Abstract
Slow troponin T (TnT) plays an indispensable role in skeletal muscle function. Alternative RNA splicing in the NH2-terminal region produces high-molecular-weight (HMW) and low-molecular-weight (LMW) isoforms of slow TnT. Normal adult slow muscle fibers express mainly HMW slow TnT. Charcot-Marie-Tooth disease (CMT) is a group of inherited peripheral polyneuropathies caused by various neuronal defects. We found in the present study that LMW slow TnT was significantly upregulated in demyelination form type 1 CMT (CMT1) but not axonal form type 2 CMT (CMT2) muscles. Contractility analysis showed an increased specific force in single fibers isolated from CMT1 but not CMT2 muscles compared with control muscles. However, an in vitro motility assay showed normal velocity of the myosin motor isolated from CMT1 and CMT2 muscle biopsies, consistent with their unchanged myosin isoform contents. Supporting a role of slow TnT isoform regulation in contractility change, LMW and HMW slow TnT isoforms showed differences in the molecular conformation in conserved central and COOH-terminal regions with changed binding affinity for troponin I and tropomyosin. In addition to providing a biochemical marker for the differential diagnosis of CMT, the upregulation of LMW slow TnT isoforms under the distinct pathophysiology of CMT1 demonstrates an adaptation of muscle function to neurological disorders by alternative splicing modification of myofilament proteins.
Keywords: muscle adaptation, demyelination, force and velocity
charcot-marie-tooth disease (CMT), also known as hereditary motor and sensory neuropathy, is a neurological disorder that causes damage to the peripheral nerves. The symptoms of CMT include muscle weakness and wasting and contractures. There are several forms of CMT. Depending on the genetic defect, the inheritance of CMT varies. CMT type 1 (CMT1) is a demyelinating form of the disease in which defects in axon myelination result in abnormalities in nerve conductive velocity; CMT type 2 (CMT2) is an axonal form of the disease in which the loss of axons reduces muscle innervations (42). Neurophysiological studies have shown an active muscle denervation-reinnervation process in CMT1 patients, whereas CMT2 patients had a low reinnervation capacity and thus compensated for the loss of motor units by increasing the amount of contractile myofibrils (5, 11).
Contraction of vertebrate skeletal muscle is regulated by Ca2+ through the troponin complex in the actin thin filament. Upon motor neuron stimulation, depolarization of the muscle cell membrane results in a rise of Ca2+ in the cytoplasm. Ca2+ binding to troponin induces a series of allosteric changes in the thin filament and activates the myosin motor ATPase and contraction (16). Troponin is a protein complex of three subunits: troponin C (TnC; the Ca2+-binding subunit), troponin I (TnI; the inhibitory subunit), and troponin T (TnT; the tropomyosin-binding subunit) (34). Anchoring the troponin complex to tropomyosin and actin, TnT is at a central position in the regulatory system of muscle (44). Higher vertebrates have evolved three types of striated muscle and three corresponding muscle type-specific TnT isoform genes: slow skeletal muscle TnT (TNNT1), fast skeletal muscle TnT (TNNT3), and cardiac TnT (TNNT2) (6, 22, 27). These homologous TnT isoform genes have differentiated structures while each of the fiber type-specific TnT isoforms is conserved in the vertebrate phylum (29), suggesting differentiated functional roles. A nonsense mutation in the slow TnT gene that causes complete loss of slow TnT in Amish nemaline myopathy has a childhood lethal phenotype (33), demonstrating the indispensable role of slow TnT in skeletal muscle function.
Molecular imaging and structural studies have suggested that TnT is an extended protein (7, 31, 52). Studies using TnT fragments have identified the functional domains of TnT. The COOH-terminal (T2) region binds to tropomyosin and interacts with TnI, TnC, and F-actin (19, 40, 43). A crystal structure of the partial troponin complex confirmed the interaction of the TnT COOH-terminal domain with TnI and TnC (48). The central region of TnT has another tropomyosin-binding site (18, 23, 46). The NH2-terminal region of TnT is a variable region and does not bind any known myofilament protein. Alternative RNA splicing in the NH2-terminal region produces multiple TnT protein isoforms (34). The alternative splicing of cardiac and fast TnT isoforms is developmentally regulated to produce large to small, acidic to basic isoform switches (25, 49). Two alternatively spliced isoforms of slow TnT are found in embryonic and adult skeletal muscle (14, 29, 45). Normal adult slow muscles express mainly high-molecular-weight (HMW) slow TnT. The more acidic HMW and less acidic low-molecular-weight (LMW) slow TnT isoforms differ by inclusion or exclusion, respectively, of 11 amino acids in the NH2-terminal region encoded by exon 5. TnT isoforms differing in the NH2-terminal region show conformational and functional differences (4, 15). Different from cardiac and fast TnT isoform expressions, the alternative splicing of slow TnT is not clearly regulated during development, and the functional significance of HMW and LMW slow TnT isoforms is not understood.
In the present study, we found that LMW slow TnT was significantly upregulated in CMT1 but not CMT2. CMT1 but not CMT2 muscle fibers increased specific force, whereas myosin motor function and myosin isoform contents were unchanged. Supporting a role of slow TnT isoform regulation in the modulation of muscle contractility, LMW and HMW slow TnT isoforms showed differences in molecular conformation and binding to TnI and tropomyosin. In addition to providing a biochemical marker for the differential diagnosis of CMT, the increase of the LMW slow TnT isoform in CMT1 demonstrates an adaptation of myofilament protein isoforms to a specific neurological disorder through alternative RNA splicing.
MATERIALS AND METHODS
All animal procedures were approved by Institutional Animal Care and Use Committees and were conducted in accordance with the “Guiding Principles in the Care and Use of Animals” as approved by the American Physiological Society.
Human muscle samples.
Biopsy samples were obtained from eight CMT1 and five CMT2 patients. Diagnosis was based on genetic analyses (chromosome 17 duplication or mutation in CMT1 patients), electrophysiology [motor and sensory neurographies, needle electromyography (EMG)], and clinical examination. In the CMT1 group, there were two men (49 and 52 yr) and six women (22–62 yr). In the CMT2 group, there were three men (46–69 yr) and two women (66 and 73 yr). Six tibialis anterior and two vastus lateralis biopsy samples from CMT1 patients with predominant slow fiber contents were analyzed. Two tibialis anterior and three vastus lateralis biopsy samples from CMT2 patients with predominant slow fiber contents were analyzed. Single muscle fiber contractile measurements were performed in muscle samples from two CMT1 patients (30 and 57 yr) and one CMT2 patient (73 yr). For comparison, contractile measurements were also performed on fibers from 1 patient with prior polio lesion (51 yr, 35) and 12 healthy controls (58 ± 6 yr; 18–84 yr). All of the patients were ambulatory and have previously been included in a study on macro EMG and muscle function in CMT1 and CMT2 patients (11). The muscle strength according to the Hendall scale did not differ between the two groups, although the average peak isokinetic torque at 30°/s was lower in CMT1 patients than in CMT2 patients (11). Informed consent was obtained from the patients and control subjects enrolled in this study. The Karolinska Institute ethics committee approved the protocol, and the experiments were carried out according to the guidelines of the Declaration of Helsinki.
Each biopsy was dissected free of fat and connective tissues and divided into two portions. One portion was frozen in isopentane cooled by liquid nitrogen and stored at −80°C for later analyses. Small bundles of 25–50 fibers were dissected from the biopsy material. These small bundles were tied to glass capillary tubes and stretched to ∼110% of the resting length. Muscle bundles were then treated with skinning solution [relaxing solution containing glycerol, 50:50 (vol/vol)] for 24 h at 4°C, after which they were transferred to −20°C. In addition, muscle bundles were treated with sucrose, a cryoprotectant, within 1–2 wk for long-term storage (52). For contractile experiments, permeabilized muscle bundles were removed from the capillary tube to isolate single muscle fibers.
Force measurement of skinned single muscle fibers.
The experimental procedure has been described in detail elsewhere (13, 35). Skinned muscle fibers used in this work had an average segment length of 2.02 ± 0.51 mm (mean ± SD, range 1.05–3.60 mm) and were exposed to solution between connectors of the force transducer. The sarcomere length (SL) of the single fiber segment was set between 2.75 and 2.85 μm by adjusting the overall segment length. SL was measured routinely in the fibers during maximal activation. Fiber depth was measured by recording the vertical displacement of the microscope nosepiece while focusing on the top and bottom surfaces of the fiber. The fiber cross-sectional area (CSA) was calculated from the width and depth, assuming an elliptical circumference. Every width and depth value represented the average of three different measurements. The accuracy of the measurements done by the same observer was verified by comparing 200 average values for depth calculated at two different SLs (r2 = 0.99). Specific tension was calculated as maximum tension (Po) normalized to CSA and was corrected for the 20% swelling that is known to occur during skinning (41). CSA, maximum force, and specific tension were calculated in a total of 223 fibers expressing the type I isoform of myosin heavy chain (MHC), i.e., in 110, 81, and 32 control, CMT1, and CMT2 fibers, respectively. A total of 107 fibers fulfilled the criteria for maximum unloaded shortening velocity (V0) measurements, i.e., 54, 34, and 19 control, CMT1, and CMT2 fibers, respectively.
Relaxing and activating solutions were prepared as previously described (35), and the apparent dissociation constants for Ca2+-EGTA were corrected for temperature and ionic strength (12).
V0 was measured by the slack test procedure (9). Fibers were activated at pCa 4.5, and, once steady tension had been reached, various amplitudes of slack (ΔL) were rapidly introduced (within 1–2 ms) at one end of the fiber. The time (Δt) required to take up the imposed slack was measured from the onset of the length step to the beginning of tension redevelopment. For each amplitude of ΔL, the fiber was reextended while relaxed to minimize the nonuniformity of SL. A straight line was fitted to the plot of ΔL versus Δt, using least-squares regression, and the slope of the line normalized to muscle fiber length was recorded as V0 for that fiber.
Po was calculated as the difference between the total tension in the activating solution (pCa 4.5) and the resting tension measured in the same segment while in the relaxing solution. All contractile measurements were carried out at 15°C. Contractile recordings were accepted in subsequent analyses if a V0 value was based on linear regressions including four or more data points, and data were discarded if r for the fitted line was <0.97, if Po changed >10% from first to final activation, or if SL during isometric tension development changed by >0.10 μm compared with SL when the fiber was relaxed (41).
After contractile analysis, fibers were detached from the force transducer and snap frozen with either Freon or isopentane cooled by liquid nitrogen and stored at −80°C for SDS-PAGE and Western blot analysis.
SDS-PAGE and Western blot analysis.
Muscle samples, bacterial extracts, or purified slow TnT proteins were prepared in SDS-PAGE sample buffer as described above and resolved by SDS-PAGE using the Laemmli buffer system. Gels were stained with silver or Coomassie brilliant blue R250 to show the resolved protein bands. Duplicated gels were electrically transferred to nitrocellulose membranes as previously described (50). After being blocked in Tris-buffered saline (TBS) containing 0.5% Triton X-100, 0.05% SDS, and 1% BSA, nitrocellulose membranes were incubated with monoclonal antibodies (mAb) [CT3 against slow and cardiac TnT but not fast TnT (30), TnI-1 against all three TnI isoforms (32), and FA2 against cardiac β-MHC/skeletal muscle type I, but not type II, MHC (26)] or a polyclonal anti-TnT antibody (RATnT) (50). Membranes were then washed in TBS containing 0.5% Triton X-100 and 0.05% SDS, incubated with alkaline phosphatase-labeled anti-mouse IgG second antibody (Sigma Chemical), and washed again to remove the unbound second antibody. Blots were developed in 5-bromo-4-chloro-3-indolylphosphate/nitroblue tetrazolium substrate solution as previously described (50) to detect TnT or TnI isoform bands.
Two-dimensional gel electrophoresis.
Total protein extracts from mouse soleus muscle were analyzed by two-dimensional gel electrophoresis as described previously (2). The first dimension was isoelectric focusing in Bio-Rad Lab mini tube gels containing pH 3.5–10 ampholine (Amersham). The second dimension was 14% Laemmli SDS-PAGE, and the resolved gel was transferred to a nitrocellulose membrane for Western blot analysis using the CT3 mAb as described above.
In vitro motility assay.
The unregulated F-actin used throughout this study was purified from rabbit skeletal muscle and fluorescently labeled with rhodamine-phalloidin (Molecular Probes, Eugene, OR). The single fiber in vitro motility assay has been described in detail elsewhere (20, 21). Briefly, a short muscle fiber segment was placed on a glass slide between two strips of grease, and a mica coverslip was placed on top, creating a flow cell of ∼2-μl volume. Myosin was extracted from the fiber segment through the addition of a high-salt buffer (0.5 M KCI, 25 mM HEPES, 4 mM MgCl2, 1 mM EGTA, and 1% 2-mercaptoethanol; pH 7.6). After 30 min of incubation on ice, a low-salt buffer (25 mM KCl, 25 mM HEPES, 4 mM MgCl2, 1 mM EGTA, and 1% 2-mercaptoethanol; pH 7.6) was applied, followed by 0.1% BSA in the same buffer. Nonfunctional myosin molecules were blocked with fragmentized F-actin, and rhodamine-phalloidin-labeled actin filaments were subsequently infused into the flow cell, followed by motility buffer (2 mM ATP, 0.1 mg/ml glucose oxidase, 23 μg/ml catalase, 2.5 mg/ml glucose, and 0.4% methyl cellulose) to initiate movement. The pH of the buffers was adjusted with KOH, and the final ionic strength of the motility buffer was 71 mM. The flow cell was placed on the stage of an inverted epifluorescence microscope (Olympus IX 70). The movements of F-actin were filmed with an image-intensified SIT camera (SIT 66, DAGE_MIT0) and recorded on tape with a videocassette recorder.
From each single fiber preparation, 13 to 20 actin filaments moving with constant speed in an oriented motion were selected for speed analysis. Recordings and analysis were only performed from preparations in which >90% of the filaments moved bidirectionally. A filament was tracked from the center of mass, and the speed was calculated from 10 to 20 frames at an acquisition rate of 5 or 1 frames/s, depending on the fiber type, using image-analysis software (OPTIMAS 6.0, Optimas). The average speed and SD of 13–20 filaments were calculated. The average motility speed of each fiber was taken as representative for the muscle fiber.
Glycerol-SDS-PAGE analysis of MHC isoforms.
As described previously (53), total protein was extracted by homogenizing isolated human skeletal muscle single fibers in Laemmli SDS-PAGE sample buffer that contained 2% SDS to inactivate proteases. Samples were heated at 80°C for 5 min and clarified by spinning in a microcentrifuge at top speed and room temperature for 5 min. MHC isoforms were resolved by glycerol-SDS-PAGE. The resolving gel contained 8% acrylamide with an acrylamide-bisacrylamide ratio of 50:1, 30% glycerol, 200 mM Tris base, 100 mM glycine (pH 8.8), and 0.4% SDS. The stacking gel contained 4% acrylamide, 70 mM Tris·HCl (pH 6.7), 4 mM EDTA, and 0.4% SDS. Gels were cast in a Bio-Rad mini-Protean II system and run at 70 V in an icebox for 24 h. The upper running buffer was composed of 100 mM Tris base, 150 mM glycine, and 0.1% SDS. The lower running buffer was a 50% dilution of the upper running buffer. The gel was stained with Coomassie brilliant blue R250 to detect protein bands. The MHC I band was confirmed by transferring parallel gels to nitrocellulose membranes for Western blot analysis using mAb FA2 as described above.
Expression and purification of human slow TnT isoforms.
cDNAs encoding HMW and LMW isoforms of human slow skeletal muscle TnT cloned in the pAED4 prokaryotic expression vector were used for protein expression in Esherichia coli as previously described (51). The two slow TnT isoform proteins expressed in bacterial culture were purified by identical procedures involving ammonium sulfate fractionation, anion-exchange, and gel filtration chromatographies as previously described (51).
mAb epitope analysis of the molecular conformation of slow TnT isoforms.
The binding affinity between an antibody and its antigenic epitope depends on its three-dimensional structural fit. Therefore, epitope affinity analysis can detect conformational differences of protein isoforms (3). ELISA epitope analysis (4) was used to examine conformational differences between HMW and LMW slow TnT isoforms. mAbs CT3 and 2C8 against epitopes in the central region and 1G9 against a COOH-terminal epitope (8) were used to examine global conformational changes in slow TnT due to alternative splicing in the NH2-terminal region.
Purified HMW and LMW slow TnT isoform proteins were dissolved in buffer A [0.1 M KCl, 3 mM MgCl2, and 10 mM PIPES (pH 7.0) or 10 mM Tris·HCl (pH 8.0)] at 2 μg/ml to coat microtiter plates at 100 μl/well and 4°C incubation overnight. After the removal of any unbound TnT and two washes with buffer A containing 0.05% Tween 20 (buffer T), plates were blocked with 150 μl/well buffer T containing 1% BSA at room temperature for 1.5 h. The immobilized TnT was incubated with 100 μl/well of serial dilutions of CT3, 2C8, or 1G9 mAb in buffer T containing 0.1% BSA at room temperature for 2 h. Following three washes with buffer T in a 10-min period to remove any unbound primary antibody, plates were incubated with 100 μl/well of horseradish peroxidase-conjugated anti-mouse immunoglobulin second antibody (Sigma) in buffer T containing 0.1% BSA (buffer B) at room temperature for 1 h. Unbound second antibody was removed by three washes as described above. The binding of mAb to their epitopes on MHW and LMW slow TnT isoforms was detected by the H2O2/2,2′-azinobis-(3-ethylbenzthiazolinesulfonic acid) substrate reaction. The enzymatic reaction in each assay well was monitored at a series of time points using an automated microplate reader (Bio-Rad Benchmark). Absorbance at 405 nm (A405 nm) values in the linear course of the color development were used to plot antibody titration curves for the quantification of the binding affinity of each mAb to its specific epitope to compare the difference due to NH2-terminal alternative splicing. All experiments were done in triplicate.
TnI and tropomyosin binding assays.
To compare the functions of HMW and LMW slow TnT isoforms within the thin filament regulatory system, ELISA solid-phase protein binding experiments (50) were performed to compare their binding affinity to TnI and tropomyosin.
HMW and LMW slow TnT proteins were dissolved individually at 5 μg/ml in buffer A and coated on triplicate wells of microtiter plates by an incubation at 4°C overnight. After washes with buffer T to remove unbound TnT, plates were blocked with buffer T containing 1% BSA. Plates were then incubated with serial dilutions of bovine cardiac TnI, purified from adult ventricular muscle as previously described (4), in buffer B at room temperature for 2 h. After washes with buffer T, bound TnI was quantified via anti-TnI mAb TnI-1 (32), horseradish peroxidase-conjugated goat anti-mouse immunoglobulin second antibody (Sigma), and H2O2/2,2′-azinobis-(3-ethylbenzthiazolinesulfonic acid) substrate reactions using ELISA procedures as previously described (50). ELISA results were recorded using an automated microplate reader (Bio-Rad Benchmark), and A405 nm values from the linear range of color development were used to plot the binding curves of TnI to HMW and LMW slow TnT isoforms. BSA-coated wells were used to produce a control curve.
Tropomyosin binding was analyzed similarly except that serial dilutions of α-tropomyosin purified from the rabbit heart, as previously described (50), and anti-tropomyosin mAb CH1 (39), a gift from Dr. Jim Lin (University of Iowa), were used.
Chronically denervated rat muscle samples.
Adult rats underwent an operation under surgical conditions to remove ∼1.0 cm of the trunk portion of the left tibial nerve. Animals were allowed to recover from anesthesia and maintained without movement restrictions. Animals were euthanized 2 wk after the operation, and gastrocnemius muscles from the denervated (left) and control (right) legs were rapidly harvested and stored at −80°C for Western blot analysis of TnT and TnI isoform expression as described above.
Data analysis.
DNA and protein sequence analyses were carried out using DNA Star computer programs. Densitometry analysis of SDS-PAGE gels and Western blots was performed on digital images scanned at 600 dpi using NIH Image program version 1.61. Statistical analysis was carried out using one-way ANOVA. In contractility analysis, a Tukey test was used for all pair-wise comparisons. If the normality test failed, Dunn's test was used for pair-wise comparisons.
RESULTS
Upregulation of alternatively spliced LMW slow TnT in muscle of CMT1 but not CMT2 patients.
Normal human tibialis anterior and vastus lateralis muscles contain both fast and slow fibers, although fibers expressing the slow myosin isoform dominate in the tibialis anterior muscle (24). In accordance with previous observations, predominantly MHC-I fibers were present in CMT muscles (5, 10). In fact, all the single fibers analyzed for contractility in the present study were type I slow fibers as identified by Western blots using FA2 mAb as well as glycerol-SDS-PAGE.
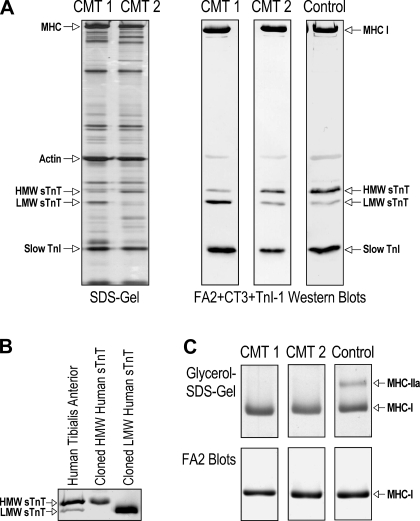
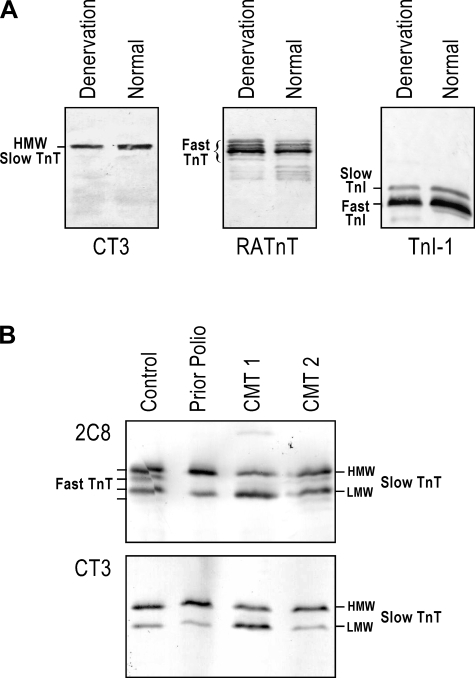
SDS-PAGE and Western blot analysis of total myofilament proteins extracted from a large number of skinned single fibers isolated from tibialis anterior muscle biopsies and frozen sections of tibialis anterior and vastus lateralis muscle biopsies revealed an upregulation of the LMW slow TnT isoform. The representative SDS gel shown in Fig. 1A demonstrated similar protein profiles in CMT2 and control muscle fibers, whereas CMT1 muscle fibers had a unique increase in a ∼30-kDa protein band. The representative Western blots shown in Fig. 1A revealed that CMT1 muscle fibers, but not CMT2 muscle fibers, had a significant increase in the level of LMW slow TnT and decrease in the level of HMW slow TnT, whereas the expression of slow TnI and MHC-I isoforms was unchanged. The identification of HMW and LMW slow TnT isoforms in CMT muscle samples was confirmed by their gel mobility in Western blots along with control human slow TnT isoform proteins expressed in E. coli from cloned cDNA (51) (Fig. 1B).
Fig. 1.
Upregulation of the low-molecular-weight (LMW) slow troponin T (sTnT) isoform in muscles of Charcot-Marie-Tooth disease (CMT) type 1 (CMT1) but not CMT type 2 (CMT2) patients. A: represent SDS-PAGE and Western blots of total myofilament protein extracts of skinned single fibers isolated from tibialis anterior muscle biopsies. The silver-stained SDS gel showed similar protein profiles in CMT2 and normal skeletal muscle fibers. In contrast, CMT1 samples showed an increased intensity of an ∼30-kDa protein band. Parallel Western blots using a mixture of monoclonal antibodies (mAb) CT3, TnI-1, and FA2 revealed that CMT1 muscle fibers had a switch of sTnT isoform expression consisting of a significant increase in the LMW sTnT isoform and a decrease in the high-molecular-weight (HMW) sTnT isoform, whereas CMT2 fibers contained mainly the HMW sTnT isoform, which was the same in control fibers. The expression of slow troponin I (TnI) and myosin heavy chain (MHC)-I isoforms was unchanged in CMT1 and CMT2 muscles. B: identities of HMW and LMW sTnT isoforms were confirmed by their gel mobility in CT3 Western blots along with control proteins expressed in Esherichia coli from cloned cDNAs encoding human sTnT isoforms. C: predominant MHC-I fibers in CMT1 and CMT2 single muscle fibers randomly selected from tibialis anterior muscle biopsies in the present study were confirmed by glycerol-SDS-PAGE and mAb FA2 Western blots of representative samples.
Table 1 shows quantification data summarized from densitometry analysis of CT3 mAb Western blots of muscle samples from CMT1, CMT2, and control subjects. The results confirmed that the LMW slow TnT isoform had a significantly increased percentage of expression in CMT1 muscle compared with control muscle. In contrast, the expression pattern of HMW and LMW slow TnT isoforms in CMT2 patients was not different from the control, in which the HMW slow TnT was predominant.
Table 1.
Increased expression of the LMW slow TnT isoform in CMT1 but not CMT2 patients
| Number of Subjects | LMW Slow TnT Isoform, % total slow TnT | |
|---|---|---|
| Control | 12 | 29.7±7.1 |
| CMT1 | 8 | 50.4±17.7* |
| CMT2 | 5 | 29.5±16.8† |
Data are means ± SD. Data are summarized from a densitometry analysis of CT3 monoclonal antibody Western blots of single muscle fibers or frozen sections of muscle biopsies from Charcot-Marie-Tooth disease (CMT) type 1 (CMT1), CMT type 2 (CMT2), and control subjects, which demonstrated that the low-molecular-weight (LMW) slow troponin T (TnT) isoform had a significantly increased expression in CMT1 muscle compared with control or CMT2 muscles
P < 0.005.
In contrast, the expression pattern of high-molecular-weight and LMW slow TnT isoforms in CMT2 was not different from the control
P > 0.75.
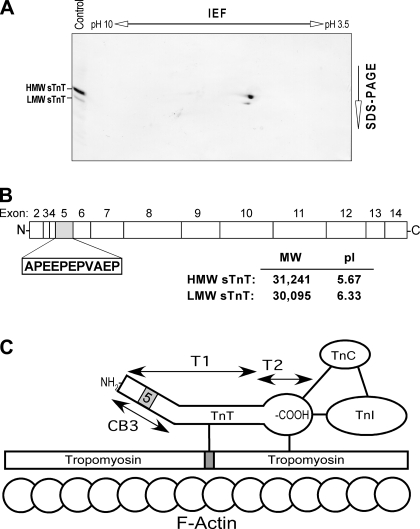
The physical properties of HMW and LMW slow TnT isoforms were analyzed by Western blot of two-dimensional gel electrophoresis-resolved mouse soleus muscle homogenates. The mAb CT3 blot shown in Fig. 2A demonstrated that the two alternatively spliced slow TnT isoforms were different in their size and isoelectric points. The linear structural map of slow TnT deduced from amino acid sequences demonstrated the difference between HMW and LMW slow TnT isoforms due to alternative splicing of exon 5, which encodes an 11-residue acidic peptide in the NH2-terminal region (Fig. 2B). Consistent with their two-dimensional gel mobility, the calculated molecular weights and isoelectric points of HMW and LMW human slow TnT isoforms (Fig. 2B) demonstrated that the splice out of exon 5 in LMW slow TnT reduces the NH2-terminal negative change. As shown in Fig. 2C, TnT occupies a central position in the thin filament Ca2+-regulatory system of striated muscle. The NH2-terminal variable region of TnT is a modulatory structure. The alternative splicing of exon 5 in the NH2-terminal region may affect the protein binding sites in central and COOH-terminal regions, conferring a specific functional adaptation by the increase in LMW slow TnT in CMT1 muscle.
Fig. 2.
HMW and LMW isoforms of sTnT generated by alternative splicing of exon 5 in the NH2-terminal variable region. A: Western blot analysis using mAb CT3 on two-dimensional gel electrophoresis-resolved mouse soleus muscle homogenate demonstrated that the alternatively spliced sTnT isoforms were different in their size and overall charge. IEF, isoelectric focusing. B: the linear structure map of human sTnT deduced from amino acid sequence alignment shows the difference between HMW and LMW isoforms by alternative splicing of exon 5 encoding an 11-residue acidic peptide in the NH2-terminus region. Molecular weights (MW) and isoelectric points (pI) of the two TnT isoforms were calculated from sequence data, demonstrating differences, consistent with their two-dimensional gel mobility. C: TnT occupies a central position in the thin filament Ca2+-regulatory system of striated muscle. The functionally characterized T1 and T2 chymotrypsin fragments, the location of alternatively spliced exon 5 in the NH2-terminal region, and the known protein binding sites in the central and COOH-terminal regions are outlined.
Increase in Ca2+-activated tension in skinned single muscle fibers from CMT1 but not CMT2 patients.
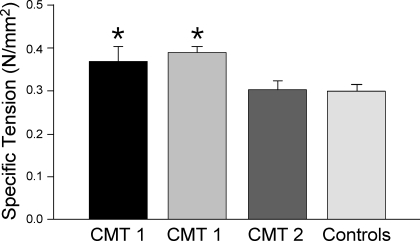
Figure 3 shows the results from contractility experiments using skinned single muscle fibers isolated from 2 CMT1, 1 CMT2, and 12 control subjects. The maximum Ca2+ activated force of CMT1 muscle fibers normalized by CSA (specific tension) was higher than those of CMT2 and control muscle fibers. No differences were observed between CMT2 and control fibers. The average CSA of individual MHC-I fibers was 3,640 ± 150 μm2 in controls, 2,670 ± 140 and 7,650 ± 300 μm2, respectively, in fibers from two CMT1 patients, and 3,860 ± 160 μm2 in CMT2 fibers. Thus, a significant muscle fiber hypertrophy was observed in one, but not the other, CMT1 patient, consistent with previous observations of a large muscle fiber size variability among CMT1 patients (11). The fibers used in the contractility analysis were recovered and examined by glycerol-SDS-PAGE and FA2 mAb Western blots. The results showed that all fibers used for the contractility analysis were MHC-I fibers. Representative data are shown in Fig. 1C.
Fig. 3.
Increase in Ca2+-activated tension in CMT1 but not CMT2 muscle fibers. Contractility experiments using skinned single muscle fibers isolated from CMT1, CMT2, and control subjects showed that Ca2+-activated specific tension of CMT 1 fibers (n = 81) normalized by cross-sectional area was higher than that of CMT2 (n = 32) and control (n = 110) fibers (*P < 0.001). Data are presented as means ± SE. All fibers used for the contractility analysis were MHC-I fibers as examined by glycerol-SDS-PAGE and FA2 mAb Western blots.
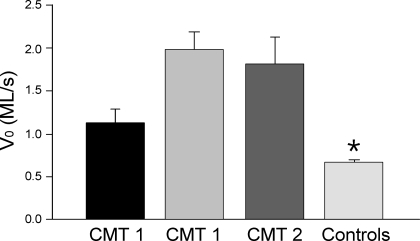
V0 in single muscle fibers increased in both CMT1 and CMT2 patients.
Although the single muscle fibers examined in the present study were all MHC-I fibers, V0 values were significantly different between groups (Fig. 4). Both CMT1 and CMT2 fibers showed higher V0 values compared with those of control fibers. Although a trend of difference was seen between the two CMT1 patients, no statistical significance was established.
Fig. 4.
Increased maximum velocity of unloaded shortening (V0) in CMT1 and CMT2 fibers. V0 values of skinned muscle fibers were higher in both CMT1 (n = 34) and CMT2 (n = 19) patients compared with control fibers (n = 54) as analyzed by ANOVA on ranks (*P < 0.001). Data are presented as means ± SE. Although there was a trend of difference between the two CMT1 patients, no statistical difference was established. All fibers analyzed were MHC-I fibers. ML, muscle lengths.
Unchanged function of myosin isolated from CMT1 and CMT2 single muscle fibers.
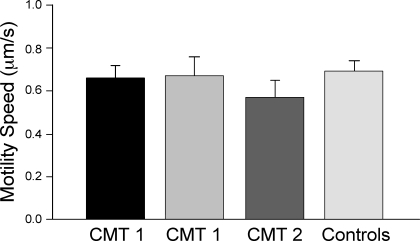
Figure 5 shows the results from a single muscle fiber in vitro motility assay using unregulated F-actin. Actin filament speed propelled by myosin from single muscle fibers in the absence of tropomyosin-troponin regulation did not differ among CMT1, CMT2, and control muscle myosins.
Fig. 5.
Unchanged intrinsic motor activity of myosin isolated from CMT1 and CMT2 muscles. Actin-activated motor activity of myosin purified from CMT1 and CMT2 single muscle fibers was examined by an in vitro motility assay using unregulated F-actin. Data are presented as means ± SE; data from 2 CMT1 and 1 CMT2 patients are shown. There were no significant differences found among CMT1, CMT2, and control muscle myosins.
Consistently, CMT1 and CMT2 single muscle fibers randomly selected for examination in the present study were all MHC-I fibers, as shown by glycerol-SDS-PAGE and mAb FA2 Western blots (Fig. 1C). The results indicate that the differences in the Ca2+-activated specific tension and V0 in CMT muscle fibers was not due to an alteration of the intrinsic activity of the myosin motor.
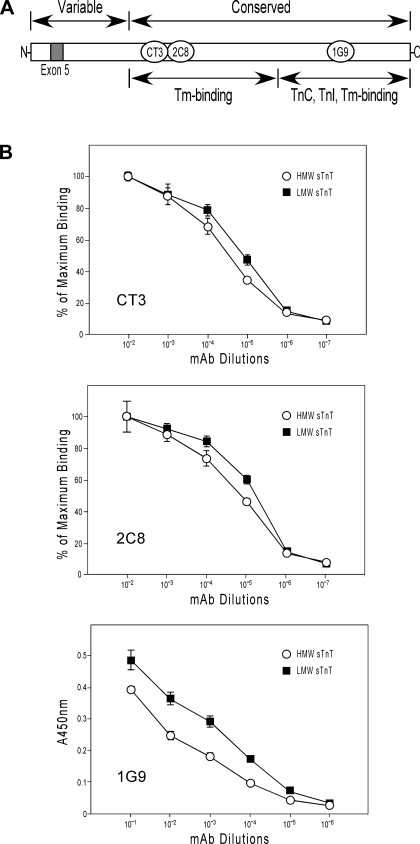
Effect of the NH2-terminal variation in HMW and LMW slow TnT on the molecular conformation of remote functional regions.
The NH2-terminal variable region is a structure that modulates the molecular conformation and function of TnT (4, 34). The conformational effect of alternative splicing of exon 5 in the NH2-terminal region on functional regions in slow TnT was analyzed by ELISA epitope affinity titration using mAb CT3, 2C8, and 1G9 against epitopes in the central and COOH-terminal regions that bind tropomyosin, TnC, and TnI (Fig. 6A). The results shown in Fig. 6B demonstrated different binding affinities of these site-specific mAb to HMW and LMW isoforms of slow TnT, indicating a conformational difference in central and COOH-terminal regions. The data suggest that the presence or absence of exon 5 in the NH2-terminal region of HMW and LMW slow TnT isoforms has a functional significance.
Fig. 6.
Effect of the NH2-terminal variation in HMW and LMW sTnT isoforms on the molecular conformation of remote functional regions. A: the effect of alternative splicing of exon 5 in the NH2-terminal region on functional regions in sTnT, which has an extended structure, was analyzed by ELISA epitope affinity titration using mAb CT3, 2C8, and 1G9 against epitopes in the central and COOH-terminal regions that bind tropomyosin (Tm), troponin C (TnC), and TnI. B: titration curves of mAb CT3 and 2C8 detected different binding affinities to HMW and LMW sTnT isoforms at pH 7.0 (P < 0.05 for both epitopes), indicating a conformational difference in the central region. Significant affinity difference was also found for mAb 1G9 at pH 8.0 (P < 0.01), demonstrating a conformational difference between HMW and LMW sTnT isoforms in the COOH-terminal region. Data are presented as means ± SD. The conformational differences in functional regions due to the presence or absence of exon 5 in the NH2-terminal region suggest a functional significance of the differential regulation of HMW and LMW sTnT isoforms.
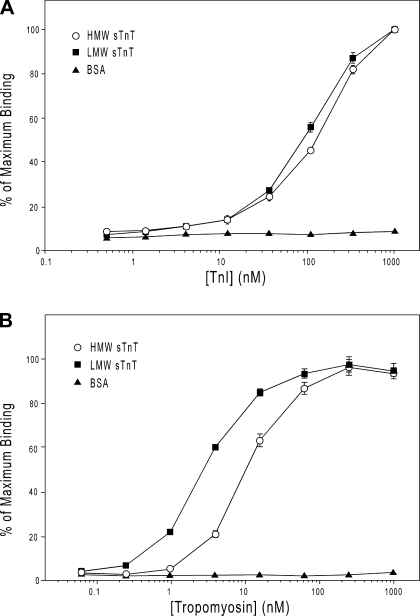
Different binding affinities of HMW and LMW slow TnT to TnI and tropomyosin.
To examine the functional significance of the conformational differences between HMW and LMW slow TnT isoforms resulting from the alternative splicing of exon 5, solid-phase protein binding assays were used to assess the interactions of HMW and LMW slow TnT isoforms to TnI and tropomyosin. The results showed that the LMW slow TnT isoform had a moderately higher binding affinity for TnI than the HMW slow TnT isoform (Fig. 7A). The LMW slow TnT isoform further showed a clearly higher binding affinity for tropomyosin than the HMW slow TnT isoform, and the difference was greater than that of their TnI-binding affinity (Fig. 7B). This observation indicates that the NH2-terminal alternative splicing of TnT more effectively affects the adjacent central region containing a tropomyosin-binding site, whereas the TnI-binding site is in the COOH-terminal region (Fig. 6A). The results suggest that the presence or absence of the NH2-terminal 11 amino acids differentially alters slow TnT conformation and function in the central and COOH-terminal regions, supporting a notion that the change in the Ca2+-activated contractility of CMT1 muscle fibers is due to the change in slow TnT isoform regulation.
Fig. 7.
Different binding affinities of HMW and LMW sTnT isoforms to TnI and Tm. Solid-phase protein binding assays were used to assess the binding of sTnT isoforms with TnI and Tm. A: normalized titration curves showed that the LMW sTnT isoform has a higher binding affinity for TnI than that of the HMW sTnT isoform (P < 0.01). B: normalized titration curves further showed that the LMW sTnT isoform had a higher binding affinity to Tm than that of the HMW sTnT isoform (P < 0.002). Data are presented as means ± SD.
Muscle denervation or overuse did not affect the ratio of HMW and LMW slow TnT isoforms.
It is very intriguing that CMT1 but not CMT2 results in the change in slow TnT isoform expression. Considering that the pathology of CMT2 is axon loss, we examined TnT isoform expression in muscle denervation. The results shown in Fig. 8A demonstrated that rat gastrocnemius muscles 2 wk after removal of the main trunk of the tibial nerve showed atrophy but no change in alternatively spliced slow TnT isoforms compared with innervated normal muscle. We have previously demonstrated that during skeletal muscle adaptation, the change in alternatively spliced TnT isoforms is a rapid response and can be detected as early as 3 days after unloading (53). Therefore, the lack of detectable change in TnT isoform expression after 2 wk of denervation indicates no detectable fiber type switching. Consistently, the expression of fast TnT and TnI isoforms also did not change.
Fig. 8.
Muscle denervation or overuse did not affect the ratio of HMW and LMW sTnT isoforms. A: 2 wk after the removal of the main trunk of the tibial nerve, rat gastrocnemius muscles showed atrophy but no change in alternatively spliced sTnT isoforms compared with innervated normal muscles. The expression of fast TnT and TnI isoforms also did not change, indicating no detectable fiber type switching after denervation. B: Western blots using mAb 2C8 (against both fast TnT and sTnT) and CT3 (against sTnT) showed that normal human tibialis anterior muscle had detectable expression of fast TnT, whereas a muscle biopsy from a prior polio patient had predominantly sTnT, similar to that found in CMT patient muscles. The switching to slow fibers after overuse of healthy muscle in prior polio patient suggests a transcriptional regulation that might also be responsible for the predominant slow fibers found in CMT muscles. However, the overuse conditions did not change the alternative splicing regulation of HMW versus LMW sTnT isoforms.
The results shown in Fig. 8B further demonstrated that normal human tibialis anterior muscle had detectable expression of fast TnT, whereas a muscle sample from a prior polio patient (36) had predominantly slow TnT, similar to that found in CMT patient muscles. The switching to slow fibers after overuse of healthy muscle in prior polio patient suggests a transcriptional regulation that might also be responsible for the predominant slow fibers found in CMT muscles. However, the overuse conditions did not change the alternative splicing pattern of the HMW versus LMW slow TnT isoforms, which may reflect a unique adaptation to the qualitative changes in neurological stimulation in CMT1.
DISCUSSION
Regulation of TnT isoforms by alternative RNA splicing in muscle diseases and adaptation.
Skeletal muscle is a highly plastic tissue that undergoes dramatic changes in contractile protein gene regulation during development and adaptation to functional demands and stress conditions (47). For example, we and others have found that the expression of slow versus fast isoforms of MHC, TnI, and TnT was highly sensitive to muscle unloading in simulated weightlessness (53). It is worth noting that these adaptations are by regulating the transcription of different genes encoding the protein isoforms. On the other hand, alternative RNA splicing has been found to regulate TnT isoforms during cardiac and fast skeletal muscle development (27, 49), primarily under the control of a systemic biological clock (28).
Nonetheless, we previously reported primary changes in cardiac TnT alternative spicing in dilated cardiomyopathy (2, 3). Alternatively spliced cardiac TnT variants have also been reported in hypertrophic and failing human hearts (1). Changes in the alternative splicing of fast TnT isoforms have been observed during hindlimb skeletal muscle unloading (53). There was no clear developmental regulation of HMW and LMW slow TnT isoforms found in mice and sheep (29). Our finding of the slow TnT isoform switch in the muscle of CMT1 patients provides a novel evidence for the role of alternatively spliced slow TnT isoforms in the modification of skeletal muscle function under disease conditions.
Specific adaptation by upregulation of LMW slow TnT in muscle of CMT1 patients with axon demyelination.
A previous study (37) has observed the role of innervations in the expression of muscle fiber type-specific TnT isoforms. A neurophysiological study (5) on muscle biopsy specimens showed an active denervation-reinnervation process in CMT1 (demyelinating form) patients, whereas CMT2 (axonal form) patients had a low reinnervation capacity and thus compensated for the loss of motor units by increasing contractile tissue. Since neuropathic abnormalities were found in CMT1 and myopathic changes due to the loss of innervation occur in CMT2, it was noted that the muscle fiber abnormalities in CMT1 patients would reflect abnormalities due to the primary nerve dysfunction and that the abnormalities seen in CMT2 patients would be secondary changes as a compensation for loss of muscle strength with an increase of contractile material (5).
Consistently, we demonstrated that the axonal loss associated with peripheral denervation in experimental animal models or in patients with prior polio lesion did not change the ratio of TnT isoforms, similar to that observed in CMT2 patients with axon loss. Furthermore, we (36) have previously reported an approximately twofold higher V0 and average CSA in muscle fibers from the prior polio patient compared with healthy control MHC-I fibers without an increase in specific tension, reflecting an adaptation to chronic overuse. Therefore, the results demonstrate that axon loss and compensatory overuse have mainly quantitative structural and functional consequences at the muscle level. In contrast, the altered splicing of slow TnT isoforms is a specific adaptation to the pathophysiology of CMT1 in which the changed quality, rather than quantity, of neurological stimuli induces adaptive changes in muscle contractile function. This novel correlation between neurological stimulation and/or muscle activation pattern and muscle contractile protein isoform regulation needs to be further investigated for the understanding of neuromuscular plasticity and functional interactions.
Potential effect of the increased LMW slow TnT isoform in CMT1 patients on muscle function.
In the present study, we demonstrated an increase in the Ca2+-activated tension normalized to the CSA of CMT1 muscle fibers compared with CMT2 or control fibers. This difference occurred on the basis of identical myosin isoforms in muscle fibers and, therefore, cannot be attributed to myosin motor function. Consistently, the activity of unregulated myosin isolated from CMT1, CMT2, and control muscles was not different in in vitro motility experiments and MHC isoform expression was identical in these muscle fibers. By excluding the role of changes in intrinsic myosin function, the correlation between the upregulation of the LMW slow TnT isoform and the increase in Ca2+-activated contractile force production suggests a plausible mechanism of functional adaptation.
A previous study (15) has shown that TnT isoforms with less NH2-terminal negative charge confer lower Ca2+ sensitivity in the activation of cardiac muscle thin filaments. The observation that the less acidic LMW slow TnT isoform correlates to higher myofibril force production in CMT1 provides a new lead to further understand the physiological and pathological significance of the NH2-terminal regulation of TnT structure and function.
V0 values were significantly increased in single muscle fibers expressing the slow (type I) MHC isoform from CMT patients irrespective the etiology of the denervation, i.e., demyelinating or axonal type. The expression of essential myosin light chain isoforms has been shown to have a significant modulatory impact on V0 in single muscle fibers from young rats and rabbits (17, 38), although this relationship has not been confirmed in old rats or in single muscle fibers from humans (35, 38). In the original study (17) reporting the modulatory influence of essential myosin light chains on V0, it was also observed that the variability in myosin light chain isoform expression covaried with TnT isoform expression. The results from the present study did not show a significant modulatory influence of the alternatively spliced HMW and LMW slow TnT isoforms on V0, i.e., increased V0 values were observed in MHC-I fibers from both CMT1 and CMT2 patients. However, the increased V0 in MHC-I fibers is consistent with previous observations of a positive impact of chronic overuse on V0 in MHC-I fibers in previously denervated muscles, such as in patients with a prior polio lesion (36), whereas the molecular mechanisms underlying this adaptation remain to be established.
Increases in the LMW slow TnT isoform may aid the differential diagnosis of CMT1.
The diagnosis and classification of CMT are currently based on clinical, electrophysiological (EMG and electroneurography), and genetic evaluations. In the present study, we demonstrated the unique increase of the LMW slow TnT isoform in CMT1 but not CMT2 muscles as well as the feasibility of determining the pattern of slow TnT isoform expression on as little biopsy materials as a single skinned muscle fiber. Therefore, a clinical test to confirm the diagnosis can be done on a spare portion of regular needle biopsy muscle samples. The Western blot procedure for such determination can be done in less than a day, making the application in routine clinical diagnosis practical. The use of standard Western blot techniques does not require specialized equipment and allows almost any diagnostic laboratory to adopt this new examination. More importantly, a differential diagnosis of CMT by the patterns of slow TnT isoform expression is based on the adaptation of muscle to the neuropathology of the disease. Therefore, this phenotype-based diagnosis will be of unique value in guiding functional evaluation and clinical management.
GRANTS
This work was supported in part by National Institutes of Health Grants AR-048816 and HD-044824 (to J.-P. Jin) and AR-45627 and AR-47318 (to L. Larsson).
Acknowledgments
We thank Dr. Jim Lin for providing the CH1 mAb.
Present address of P. Höök: Dept. of Pathology, Columbia Univ., Columbia, NY 10027.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Anderson PA, Greig A, Mark TM, Malouf NN, Oakeley AE, Ungerleider RM, Allen PD, Kay BK. Molecular basis of human cardiac troponin T isoforms expressed in the developing, adult, and failing heart. Circ Res 76: 681–686, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Biesiadecki BJ, Elder BD, Yu ZB, Jin JP. Cardiac troponin T variants produced by aberrant splicing of multiple exons in animals with high instances of dilated cardiomyopathy. J Biol Chem 277: 50275–50285, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Biesiadecki BJ, Jin JP. Exon skipping in cardiac troponin T of turkeys with inherited dilated cardiomyopathy. J Biol Chem 277: 18459–18468, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Biesiadecki BJ, Chong SM, Nosek TM, Jin JP. Troponin T core structure and the regulatory NH2-terminal variable region. Biochemistry 46: 1368–1379, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borg K, Ericson-Gripenstedt U. Muscle biopsy abnormalities differ between Charcot-Marie-Tooth type 1 and 2: reflect different pathophysiology? Exerc Sport Sci Rev 30: 4–7, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Breitbart RE, Nadal-Ginard B. Complete nucleotide sequence of the fast skeletal troponin T gene. Alternatively spliced exons exhibit unusual interspecies divergence. J Mol Biol 188: 313–324, 1986. [DOI] [PubMed] [Google Scholar]
- 7.Cabral-Lilly D, Tobacman LS, Mehegan JP, Cohen C. Molecular polarity in tropomyosin-troponin T co-crystals. Biophys J 73: 1763–1770, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chong SM, Hossain MM, Jin JP. Hidden ancestral traits in the three dimensional structure of modern proteins (Abstract). Biophys J 92: 19a, 2007.
- 9.Edman KA The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol 291: 143–159, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ericson U, Ansved T, Borg K. Charcot-Marie-Tooth disease–muscle biopsy findings in relation to neurophysiology. Neuromuscul Disord 8: 175–181, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Ericsson U, Borg J, Borg K. Macro-EMG and muscle biopsy of paretic foot dorsiflexors in Charcot-Marie-Tooth disease. Muscle Nerve 23: 217–222, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Fabiato A Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol 157: 378–417, 1988. [DOI] [PubMed] [Google Scholar]
- 13.Frontera WR, Larsson L. Contractile studies of single human skeletal muscle fibers: a comparison of different muscles, permeabilization procedures, and storage techniques. Muscle Nerve 20: 948–952, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Gahlmann R, Troutt AB, Wade RP, Gunning P, Kedes L. Alternative splicing generates variants in important functional domains of human slow skeletal troponin T. J Biol Chem 262: 16122–16126, 1987. [PubMed] [Google Scholar]
- 15.Gomes AV, Guzman G, Zhao J, Potter JD. Cardiac troponin T isoforms affect the Ca2+ sensitivity and inhibition of force development. Insights into the role of troponin T isoforms in the heart. J Biol Chem 277: 35341–35349, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev 80: 853–924, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Greaser ML, Moss RL, Reiser PJ. Variations in contractile properties of rabbit single muscle fibres in relation to troponin T isoforms and myosin light chains. J Physiol 406: 85–98, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heeley DH, Golosinska K, Smillie LB. The effects of troponin T fragments T1 and T2 on the binding of nonpolymerizable tropomyosin to F-actin in the presence and absence of troponin I and troponin C. J Biol Chem 262: 9971–9978, 1987. [PubMed] [Google Scholar]
- 19.Heeley DH, Smillie LB. Interaction of rabbit skeletal muscle troponin T and F-actin at physiological ionic strength. Biochemistry 27: 8227–8232, 1988. [DOI] [PubMed] [Google Scholar]
- 20.Hook P, Li X, Sleep J, Hughes S, Larsson L. In vitro motility speed of slow myosin extracted from single soleus fibres from young and old rats. J Physiol 520: 463–471, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hook P, Larsson L. Actomyosin interactions in a novel single muscle fiber in vitro motility assay. J Muscle Res Cell Motil 21: 357–365, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Huang QQ, Chen A, Jin JP. Genomic sequence and structural organization of mouse slow skeletal muscle troponin T gene. Gene 229: 1–10, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Ishii Y, Lehrer SS. Two-site attachment of troponin to pyrene-labeled tropomyosin. J Biol Chem 266: 6894–6903, 1991. [PubMed] [Google Scholar]
- 24.Jakobsson F, Borg K, Edstrom L. Fibre-type composition, structure and cytoskeletal protein location of fibres in anterior tibial muscle. Comparison between young adults and physically active aged humans. Acta Neuropathol 80: 459–468, 1990. [DOI] [PubMed] [Google Scholar]
- 25.Jin JP, Lin JJ. Rapid purification of mammalian cardiac troponin T and its isoform switching in rat hearts during development. J Biol Chem 263: 7309–7315, 1988. [PubMed] [Google Scholar]
- 26.Jin JP, Malik ML, Lin JJ. Monoclonal antibodies against cardiac myosin heavy chain. Hybridoma 9: 597–608, 1990. [DOI] [PubMed] [Google Scholar]
- 27.Jin JP, Huang QQ, Yeh HI, Lin JJ. Complete nucleotide sequence and structural organization of rat cardiac troponin T gene. A single gene generates embryonic and adult isoforms via developmentally regulated alternative splicing. J Mol Biol 227: 1269–1276, 1992. [DOI] [PubMed] [Google Scholar]
- 28.Jin JP Alternative RNA splicing-generated cardiac troponin T isoform switching: a non-heart-restricted genetic programming synchronized in developing cardiac and skeletal muscles. Biochem Biophys Res Commun 225: 883–889, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Jin JP, Chen A, Huang QQ. Three alternatively spliced mouse slow skeletal muscle troponin T isoforms: conserved primary structure and regulated expression during postnatal development. Gene 214: 121–129, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Jin JP, Chen A, Ogut O, Huang QQ. Conformational modulation of slow skeletal muscle troponin T by an NH2-terminal metal-binding extension. Am J Physiol Cell Physiol 279: C1067–C1077, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Jin JP, Root DD. Modulation of troponin T molecular conformation and flexibility by metal ion binding to the NH2-terminal variable region. Biochemistry 39: 11702–11713, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Jin JP, Yang FW, Yu ZB, Ruse CI, Bond M, Chen A. The highly conserved COOH terminus of troponin I forms a Ca2+-modulated allosteric domain in the troponin complex. Biochemistry 40: 2623–2631, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Jin JP, Brotto MA, Hossain MM, Huang QQ, Brotto LS, Nosek TM, Morton DH, Crawford TO. Truncation by Glu180 nonsense mutation results in complete loss of slow skeletal muscle troponin T in a lethal nemaline myopathy. J Biol Chem 278: 26159–26165, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Jin JP, Zhang Z, Bautista JA. Isoform diversity, regulation, and functional adaptation of troponin and calponin. Crit Rev Euk Gene Expr 18: 92–124, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Larsson L, Moss RL. Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J Physiol 472: 595–614, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larsson L, Li X, Tollback A, Grimby L. Contractile properties in single muscle fibres from chronically overused motor units in relation to motoneuron firing properties in prior polio patients. J Neurol Sci 132: 182–192, 1995. [DOI] [PubMed] [Google Scholar]
- 37.Leeuw T, Kapp M, Pette D. Role of innervation for development and maintenance of troponin subunit isoform patterns in fast- and slow-twitch muscles of the rabbit. Differentiation 55: 193–201, 1994. [DOI] [PubMed] [Google Scholar]
- 38.Li X, Larsson L. Maximum shortening velocity and myosin isoforms in single muscle fibers from young and old rats. Am J Physiol Cell Physiol 270: C352–C360, 1996. [DOI] [PubMed] [Google Scholar]
- 39.Lin JJ, Chou CS, Lin JL. Monoclonal antibodies against chicken tropomyosin isoforms: production, characterization, and application. Hybridoma 4: 223–242, 1985. [DOI] [PubMed] [Google Scholar]
- 40.Mak AS, Smillie LB. Structural interpretation of the two-site binding of troponin on the muscle thin filament. J Mol Biol 149: 541–550, 1981. [DOI] [PubMed] [Google Scholar]
- 41.Moss RL Sarcomere length-tension relations of frog skinned muscle fibres during calcium activation at short lengths. J Physiol 292: 177–192, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicholson GA The dominantly inherited motor and sensory neuropathies: clinical and molecular advances. Muscle Nerve 33: 589–597, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Pearlstone JR, Smillie LB. Binding of troponin-T fragments to several types of tropomyosin. Sensitivity to Ca2+ in the presence of troponin-C. J Biol Chem 257: 10587–10592, 1982. [PubMed] [Google Scholar]
- 44.Perry SV Troponin T: genetics, properties and function. J Muscle Res Cell Motil 19: 575–602, 1998. [DOI] [PubMed] [Google Scholar]
- 45.Samson F, Mesnard L, Mihovilovic M, Potter TG, Mercadier JJ, Roses AD, Gilbert JR. A new human slow skeletal troponin T (TnTs) mRNA isoform derived from alternative splicing of a single gene. Biochem Biophys Res Commun 199: 841–847, 1994. [DOI] [PubMed] [Google Scholar]
- 46.Schaertl S, Lehrer SS, Geeves MA. Separation and characterization of the two functional regions of troponin involved in muscle thin filament regulation. Biochemistry 34: 15890–15894, 1995. [DOI] [PubMed] [Google Scholar]
- 47.Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev 76: 371–423, 1996. [DOI] [PubMed] [Google Scholar]
- 48.Takeda S, Yamashita A, Maeda K, Maeda Y. Structure of the core domain of human cardiac troponin in the Ca2+-saturated form. Nature 424: 35–41, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Wang J, Jin JP. Primary structure and developmental acidic to basic transition of 13 alternatively spliced mouse fast skeletal muscle troponin T isoforms. Gene 193: 105–114, 1997. [DOI] [PubMed] [Google Scholar]
- 50.Wang J, Jin JP. Conformational modulation of troponin T by configuration of the NH2-terminal variable region and functional effects. Biochemistry 37: 14519–14528, 1998. [DOI] [PubMed] [Google Scholar]
- 51.Wang X, Huang QQ, Breckenridge MT, Chen A, Crawford TO, Morton DH, Jin JP. Cellular fate of truncated slow skeletal muscle troponin T produced by Glu180 nonsense mutation in Amish nemaline myopathy. J Biol Chem 280: 13241–13249, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Wendt T, Guenebaut V, Leonard KR. Structure of the Lethocerus troponin-tropomyosin complex as determined by electron microscopy. J Struct Biol 118: 1–8, 1997. [DOI] [PubMed] [Google Scholar]
- 53.Yu ZB, Gao F, Feng HZ, Jin JP. Differential regulation of myofilament protein isoforms underlying the contractility changes in skeletal muscle unloading. Am J Physiol Cell Physiol 292: C1192–C1203, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]