Abstract
The nuclear factor of activated T-cells (NFAT) is a Ca2+-dependent transcription factor that has been reported to regulate the expression of smooth muscle contractile proteins and ion channels. Here we report that large conductance Ca2+-sensitive potassium (BK) channels and voltage-gated K+ (KV) channels may be regulatory targets of NFATc3 in urinary bladder smooth muscle (UBSM). UBSM myocytes from NFATc3-null mice displayed a reduction in iberiotoxin (IBTX)-sensitive BK currents, a decrease in mRNA for the pore-forming α-subunit of the BK channel, and a reduction in BK channel density compared with myocytes from wild-type mice. Tetraethylammonium chloride-sensitive KV currents were elevated in UBSM myocytes from NFATc3-null mice, as was mRNA for the Shab family member KV2.1. Despite KV current upregulation, bladder strips from NFATc3-null mice displayed an elevated contractile response to electrical field stimulation relative to strips from wild-type mice, but this difference was abrogated in the presence of the BK channel blocker IBTX. These results support a role for the transcription factor NFATc3 in regulating UBSM contractility, primarily through an NFATc3-dependent increase in BK channel activity.
Keywords: large conductance potassium channel, voltage-gated potassium, nuclear factor of activated T-cell, contractility, urinary bladder
stimulation of parasympathetic nerves in the urinary bladder induces the corelease of the neurotransmitters ATP and ACh, which act directly on the detrusor smooth muscle (SM) purinergic and cholinergic receptors, respectively, to generate the force necessary to expel urine. Electrical stimulation initiates excitatory junction potentials that trigger SM action potentials (18). Action potentials consist of a depolarizing upstroke followed by a repolarizing phase and an afterhyperpolarization (5, 17, 19). Ca2+ influx through L-type voltage-gated Ca2+ channels (L-VDCC) mediates the upstroke of the action potential (7, 17, 20). K+ efflux through two types of voltage-sensitive K+ channels [large-conductance, Ca2+-sensitive K+ (BK) channels and voltage-gated K+ (KV) channels] contributes to the repolarization phase of the action potential (16, 17, 20, 45). In addition to their role in repolarization, KV channels, as well as small conductance, Ca2+-sensitive K+ (SK) channels, likely play a role in the afterhyperpolarization (11, 17, 45).
Nerve-evoked contractions of urinary bladder smooth muscle (UBSM) are greatly enhanced by blocking BK channels or by targeted disruption of the gene for the pore-forming α-subunit of the BK channel (Slo or Kcnma1) (33, 43, 46). These effects likely reflect actions on UBSM BK channels because nerves in UBSM strips do not express BK channels (43, 46). BK channels in SM are composed of four pore-forming α-subunits and accessory β1-subunits, which increase the apparent voltage and Ca2+ sensitivity of the α-subunits (13, 32, 44). Block of BK channels by the scorpion toxins iberiotoxin (IBTX) or charybdotoxin increases the duration and peak amplitude of UBSM action potentials (20, 30), resulting in an increase in the force of muscle contraction (16, 17, 22, 27). Mice lacking the pore-forming α-subunit exhibit significantly elevated bladder pressures, increased oscillations of intravesicular pressure, reduced bladder capacity, reduced volume per void, and urinary incontinence (33, 43), suggesting that a decrease in BK channel activity may contribute to the symptoms associated with urinary bladder dysfunction.
KV channels are composed of four α-subunits arranged radially around a central pore (29, 48, 49). We (45) have previously evaluated the biophysical, pharmacological, and molecular properties of the murine UBSM KV current. RT-PCR analysis of UBSM tissue, combined with biophysical comparisons of the murine UBSM KV current to values reported in the literature for other KV channel complexes, suggests that the UBSM KV channel is most likely a heteromultimeric assemblage of KV2.1 subunits and KV5.1 and/or KV6.1 subunits (45). This stands in contrast to vascular smooth muscle KV currents, which are carried primarily by channels composed of members of the 4-aminopyridine (4-AP)-sensitive KV1 subfamily (42, 50) and, as suggested by recent work, through KV2.1 channels (2, 3).
Recent reports have shown that the expression of BK and KV channels can be modulated by Ca2+-dependent transcription factors, providing a potential link between alterations in Ca2+ signaling patterns and changes in K+ channel activity. Specifically, the transcription factor nuclear factor of activated T cells (NFAT) has been reported to regulate the expression of KV2.1 channels in arterial SM and in cardiac myocytes (2, 39) and to regulate the expression of the modulatory β1-subunit of the BK channel in arterial SM (34).
Four members of the NFAT family (NFAT1/c2, NFAT2/c1, NFAT3/c4, and NFAT4/c3) are Ca2+ dependent. The transcriptional activity of these NFAT isoforms is controlled primarily through regulation of NFAT subcellular localization (9, 12, 52). A fifth isoform, NFAT5/TonEBP, is Ca2+ independent and shares limited homology with the other family members. Elevations in intracellular Ca2+ promote the nuclear localization of NFAT by activating the Ca2+- and calmodulin-dependent protein phosphatase calcineurin, which dephosphorylates NH2-terminal NFAT serine residues, inducing a conformational change that exposes nuclear localization signals (4, 8, 10, 28, 36, 38). Once in the nucleus, NFAT acts as a transcriptional coregulator associating with cofactors at composite regulatory elements to modulate the transcription of target genes (10, 26, 38).
K+ channel expression and activity is a crucial factor in determining the contractile status of SM, including UBSM. The fact that KV channels are putative regulatory targets of NFAT, in turn, implies that NFAT transcriptional activity might modulate UBSM contractility. There are currently no reports of NFAT activity in UBSM. However, in cultured UBSM myocytes, overexpression of a constitutively active form of calcineurin has been shown to induce a hypertrophic phenotype (35), suggesting a possible role for NFAT activity in the urinary bladder.
The goal of the present study was to examine the role of NFAT in regulating nerve-evoked UBSM contractility. We focused on NFATc3 since this isoform is prominently expressed in SM (14, 15, 25, 41) and has been previously reported to modulate the expression of proteins involved in SM contractility, including K+ channels and α-actin (2, 15, 34). We found that UBSM strips from NFATc3-null mice exhibited increased force generation in response to electrical field stimulation (EFS) compared with strips from wild-type mice. We attribute the increased nerve-evoked contractility of the UBSM from NFATc3-null mice to decreased expression of the BK channel pore-forming α-subunit and an associated decrease in overall BK channel activity. These results indicate that NFATc3 may play an important role in regulating UBSM excitability and contractility and further suggest that UBSM disorders, which are characterized by alterations in Ca2+ regulation, may reflect NFATc3-dependent alterations in BK channel expression and function.
METHODS
Isolation of myocytes.
Adult mice were euthanized with an overdose of pentobarbital sodium followed by exsanguinations, according to a protocol approved by the University of Vermont Office of Animal Care and Management. NFATc3-null mice, originally supplied by Dr. Laurie Glimcher, and wild-type background strain mice (Balb/C) were used in all experiments. Other researchers working with these NFATc3-null mice have reported that the loss of NFATc3 did not result in compensatory changes in the expression of remaining NFAT isoforms at the mRNA level (47).
The bladders were removed and transferred to an ice-cold Ca2+-free dissection solution (DS; in mM: 55 NaCl, 5.6 KCl, 2 MgCl2, 80 sodium glutamate, and 10 glucose) buffered to pH 7.3 with 10 mM HEPES. After the external fatty and connective tissues were removed, the bladders were cut open and rinsed free of urine, and the urothelial layer was carefully peeled away. The detrusor was cut into small sections (∼2 mm × 2 mm) and digested for 20–30 min in DS containing 1 mg/ml papain (Worthington, Lakewood, NJ) and 1 mg/ml dithioerythritol (Sigma, St. Louis, MO), rinsed briefly with DS, and then further digested in DS containing 1 mg/ml collagenase type II (Sigma) and 100 μm CaCl2 for 6–10 min. Both digestions were performed at 37°C. The digested tissue sections were gently triturated using a fire-polished glass Pasteur pipette to release individual UBSM myocytes.
Electrophysiology.
Whole cell currents were recorded using the perforated-patch configuration of the whole cell patch-clamp technique. Aliquots of dissociated myocytes were transferred to a 0.5-ml electrophysiology chamber and allowed to adhere. After 15–20 min, the cells were thoroughly rinsed in a bath solution consisting of (in mM) 134 NaCl, 6 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES, pH 7.4. The pipette solution consisted of (in mM) 110 potassium aspartate, 10 NaCl, 30 KCl, 1 MgCl2, 0.05 EGTA, and 10 HEPES, pH 7.2. Amphotericin, initially solubilized in DMSO, was added to the pipette solution at a final concentration of 200 μg/ml. Iberiotoxin (IBTX; 100 nM final concentration) and tetraethylammonium chloride (TEA; 5 mM final concentration) were both purchased from Sigma and solubilized in the bath solution. All recordings were made at 22°C.
Single-channel BK currents (IBK) were recorded in excised inside-out patches using the patch-clamp technique. The patches were exposed to a 10 μM buffered Ca2+ solution. Single-channel currents were recorded for 2–5 min at potentials of −40 to + 40 mV at 22°C using pipettes fire polished to a final tip resistance of ∼4–6 MΩ. The bath and pipette solution consisted of (in mM) 140 KCl, 1.8 MgCl2, 1 5-hydroxyethylene-diaminetriacetic acid, and 0.13 CaCl2 (10 μM free), adjusted to pH 7.2 with NaOH. Data were analyzed using pCLAMP software (Axon Instruments). The number of channels per patch was determined at a holding potential of +60 mV. Channel open probability (Po) was determined by dividing channel activity (NPo) derived using the pCLAMP software by the number of channels (N) as in the respective patch.
UBSM contractility studies.
Bladders were harvested and rinsed, and the urothelium was removed, as detailed above. Detrusor strips (1 mm × 3 mm), cut longitudinally from the dome to the bladder base, were suspended in jacketed water baths (7 ml volume, 37°C) in an aerated bath solution (in mM: 119 NaCl, 4.7 KCl, 24 NaHCO3, 1.2 KH2PO4, 2.5 CaCl2, 1.2 MgSO4, 0.023 EDTA, and 11 glucose, pH 7.3). Contractility was measured after strips were prestretched to 5 mN of tension for 45 min using a myograph (MyoMED, MED Associates). The EFS protocol consisted of sequential pulses from 0.5 to 50 Hz (20-V pulse amplitude, 0.2-ms pulse width, 2-s pulse duration) at 3-min intervals. All recording were made at 37°C.
Immunohistochemistry and nuclear localization assay.
Dissociated myocytes were placed into eight-well plates and the cells were allowed to adhere for 1 h at 37°C. The medium was then replaced with growth media consisting of Dulbecco's modified Eagle's medium (Invitrogen) with 10% fetal bovine serum (Invitrogen, Carlsbad, CA) and penicillin-streptomycin (10 U/ml, Invitrogen). The plates were maintained at 37°C, 5% CO2 for 24 h, at which time the cells were ∼60% confluent. Stimulation with platelet-derived growth factor (PDGF; 20 ng/ml), endothelin (ET-1, 100 nM), or pretreatment with FK506 and cyclosporine A (CsA, 1 μM each) was performed as detailed in results. Immunostaining and quantitation of NFAT nuclear localization was performed as detailed previously (15). All NFAT antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Semiquantitative RT-PCR.
Approximately 100-mg sections of mouse urinary bladder detrusor muscle, denuded of urothelium, were homogenized in TRIzol reagent (Sigma) using a motorized homogenizer, and the RNA was isolated as per the manufacturer's protocol. cDNA was generated using the Superscript First-Strand cDNA kit (Invitrogen) according to the manufacturer's protocol. Primers for KV2.1 (forward: 5′-TGGACAT CGTGGTGGAGAA-3′, reverse: 5′-CAGATACTCTGATCCCGAG-3′) were described previously (45). Primers for the α- and β-subunits of the BK channel (GenBank accession numbers L16912 and AF020711, respectively) were α-BK forward: 5′-AGTCTGCATCTTTGGGGATG-3′, reverse: 5′-TGTCCATTCCAGGAGGTGT-3′; β-BK forward: 5′-TTCTGCACCTCAAGTCAACG-3′, reverse: 5′-GACAGCGTCATTGC-AGG TAA-3′. RT-PCR was performed using the Fail-Safe PCR kit (Epicenter) with Amplitaq Gold DNA polymerase. For semiquantitative analysis of K+ channel expression, PCR reactions were terminated at a point empirically determined to lie within the logarithmic phase (33 cycles for KV2.1 and the BK α- and β-subunits) and expressed relative to the housekeeping gene β-actin (23 cycles). Densitometry of bands in ethidium bromide-stained agarose gels was determined using Quantity One imaging analysis software (Bio-Rad).
Statistical analyses.
The summary data are presented as means ± SE unless indicated otherwise. Statistical significance was determined using Student's t-test (unpaired or paired) with a P value <0.05 being considered statistically significant.
RESULTS
Expression and stimulation of NFAT nuclear localization in UBSM.
We have previously reported that NFATc3 and NFATc4 are expressed in cerebral vascular and ileal SM (14, 41). In primary cultured UBSM myocytes, immunohistochemistry revealed the expression of NFATc1, NFATc3, and NFATc4 but not NFATc2 (Fig. 1). Similarly, RT-PCR analysis of fresh UBSM detrusor tissue indicated that NFATc1, NFATc3, and NFATc4 were present (Fig. 1, inset). The lack of NFATc2 expression in UBSM is consistent with previously published reports on the absence of NFATc2 expression in other SM tissues (14, 41).
Fig. 1.
Nuclear factor of activated T cell (NFAT) expression in murine urinary bladder smooth muscle (UBSM) cultured cells and tissue. Immunostaining of primary cultures of UBSM myocytes indicates that murine UBSM expresses NFATc1, NFATc3, and NFATc4 but not NFATc2. NFAT expression is shown in red, nuclear staining in green, and regions of NFAT nuclear localization in white. Some nuclei exhibit NFATc3 nuclear localization under these basal conditions. Inset: RNA extracted from UBSM tissues was probed for the presence of mRNA for NFATc1-c4 using RT-PCR (H, heart; B, bladder; T, thymus).
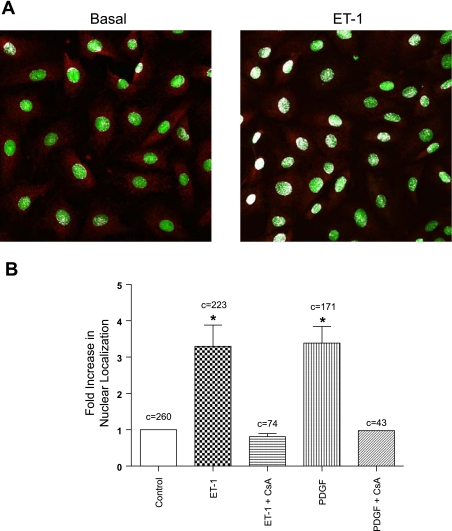
The SM mitogens PDGF and ET-1 are capable of stimulating NFATc3 nuclear translocation in ileal and cerebral vascular SM tissues (14, 41) and have been suggested to play a role in the physiology and pathophysiology of the urinary bladder (1, 31, 40). To determine whether these growth factors activate NFATc3 in UBSM, we treated primary cultured UBSM myocytes with either ET-1 (100 nM) or PDGF (20 ng/ml) for 30 min and quantified NFATc3 nuclear accumulation using confocal microscopy. Under nonstimulated, basal conditions, NFATc3 was found primarily in the cytosol. Upon stimulation with ET-1 (Fig. 2A) or PDGF (not shown), NFATc3 displayed prominent nuclear localization. As summarized in Fig. 2B, NFATc3 nuclear localization was increased 3.3 ± 0.6- and 3.4 ± 0.5-fold by exposure to ET-1 and PDGF, respectively (P < 0.05). ET-1- and PDGF-dependent nuclear translocation was blocked by preincubation with the calcineurin inhibitors FK506 and CsA (1 μM each). Thus NFAT is expressed in intact and cultured UBSM tissue, and nuclear translocation of NFATc3 can be induced by physiologically relevant agents.
Fig. 2.
Stimulation of NFATc3 nuclear translocation in UBSM. A: example micrographs showing the subcellular distribution pattern of NFATc3 under basal conditions and after stimulation with endothelin-1 (ET-1). B: treatment with either ET-1 or platelet-derived growth factor (PDGF) led to a significant increase in NFATc3 nuclear localization compared with basal conditions (*P < 0.05; c = number of cells analyzed). Treatment with FK506 and cyclosporine A (CsA, 1 μM; 30 min) before the addition of the PDGF or ET-1 blocked NFATc3 nuclear translocation.
UBSM strips from NFATc3-null mice exhibit enhanced nerve-evoked contractility but reduced responses to the BK channel blocker IBTX.
We have previously reported that the NFATc3 isoform is expressed in vascular and ileal SM (14, 41) and regulates the expression of the contractile protein SM α-actin (15). NFATc3 has also been implicated in the regulation of both BK and KV channels in vascular smooth muscle (2, 34), suggesting that the NFATc3 isoform may be important in the regulation of SM function.
To determine whether NFATc3 modulates UBSM contractility, we compared the force generated by UBSM strips taken from NFATc3-null and wild-type mice in response to EFS using wire myography. A representative recording is presented in Fig. 3A, and the averaged force-frequency plot is presented in Fig. 3B. UBSM strips from NFATc3-null mice generated more force than strips from wild-type mice at all stimulation frequencies >5 Hz (Fig. 3B; n = 16 strips from 3 animals each; P < 0.05). At 20 Hz, the mean force generated by NFATc3-null UBSM strips was ∼1.8-fold that of strips from wild-type animals. The contractile responses of UBSM strips from wild-type and NFATc3-null animals to direct membrane potential depolarization with 60 mM K+ were not significantly different (10.6 ± 2.9 mN for wild-type and 8.5 ± 1.5 mN for NFATc3-null mice; P > 0.05; n = 13), suggesting that the L-VDCCs, which mediate depolarization-induced Ca2+ influx, are unchanged in NFATc3-null mice. The enhanced contractile response to EFS of UBSM strips taken from NFATc3-null mice suggests that the NFATc3 isoform contributes to the regulation of detrusor SM contractility, independent of L-VDCCs.
Fig. 3.
UBSM strips from NFATc3-null mice are more contractile than strips from wild-type mice and display a reduced responsiveness to IBTX. A: representative myograph recordings obtained from UBSM strips from wild-type and NFATc3-null mice subjected to EFS from 1 to 50 Hz. B: UBSM tissues strips from NFATc3-null mice exhibited a greater contractile response than UBSM strips from wild-type mice at all frequencies of stimulation >5 Hz (*P < 0.05; n = 3 animals and 16 strips each). C: in the presence of iberiotoxin (IBTX), there was no significant difference (P > 0.05) in the amplitude of electrical field stimulation (EFS)-induced contractions of UBSM strips from wild-type and NFATc3-null mice. Note that the results presented in A and B above are from a separate series of experiments from those in C.
BK channels play a central role in the repolarization of the UBSM action potential (20) and figure prominently in nerve-evoked contractility of UBSM (33, 43, 46). Blocking BK channels with IBTX increases the contractility of UBSM strips by shifting the force-frequency relationship to the left and by increasing the maximum force generated in response to muscarinic or purinergic stimulation or to EFS (20, 23, 37, 43, 46). To determine whether a decrease in BK channel activity might account for the increased contractility of bladder strips from NFATc3-null mice, we evaluated the effects of IBTX on the EFS-induced contractions of UBSM strips from NFATc3 -null and wild-type mice. UBSM tissue strips were stimulated at frequencies from 1 to 50 Hz before and after treatment with IBTX. The averaged force-frequency plot in the presence of IBTX is presented in Fig. 3C. IBTX increased the amplitude of EFS-induced contractions in UBSM strips from both wild-type and NFATc3-null mice. However, IBTX treatment had a greater effect on the contractile response of UBSM strips taken from wild-type mice than on strips taken from NFATc3-null mice. For example, at 5 Hz the contraction amplitude in the wild-type UBSM strips was increased 2.2 ± 0.07-fold by treatment with IBTX but was increased only 1.5 ± 0.02-fold in the NFATc3-null mice (n = 6; P < 0.05). The decreased responsiveness of NFATc3 null UBSM strips to IBTX treatment suggests that BK channel activity is downregulated in these animals, which may account for the increase contractility in UBSM strips from NFATc3-null mice. In support of this, the force-frequency relationships of the NFATc3-null and wild-type mice were not significantly different when BK channels were blocked with IBTX (Fig. 3C).
SK channel activity has also been previously demonstrated to play a significant role in the contractility of UBSM tissues (11, 17, 21, 24). When we exposed UBSM strips to the SK channel blocker apamin (320 nM), the force generated by EFS was increased at all frequencies, but there was no significant difference in the magnitude of the increase between strips from wild-type and NFATc3-null mice (data not shown). At 5 Hz stimulation, for example, apamin treatment led to a 1.7 ± 0.2-fold increase in force in the wild-type UBSM strips and a 1.6 ± 0.1-fold increase in the strips from NFATc3-null mice (n = 6; P > 0.05). We conclude from these experiments that the role of SK channels in the regulation of the force response to EFS is similar in wild-type and NFATc3-null mice.
IBK is reduced in UBSM myocytes from NFATc3-null mice.
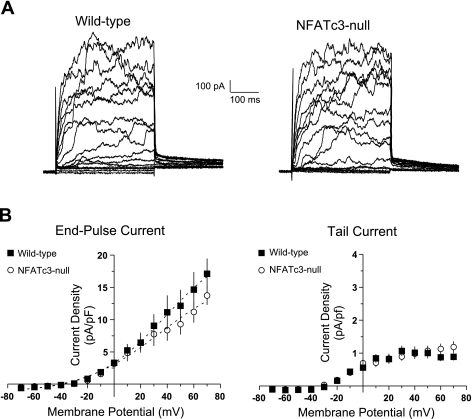
UBSM strips from NFATc3-null mice displayed a reduced responsiveness to IBTX, which may reflect a decrease in the activity or expression of BK channels in detrusor SM cells. To explore this possibility, we compared whole cell currents elicited by 10-mV voltage steps from −70 to +70 mV from UBSM cells obtained from NFATc3 null and wild-type mice. Currents were recorded before and after the application of IBTX to selectively block BK channels. An evaluation of the whole cell K+ currents (IK) recorded before the application of IBTX revealed no significant difference in either end-pulse or tail currents between the myocytes isolated from wild-type and NFATc3-null mice. Representative recordings are presented in Fig. 4A and averaged current-voltage plots for the end-pulse and tail currents are presented in Fig. 4B.
Fig. 4.
UBSM myocytes from wild-type and NFATc3-null mice have similar whole cell K+ currents (IK). A: representative whole cell current recordings elicited by 10-mV membrane potential steps in freshly isolated UBSM myocytes from wild-type and NFATc3-null mice. B: analyses of the resulting end-pulse and tail currents revealed no significant difference between the myocytes isolated from wild-type (▪) and NFATc3-null mice (○) (P > 0.05).
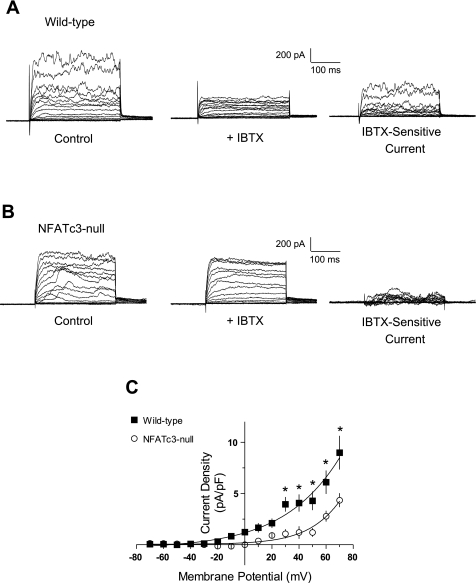
Although there was no significant difference in IK, the IBTX-sensitive current (IBK) was significantly reduced in UBSM cells from NFATc3-null mice (Fig. 5, A and B). As shown in Fig. 5C, IBK was significantly lower in UBSM myocytes from NFATc3-null animals at all membrane potentials more positive than −20 mV (P < 0.05). At +40 mV, IBK was 4.1 ± 0.8 pA/pF (n = 9) in wild-type myocytes but was only 1.2 ± 0.6 pA/pF (n = 10) in NFATc3-null myocytes, a ∼70% reduction in IBK. Thus despite the similarity in IK, IBK was reduced in myocytes isolated from NFATc3-null mice. The reduction of IBK in UBSM myocytes from NFATc3-null mice is consistent with the contractility data and supports the hypothesis that the enhanced contractile response of UBSM strips from NFATc3-null mice is due to a reduction in BK channel activity.
Fig. 5.
UBSM myocytes from NFATc3-null mice have reduced IBTX-sensitive current (IBK). A and B: representative whole cell current recordings elicited by 10-mV membrane potential steps in freshly isolated UBSM myocytes from wild-type (A) and NFATc3-null mice (B) before and after addition of IBTX to block BK channels. C: UBSM myocytes obtained from NFATc3-null mice (○) displayed reduced IBTX-sensitive IBK compared with myocytes obtained from control mice (▪) (*P < 0.05).
BK channel expression is downregulated in NFATc3-null mice.
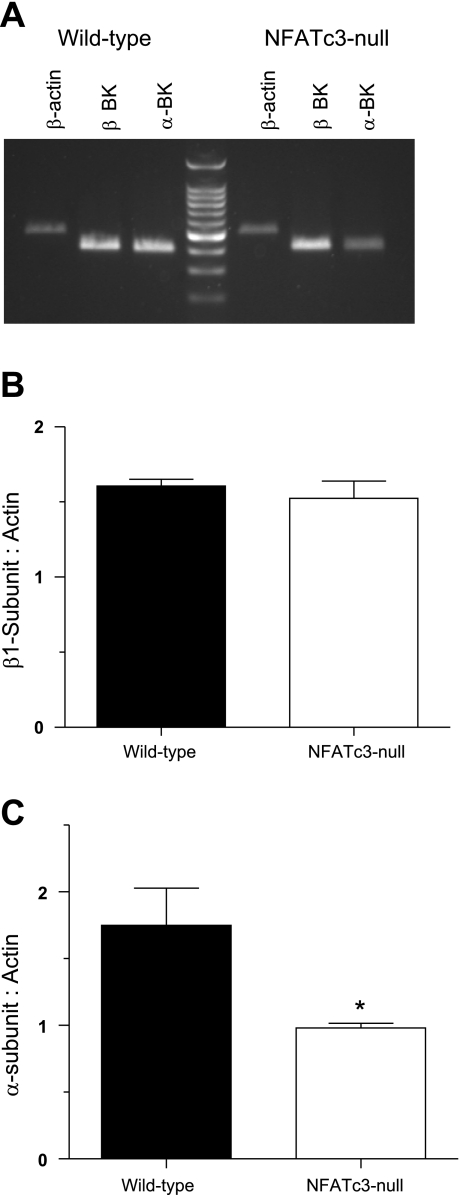
It is possible that expression of either or both pore-forming α- and modulatory β1-subunits are altered in the NFATc3-null mice. To address this, we used semiquantitative RT-PCR to determine the expression levels of BKα- and β1-subunit mRNA (Fig. 6A). There was no significant difference in the expression of mRNA for the β1-subunit between wild-type and NFATc3-null mice (P > 0.05; Fig. 6B). However, the expression of mRNA for the pore-forming α-subunit was ∼44% lower in UBSM from NFATc3-null mice than in wild-type mice (α-subunit: β-actin ratio of 1.78 ± 0.28 for wild-type mice vs. 0.98 ± 0.03 in NFATc3-null mice P < 0.05; n = 5 wild-type and 4 for NFATc3-null mice; Fig. 6C). Thus the reduction in IBK in UBSM myocytes from NFATc3-null mice is supported at the molecular level by a decrease in the mRNA for the pore-forming α-subunit of the BK channel.
Fig. 6.
Reduced expression of the large conductance K+ channel (BK) α-subunit in UBSM from NFATc3-null mice. A: representative agarose gel of semiquantitative RT-PCR analysis of RNA isolated from wild-type and NFATc3-null mice probed for β-actin, BK α-subunit, and BK β-subunits. B: there was no difference in the expression of mRNA for the β1-subunit (relative to β-actin) in UBSM from wild-type and NFATc3-null mice (n = 3 animals each). C: UBSM myocytes isolated from NFATc3-null mice expressed significantly lower levels of the pore-forming BK α-subunit (relative to β-actin levels) (*P < 0.05; n = 5 wild-type and 3 NFATc3-null mice).
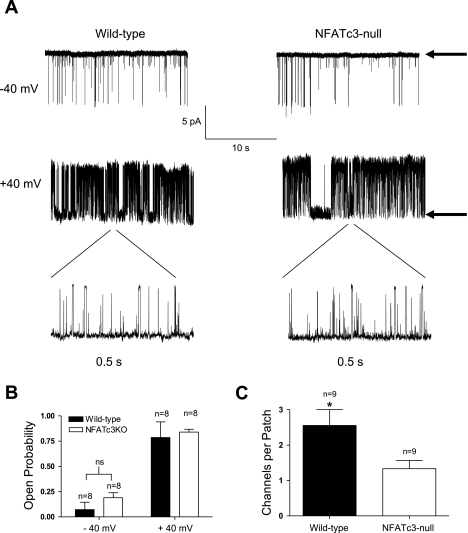
A reduction in the cell membrane expression of the pore-forming α-subunit of the BK channel should not affect the channel Po but should lead to a reduction in the channel density; that is, there should be fewer channels per patch. In contrast, a loss of β1-subunit expression should dramatically lower the single channel Po (6, 37). To definitively rule out the possibility that altered β1-subunit expression contributes to the reduction in BK channel current, we determined the Po of BK channels in wild-type and NFATc3-null UBSM myocytes by evaluating single BK channel currents measured in excised inside-out patches (Fig. 7A). All recordings were made in symmetrical 140 mM K+ with 10 μM intracellular Ca2+. Under these conditions, we have previously shown that loss of the β1-subunit in UBSM decreased BK channel Po at −40 mV and +40 mV 40-fold and 1.6-fold, respectively (37). There was no significant difference in Po between wild-type and NFATc3-null myocytes at negative (−40 mV) or positive (+40 mV) membrane potentials (Fig. 7B; P > 0.05; n = 9). However, the BK channel density was significantly lower (Fig. 7C; P < 0.05; n = 9) in excised patches isolated from NFATc3-null myocytes (1.3 ± 0.7 channels per patch) than in wild-type UBSM myocytes (2.6 ± 1.3 channels per patch). These data are in accord with the whole cell BK current noted previously (Fig. 5C) and indicate that the number of functional channels is significantly reduced in UBSM from NFATc3-null mice.
Fig. 7.
Single channel BK recordings reveal a reduced number of BK channels per patch in NFATc3-null UBSM myocytes. A: representative single channel recordings from excised patches held at −40 and +40 mV. Arrows indicate closed state. B: there was no significant difference in open probability (Po) of excised BK channels isolated from wild-type or NFATc3-null UBSM myocytes. C: number of channels per patch was significantly reduced in UBSM myocytes from NFATc3-null mice (P < 0.05; n = 9; error bars represent means ± SD).
UBSM myocytes isolated from NFATc3-null mice have increased KV currents.
Our observation that IK in wild-type and NFATc3-null mice was similar (Fig. 4B), despite a significant decrease in IBK in the NFATc3-null mice (Fig. 5C), was initially puzzling. However, IK in UBSM is composed of currents through multiple families of K+ channels (7, 20, 24, 27), so it was conceivable that upregulation of another class of K+ channels might have offset the reduction in IBK in NFATc3-null mice. Whereas our molecular, electrophysiological, and functional data indicate that BK expression is NFATc3 dependent, there are reports that NFATc3 may also regulate KV channel expression (2, 39). Thus we investigated the possibility that upregulation of KV channel activity in UBSM myocytes from NFATc3-null mice might account for the observed similarities in IK.
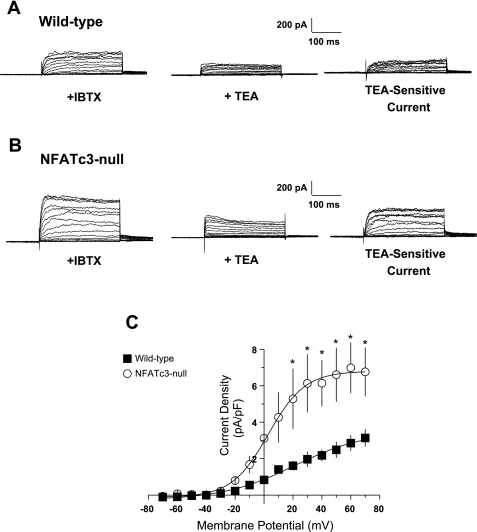
The wild-type murine UBSM KV current is insensitive to block by 4-AP but is sensitive to block by TEA with an IC50 of 5.2 mM (45). Thus, to determine whether the KV component (IKV) of IK was altered in UBSM myocytes from NFATc3-null mice, we evaluated whole cell currents elicited by 10-mV membrane potential steps before and after application of 5 mM TEA (Fig. 8, A and B, for wild-type and NFATc3-null myocytes, respectively). All recordings were made in the presence of IBTX to block BK channels. As shown in the Fig. 8C, IKV, defined as the current blocked by the addition of 5 mM TEA in the presence of IBTX, is significantly larger in the NFATc3-null UBSM myocytes at all membrane potentials more positive than −20 mV (P < 0.05; Fig. 4B). At +40 mV, IKV was 5.40 ± 1.31 pA/pF in myocytes from NFATc3-null mice and only 2.18 ± 0.32 pA/pF in wild-type myocytes (n = 7). These results indicate that the reduction in IBK in NFATc3 null myocytes is offset by an increase in IKV, resulting in an IK that is essentially the same in both NFATc3-null and wild-type UBSM myocytes.
Fig. 8.
UBSM myocytes from NFATc3-null mice have elevated KV ccurrent (IKV). A and B: representative whole cell current recordings elicited by 10-mV membrane potential steps from freshly isolated UBSM myocytes from wild-type (A) and NFATc3-null mice (B) before and after addition of tetraethylammonium chloride (TEA) to block voltage-gated (KV) channels. All cells were also pretreated with IBTX to block BK channels. C: analysis of the averaged difference currents from wild-type (▪) and NFATc3-null mice (○) after treatment with 5 mM TEA indicates that UBSM myocytes from NFATc3- null mice displayed significantly elevated IKV (*P < 0.05).
Biophysical and molecular evidence for increased KV2.1 expression in NFATc3-null mice.
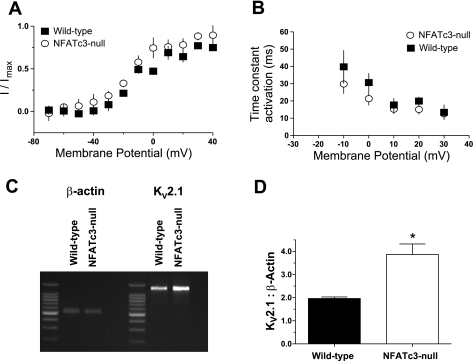
The simplest explanation for the observed increase in IKV in NFATc3-null mice would be an upregulation of the native KV channel complexes, without any change in subunit composition. However, it is also possible that the changes in KV activity may be due to alterations in the subunit composition of the channels. To distinguish between these two possibilities, we compared some of the biophysical and pharmacological properties of IKV in isolated myocytes from wild-type and NFATc3-null mice. There was no significant difference in IKV steady-state activation curves obtained in myocytes isolated from NFATc3-null or wild-type mice (Fig. 9A). The slope factors (k) of the activation curves for wild-type (n = 5) and NFATc3-null mice (n = 7) were 16.3 ± 3.3 and 17.6 ± 4.1, respectively (P > 0.05). Similarly, a comparison of the activation time constants (Fig. 9B) also revealed no significant difference between UBSM myocytes from NFATc39-null and wild-type mice (P < 0.05). In addition, neither the wild-type nor the NFATc39-null UBSM tissues strips responded to treatment with 4-AP (data not shown), indicating that expression of 4-AP9-sensitive KV family members (KV1 and KV4) was not induced in NFATc39-null UBSM myocytes. Thus the biophysical similarities and the lack of responsiveness to 4-AP suggest that the upregulation of IKV in the NFATc39-null myocytes is likely due to increased expression of the native KV2.1-KV5.1/KV6.1 channel complex rather than the expression of an alternate channel assemblage.
Fig. 9.
Molecular and electrophysiological evidence for elevated KV2.1 expression in NFATc3-null mice. There was no significant difference in the steady-state activation curves (A) or activation time constants (B) between UBSM myocytes obtained from wild-type and NFATc3-null mice (P < 0.05). C: UBSM tissue from wild-type and NFATc3-null mice was probed for KV2.1 and β-actin mRNA expression. D: comparison of the averaged ratios of KV2.1:β-actin in UBSM tissues indicates that the UBSM from NFATc3-null mice contained significantly higher levels of mRNA for KV2.1 than did UBSM tissues from wild-type mice (D) (*P < 0.05; n = 3 animals each).
To determine whether evidence for IKV upregulation was supported at the molecular level, we performed semiquantitative RT-PCR on UBSM from wild-type and NFATc3-null mice to compare the levels of mRNA for KV2.1, which has previously been reported to be regulated by NFATc3 (2, 39) (Fig. 9C). Consistent with the increase in IKV, UBSM from NFATc3-null mice exhibited a 40% increase in the expression of mRNA for KV2.1 relative to that from wild-type mice (P < 0.05; n = 3 animals each; Fig. 9D). Thus these data suggest that IK in NFATc3-null and wild-type mice are similar despite the downregulation of IBK in the NFATc3-null mice because the expression of KV2.1 is increased.
DISCUSSION
In this study, we have found that NFATc3-dependent changes in ion channel expression play a role in the regulation of UBSM contractility. UBSM tissue strips from NFATc3-null mice generated more force in response to EFS compared with strips from wild-type mice. We attribute this enhanced contractility to a reduction in BK channel activity. This interpretation is supported at the molecular level by a reduction in BK α-subunit expression in the NFATc3-null UBSM myocytes and at the electrophysiological level by a significant reduction in IBK and BK channel density. However, IK was similar in both NFATc3-null and wild-type mice, an observation that can be explained by an upregulation of IKV in the NFATc3-null mice that is supported at the molecular level by an increase in the expression of mRNA for the KV2.1 subunit, a major component of the mouse UBSM KV channel complex.
Our observation that nerve-evoked contractility in NFATc3-null mice was increased despite the presence of an enhanced IKV suggests that, at least in response to EFS, IBK downregulation has a more significant impact than IKV upregulation. This is presumably due to the prominent role that the BK channel plays in the repolarization phase of the action potential (20, 22, 24), where a small decrease in BK channel activity can lead to a relatively large increase in force. Furthermore, the observation that the force-frequency relationships in UBSM strips from NFATc3-null and wild-type mice were identical in the presence of the BK channel blocker IBTX suggests that modulation of other channel types (e.g., KV) does not significantly contribute to NFATc3-dependent differences in EFS-induced contractions. We also found no significant difference in the amplitude of the contractile response to K+ (60 mM)-mediated depolarization between UBSM muscle strips isolated from NFATc3-null and wild-type mice, suggesting that L-VDCC expression was unchanged.
Several families of K+ channels are expressed in UBSM, including KV, BK, SK, and ATP-dependent K+ (KATP) channels. In the absence of a channel opener, KATP channels make little or no contribution to UBSM contractility (27) and, in any case, would not contribute to voltage-dependent K+ current since their activity is independent of voltage. In contrast, blocking BK channels has been demonstrated to cause very significant increases in nerve-evoked contractions (21, 33, 43, 46). The absence of a significant difference in IK between NFATc3-null and wild-type mice seems to be explained by reciprocal changes in BK and KV channel expression and activity that we have described. Quantitatively, our data would support this hypothesis given that, at +40 mV, the IBK was reduced by ∼3 pA/pF, whereas the IKV was elevated by approximately the same amount.
Our observation that IKV and KV2.1 channel expression are upregulated in UBSM from NFATc3-null mice is consistent with previous reports. In rats, sustained administration of ANG II leads to a reduction in IKV and KV2.1 protein levels in cerebral arteries (2). This downregulation is blocked by the calcineurin-NFAT inhibitor CsA and is also abrogated in NFATc3-null mice. Similarly, Santana and colleagues (39) found that IKV and KV2.1 expression levels are reduced in mouse cardiac muscle after surgically induced myocardial infarction (MI). In these studies, administration of CsA prevented the post-MI reduction in IKV, and there was no post-MI decrease in NFATc3-null mice. Conversely, the expression of a constitutively active form of NFATc3 recapitulated these changes in IKV in the absence of MI. Thus these previous reports strongly suggest that NFATc3 mediates the downregulation of KV2.1 channel expression and activity, similar to what we have reported in the current study. However, we found that EFS-induced contractions were the same in UBSM strips from wild-type and NFATc3-null mice in the presence of the BK channel blocker IBTX, suggesting that a decrease in BK channel activity can explain the increase in EFS-induced contractions in NFATc3- null mice. Although the current study does not provide evidence for a functional consequence of KV channel upregulation, it is conceivable that modulation of this channel type may play a role under other circumstances.
We have found evidence for the downregulation of basal BK channel expression and activity in UBSM from NFATc3-null mice at multiple levels, including a reduction in BK α-subunit expression and channel density, a reduction in IBTX-sensitive IBK, and a reduced responsiveness of UBSM muscle strips to IBTX treatment. Recently, Santana's laboratory (34) reported that activation of the calcineurin-NFATc3 pathway in arterial SM by ANG II stimulation leads to a decrease in β1-subunit expression with no change in the expression of the α-subunit. In contrast to our current work, where we found that the α-subunit, but not the β1-subunit, is constitutively downregulated in UBSM of NFATc3-null mice, this previous study found that neither α- nor β1-subunit expression was changed in the arteries of NFATc3-null mice under basal conditions. Although it is tempting to speculate that these different outcomes reflect tissue-specific differences in NFATc3 regulatory patterns, an alternative explanation might be that engagement of the ANG II signaling pathway modulates NFAT target specificity, perhaps through effects on the relative abundance of potential NFAT cofactors. Additional studies will be required to resolve the molecular questions raised by these results, but collectively they indicate that, directly or indirectly, NFATc3 is capable of regulating the expression of both the α- and β1-subunits of the BK channel, and thus, may be an important regulator of BK channel activity.
Our semiquantitative PCR data indicated that the mRNA for the α-subunit of the BK channel was reduced ∼44%, a value that is similar to the decrease in single BK channel density (∼50%) at +40 mV. However, at +40 mV, the ∼70% decrease in IBK was much greater than would be expected based on the PCR and single-channel data. It should be noted that, at +70 mV, the reduction in IBK was ∼50%, which is more consistent with the PCR and single-channel data. The possible discrepancy between the magnitude of the NFATc3-dependent decrease in BK-α message and the magnitude of the resulting decrease in the IBTX-sensitive current suggests that mechanisms in addition to decreased mRNA expression may contribute to the total reduction in functional channels. In this context, Toro's laboratory (51) found that a splice variant of the α-subunit of the BK channel (SV1), when coexpressed with the human BK α-subunit and β1-subunits in HEK293 cells, reduced surface expression of the BK channel complex by ∼80%. Accordingly, it is possible that, in addition to inducing a decrease in BK α-subunit expression, the absence of NFATc3 might result in the expression of an alternative splice variant of the α-subunit capable of inhibiting surface expression of the native BK channel. Sequence analysis of putative 5′ upstream regulatory regions of the KV2.1 gene from mouse, human, and rat genomes reveals 15 potential NFAT consensus binding sites (GGAAA) within 3600 base pairs of the transcriptional initiation site. Analysis of the promoter regions for the human and mouse BK channel α-subunit reveals eight potential NFAT binding sites within 2600 base pairs of the initiation site, two of which are conserved in both the human and mouse genome. In addition, it has been reported that the BK β-subunit, which is also a potential target of NFAT, contains putative NFAT recognition sites (34). Thus the KV2.1, BK α-subunit and BK β-subunits all represent potential targets of NFAT regulation. In theory, NFATc3 might directly regulate the expression of both KV2.1 and BK α-subunit genes. However, this possibility seems unlikely since the opposing influences of these two transcriptional events create an outcome that is physiologically contradictory. More likely, NFATc3 is directly involved in regulating either KV2.1 or the BK α-subunit, but not both. In this scenario, the change in the non-NFATc3-regulated gene might constitute an example of developmental compensation that would have the effect of maintaining membrane potential within a normal physiological range. Although it has been previously shown that NFATc3 may directly downregulate KV2.1 (2, 39), our data does not definitively rule out the possibility that the changes in KV2.1 expression are an indirect consequence of NFATc3 action on an alternative target. Further studies will employ additional experimental approaches to deconvolute the underlying molecular events. These include the use of promoter-reporter constructs and chromatin binding assays to establish functional significance of putative NFAT binding sites, and temporally controlled disruptions of NFATc3 using small interfering RNA/antisense or dominant negative approaches to determine immediate and secondary consequences of NFAT inhibition. In vitro experiments employing cultured UBSM might also be helpful in this regard, for example, by demonstrating that NFAT-activating stimuli (e.g., endothelin, PDGF) induce the predicted changes in KV2.1 and BK expression. However, UBSM undergoes significant functional changes when cultured for as little as 24 h, so results obtained using cultured cells would need to be considered in context with additional, more physiologically relevant, experimental approaches.
In conclusion, our data indicate that NFATc3 plays a role in regulating the contractility of UBSM by directly or indirectly regulating BK channel expression and function. NFATc3-dependent changes in KV channel activity, which might be expected to modulate UBSM contractility, were also observed, although these changes had no significant effect on the functional parameters measured in this study. UBSM strips from NFATc3-null mice exhibited greater nerve-evoked contractions than strips from wild-type mice, and this enhanced contractility was attributable to a decrease in the expression and activity of BK channels in UBSM cells. IK in NFATc3-null and wild-type mice were statistically indistinguishable, an observation that is largely explained by a concomitant upregulation in IKV and KV2.1 channel expression. At present, it is unclear whether BK and KV2.1 channels are direct targets of NFATc3 or whether the expression of one is indirectly regulated by reciprocal change in the other channel in a kind of compensatory event. Notwithstanding these unresolved questions, our studies strongly support a role for NFATc3 in the regulation of nerve-evoked contractions through modulation of BK channel activity.
GRANTS
This research was funded by the Totman Trust for Medical Research, the National Heart, Lung, and Blood Institute Training Program in the Molecular Basis of Cardiovascular Disease Grants T32 HL-007944 and HL-007647-19, and National Institutes of Health Grants DK-53832, DK-65947, HL-44455, and HL-77378.
Acknowledgments
We gratefully thank Dr. Adrian Bonev for advice and guidance on the single-channel recordings and Dr. Laurie Glimcher for providing the NFATc3-null mice used in these experiments. We also thank Dr. Stephen Straub for insightful and helpful discussions and Bernhard Nausch for help with electrophysiological analyses.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adam RM, Roth JA, Cheng HL, Rice DC, Khoury J, Bauer SB, Peters CA, Freeman MR. Signaling through PI3K/Akt mediates stretch and PDGF-BB-dependent DNA synthesis in bladder smooth muscle cells. J Urol 169: 2388–2393, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Amberg GC, Rossow CF, Navedo MF, Santana LF. NFATc3 regulates Kv2.1 expression in arterial smooth muscle. J Biol Chem 279: 47326–47334, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Amberg GC, Santana LF. Kv2 channels oppose myogenic constriction of rat cerebral arteries. Am J Physiol Cell Physiol 291: C348–C356, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Beals CR, Clipstone NA, Ho SN, Crabtree GR. Nuclear localization of NF-ATc by a calcineurin-dependent, cyclosporin-sensitive intramolecular interaction. Genes Dev 11: 824–834, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Brading AF Ion channels and control of contractile activity in urinary bladder smooth muscle. Jpn J Pharmacol 58, Suppl 2: 120P–127P, 1992. [PubMed] [Google Scholar]
- 6.Brenner R, Perez GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature 407: 870–876, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Buckner SA, Milicic I, Daza AV, Coghlan MJ, Gopalakrishnan M. Spontaneous phasic activity of the pig urinary bladder smooth muscle: characteristics and sensitivity to potassium channel modulators. Br J Pharmacol 135: 639–648, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clipstone NA, Crabtree GR. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature 357: 695–697, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Crabtree GR, Clipstone NA. Signal transmission between the plasma membrane and nucleus of T lymphocytes. Annu Rev Biochem 63: 1045–1083, 1994. [DOI] [PubMed] [Google Scholar]
- 10.Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell 109, Suppl: S67–S79, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Creed KE, Ishikawa S, Ito Y. Electrical and mechanical activity recorded from rabbit urinary bladder in response to nerve stimulation. J Physiol 338: 149–164, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flanagan WM, Corthesy B, Bram RJ, Crabtree GR. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature 352: 803–807, 1991. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Calvo M, Knaus HG, McManus OB, Giangiacomo KM, Kaczorowski GJ, Garcia ML. Purification and reconstitution of the high-conductance, calcium-activated potassium channel from tracheal smooth muscle. J Biol Chem 269: 676–682, 1994. [PubMed] [Google Scholar]
- 14.Gomez MF, Stevenson AS, Bonev AD, Hill-Eubanks DC, Nelson MT. Opposing actions of inositol 1,4,5-trisphosphate and ryanodine receptors on nuclear factor of activated T-cells regulation in smooth muscle. J Biol Chem 277: 37756–37764, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez Bosc LV, Layne JJ, Nelson MT, Hill-Eubanks DC. Nuclear factor of activated T cells and serum response factor cooperatively regulate the activity of an alpha-actin intronic enhancer. J Biol Chem 280: 26113–26120, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Hashitani H, Brading AF. Electrical properties of detrusor smooth muscles from the pig and human urinary bladder. Br J Pharmacol 140: 146–158, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashitani H, Brading AF. Ionic basis for the regulation of spontaneous excitation in detrusor smooth muscle cells of the guinea-pig urinary bladder. Br J Pharmacol 140: 159–169, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashitani H, Bramich NJ, Hirst GD. Mechanisms of excitatory neuromuscular transmission in the guinea-pig urinary bladder. J Physiol 524: 565–579, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashitani H, Fukuta H, Takano H, Klemm MF, Suzuki H. Origin and propagation of spontaneous excitation in smooth muscle of the guinea-pig urinary bladder. J Physiol 530: 273–286, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heppner TJ, Bonev AD, Nelson MT. Ca2+-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am J Physiol Cell Physiol 273: C110–C117, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Herrera GM, Etherton B, Nausch B, Nelson MT. Negative feedback regulation of nerve-mediated contractions by KCa channels in mouse urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol 289: R402–R409, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Herrera GM, Heppner TJ, Nelson MT. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am J Physiol Regul Integr Comp Physiol 279: R60–R68, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Herrera GM, Heppner TJ, Nelson MT. Voltage dependence of the coupling of Ca2+ sparks to BK(Ca) channels in urinary bladder smooth muscle. Am J Physiol Cell Physiol 280: C481–C490, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Herrera GM, Nelson MT. Differential regulation of SK and BK channels by Ca(2+) signals from Ca(2+) channels and ryanodine receptors in guinea-pig urinary bladder myocytes. J Physiol 541: 483–492, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hill-Eubanks DC, Gomez MF, Stevenson AS, Nelson MT. NFAT regulation in smooth muscle. Trends Cardiovasc Med 13: 56–62, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Im SH, Rao A. Activation and deactivation of gene expression by Ca2+/calcineurin-NFAT-mediated signaling. Mol Cell 18: 1–9, 2004. [PubMed] [Google Scholar]
- 27.Imai T, Okamoto T, Yamamoto Y, Tanaka H, Koike K, Shigenobu K, Tanaka Y. Effects of different types of K+ channel modulators on the spontaneous myogenic contraction of guinea-pig urinary bladder smooth muscle. Acta Physiol Scand 173: 323–333, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Jain J, McCaffrey PG, Valge-Archer VE, Rao A. Nuclear factor of activated T cells contains Fos and Jun. Nature 356: 801–804, 1992. [DOI] [PubMed] [Google Scholar]
- 29.Jan LY, Jan YN. Voltage-gated and inwardly rectifying potassium channels. J Physiol 505: 267–282, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaczorowski GJ, Knaus HG, Leonard RJ, McManus OB, Garcia ML. High-conductance calcium-activated potassium channels; structure, pharmacology, and function. J Bioenerg Biomembr 28: 255–267, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Khan MA, Dashwood MR, Thompson CS, Mumtaz FH, Mikhailidis DP, Morgan RJ. Up-regulation of endothelin ET(A) and ET(B) receptors and down-regulation of nitric oxide synthase in the detrusor of a rabbit model of partial bladder outlet obstruction. Urol Res 27: 445–453, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Knaus HG, Garcia-Calvo M, Kaczorowski GJ, Garcia ML. Subunit composition of the high conductance calcium-activated potassium channel from smooth muscle, a representative of the mSlo and slowpoke family of potassium channels. J Biol Chem 269: 3921–3924, 1994. [PubMed] [Google Scholar]
- 33.Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem 279: 36746–36752, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Nieves-Cintron M, Amberg GC, Nichols CB, Molkentin JD, Santana LF. Activation of NFATc3 down-regulates the beta1 subunit of large conductance, calcium-activated K+ channels in arterial smooth muscle and contributes to hypertension. J Biol Chem 282: 3231–3240, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Nozaki K, Tomizawa K, Yokoyama T, Kumon H, Matsui H. Calcineurin mediates bladder smooth muscle hypertrophy after bladder outlet obstruction. J Urol 170: 2077–2081, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Okamura H, Aramburu J, Garcia-Rodriguez C, Viola JP, Raghavan A, Tahiliani M, Zhang X, Qin J, Hogan PG, Rao A. Concerted dephosphorylation of the transcription factor NFAT1 induces a conformational switch that regulates transcriptional activity. Mol Cell 6: 539–550, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Petkov GV, Bonev AD, Heppner TJ, Brenner R, Aldrich RW, Nelson MT. Beta1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J Physiol 537: 443–452, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol 15: 707–747, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Rossow CF, Minami E, Chase EG, Murry CE, Santana LF. NFATc3-induced reductions in voltage-gated K+ currents after myocardial infarction. Circ Res 94: 1340–1350, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Saenz de Tejada I, Mueller JD, de Las Morenas A, Machado M, Moreland RB, Krane RJ, Wolfe HJ, Traish AM. Endothelin in the urinary bladder. I Synthesis of endothelin-1 by epithelia, muscle and fibroblasts suggests autocrine and paracrine cellular regulation. J Urol 148: 1290–1298, 1992. [DOI] [PubMed] [Google Scholar]
- 41.Stevenson AS, Gomez MF, Hill-Eubanks DC, Nelson MT. NFAT4 movement in native smooth muscle. A role for differential Ca(2+) signaling. J Biol Chem 276: 15018–15024, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Thorneloe KS, Chen TT, Kerr PM, Grier EF, Horowitz B, Cole WC, Walsh MP. Molecular composition of 4-aminopyridine-sensitive voltage-gated K(+) channels of vascular smooth muscle. Circ Res 89: 1030–1037, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Thorneloe KS, Meredith AL, Knorn AM, Aldrich RW, Nelson MT. Urodynamic properties and neurotransmitter dependence of urinary bladder contractility in the BK channel deletion model of overactive bladder. Am J Physiol Renal Physiol 289: F604–F610, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Thorneloe KS, Nelson MT. Ion channels in smooth muscle: regulators of intracellular calcium and contractility. Can J Physiol Pharmacol 83: 215–242, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Thorneloe KS, Nelson MT. Properties and molecular basis of the mouse urinary bladder voltage-gated K+ current. J Physiol 549: 65–74, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Werner ME, Knorn AM, Meredith AL, Aldrich RW, Nelson MT. Frequency encoding of cholinergic- and purinergic-mediated signaling to mouse urinary bladder smooth muscle: modulation by BK channels. Am J Physiol Regul Integr Comp Physiol 292: R616–R624, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Wilkins BJ, De Windt LJ, Bueno OF, Braz JC, Glascock BJ, Kimball TF, Molkentin JD. Targeted disruption of NFATc3, but not NFATc4, reveals an intrinsic defect in calcineurin-mediated cardiac hypertrophic growth. Mol Cell Biol 22: 7603–7613, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yellen G The voltage-gated potassium channels and their relatives. Nature 419: 35–42, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Yi BA, Minor DL Jr, Lin YF, Jan YN, Jan LY. Controlling potassium channel activities: interplay between the membrane and intracellular factors. Proc Natl Acad Sci USA 98: 11016–11023, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan XJ, Wang J, Juhaszova M, Golovina VA, Rubin LJ. Molecular basis and function of voltage-gated K+ channels in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 274: L621–L635, 1998. [DOI] [PubMed] [Google Scholar]
- 51.Zarei MM, Zhu N, Alioua A, Eghbali M, Stefani E, Toro L. A novel MaxiK splice variant exhibits dominant-negative properties for surface expression. J Biol Chem 276: 16232–16239, 2001. [DOI] [PubMed] [Google Scholar]
- 52.Zhu J, McKeon F. Nucleocytoplasmic shuttling and the control of NF-AT signaling. Cell Mol Life Sci 57: 411–420, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]