Abstract
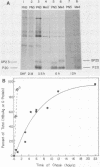
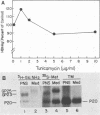
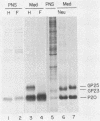
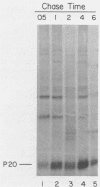
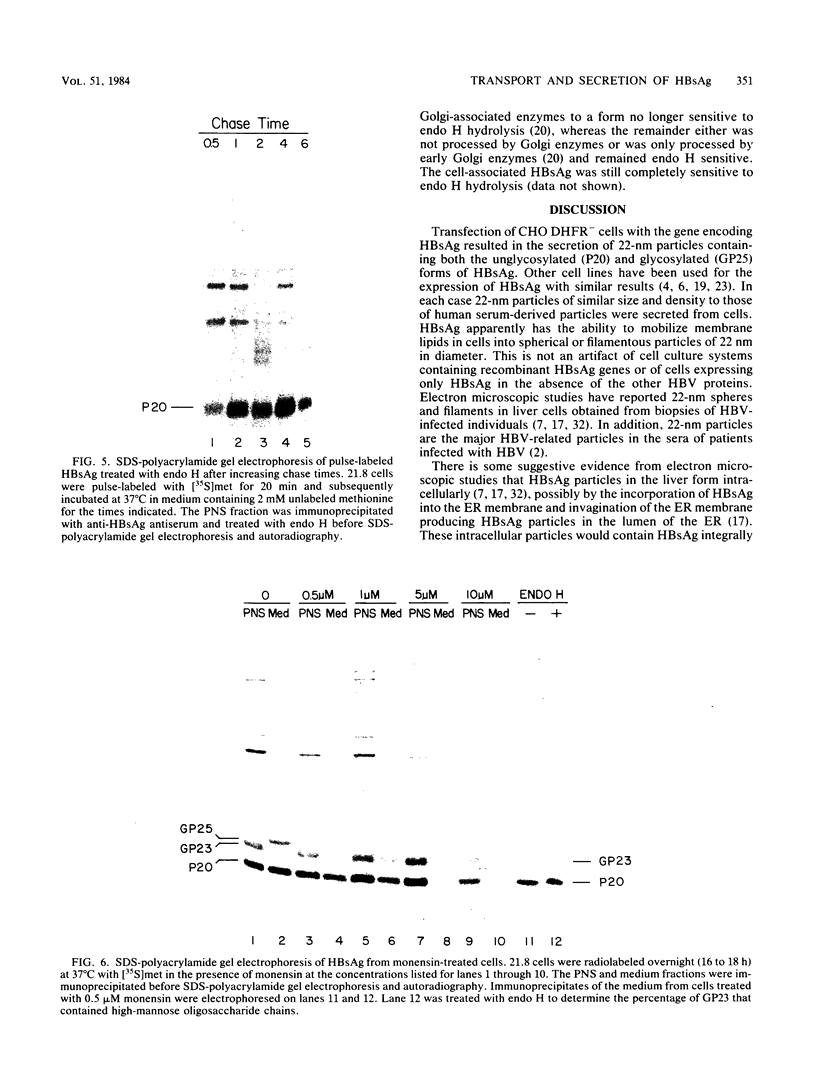
The oligosaccharide processing and secretion of hepatitis B surface antigen (HBsAg) was studied in Chinese hamster ovary cells stably transfected with the gene coding HBsAg. HBsAg was secreted from cells with a relatively long half time (ca. 5 h). This appeared to be a characteristic of HBsAg itself, since HBsAg-producing cells infected with vesicular stomatitis virus transported the viral envelope glycoprotein to the cell surface with normal kinetics (half time of ca. 30 min). The secreted HBsAg was comprised of both the unglycosylated (P20) and the glycosylated (G25) polypeptides, characteristic of HBsAg isolated from human serum or secreted from other cell lines (C. W. Crowley, C.-C. Liu, and A. D. Levinson, Mol. Cell. Biol. 3:44-55, 1983; M. F. Dubois, C. Pourcel, S. Rousset, C. Chang, and P. Tiollais, Proc. Natl. Acad. Sci. U.S.A. 77:4549-4553, 1980; C.-C. Liu, D. Yansura, and A. D. Levinson, DNA, 1:213-221, 1982; G. M. Macnab, J. J. Alexander, G. Lecatsas, E. M. Bey, and J. M. Urbanocvicz, Br. J. Cancer, 24:509-515, 1976; A. M. Moriarity, B. H. Hoyer, J. W.-K. Shih, J. L. Gerin, and D. H. Hamer, Proc. Natl. Acad. Sci. U.S.A. 78:2606-2610, 1981; D. L. Peterson, J. Biol. Chem., 256:6975-6983, 1981). The glycosylated polypeptide (GP25) contained complex oligosaccharide chains. Cell-associated HBsAg also was comprised of both an unglycosylated and a glycosylated polypeptide; however, the glycosylated form (GP23) contained only high-mannose oligosaccharide chains. No oligosaccharide processing of the high-mannose chains could be detected within the cells. Thus, most of the time before secretion of HBsAg from cells must have been spent in a pre-Golgi or early Golgi compartment. Glycosylation was inhibited completely by tunicamycin, although unglycosylated particles were still secreted from cells and were antigenic. The secretion and oligosaccharide processing of HBsAg were inhibited with high concentrations of monensin, but at lower concentrations of monensin HBsAg was still secreted, although only half of the oligosaccharide chains were processed to the complex form.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberti A., Realdi G., Tremolada F., Spina G. P. Liver cell surface localization of hepatitis B antigen and of immunoglobulins in acute and chronic hepatitis and in liver cirrhosis. Clin Exp Immunol. 1976 Sep;25(3):396–402. [PMC free article] [PubMed] [Google Scholar]
- Almeida J. D. Electron microscopic observations and speculations on Australia antigen. Postgrad Med J. 1971 Jul;47(549):484–487. doi: 10.1136/pgmj.47.549.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braell W. A., Lodish H. F. Biosynthesis of the erythrocyte anion transport protein. J Biol Chem. 1981 Nov 10;256(21):11337–11344. [PubMed] [Google Scholar]
- Crowley C. W., Liu C. C., Levinson A. D. Plasmid-directed synthesis of hepatitis B surface antigen in monkey cells. Mol Cell Biol. 1983 Jan;3(1):44–55. doi: 10.1128/mcb.3.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreesman G. R., Hollinger F. B., Suriano J. R., Fujioka R. S., Brunschwig J. P., Melnick J. L. Biophysical and biochemical heterogeneity of purified hepatitis B antigen. J Virol. 1972 Sep;10(3):469–476. doi: 10.1128/jvi.10.3.469-476.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois M. F., Pourcel C., Rousset S., Chany C., Tiollais P. Excretion of hepatitis B surface antigen particles from mouse cells transformed with cloned viral DNA. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4549–4553. doi: 10.1073/pnas.77.8.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn A. E., Peters R. L., Schweitzer I. L. An ultrastructural study of liver explants from infants with vertically transmitted hepatitis. Exp Mol Pathol. 1973 Aug;19(1):113–126. doi: 10.1016/0014-4800(73)90045-2. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting T., Kabat D. Evidence for a glycoprotein "signal" involved in transport between subcellular organelles. Two membrane glycoproteins encoded by murine leukemia virus reach the cell surface at different rates. J Biol Chem. 1982 Dec 10;257(23):14011–14017. [PubMed] [Google Scholar]
- Furuta S., Kiyosawa K., Nagata A., Akahane Y., Oda M. Letter: HBsAg on cell membrane in symptom-free carrier. Lancet. 1975 Aug 2;2(7927):227–227. doi: 10.1016/s0140-6736(75)90693-5. [DOI] [PubMed] [Google Scholar]
- Gavilanes F., Gonzalez-Ros J. M., Peterson D. L. Structure of hepatitis B surface antigen. Characterization of the lipid components and their association with the viral proteins. J Biol Chem. 1982 Jul 10;257(13):7770–7777. [PubMed] [Google Scholar]
- Gerlich W. H., Feitelson M. A., Marion P. L., Robinson W. S. Structural relationships between the surface antigens of ground squirrel hepatitis virus and human hepatitis B virus. J Virol. 1980 Dec;36(3):787–795. doi: 10.1128/jvi.36.3.787-795.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Quinn P., Warren G. Dissection of the Golgi complex. I. Monensin inhibits the transport of viral membrane proteins from medial to trans Golgi cisternae in baby hamster kidney cells infected with Semliki Forest virus. J Cell Biol. 1983 Mar;96(3):835–850. doi: 10.1083/jcb.96.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B., Kelly R. B. Two distinct intracellular pathways transport secretory and membrane glycoproteins to the surface of pituitary tumor cells. Cell. 1982 Jan;28(1):51–59. doi: 10.1016/0092-8674(82)90374-9. [DOI] [PubMed] [Google Scholar]
- Heifetz A., Keenan R. W., Elbein A. D. Mechanism of action of tunicamycin on the UDP-GlcNAc:dolichyl-phosphate Glc-NAc-1-phosphate transferase. Biochemistry. 1979 May 29;18(11):2186–2192. doi: 10.1021/bi00578a008. [DOI] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Kamimura T., Yoshikawa A., Ichida F., Sasaki H. Electron microscopic studies of Dane particles in hepatocytes with special reference to intracellular development of Dane particles and their relation with HBeAg in serum. Hepatology. 1981 Sep-Oct;1(5):392–397. doi: 10.1002/hep.1840010504. [DOI] [PubMed] [Google Scholar]
- Knipe D. M., Lodish H. F., Baltimore D. Localization of two cellular forms of the vesicular stomatitis viral glycoprotein. J Virol. 1977 Mar;21(3):1121–1127. doi: 10.1128/jvi.21.3.1121-1127.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. C., Yansura D., Levinson A. D. Direct expression of hepatitis B surface antigen in monkey cells from an SV40 vector. DNA. 1982;1(3):213–221. doi: 10.1089/dna.1.1982.1.213. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Kong N., Snider M., Strous G. J. Hepatoma secretory proteins migrate from rough endoplasmic reticulum to Golgi at characteristic rates. Nature. 1983 Jul 7;304(5921):80–83. doi: 10.1038/304080a0. [DOI] [PubMed] [Google Scholar]
- MacNab G. M., Alexander J. J., Lecatsas G., Bey E. M., Urbanowicz J. M. Hepatitis B surface antigen produced by a human hepatoma cell line. Br J Cancer. 1976 Nov;34(5):509–515. doi: 10.1038/bjc.1976.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishiro S., Imai M., Takahashi K., Machida A., Gotanda T., Miyakawa Y., Mayumi M. A 49,000-dalton polypeptide bearing all antigenic determinants and full immunogenicity of 22-nm hepatitis B surface antigen particles. J Immunol. 1980 Apr;124(4):1589–1593. [PubMed] [Google Scholar]
- Moriarty A. M., Hoyer B. H., Shih J. W., Gerin J. L., Hamer D. H. Expression of the hepatitis B virus surface antigen gene in cell culture by using a simian virus 40 vector. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2606–2610. doi: 10.1073/pnas.78.4.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D. L. Isolation and characterization of the major protein and glycoprotein of hepatitis B surface antigen. J Biol Chem. 1981 Jul 10;256(13):6975–6983. [PubMed] [Google Scholar]
- Peterson D. L., Nath N., Gavilanes F. Structure of hepatitis B surface antigen. Correlation of subtype with amino acid sequence and location of the carbohydrate moiety. J Biol Chem. 1982 Sep 10;257(17):10414–10420. [PubMed] [Google Scholar]
- Rose J. K., Bergmann J. E. Expression from cloned cDNA of cell-surface secreted forms of the glycoprotein of vesicular stomatitis virus in eucaryotic cells. Cell. 1982 Oct;30(3):753–762. doi: 10.1016/0092-8674(82)90280-x. [DOI] [PubMed] [Google Scholar]
- Ruddon R. W., Hanson C. A., Addison N. J. Synthesis and processing of human chorionic gonadotropin subunits in cultured choriocarcinoma cells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5143–5147. doi: 10.1073/pnas.76.10.5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen C. C., Levinson A. D. Isolation and expression of an altered mouse dihydrofolate reductase cDNA. Proc Natl Acad Sci U S A. 1983 May;80(9):2495–2499. doi: 10.1073/pnas.80.9.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous G. J., Lodish H. F. Intracellular transport of secretory and membrane proteins in hepatoma cells infected by vesicular stomatitis virus. Cell. 1980 Dec;22(3):709–717. doi: 10.1016/0092-8674(80)90547-4. [DOI] [PubMed] [Google Scholar]
- Tartakoff A. M. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell. 1983 Apr;32(4):1026–1028. doi: 10.1016/0092-8674(83)90286-6. [DOI] [PubMed] [Google Scholar]
- Urlaub G., Chasin L. A. Isolation of Chinese hamster cell mutants deficient in dihydrofolate reductase activity. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4216–4220. doi: 10.1073/pnas.77.7.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada G., Feinberg L. E., Nakane P. K. Hepatitis B. Cytologic localization of virus antigens and the role of the immune response. Hum Pathol. 1978 Jan;9(1):93–109. doi: 10.1016/s0046-8177(78)80011-2. [DOI] [PubMed] [Google Scholar]