgren AS, Pedersen L, Petersen MA, Hoermann L, Groenvold M. Multidimensional measurement of fatigue in advanced cancer patients in palliative care: an application of the Multidimensional Fatigue Inventory. J Pain Symptom Manage. 2006;31:533–41. doi: 10.1016/j.jpainsymman.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 41.Holzner B, Kemmler G, Meraner V, et al. Fatigue in ovarian carcinoma patients: a neglected issue? Cancer. 2003;97:1564–72. doi: 10.1002/cncr.11253. [DOI] [PubMed] [Google Scholar]
- 42.Holzner B, Kemmler G, Greil R, et al. The impact of hemoglobin levels on fatigue and quality of life in cancer patients. Ann Oncol. 2002;13:965–73. doi: 10.1093/annonc/mdf122. [DOI] [PubMed] [Google Scholar]
- 43.Schneider RA. Concurrent validity of the Beck Depression Inventory and the Multidimensional Fatigue Inventory-20 in assessing fatigue among cancer patients. Psychol Rep. 1998;82:883–6. doi: 10.2466/pr0.1998.82.3.883. [DOI] [PubMed] [Google Scholar]
- 44.Meek PM, Nail LM, Barsevick A, et al. Psychometric testing of fatigue instruments for use with cancer patients. Nurs Res. 2000;49:181–90. doi: 10.1097/00006199-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Bartsch HH, Weis J, Moser MT. Cancer-related fatigue in patients attending oncological rehabilitation programs: prevalence, patterns and predictors. Onkologie. 2003;26:51–7. doi: 10.1159/000069864. [DOI] [PubMed] [Google Scholar]
- 46.Visser MR, Smets EM. Fatigue, depression and quality of life in cancer patients: how are they related? Support Care Cancer. 1998;00:6101–8. doi: 10.1007/s005200050142. [DOI] [PubMed] [Google Scholar]
- 47.Ruffer JU, Flechtner H, Tralls P, et al. Fatigue in long-term survivors of Hodgkin's lymphoma; a report from the German Hodgkin Lymphoma Study Group (GHSG) Eur J Cancer. 2003;39:2179–86. doi: 10.1016/s0959-8049(03)00545-8. [DOI] [PubMed] [Google Scholar]
- 48.van Weert E, Hoekstra-Weebers J, Otter R, Postema K, Sanderman R, van der Schans C. Cancer-related fatigue: predictors and effects of rehabilitation. Oncologist. 2006;11:184–96. doi: 10.1634/theoncologist.11-2-184. [DOI] [PubMed] [Google Scholar]
- 49.Havlikova E, Rosenberger J, Nagyova I, et al. Clinical and psychosocial factors associated with fatigue in patients with Parkinson's disease. Parkinsonism Relat Disord. 2008;14:187–92. doi: 10.1016/j.parkreldis.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 50.Trojan D, Arnold D, Collet JP, et al. Fatigue in multiple sclerosis: association with disease-related, behavioural and psychosocial factors. Mult Scler. 2007;13:985–95. doi: 10.1177/1352458507077175. [DOI] [PubMed] [Google Scholar]
- 51.Falk K, Swedberg K, Gaston-Johansson F, Ekman I. Fatigue is a prevalent and severe symptom associated with uncertainty and sense of coherence in patients with chronic heart failure. Eur J Cardiovasc Nurs. 2007;6:99–104. doi: 10.1016/j.ejcnurse.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 52.Wagner D, Nisenbaum R, Heim C, Jones JF, Unger ER, Reeves WC. Psychometric properties of the CDC symptom inventory for assessment of chronic fatigue syndrome. Popul Health Metr. 2005;22:8. doi: 10.1186/1478-7954-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oh EG, Kim CJ, Lee WH, Kim SS. Correlates of fatigue in Koreans with chronic lung disease. Heart Lung. 2004;33:13–20. doi: 10.1016/j.hrtlng.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 54.Schneider RA. Reliability and validity of the Multidimensional Fatigue Inventory (MFI-20) and the Rhoten Fatigue Scale among rural cancer outpatients. Cancer Nurs. 1998;21:370–3. doi: 10.1097/00002820-199810000-00009. [DOI] [PubMed] [Google Scholar]
- 55.Dagnelie PC, Pijls-Johannesma MC, Pijpe A, et al. Psychometric properties of the revised Piper Fatigue Scale in Dutch cancer patients were satisfactory. J Clin Epidemiol. 2006;59:642–9. doi: 10.1016/j.jclinepi.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 56.Hagelin CL, Wengstrom Y, Runesdotter S, Furst CJ. The psychometric properties of the Swedish Multidimensional Fatigue Inventory: MFI-20 in four different populations. Acta Oncol. 2007;46:97–104. doi: 10.1080/02841860601009430. [DOI] [PubMed] [Google Scholar]
- 57.Strauss B, Brix C, Fischer S, et al. The influence of resilience on fatigue in cancer patients undergoing radiation therapy (RT) J Cancer Res Clin Oncol. 2007;133:511–8. doi: 10.1007/s00432-007-0195-z. [DOI] [PubMed] [Google Scholar]
- 58.Cella D, Peterman A, Passik S, Jacobsen P, Breitbart W. Progress toward guidelines for the management of fatigue. Oncology. 1998;12:369–77. [PubMed] [Google Scholar]
- 59.Hewlett S, Hehir M, Kirwan JR. Measuring fatigue in rheumatoid arthritis: a systematic review of scales in use. Arthritis Rheum. 2007;57:429–39. doi: 10.1002/art.22611. [DOI] [PubMed] [Google Scholar]
- 60.Kirwan JR, Minnock P, Adebajo A, et al. Patient perspective: fatigue as a recommended patient centered outcome measure in rheumatoid arthritis. J Rheumatol. 2007;34:1174–7. [PubMed] [Google Scholar]
- 61.Van Belle S, Paridaens R, Evers G, et al. Comparison of proposed diagnostic criteria with FACT-F and VAS for cancer-related fatigue: proposal for use as a screening tool. Support Care Cancer. 2005;13:246–54. doi: 10.1007/s00520-004-0734-y. [DOI] [PubMed] [Google Scholar]
- 62.Williams JW, Jr, Pignone M, Ramirez G, Perez Stellato C. Identifying depression in primary care: a literature synthesis of case-finding instruments. Gen Hosp Psychiatry. 2002;24:225–37. doi: 10.1016/s0163-8343(02)00195-0. [DOI] [PubMed] [Google Scholar]
- 63.Mitchell AJ, Coyne JC. Do ultra-short screening instruments accurately detect depression in primary care? A pooled analysis and meta-analysis of 22 studies. Br J Gen Pract. 2007;57:144–51. [PMC free article] [PubMed] [Google Scholar]