Abstract
We previously found that the phosphorylation of ERK1/2 by submaximal concentrations of the muscarinic receptor ligand carbachol was potentiated in rat parotid acinar cells exposed to ouabain, a cardiac glycoside that inhibits the Na-K-ATPase. We now report that this signaling phenomenon involves the prevention of negative regulation of extracellular signal-regulated kinase-1/2 (ERK1/2) that is normally mediated by AMP-activated protein kinase (AMPK). Carbachol increases the turnover of the ATP-consuming Na-K-ATPase, reducing intracellular ATP and promoting the phosphorylation/activation of the energy sensor AMPK. Ouabain blocks the reduction in ATP and subsequent AMPK phosphorylation, which is regulated by the AMP-to-ATP ratio. The ouabain-promoted enhancement of ERK1/2 phosphorylation was not reproduced in Par-C10 cells, an immortalized rat parotid cell line that did not respond to carbachol with an ATP reduction and that employs an upstream AMPK kinase (Ca2+/calmodulin-dependent protein kinase kinase, CaMKK) different from that (LKB1) in native cells. In native parotid cells, inhibitory effects of AMPK on ERK1/2 signaling were examined by activating AMPK with 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR), which is converted to an AMP mimetic but does not alter parotid ATP levels. AICAR-treated cells display increases in AMPK phosphorylation and a reduced phosphorylation of ERK1/2 subsequent to activation of muscarinic and P2X7 receptors, which promote increases in Na-K-ATPase turnover, but not upon epidermal growth factor receptor activation. These results suggest that carbachol-initiated AMPK activation can produce a negative feedback on ERK1/2 signaling in response to submaximal muscarinic receptor activation and that increases in fluid secretion can modulate receptor-initiated signaling events indirectly by producing ion transport-dependent decreases in ATP.
Keywords: adenosine 5′-triphosphatase; calmodulin-dependent protein kinase kinase; 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside; carbachol; 2′,3′-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate
the na-k-atpase (Na Pump) is an integral membrane protein that mediates the electrogenic exchange of two extracellular K ions for three intracellular Na ions, a reaction that consumes one molecule of ATP. It plays a critical role in maintaining the Na and K gradients across the plasma membrane, thereby contributing to the membrane potential in most mammalian cells. The receptor-mediated stimulation of net ion movement across epithelia increases the Na-K-ATPase turnover due to cellular ionic changes that accompany net absorption and secretion. This increases ATP hydrolysis and this promotes increases in ATP production by oxidative phosphorylation (21, 31). The Na-K-ATPase ion pumping activity and ATP consumption is blocked by the cardiac glycoside ouabain, which binds to the α-subunit of the enzyme. During the last decade ouabain was discovered to have a hormonal type of effect in addition to acting as an inhibitor of enzyme activity, and it initiates many signaling effects. The binding of ouabain to the α-subunit of the Na-K-ATPase can initiate extracellular signal-regulated kinase-1/2 (ERK1/2) activation and multiple signaling events by promoting the formation of a signalplex of proteins that use the Na-K-ATPase as a platform in cardiac, epithelial, and other cells. This signaling protein complex involves Src and other proteins, including phospholipase C and inositol 1,4,5-trisphosphate (IP3) receptors, and is often initiated by low concentrations of ouabain that do not produce a substantial inhibition of the Na-K-ATPase activity (28, 37, 43). In fact, a low concentration of ouabain stimulated Na-K-ATPase-mediated 86Rb uptake via an ERK-dependent pathway (15). There is specificity in the α-isoforms that affect the degree of Na-K-ATPase-mediated signaling to ERK (27). Also, the actions of ouabain are specific to its binding to the Na-K-ATPase, not promiscuously to other proteins; therefore, these newly described actions of ouabain originate from specific binding to one protein.
In contrast to the effect of low concentrations of ouabain to initiate ERK1/2 activation and signalplex formation, we found that ouabain (10–1,000 μM) did not produce a large stimulation of ERK1/2 phosphorylation in freshly isolated rat parotid acinar salivary gland cells; however, high concentrations of ouabain potentiated the phosphorylation of ERK1/2 downstream of the stimulation of the parotid muscarinic type 3 receptor (M3R) by submaximal concentrations (3 × 10−7 and 1 × 10−6 M) of the ligand carbachol (29). The EC50 of ouabain in enhancing the activation of ERK1/2 by 10−6 M carbachol was ∼100 μM, and this was similar to the inhibitor constant (Ki) of ouabain in blocking the Na-K-ATPase enzyme activity of rodent cells that express the α1-subunit of this enzyme. Ouabain did not appear to exert its effects directly on the activation of the M3R itself or on the coupling of the receptor to phospholipase C, since the IP3-dependent release of intracellular Ca was not affected in ouabain-treated cells. Src and PKC contributed positively to the activation of ERK1/2 by maximal concentrations of carbachol as well as to the potentiation by ouabain of submaximal carbachol concentrations.
The focus of the present investigation was to determine how ouabain contributed in a positive manner to the activation of ERK1/2 by the carbachol-initiated activation of the M3R in parotid acinar epithelial cells. Muscarinic ligands promote an increase in fluid secretion by these cells that initiates the production of saliva. This process involves the concerted activation of ion channels and other ion transport proteins, including the ATP-dependent Na-K-ATPase (22). This increases the turnover of ATP, and this activates the energy-sensor AMP-activated protein kinase (AMPK) in parotid cells (32). AMPK is a heterotrimeric complex consisting of a catalytic α-subunit and regulatory β- and γ-subunits, and phosphorylation of Thr172 in the kinase domain at the NH2-terminus of AMPKα is required for activation. AMPK is allosterically activated by increases in AMP, and increases in the AMP-to-ATP ratio enhance the phosphorylation of AMPK by the upstream AMPK kinase LKB1 (38). AMPK plays a central role in many metabolic effects, including those involved in insulin signaling as well as responses to stress, and has an inhibitory effect on cell growth, proliferation, and cell survival (20, 38). AMPK has been found to have both positive and negative effects on ERK signaling (4, 7, 16, 17). Since we previously found that carbachol increased AMPK phosphorylation in parotid acinar cells, we examined carbachol-stimulated ERK1/2 signaling more closely to determine whether AMPK and upstream signaling molecules in the ERK1/2 cascade contributed to the potentiation of ERK1/2 phosphorylation by ouabain. Our findings suggest that stimulation of the Na-K-ATPase turnover can dampen the activation of ERK1/2 by carbachol in native parotid acinar cells under some conditions. In parallel experiments, the effects of AMPK on ERK1/2 signaling were examined by activating AMPK with AICAR, a cell-permeable compound that is converted to an AMP mimetic. We compared the effects of AICAR on ERK1/2 phosphorylation by ligands to three different types of receptors, including carbachol (G protein-coupled muscarinic receptors), 2′,3′-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate (BzATP; P2X7 receptors, which also are ion channels), and epidermal growth factor (EGF, growth factor receptors). The results suggest that receptor-mediated Na-K-ATPase-dependent increases in ATP hydrolysis can affect the activities of protein kinases in the ERK1/2 signaling cascade.
We also conducted similar studies using Par-C10 cells, an immortalized cell line of rat parotid acinar cells that share many of the receptor types, second messenger production, cell signaling events, and ion transport characteristics of native rat parotid cells (39). This cell line is also amenable to transfections and alterations of protein expression and ideally could be used for directed studies of the proteins involved in the potentiation of ERK1/2 by ouabain in carbachol-treated cells. However, we were unable to replicate our findings concerning ouabain and ERK1/2 activation. We explored the reasons for the different responses between native and immortalized cells and found this to be due to differences in cell metabolism and the regulation of key signaling molecules, including the kinases upstream of AMPK.
MATERIALS AND METHODS
Chemicals.
Carbamylcholine (carbachol) (C-4382), ATP (A-2383), carbonyl cyanide-p-trifluoromethoxyphenyl hydrazone (FCCP) (C-2920), and ouabain (O-3125) were purchased from Sigma. STO-609 (1551) was obtained from Tocris. Rabbit polyclonal ERK2 (SC-154) and mouse monoclonal ERK2 (SC-1647) were purchased from Santa Cruz Biotechnology. The following phospho-specific antibodies were purchased from Cell Signal Technology: Thr202/Tyr204-ERK1/2 (9101), Ser217/221-MEK (9121), Thr172-AMPKα (2532), Ser79-ACC (3661), Ser338-C-Raf (9427), and LKB1 (3052). Ca2+/calmodulin-dependent protein kinase kinase (CaMKK) antibody (610544) was purchased from BD Transduction Laboratories. α-Isoform-specific (α1, -2, and -3) Na-K-ATPase antibodies were provided by Dr. Thomas A. Pressley (Texas Tech University Health Sciences Center). Secondary antibodies used for the Odyssey Infrared Imaging System were IRDye 800 conjugated goat anti-rabbit IgG (Rockland, no. 611-632-122) and Alexa Fluor 680 goat anti-mouse IgG (Invitrogen A-21058). Goat anti-rabbit IgG (AP307P) and goat anti-mouse IgG (AP124P) secondary antibodies used for Western blot analysis using film were obtained from Chemicon. Igepal was purchased from ICN Biomedicals. All other chemicals were reagent grade or better.
Parotid acinar cell preparation.
Parotid acinar cells were prepared from male Sprague-Dawley rats (Charles River Laboratories, Kingston, NY, or Taconic, Germantown, NY; 175–225 g) using previously established techniques (34). The Institutional Animal Care and Use Committee of Beth Israel Deaconess Medical Center approved this study. In brief, rat parotid glands were removed and treated with trypsin and collagenase to get a suspension of single cells and small groups of cells. Cells were suspended at ∼0.5–1 mg protein/ml in solution A composed of the following (in mM: 116.4 NaCl, 5.4 KCl, 1 NaH2PO4, 25 Na HEPES, 1.8 CaCl2, 0.8 MgCl2, 5 Na β-hydroxybutyrate, and 5.6 glucose, pH 7.4). Cells were maintained on ice before use.
All treatments of cells were performed at 37°C in a water-jacketed chamber using a magnetic flea to stir the suspended cells. Aliquots of cells (usually 1.5 ml) were equilibrated for ∼10 min before treatment with various agents or vehicles [water or 0.1–0.2% dimethylsulfoxide (DMSO)]. At the end of the treatment period, parotid cells were collected by rapid sedimentation in a microcentrifuge (Brinkmann 5414). The supernatant was removed, and cells were lysed in ∼500 μl of ice-cold lysis buffer [137 mM NaCl, 20 mM Tris base, pH 7.5, 1 mM EGTA, 1 mM EDTA, 10% (vol/vol) glycerol, and 1% (vol/vol) Igepal] containing the following reagents: 1 mM vanadate, 4.5 mM sodium pyrophosphate, 47.6 mM NaF, 9.26 mM β-glycerophosphate, 0.5 mM dithiothreitol, 2 mg/ml leupeptin, 2 mg/ml pepstatin, 2 mg/ml aprotenin, and 2 mg/ml 4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride (AEBSF). The lysates were vortexed extensively and then sedimented at 15,000 g for 15 min at 4°C. The cleared supernatants were transferred to fresh microfuge tubes and diluted with 5× Laemmli sample buffer [312.5 mM Tris·HCl (pH 6.8), 25% glycerol, 5% sodium dodecyl sulfate, 1.8 M β-mercaptoethanol, and bromophenol blue (trace)], boiled for 5 min, and stored at −20°C before electrophoresis.
Par-C10 cells.
Cells were grown to near confluence in DMEM-F12 (1:1) medium containing 2.5% fetal bovine serum (FBS) and supplements similar to those specified in Turner et al. (39). Cells were cultured on 100-mm BD Falcon tissue culture dishes in a humidified atmosphere of 95% air-5% CO2 at 37°C.
Western blot analysis.
Samples were separated using SDS-polyacrylamide gel electrophoresis with a 10% separating gel and a 3% stacking gel as described previously (29). Proteins were transferred from the gels to nitrocellulose. Immunoblots were probed overnight with various antibodies according to the supplier's specifications. Proteins were visualized using enhanced chemiluminescence (ECL) reagents and X-ray film; alternatively, in some experiments proteins were visualized and quantified by direct infrared fluorescence using an Odyssey Imaging System (Li-Cor Biosciences). Blots were stripped and reprobed to identify changes in the phosphorylation status of multiple signaling proteins.
Quantification of protein phosphorylation.
For most ERK and AMPK studies, the blots were probed for phosphoproteins and total protein levels using various protein-selective primary antibodies, and fluorescent anti-rabbit and anti-mouse antibodies were used for visualization and subsequent quantification using the Odyssey Imaging System, as reported previously (29). The phosphorylation status of mitogen-activated protein kinase kinase (MEK), RAF, and some AMPK and ERK proteins visualized on film using ECL techniques were quantified by densitometry using the ImageJ software program of the National Institutes of Health. Changes in the phosphorylation status of these proteins were evaluated as an indication of changes in their activation status. For each sample, the level of phosphorylated protein was normalized to the total protein level of the signaling protein being analyzed or to total ERK2 to account for gel loading/transfer variations. Changes in protein phosphorylation for various conditions were compared with the phosphorylation status under basal (nonstimulated) conditions in the absence of inhibitors.
Cellular ATP measurements.
ATP was measured in parotid acinar cells as described previously (32). In general, cells suspended in solution A were equilibrated for ∼10 min at 37°C before treatment with various agents or vehicles (water or 0.1–0.2% DMSO), as indicated. ATP was extracted from the cells using 6% perchloric acid (PCA), the acid extract was neutralized using K2CO3, and the sample was frozen at −80°C until it was analyzed spectrophometrically in a hexokinase-linked enzyme assay using authentic ATP as a standard. The ATP content of each sample was normalized to the amount of PCA-precipitated protein. Multiple samples (generally 2–4) for each condition were collected from each cell preparation, and the results from one preparation were averaged and treated as n = 1. The numbers of independent experiments are presented for each condition.
For experiments with Par-C10 cells, the medium was switched to DMEM plus 0.1% FBS overnight or several hours before the experiment. ATP was extracted and quantified as for native parotid acinar cells. Multiple samples (generally 2–4) for each condition were collected from each experiment, and the results from one experiment were averaged and treated as n = 1. The numbers of independent experiments are as presented.
Data.
Values were calculated as the means ± SE of n number of independent experiments (each n from a different experiment). Differences between control/basal and experimental samples for the accumulated data were evaluated using a Student's t-test. All experiments, including Western blot analyses, were performed at least three different times. Within each experiment designed to quantify changes in protein phosphorylation using the Odyssey system or film densitometry, multiple (duplicate or triplicate) samples of cells were collected for each condition and were subjected to SDS-PAGE. For the quantification of protein phosphorylation, the average of the values obtained for each condition within each individual experiment was treated as n = 1.
RESULTS
Effect of ouabain on signaling proteins upstream and downstream of ERK1/2.
In previous studies we observed that ouabain potentiated the activation of ERK1/2 in rat parotid acinar cells by low concentrations of the muscarinic ligand carbachol (29). The relatively low potency of ouabain on ERK1/2 enhancement suggested that this effect was mediated by the ouabain-binding α1-subunit, which is known to be ouabain-insensitive in rodents. To examine the α-subunit isoforms, we probed parotid gland lysates with isoform-specific α antibodies, and found that the parotid gland expresses the α1-subunit (Fig. 1). Brain tissue expresses all three isoforms and served as a positive control for the antibodies. These results were consistent with the high concentration dependence of ouabain on ERK1/2 potentiation being mediated via the α1-subunit.
Fig. 1.
Western blot of α1-, α2-, and α3-subunits of the Na-K-ATPase. α1 was detectible in brain, parotid gland, and a rat parotid cell line (Par-C10). α2 and α3 expression were readily detectible in brain but not in parotid or Par-C10 cells. All lanes were loaded with 100 μg protein.
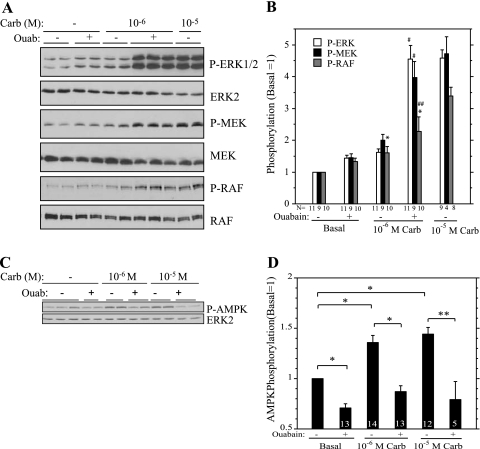
Since the enhanced muscarinic effect produced by ouabain did not appear to be due to an effect at the M3 receptor level (29), we examined the signaling cascade downstream of the receptor to gain insights into the mechanism of this effect. Ouabain (1 mM) increased the phosphorylation of ERK1/2 promoted by 10−6 M carbachol to a level similar to that produced by 10−5 M carbachol (Fig. 2, A and B), as observed previously. Not unexpectedly, increases in the phosphorylation of MEK, the kinase immediately upstream of ERK1/2, mimicked that of ERK1/2 (Fig. 2, A and B). We also found that ouabain also enhanced the phosphorylation of the ERK1/2 effector p90RSK in cells treated with 10−6 M carbachol (not shown). Previously we found that 10−5 M carbachol increased p90RSK phosphorylation (29). We also examined the phosphorylation of C-RAF on several sites using phospho-specific antibodies. C-RAF activation is complicated and potentially involves five residues within the kinase domain (5, 13) and sometimes involves B-RAF immediately upstream of C-RAF activation (8). C-RAF was constitutively phosphorylated on S259 (not shown), a site to which 14-3-3 binds. However, changes in the phosphorylation of Ser338 on the NH2-terminus of the kinase domain, a site important to C-RAF activation by G protein-coupled receptors (GPCRs) (5), were similar to those of MEK and ERK1/2 in that the effects of 10−6 M carbachol were increased by ouabain, although not to the same degree (Fig. 2, A and B). The increases in ERK1/2, MEK, and RAF phosphorylation produced by 10−6 M carbachol plus ouabain were similar to that produced by 10−5 M carbachol.
Fig. 2.
Ouabain (Ouab) increases the effect of carbachol (Carb) on the phosphorylation of proteins in the extracellular signal-regulated kinase-1/2 (ERK1/2) signaling cascade but blocks AMP-activated protein kinase (AMPK) phosphorylation. A: parotid acinar cells were treated with 10−6 M Carb for 2 min after a 1-min exposure to DMSO (−) or 1 mM Ouab and to 10−5 M Carb after a 1-min exposure to DMSO. Cells in basal (−) condition were exposed for Ouab or DMSO for 3 min. Lysates were subjected to immunoblot analysis as indicated. Representative Western blot analysis of replicate samples from one experiment. Ouab increases the phosphorylation of RAF-S338, mitogen-activated protein kinase kinase (MEK), and ERK1/2 in cells treated with 10−6 M Carb. B: quantification of changes in phosphorylation of ERK1/2, MEK, and RAF. For each condition, the level of phosphoprotein was quantified and normalized to the total individual protein (ERK2, MEK, RAF), and values are presented relative to basal (no Ouab). Number of individual experiments is indicated below bars. *P < 0.02 vs. basal (no Ouab). #P < 0.01 vs. 10−6 M Carb (no Ouab). ##P ≤ 0.05 vs. 10−6 M Carb (no Ouab). All other conditions are P ≤ 0.01 vs. basal (no ouabain). C: parotid acinar cells were treated with 10−6 and 10−5 M Carb for 2 min after a 1-min exposure to DMSO (−) or 1 mM Ouab. Cells in basal (−) condition were exposed for Ouab or DMSO for 3 min. Lysates were subjected to immunoblot analysis using a P-AMPK antibody. Representative Western blot analysis of replicate samples from one experiment. Ouab reduces/blocks the phosphorylation of AMPK. D: quantification of AMPK phosphorylation. For each condition, the phosphorylation of AMPK was quantified and normalized to total AMPK (or ERK2) as a loading control, and values are shown relative to basal (no Ouab). Number of individual experiments is indicated at base of bars. *P < 0.01; **P < 0.05. P values were calculated based on matched pairs from same experiment.
AMPK phosphorylation is blocked by inhibition of the Na-K-ATPase.
Since receptor-initiated increases in the parotid Na-K-ATPase activity enhance ATP turnover, we examined the activation of the energy sensor AMPK (by monitoring changes in its phosphorylation on T172), which is sensitive to alterations in the cellular energy status (AMP-to-ATP ratio). Both 10−6 M and 10−5 M carbachol increased the phosphorylation/activation of AMPK, and these increases were blocked by ouabain (Fig. 2, C and D). Both concentrations of carbachol produced similar increases in AMPK phosphorylation. The effects of ouabain to block the activation of AMPK by carbachol are consistent with its inhibition of the large energy (ATP) utilization by the Na-K-ATPase when fluid secretion is stimulated in parotid cells. Notably, the block of AMPK phosphorylation coincided with the increased phosphorylation of proteins in the ERK-signaling cascade when 10−6 M carbachol was added to ouabain-treated cells. This is of interest because AMPK can be a negative regulator of ERK1/2 (7, 16, 17).
AMPK activation reduces ERK1/2 phosphorylation.
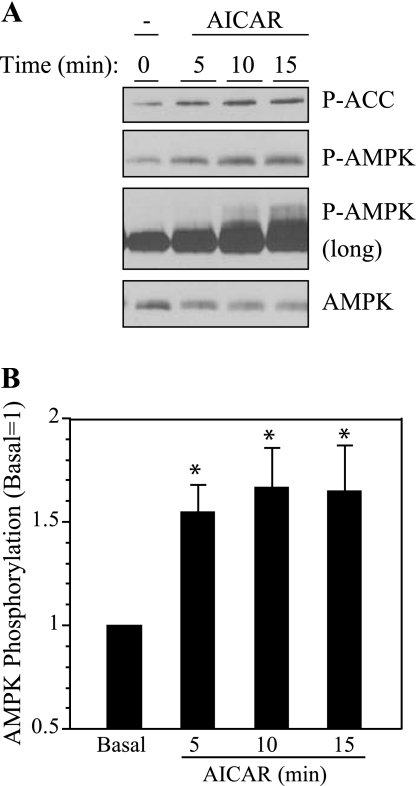
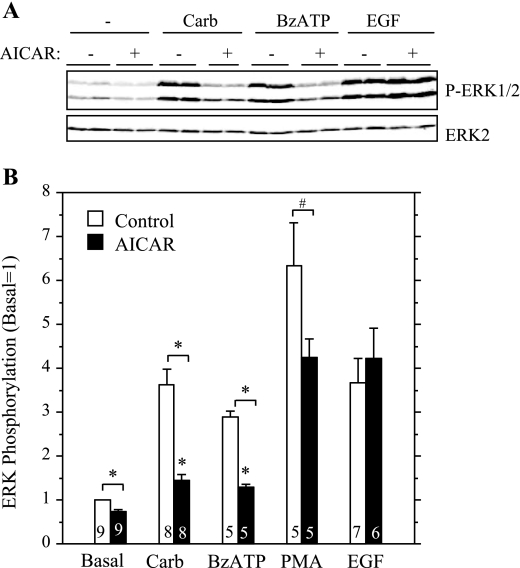
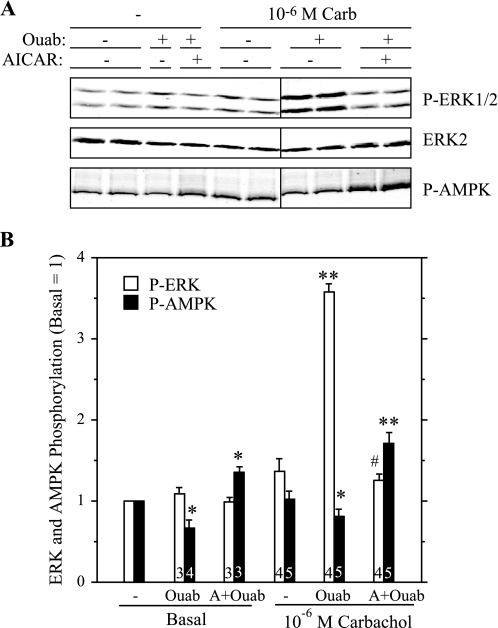
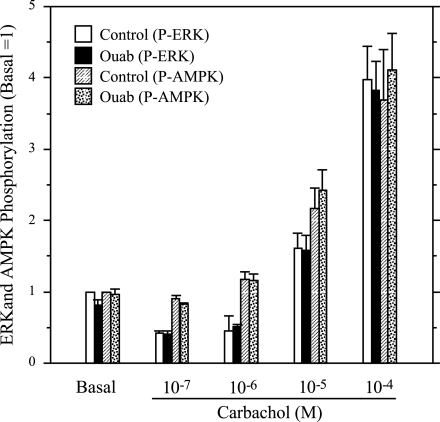
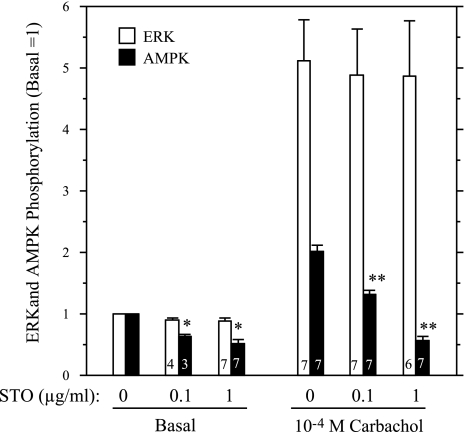
To examine the involvement of AMPK in ERK1/2 activation in a more direct manner, we treated parotid cells with AICAR, a cell-permeable compound that is converted to an AMP mimetic that is used to activate AMPK (42), and we tested its effect on the stimulation of ERK1/2 by various receptor ligands in rat parotid acinar cells. First, we confirmed that AICAR affected AMPK in parotid acinar cells. When cells were exposed to AICAR for 5–15 min (Fig. 3), there was an increase in AMPK phosphorylation, indicating the activation of this kinase. Consistent with this, the phosphorylation of acetyl CoA carboxylase (ACC), a downstream substrate of AMPK, also was increased in AICAR-treated cells. To test the effect of AMPK on ERK1/2 activation, we exposed parotid cells to various stimuli with or without pretreatment with AICAR (Fig. 4). The treatment of cells with AICAR significantly reduced the phosphorylation of ERK1/2 promoted by carbachol and BzATP, which activates P2X7 receptors in parotid acinar cells. AICAR also reduced the phosphorylation of ERK1/2 promoted by the phorbol ester PMA, but it had no effect on the phosphorylation promoted by the growth factor EGF. To examine the involvement of AMPK in the enhancement of ERK1/2 phosphorylation by ouabain, cells were pretreated with vehicle, ouabain alone, or AICAR followed by ouabain; and subsequently they were treated with vehicle or carbachol (Fig. 5). The ouabain-dependent increases in ERK1/2 phosphorylation in cells exposed to 10−6 M carbachol were not observed in cells that were pretreated with AICAR. AMPK phosphorylation was reduced in cells exposed to ouabain alone or exposed to carbachol subsequent to ouabain, as observed in previous studies (Fig. 2). In contrast, AMPK phosphorylation was elevated above basal levels when ouabain (+/− carbachol) was added subsequent to AICAR. These results along with those in Fig. 4 suggest that the activation of AMPK by AICAR blocked ERK1/2 activation, and conversely, that the reduction in AMPK activation by ouabain (in the absence of AICAR treatment) contributed to the enhanced phosphorylation of ERK1/2 by 10−6 M carbachol in the presence of ouabain.
Fig. 3.
5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) increases the phosphorylation of AMPK and acetyl CoA carboxylase (ACC) in parotid acinar cells. A: representative Western blot analysis of parotid acinar cells exposed to 2 mM AICAR for 5–15 min. In one panel the blot was exposed to P-AMPK antibody (long) for an extended period of time. AICAR did not have a consistent affect on AMPK levels. B: quantification of AMPK phosphorylation in cells treated with 2 mM AICAR as indicated. For each condition, the phosphorylation of AMPK was quantified and normalized to total ERK2, and values were calculated relative to basal (no AICAR). n = 3 experiments. P ≤ 0.05.
Fig. 4.
Effects of AICAR on ERK1/2 phosphorylation in parotid cells exposed to different stimuli. Cells were pretreated with AICAR (2 mM) or vehicle for 10–15 min and then exposed to Carb (10−5 M), 2′,3′-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate (BzATP, 10 μM), phorbol 12-myristate 13 acetate (PMA, 100 nM), and epidermal growth factor (EGF, 100 μg/ml) for 2 min. A: representative Western blot analysis of different samples from one experiment. Blot was probed with P-ERK1/2 and ERK2 antibodies. B: quantification of ERK1/2 phosphorylation. For each condition, the phosphorylation of ERK1/2 was quantified and normalized to total ERK2, and values are shown relative to basal (no AICAR). AICAR had an inhibitory effect on all stimuli except EGF. Number of individual experiments is indicated at base of bars. *P < 0.01; #P < 0.03.
Fig. 5.
AICAR blocks the action of ouabain to increase ERK1/2 phosphorylation by 10−6 M Carb. Cells were pretreated with AICAR (2 mM, 10–15 min) before exposure to 10−6 M Carb for 2 min. Cells were treated with ouabain (1 mM) for 1 min before Carb where indicated; cells were exposed to ouabain for 3 min if Carb was not added. A: blot was probed with P-ERK1/2, ERK2, and P-AMPK antibodies. Representative Western blot analysis of samples collected from one experiment. Vertical line indicates location on immunoblot in which nonrelevant samples were excised. B: quantification of ERK and AMPK phosphorylation from multiple experiments. Number of individual experiments shown at bottom of bars. *P ≤ 0.05 vs. basal (no additions). **P < 0.01 vs. basal (no additions). #P < 0.01 vs. Carb + Ouab.
Ouabain does not affect ERK1/2 phosphorylation in an immortalized rat parotid cell line.
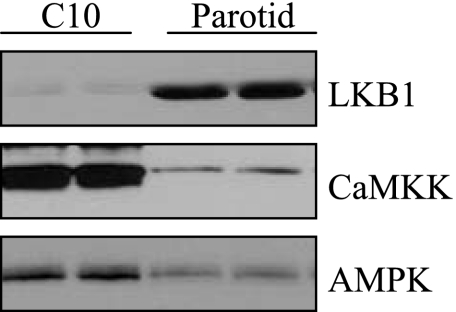
We also examined the effects of ouabain on ERK1/2 activation by carbachol in Par-C10 cells, a simian virus 40-transformed cell line of rat parotid acinar cells that has many signaling and fluid secretion characteristics of native rat parotid cells (39). Par-C10 cells were treated with ouabain (1 mM), followed by various concentrations (10−7–10−4 M) of carbachol. However, unlike in native cells, ouabain did not enhance the carbachol-promoted phosphorylation of ERK1/2, and ouabain also did not block the phosphorylation of AMPK promoted by carbachol in Par-C10 cells (Fig. 6). One reason for this could be the relatively lower amount of Na-K-ATPase in Par-C10 cells compared with that in native cells (Fig. 1); but, since AICAR did not have any effect on AMPK phosphorylation in C10 cells (not shown), we also examined whether this might involve differences immediately upstream of AMPK. Two different proteins, LKB1 and CaMKK, can act as AMPK kinases (14), and the lack of effect of AICAR on AMPK phosphorylation is found in cells lacking LKB1 (30). In fact, we found that Par-C10 cells have much less LKB1 than native cells (Fig. 7), and Par-C10 cells have much more CaMKK than native parotid acinar cells. We investigated a functional role for CaMKK by treating Par-C10 cells with STO-609, a CaMKK inhibitor. AMPK phosphorylation initiated by 10−4 M carbachol was blocked by STO-609 in a concentration-dependent manner (Fig. 8). Moreover, STO-609 did not inhibit ERK1/2 phosphorylation, consistent with a lack of effect of AMPK on ERK1/2 in Par-C10 cells. These results suggest that CaMKK rather than LKB1 is the upstream kinase of AMPK in Par-C10 cells.
Fig. 6.
Ouabain does not block the stimulation of AMPK phosphorylation or enhance ERK1/2 phosphorylation in Carb-treated Par-C10 cells. Cells were pretreated with 1 mM ouabain or vehicle (DMSO) for 2 min before exposure to 10−4−10−7 M concentrations of Carb (3 min). Samples were lysed and subjected to SDS-PAGE electrophoresis. For each condition, the phosphorylations of ERK1/2 and AMPK were quantified and normalized to total ERK2 and are shown relative to basal (no Ouab). N = 4 for all values, except n = 3 for 10−4 M Carb. At all concentrations of Carb, the comparison between control and ouabain-treated cells were not statistically different.
Fig. 7.
Relative levels of CaMKK and LKB1 in Par-C10 and native parotid acinar cells. Lysates (50 μg/lane) of each cell type were subjected to Western blot analysis and probed with CaMKK and LKB1 antibodies. Native cells have much more LKB1 protein than Par-C10 cells, and Par-C10 cells have more CaMKK protein than native cells.
Fig. 8.
STO-609 blocks the stimulation AMPK phosphorylation but not ERK1/2 phosphorylation in Par-C10 cells. Cells were pretreated with various concentrations of the CaMKK inhibitor STO-609 for 60 min and then exposed to 10−4 M Carb (3 min). Samples were lysed and subjected to SDS-PAGE electrophoresis. For each condition, the phosphorylations of ERK1/2 and AMPK were quantified and normalized to total ERK2 levels and are shown relative to control (no STO-609). Number of individual experiments is indicated at base of bars. *P < 0.01 vs. basal (no STO-609); **P < 0.01 vs. carbachol (no STO-609).
Ouabain blocks carbachol-initiated decreases in ATP; AICAR does not affect ATP.
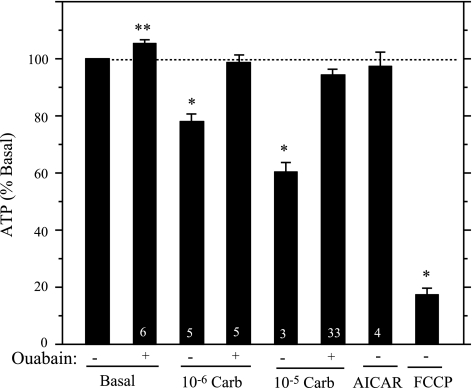
We previously found that 10−5 M carbachol reduced the ATP level of rat parotid acinar cells (32), consistent with this serving as an event upstream of AMPK activation, but we had not determined whether this effect was blocked by ouabain. Therefore, we examined this as well as whether 10−6 M carbachol reduced ATP levels in an ouabain-sensitive manner. The treatment of cells with 10−6 M and 10−5 M carbachol (each for 2 min) reduced the ATP level by 22.1 ± 2.8% (5) and 39.8 ± 3.4% (3), respectively (Fig. 9). Moreover, these reductions were largely prevented in cells pretreated with 1 mM ouabain for 1 min, conditions identical to those used for the studies of the effects of ouabain on ERK1/2 (Fig. 2). The cellular ATP content exhibited a small but significant increase in cells treated with ouabain (1 mM, 3 min) alone, consistent with inhibition of an energy demand to support the basal activity of the Na-K-ATPase. In comparison, the mitochondrial uncoupler carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP, 10 μM, 2 min) reduced ATP levels by 82.7 ± 2.3% (3). These results suggest that the carbachol-dependent alterations of the AMP-to-ATP ratio contribute to the activation of AMPK in rat parotid acinar cells. The data also suggest that by preventing these changes, ouabain prevents the activation of AMPK, thereby preventing its inhibitory effects on ERK1/2 activation by 10−6 M carbachol.
Fig. 9.
Ouabain blocks the Carb-promoted decrease of the cellular ATP content of rat parotid acinar cells. Cells were pretreated with DMSO or ouabain (1 mM) for 1 min and then treated with vehicle (basal) or 10−6 and 10−5 M carbachol for 2 min. Other cells were treated with AICAR (2 mM, 15 min) and FCCP (10 μM, 2 min). In each experiment, the ATP contents were calculated relative to the basal (no Ouab) content (4.5 ± 0.3 nmol ATP/mg protein, n = 6). Number of individual experiments is indicated at base of bars. *P < 0.01 vs. basal (no ouabain); **P ≤ 0.02 vs. basal (no Ouab).
We also examined whether carbachol produced changes in ATP in immortalized Par-C10 cells. We did not see any changes in ATP when cells were treated with 10−3 M carbachol for 2 and 5 min [99.8 ± 4.6% (4) and 100.9 ± 2.3% (6) basal levels, respectively]. The mitochondrial uncoupler FCCP (10 μM, 15 min) produced a 12.9 ± 3.1% (3) decrease in ATP. The lack of effect of carbachol on the ATP content of Par-C10 cells could be due to a number of reasons (see DISCUSSION).
Recently, it was suggested that AICAR itself can have negative effects on mitochondrial oxidative phosphorylation and can activate AMPK in some cells by reducing ATP and changing adenine nucleotide levels (9). To investigate this, we exposed rat parotid acinar cells to AICAR (2 mM, 15 min) and examined the ATP levels. Under these conditions AICAR did not produce any significant change (Fig. 9), suggesting that it did not activate AMPK indirectly by decreasing ATP levels.
DISCUSSION
The present study was based on previous experiments indicating that inhibition of the Na-K-ATPase by ouabain potentiated the activation of ERK1/2 in parotid cells exposed to low concentrations of carbachol. In new studies we observed the following: 1) ouabain enhanced the actions of carbachol on the phosphorylation of MEK and C-RAF, kinases that are immediately upstream of ERK1/2; 2) carbachol promotes an increase in AMPK phosphorylation and this is reduced in cells exposed to ouabain; 3) ouabain blocks the carbachol-promoted decreases in cellular ATP levels; 4) the activation of AMPK by AICAR reduces the phosphorylation of ERK1/2 in response to carbachol and BzATP, ligands to parotid M3 and P2X7 receptors, respectively, that increase the Na-K-ATPase turnover; and 5) ouabain did not enhance the phosphorylation of ERK1/2 by carbachol in cells in which AMPK was preactivated by AICAR. These data suggest that AMPK is activated by the enhanced energy utilization (ATP consumption) by the Na-K-ATPase in carbachol-treated cells, and that under some conditions this produces an ouabain-sensitive negative effect on ERK1/2 activation.
In previous studies (29) we investigated whether ouabain itself promoted a substantial increase in ERK1/2 signaling in freshly dispersed parotid acinar cells in the manner in which it does in many cultured epithelial and nonepithelial cells. Although ouabain produced a modest increase in ERK1/2 phosphorylation in parotid cells within several minutes, there was generally less than a 50% increase in the basal state of ERK1/2 phosphorylation (as in Fig. 2B), and this required large ouabain concentrations. In contrast, low ouabain concentrations (≤10 μM) are often sufficient to activate ERK1/2 in many cell lines or primary cells in culture. This response to ouabain involves the Na-K-ATPase α-subunits serving as signaling receptors/signalplexes that are localized in caveolae, where they associate with many signaling proteins (23, 28, 37). Recently, it was shown that there are two pools of Na-K-ATPase proteins in the plasma membrane of LLC-PK1 (pig kidney) cells. One pool is the ion pumping and energy-transducing enzyme, and the other is the signaling protein (19).
The Na-K-ATPase is made up of a heterodimer of an α-subunit, which binds ouabain, and a β-subunit, and there are multiple isoforms of each subunit (2). In some systems, a γ-subunit can act in a regulatory manner (36). Many cells in which low concentrations of ouabain promote signal transduction events have an α-subtype with a high sensitivity (low micromolar or less) to ouabain for ion transport inhibition as well as cell signaling. Differences in ouabain sensitivity can be due to different α-isoforms as well as to differences among species. The different sensitivities of pig (LLC-PK1) and rat (A75R) cells responding to the activation of ERK and Src by ouabain were also observed for its effects on Na-K-ATPase inhibition: pig cells were ∼100 times more sensitive for both signaling and Na-K-ATPase inhibition (10), However, the Ki (∼100 μM) of a rat α1/β1 dimer is 100-fold less sensitive to ouabain than rat α2- and α3-subunits paired in different combinations with β1 and β2 (2). Nonetheless, even in cultured rat cells, very low (10–100 nM) concentrations of ouabain were sufficient to promote Ca2+ oscillations (1) and to activate ERK and promote cell growth (6) at concentrations that did not produce significant increases in intracellular Na due to Na-K-ATPase inhibition. In contrast, in primary cultures of rat myocytes, ERK activation and PKC translocation was promoted by a concentration of ouabain (100 μM) that blocked about 50% of the ion transporting activity of the Na-K-ATPase (24). The rat α specificity of ouabain signaling to ERK was examined by expressing rat β1 and different rat α-isoforms in insect sf9 cells (27). The α1/β1-isozyme was 100–1,000-fold less sensitive than α3/β1- and α4/β1-, and α2/β1-isozymes did not respond to ouabain,. Rat parotid gland expresses primarily the ouabain-insensitive α1-isoform (Fig. 1). Recently, very low mRNA levels of the α2-isoform also were detected in rat parotid glands using quantitative PCR (18).
These studies were designed to investigate how the Na-K-ATPase inhibitor ouabain acted in concert with carbachol to enhance the activation of ERK1/2. Unlike most studies of ouabain on signaling events, the potentiation by ouabain of ERK1/2 phosphorylation in cells treated with 10−6 M carbachol was dependent on its inhibition of the Na-K-ATPase enzyme activity, since the effects of ouabain on ERK signaling and Na-K-ATPase inhibition both displayed similar concentration dependencies (29). Our new findings suggested that this involves AMPK, an energy sensor that responds to agents that alter the AMP-to-ATP ratio (12, 38) and which can negatively affect ERK1/2 activation in some cells. In fact, the inhibitory effect of AICAR on the M3R-promoted increases in ERK1/2 phosphorylation (Fig. 4) suggested that activated AMPK can block ERK1/2 activation downstream of this receptor in parotid cells. Moreover, since ouabain blocked the decrease in ATP (Fig. 9) and the increase in AMPK phosphorylation (Fig. 2) promoted by carbachol, this suggested that AMPK was activated by the alteration of ATP and other nucleotides subsequent to the increases of the Na-K-ATPase turnover that accompany increases in fluid secretion/saliva formation. Thus the data suggest that by preventing AMPK activation by 10−6 M carbachol, ouabain blocked an inhibitory effect of AMPK on ERK1/2. These findings suggest that that AMPK can have a central role in the regulation of ERK1/2 activation under physiological conditions that are related to the relatively high energy demands necessary to support ion movement, which is accompanied by large increases in oxidative phosphorylation in salivary and other epithelial cells (21, 25, 34). We have interpreted our data in terms of the tight coupling between the Na-K-ATPase and mitochondria for the replenishment of ATP utilized to support ion movement in many types of epithelial tissues (21). However, in rat submandibular salivary glands, the creatine phosphokinase system also may be involved in the replenishment of ATP from phosphocreatine during rapid energy utilization when fluid secretion is stimulated (26). Under such conditions, creatine phosphate could indirectly modulate AMPK activity, as has been suggested upon skeletal muscle contraction (35).
The effectiveness of AICAR in negatively regulating ERK1/2 activation in parotid acinar cells downstream of both the P2X7 ion channel/receptor and the G protein-coupled muscarinic receptor (Fig. 4) is consistent with our observations that these two diverse receptor types activate similar signaling molecules and cascades including those leading to ERK1/2 (3). Related to this, the inhibitory effect of AICAR on ERK1/2 phosphorylation promoted by PMA, which activates PKC family members in parotid and other cells, also is consistent with previous findings that PKC contributes to ERK1/2 activation by both M3 and P2X7 receptors (3). Carbachol and BzATP also both increase the Na-K-ATPase turnover in parotid cells (33). Previously, we (32) observed that there was an increase in AMPK phosphorylation in parotid cells exposed to carbachol and BzATP and to mitochondrial uncouplers. Ouabain blocked the phosphorylation promoted by the receptor ligands but not by the uncouplers, since the reductions in ATP promoted by the latter were not due to activation of the Na-K-ATPase. Of interest, the increases in cellular ATP produced by ouabain in secretory epithelia, such as parotid cells (Fig. 9), as well as in absorptive epithelia (40) attest to the common nature of the large demand that the Na-K-ATPase makes on ATP.
The involvement of AMPK in ERK1/2 activation was selective for the particular stimulating agent, since AICAR did not block EGF-promoted ERK1/2 phosphorylation. Other investigators also reported that AICAR was ineffective in blocking ERK1/2 activation by EGF, although it did block the stimulation by IGF-1 at the level of Ras (16). Activation of AMPK by AICAR also blocked ERK activation by serum in cultured rat cardiac fibroblast (7). In contrast, AICAR increased ERK activity in rat muscle (4) and promoted the phosphorylation of ERK1/2 in an AMPK-dependent manner in HepG2 cells (41), a phenomenon that we did not observe in parotid acinar cells (Fig. 4). In liver cells AICAR lowered ATP levels by blocking oxidative phosphorylation in an AMPK-independent manner (9). AICAR did not have this effect on parotid cells, since it did not lower ATP levels (Fig. 9). Potentially, such an action would have a negative effect on the Na-K-ATPase in an indirect manner (i.e., ATP limitation). However, AMPK also could have a more direct role in modifying salivary secretion under various conditions, including metabolic stress, hypoxia, and cellular work. Various ion transport systems, including the cystic fibrosis transmembrane conductance regulator (CFTR), epithelial Na channel (ENaC), and others, can be downregulated via AMPK when energy levels decline in cells (11).
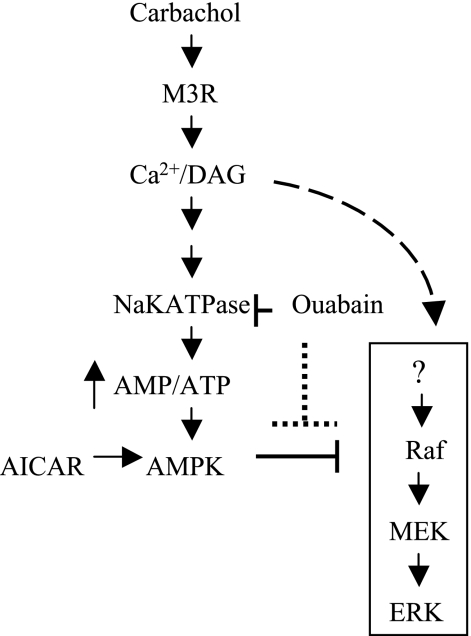
A schematic model of the effect of ouabain on signaling in rat parotid acinar cells stimulated with carbachol is shown in Fig. 10. Carbachol stimulates G protein-coupled M3 muscarinic receptors linked to phospholipase C, generating diacylglycerol and second messengers that increase intracellular Ca2+ concentration ([Ca2+]i). The rise in [Ca2+]i opens ion channels and stimulates other ion transporters that increase intracellular Na and reduce intracellular K, thereby activating the Na-K-ATPase and leading to an increase in ATP hydrolysis and the activation of AMPK. The production of second messengers also leads to the activation of ERK1/2 in a cascade that involves MEK and C-RAF activation. When cells are exposed to low (<10−5 M) concentrations of carbachol, the stimulation of AMPK dampens the activation of ERK1/2, probably acting at a level of C-RAF or above. This inhibitory effect can be blocked by ouabain, which blocks AMPK activation by blocking the increased consumption of ATP by the Na-K-ATPase.
Fig. 10.
Schematic model of the effect of ouabain on signaling in rat parotid acinar cells treated with carbachol. Carbachol stimulates the G protein-coupled M3 muscarinic receptor, generating second messengers that initiate the ERK1/2 activation cascade (right). Carbachol also promotes ionic alterations that increase the Na-K-ATPase activity, which increases ATP hydrolysis and the activation of AMPK, which can have inhibitory effects on ERK1/2 activation under some conditions. Ouabain inhibits ATP hydrolysis by the Na-K-ATPase, thereby indirectly blocking AMPK activation. AMPK can also be activated by AICAR, which also blocks ERK1/2 activation. See text for more details.
One finding to be noted is that both 10−6 and 10−5 M carbachol increase the phosphorylation of AMPK in an ouabain-sensitive manner (Fig. 2), yet ouabain usually enhances only the phosphorylation of ERK1/2 by 10−6 M carbachol (29). Thus the Na-K-ATPase-linked AMPK activation does not appear to negatively effect the activation of ERK1/2 by 10−5 M carbachol. This suggests that a stronger positive input into the ERK1/2 activation cascade by high concentrations of carbachol, presumably downstream of the receptor and at the level of C-RAF or above, can overwhelm a negative effect of Na-K-ATPase-initiated AMPK activation. This was not found to be the case when AMPK was activated by AICAR rather than by carbachol, since AICAR blocked the phosphorylation of ERK1/2 promoted by 10−5 M carbachol. One explanation may be that AICAR can activate AMPK to a greater degree than 10−5 M carbachol, and that this is sufficient to block ERK1/2 under these conditions, although we did not find substantial differences in AMPK phosphorylation by these agents (e.g., compare Figs. 2 and 3). A more likely explanation may have to do with the temporal nature of the modification of signaling proteins downstream of the receptor. AICAR was added 10–15 min before the addition of carbachol and had sufficient time to activate AMPK before the addition of receptor ligand (Fig. 4). In contrast, the addition of carbachol to cells would be expected to produce overlapping temporal effects on the stimulatory and inhibitory arms of the cascades, conditions that appear to be weighted toward the stimulatory effect for 10−5 M carbachol.
We employed Par-C10 cells in an attempt to duplicate the ouabain studies in a cell culture system that could be manipulated and evaluated by molecular approaches. However, relevant components of the signaling effects observed in native parotid cells were not obtained in these cells: ouabain did not enhance the effects of low concentrations of carbachol on ERK1/2 phosphorylation, carbachol did not alter the ATP content, and ouabain did not block increases in AMPK phosphorylation promoted by carbachol. Differences in the cellular responses in native and PAR-C10 cells were due to various factors. Perhaps the foremost difference is that of cell metabolism, which is oxidative in native cells and likely glycolytic in Par-C10 cells, consistent with the relative differences in the actions of mitochondrial uncouplers on ATP in the two parotid cell types. Also, the density of the Na-K-ATPase α1-protein is much less in Par-C10 cells compared with native parotid acinar cells (Fig. 1). Finally, AMPK activation in Par-C10 cells appears to be due to the activation of CaMKK (Fig. 8) rather than LKB1, which is found in much lower levels in Par-C10 cells than in native cells (Fig. 7). These findings demonstrate that there are important differences between native and immortalized parotid cells regarding the regulation of key signaling molecules.
Although the rationale for our studies was to an attempt to understand how ouabain enhanced the effects of carbachol on ERK1/2 phosphorylation, our results also suggest a novel way that intrinsic metabolic aspects of cell functions can regulate signaling downstream of receptor activation in some cells. This new paradigm potentially has a more widespread application and greater significance to signaling biology than simply offering an explanation of our previous findings. Although numerous studies have documented how metabolic events can affect signaling by insulin and other growth factors and hormones, far fewer studies have described cell signaling interactions involving a cast of receptors, oxidative metabolism, and ion transport proteins. The data presented here offer new details to explain functional signaling links between the Na-K-ATPase and ERK1/2 that involve metabolic changes that are initiated when fluid secretion is initiated or modified. As demonstrated here for ERK1/2, the energy utilization by the Na-K-ATPase can impose a unique regulation of cell signaling and this doubtlessly has biological ramifications.
GRANTS
This work was supported by the National Institute of Dental and Craniofacial Research Grant DE-10877.
Acknowledgments
We thank Bin Zheng and Atsuo Sasaki (Beth Israel Deaconess Medical Center) for helpful comments and discussions. We also thank David Quissell (University of Colorado Health Sciences Center, Boulder) and Jean Camden (University of Missouri, Columbia) for providing Par-C10 cells. We thank Thomas A. Pressley (Texas Tech University Health Sciences Center) for generously providing antibodies to the Na-K-ATPase α-subunits.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aizman O, Uhlen P, Lal M, Brismar H, Aperia A. Ouabain, a steroid hormone that signals with slow calcium oscillations. Proc Natl Acad Sci USA 98: 13420–13424, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol Renal Physiol 275: F633–F650, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Bradford MD, Soltoff SP. P2X7 receptors activate protein kinase D and p44/p42 mitogen-activated protein kinase (MAPK) downstream of protein kinase C. Biochem J 366: 745–755, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen HC, Bandyopadhyay G, Sajan MP, Kanoh Y, Standaert M, Farese RV Jr, and Farese RV. Activation of the ERK pathway and atypical protein kinase C isoforms in exercise- and aminoimidazole-4-carboxamide-1-beta-d-riboside (AICAR)-stimulated glucose transport. J Biol Chem 277: 23554–23562, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Chong H, Lee J, Guan KL. Positive and negative regulation of Raf kinase activity and function by phosphorylation. EMBO J 20: 3716–3727, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dmitrieva RI, Doris PA. Ouabain is a potent promoter of growth and activator of ERK1/2 in ouabain-resistant rat renal epithelial cells. J Biol Chem 278: 28160–28166, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Du J, Guan T, Zhang H, Xia Y, Liu F, Zhang Y. Inhibitory crosstalk between ERK and AMPK in the growth and proliferation of cardiac fibroblasts. Biochem Biophys Res Commun 368: 402–407, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Garnett MJ, Rana S, Paterson H, Barford D, Marais R. Wild-type and mutant B-RAF activate C-RAF through distinct mechanisms involving heterodimerization. Mol Cell 20: 963–969, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Guigas B, Taleux N, Foretz M, Detaille D, Andreelli F, Viollet B, Hue L. AMP-activated protein kinase-independent inhibition of hepatic mitochondrial oxidative phosphorylation by AICA riboside. Biochem J 404: 499–507, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haas M, Wang H, Tian J, Xie Z. Src-mediated inter-receptor cross-talk between the Na+/K+-ATPase and the epidermal growth factor receptor relays the signal from ouabain to mitogen-activated protein kinase. J Biol Chem 277: 18694–18702, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Hallows KR Emerging role of AMP-activated protein kinase in coupling membrane transport to cellular metabolism. Curr Opin Nephrol Hypertens 14: 464–471, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Hardie DG The AMP-activated protein kinase pathway–new players upstream and downstream. J Cell Sci 117: 5479–5487, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Hekman M, Wiese S, Metz R, Albert S, Troppmair J, Nickel J, Sendtner M, Rapp UR. Dynamic changes in C-Raf phosphorylation and 14-3-3 protein binding in response to growth factor stimulation: differential roles of 14-3-3 protein binding sites. J Biol Chem 279: 14074–14086, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Hurley RL, Anderson KA, Franzzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/Calmodulin-dependent protein kinases are AMP-activated protein kinase kinases. J Biol Chem 280: 29060–29066, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Khundmiri SJ, Amin V, Henson J, Lewis J, Ameen M, Rane MJ, Delamere NA. Ouabain stimulates protein kinase B (Akt) phosphorylation in opossum kidney proximal tubule cells through an ERK-dependent pathway. Am J Physiol Cell Physiol 293: C1171–C1180, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J, Yoon MY, Choi SL, Kang I, Kim SS, Kim YS, Choi YK, Ha J. Effects of stimulation of AMP-activated protein kinase on insulin-like growth factor 1- and epidermal growth factor-dependent extracellular signal-related kinase pathway. J Biol Chem 276: 19102–19110, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Kim JE, Ahn MW, Baek SH, Lee IK, Kim YW, Kim JY, Dan JM, Park SY. AMPK activator, AICAR, inhibits palmitate-induced apoptosis in osteoblast. Bone 43: 394–404, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Kurihara K, Nakanishi N, Amano O, Tonosaki K. Expression of Na(+)/K(+)-ATPase alpha subunit isoforms in rat salivary glands: occurrence of sense and antisense RNAs of the alpha3 isoform in the sublingual gland. Arch Oral Biol 53: 593–604, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Liang M, Tian J, Liu L, Pierre S, Liu J, Shapiro J, Xie ZJ. Identification of a pool of non-pumping Na/K-ATPase. J Biol Chem 282: 10585–10593, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Luo Z, Saha AK, Xiang X, Ruderman NB. AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci 26: 69–76, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Mandel LJ, Balaban RS. Stoichiometry and coupling of active transport to oxidative metabolism in epithelial tissues. Am J Physiol Renal Fluid Electrolyte Physiol 240: F357–F371, 1981. [DOI] [PubMed] [Google Scholar]
- 22.Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol 67: 445–469, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Miyakawa-Naito A, Uhlen P, Lal M, Aizman O, Mikoshiba K, Brismar H, Zelenin S, Aperia A. Cell signaling microdomain with Na,K-ATPase and inositol 1,4,5-triphosphate receptor generates calcium oscillations. J Biol Chem 278: 50355–50361, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Mohammadi K, Kometiani P, Xie Z, Askari A. Role of Protein Kinase C in the signal pathways that link Na+/K+-ATPase to ERK1/2. J Biol Chem 276: 42050–42056, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Murakami M, Miyamoto S, Imai Y. Oxygen consumption for K+ uptake during post-stimulatory activation of Na+,K+-ATPase in perfused rat mandibular gland. J Physiol 426: 127–143, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murakami M, Seo Y, Watari H. Dissociation of fluid secretion and energy supply in rat mandibular gland by high dose of ACh. Am J Physiol Gastrointest Liver Physiol 254: G781–G787, 1988. [DOI] [PubMed] [Google Scholar]
- 27.Pierre SV, Sottejeau Y, Gourbeau JM, Sanchez G, Shidyak A, Blanco G. Isoform specificity of Na-K-ATPase-mediated ouabain signaling. Am J Physiol Renal Physiol 294: F859–F866, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Pierre SV, Xie Z. The Na,K-ATPase receptor complex: its organization and membership. Cell Biochem Biophys 46: 303–316, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Plourde D, Soltoff SP. Ouabain potentiates the activation of ERK1/2 by carbachol in parotid gland epithelial cells; inhibition of ERK1/2 reduces Na+-K+-ATPase activity. Am J Physiol Cell Physiol 290: C702–C710, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Shaw RJ, Kosmata M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor supressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA 101: 3329–3335, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soltoff SP Coupling renal metabolism to ion transport in renal epithelia. Sem Nephrol 7: 20–28, 1987. [PubMed] [Google Scholar]
- 32.Soltoff SP Evidence that tyrphostins AG10 and AG18 are mitochondrial uncouplers that alter phosphorylation-dependent cell signaling. J Biol Chem 279: 10910–10918, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Soltoff SP, McMillian MK, Cragoe EJ Jr, Cantley LC, Talamo BR. Effects of extracellular ATP on ion transport systems and [Ca2+]i in rat parotid acinar cells. Comparison with the muscarinic agonist carbachol. J Gen Physiol 95: 319–346, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soltoff SP, McMillian MK, Cantley LC, Cragoe EJ Jr, Talamo BR. Effects of muscarinic, alpha-adrenergic, and substance P agonists and ionomycin on ion transport mechanisms in the rat parotid acinar cell. The dependence of ion transport on intracellular calcium. J Gen Physiol 93: 285–319, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor EB, Ellingson WJ, Lamb JD, Chesser DG, Compton CL, Winder WW. Evidence against regulation of AMP-activated protein kinase and LKB1/STRAD/MO25 activity by creatine phosphate. Am J Physiol Endocrinol Metab 290: E661–E669, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Therien AG, Blostein R. Mechanisms of sodium pump regulation. Am J Physiol Cell Physiol 279: C541–C566, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Tian J, Cai T, Yuan Z, Wang H, Liu L, Haas M, Maksimova E, Huang XY, Xie ZJ. Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol Biol Cell 17: 317–326, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res 100: 328–341, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Turner JT, Redman RS, Camden JM, Landon LA, Quissell DO. A parotid gland cell line, Par-C10, exhibits neurotransmitter-regulated transepithelial anion secretion. Am J Physiol Cell Physiol 275: C367–C374, 1998. [DOI] [PubMed] [Google Scholar]
- 40.Urbach V, Van Kerkhove E, Maguire D, Harvey BJ. Cross-talk between ATP-regulated K+ channels and Na+ transport via cellular metabolism in frog skin principal cells. J Physiol 491: 99–109, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang HM, Mehta S, Bansode R, Huang W, Mehta KD. AICAR positively regulate glycogen synthase activity and LDL receptor expression through Raf-1/MEK/p42/44(MAPK)/p90(RSK)/GSK-3 signaling cascade. Biochem Pharmacol 75: 457–467, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woollhead AM, Scott JW, Hardie DG, Baines DL. Phenformin and 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) activation of AMP-activated protein kinase inhibits transepithelial Na+ transport across H441 lung cells. J Physiol 566: 781–792, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan Z, Cai T, Tian J, Ivanov AV, Giovannucci DR, Xie Z. Na/K-ATPase tethers phospholipase C and IP3 receptor into a calcium-regulatory complex. Mol Biol Cell 16: 4034–4045, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]