Abstract
A growing body of data supports a view of the actin cytoskeleton of smooth muscle cells as a dynamic structure that plays an integral role in regulating the development of mechanical tension and the material properties of smooth muscle tissues. The increase in the proportion of filamentous actin that occurs in response to the stimulation of smooth muscle cells and the essential role of stimulus-induced actin polymerization and cytoskeletal dynamics in the generation of mechanical tension has been convincingly documented in many smooth muscle tissues and cells using a wide variety of experimental approaches. Most of the evidence suggests that the functional role of actin polymerization during contraction is distinct and separately regulated from the actomyosin cross-bridge cycling process. The molecular basis for the regulation of actin polymerization and its physiological roles may vary in diverse types of smooth muscle cells and tissues. However, current evidence supports a model for smooth muscle contraction in which contractile stimulation initiates the assembly of cytoskeletal/extracellular matrix adhesion complex proteins at the membrane, and proteins within this complex orchestrate the polymerization and organization of a submembranous network of actin filaments. This cytoskeletal network may serve to strengthen the membrane for the transmission of force generated by the contractile apparatus to the extracellular matrix, and to enable the adaptation of smooth muscle cells to mechanical stresses. Better understanding of the physiological function of these dynamic cytoskeletal processes in smooth muscle may provide important insights into the physiological regulation of smooth muscle tissues.
Keywords: actin polymerization, adhesion junction, contractile activation, cytoskeletal signaling, smooth muscle tissue
the role of filamentous actin in the activation of myosin ATPase activity and cross-bridge cycling is well established, and actomyosin cross-bridge cycling is recognized as the fundamental mechanism for tension development and shortening in all forms of muscle, as well as in contractile nonmuscle cells. The activation of myosin by a contractile stimulus enables myosin filaments to crawl along actin filaments through the ATPase activity of the myosin head, thus resulting in shortening or tension generation by the cell. This well-established paradigm for smooth muscle contraction has relied on the assumption that the structure and organization of filamentous actin remains relatively constant during a contractile event, and that actin filaments anchored at adhesion sites at the plasma membrane and at dense bodies within the cytosol provide a fixed and stable network on which the myosin or thick filaments move during shortening and tension development.
Recent studies have documented a critical role for actin polymerization and cytoskeletal dynamics in the regulation of active tension development in smooth muscle. There is mounting evidence that smooth muscle contraction requires the polymerization of actin filaments and a range of other cytoskeletal processes that extend well beyond the actomyosin interaction and cross-bridge cycling. A complex set of cytoskeletal events is triggered concurrently with activation of the actomyosin system that appear to play a fundamental role in the mechanical response of the muscle tissue. This has prompted the formulation of new paradigms for smooth muscle contraction to encompass observations that the activation of the actomyosin system is not the only cellular mechanism involved in the regulation of smooth muscle contraction and tension development. These dynamic cytoskeletal processes may underlie the unique adaptive properties of many smooth muscle tissues that enable them to modulate their contractile and mechanical properties to accommodate to changes in their surrounding environment. Growing evidence suggests that the cytoskeletal processes that occur during the contractile activation of smooth muscle cells may have much in common with the cytoskeletal mechanisms that govern cell motility and migration, and that tension generation in smooth muscle requires a more complex array of physiological processes than previously supposed.
Actin Polymerization is Necessary for Contraction and Tension Development in Smooth Muscle Tissues
Actin is the most abundant protein in cells, and it exists in both a soluble and filamentous state. Filamentous actin is a polymeric structure made up of asymmetric bilobed 42-kDa actin monomers that are organized into a double-stranded helical array. Soluble cytosolic actin monomers are in constant exchange with actin monomers within the actin filaments. In smooth muscle cells, actin filaments are anchored to the membrane via a complex of adhesion proteins that associate with the cytoplasmic tails of integrin proteins, where the cytoskeleton links to the extracellular matrix (54, 142). Actin filaments also anchor within the cytosol of smooth muscle cells at cytosolic dense bodies that are composed primarily of the actin cross-linking protein, α-actinin (44, 45).
The critical role of actin polymerization in tension development has been documented in smooth muscle tissues and cells by numerous studies that have evaluated the effects of inhibiting the actin polymerization process on tension generation in response to contractile stimulation. The pharmacologic agents latrunculin and cytochalasin, which inhibit actin polymerization by sequestering G-actin monomers and by capping actin filaments, respectively (17, 32, 35), have been widely employed in a variety of smooth muscle tissue and cell types to evaluate the effects of inhibiting actin polymerization on contractile responses to agonist stimulation. Studies of airway smooth muscle (6, 43, 84, 115, 131, 140), vascular smooth muscle (1, 25, 27, 28, 92, 93, 104, 107, 112, 138), and uterine (112) and intestinal smooth muscle (81, 91) have all shown that the short-term exposure of smooth muscle tissues to inhibitors of actin polymerization causes a profound suppression of tension development and an inhibition of shortening or constriction. Additional evidence that actin polymerization plays a critical role in the process of mechanical tension development in smooth muscle comes from studies showing that molecular constructs or peptides that disrupt specific steps in the actin polymerization process also inhibit tension development in smooth muscle tissues in response to contractile stimuli (7, 123, 127, 128, 143, 144). Cellular imaging studies have documented that the depression of tension development by interventions that inhibit actin polymerization does not result from disruption of the organization or integrity of the contractile apparatus (1, 84, 143). Collectively, this large body of studies provides overwhelming evidence that dynamic changes in the actin cytoskeleton play a fundamental role in the regulation of tension development during smooth muscle contraction. However, the mechanism by which actin polymerization regulates tension development in smooth muscle is currently an unresolved question.
A Small Proportion of Total Actin Undergoes Polymerization During the Contractile Activation of Smooth Muscle
The contraction of smooth muscle tissues and cells causes an increase in the pool of filamentous (F) actin and a decrease in the pool of monomeric (globular) (G) actin (12, 27, 48, 65, 68, 72, 84, 120, 130, 143). These transitions in the state of actin have been documented in diverse smooth muscle cell and tissue types using a number of different approaches to estimate monomeric and filamentous pools of actin. These include the use of DNase inhibition assays to measure the amount of globular (G) actin (84), measurements of soluble (G) actin and insoluble (F) actin by cell fractionation (104, 120, 127, 143), fluorescence imaging to visualize G- and F-actin in isolated smooth muscle cells or tissues using G- and F-actin-specific stains (27, 48, 68, 72, 130), assessments of the rate of ADP-ATP actin monomer exchange (12), and electron microscopic studies that quantify actin filament density (65). All of these approaches have consistently shown that an increase in F-actin and a decrease in G-actin occurs when smooth muscle cells or tissues are activated by a contractile stimulus.
Small and colleagues (44, 52, 90) proposed that the smooth muscle actin isoforms α and γ preferentially associate with myosin and caldesmon and participate in contraction, whereas the β-actin isoform associates with calponin and plays a structural role in smooth muscle cells. Although this idea has been challenged (47), it is of interest to consider whether the polymerization of actin is restricted to a specific actin isoform. Parker and colleagues (99, 100) observed β-actin to be preferentially localized to the periphery of dissociated portal vein cells, and they suggested that β-actin may comprise a pool of cortical actin distinct from the contractile apparatus. However, when immunoblots were used to quantify the relative amounts of α-actin and β-actin isoforms that undergo polymerization induced by the contractile stimulation of tracheal muscle, both isoforms of actin were found to participate comparably in the polymerization process (143).
The proportions of cellular G- and F-actin that undergo polymerization during contraction have been quantified in different smooth muscle tissues. We estimated the proportions of G- and F-actin in tracheal smooth muscle by quantifying the amount of actin in the insoluble and soluble fractions of muscle extracts after separation by high-speed centrifugation (142, 143). Using this approach, we found actin in the insoluble fraction (primarily F-actin) to constitute 70–80% of the total actin in the unstimulated smooth muscle tissue, whereas approximately 20–30% of the actin was found in the soluble fraction (primarily G-actin). We obtained a comparable result when the method of DNase inhibition was used to quantitate the amount of G-actin (84). Our studies have consistently shown that the contractile stimulation of tracheal smooth muscle causes a 30–40% decrease in the pool of G-actin (143). On the basis of this analysis, we calculate that contractile stimulation increases the proportion of filamentous actin in the smooth muscle cells by approximately 10–12%, a relatively small proportion of total actin in the muscle cell (142, 143).
These estimates are consistent with estimates obtained in a number of laboratories using different experimental approaches in other tissues and preparations. Hirshman and colleagues (67) measured the ratio of F- to G-actin by fluorescence staining of G- and F-actin in primary cultures of tracheal smooth muscle cells and obtained ratios of F- to G-actin that were similar to the measurements obtained in the tracheal tissue extracts. Barany et al. (12) made comparable estimates of the size of F- and G-actin pools in arterial muscle, uterus, and urinary bladder smooth muscle tissues based on measurements of the rate of ADP-actin/ATP-actin exchange.
The conclusion that the total amount of actin that undergoes polymerization is relatively small is also supported by electron microscopic and fluorescence images of smooth muscle tissues and differentiated cells that were treated with actin polymerization inhibitors (1, 84). These images show that most of the filamentous actin in the smooth muscle cell is unaffected by treating the muscles with inhibitors of actin polymerization at concentrations sufficient to cause marked inhibition of tension development. The results of all of these studies raise the critical question of how the polymerization of a relatively small proportion of actin in the smooth muscle cell can exert such a profound effect on the development of contractile tension.
Stimulus-Induced Actin Polymerization Does Not Regulate Activation of the Contractile Apparatus in Smooth Muscle
The issue of whether actin polymerization regulates tension development by directly or indirectly contributing to the processes that mediate activation of contractile proteins and cross-bridge cycling is critical to understanding the function of actin polymerization during smooth muscle contraction. Studies conducted on a variety of different smooth muscle tissue and cell types have evaluated the effect of inhibiting actin polymerization on agonist-induced increases in myosin light chain phosphorylation and/or intracellular Ca2+. The results of almost all of these studies support the conclusion that actin polymerization does not regulate changes in intracellular Ca2+ or myosin light chain phosphorylation that are activated by contractile stimulation.
Saito et al. (107) first evaluated the effect of cytochalasin on contractile protein activation in rat aorta smooth muscle during contractions induced by either norepinephrine or K+ depolarization. They found that although treatment of the tissues with cytochalasin inhibited contractile tension development, it did not affect intracellular Ca2+, myosin light chain phosphorylation, or myosin ATPase activity. They therefore proposed that cytochalasin D inhibits smooth muscle contraction by uncoupling force generation from the activated actomyosin Mg2+-ATPase system. Subsequent studies of the effects of inhibiting actin polymerization in a number of other smooth muscle tissues have led to similar conclusions. Studies of rat mesenteric arteries (25, 93), guinea pig taenia coli (91), and airway smooth muscle tissues (84) have demonstrated that tension development can be substantially inhibited by cytochalasin and/or latrunculin with no detectable effect on myosin light chain phosphorylation. Furthermore, Shaw et al. (112) showed that phenylephrine-induced increases in intracellular Ca2+ in rat mesenteric arteries were not inhibited when constriction was inhibited by cytochalasin or latrunculin.
Myosin light chain phosphorylation has also been measured after inhibiting actin polymerization using molecular genetic interventions that inhibit the activation of proteins involved in catalyzing the actin polymerization process, or that block steps in the signaling pathway that regulates actin polymerization (7, 126–128, 143, 144). In airway smooth muscle, the expression of dominant-negative peptides that prevent the activation of the actin nucleation initiating protein, neuronal Wiskott-Aldrich syndrome protein (N-WASp), inhibits actin polymerization and tension development without affecting myosin light chain phosphorylation (143). Interventions that inhibit the specific molecular steps required for the activation of N-WASp also inhibit actin polymerization without affecting myosin light chain phosphorylation (126–128, 144) (see Activation of the Arp2/3 Complex by WASp Family Proteins Regulates Actin Polymerization During Smooth Muscle Contraction). There have been several studies in airway smooth muscle in which treatment of the tissues with cytochalasin at relatively high concentrations did cause a small depression of myosin light chain phosphorylation (84, 131, 140). As other inhibitors or interventions that inhibit actin polymerization in these tissues do not affect myosin light chain phosphorylation, it seems unlikely that this effect on myosin light chain phosphorylation resulted from the inhibitory effect of cytochalasin on actin polymerization; more likely it was due to nonspecific secondary effects of cytochalasin on other processes in the cell that contribute to the regulation of myosin.
The converse question of the potential role of myosin light chain phosphorylation and contractile protein activation on the regulation of actin polymerization has also been evaluated. These studies, though fewer in number, also provide evidence for the independence of these two processes. An et al. (6) found no effect of the myosin light chain kinase inhibitor, ML-7, on agonist-induced increase in actin polymerization in primary cultures of airway smooth muscle cells, as assessed by phalloidin staining of F-actin. Smith et al. (115) came to a similar conclusion using atomic force microscopy to probe agonist-induced increases in cell surface stiffness and rigidity in cultured rat tracheal smooth muscle cells. They found that the changes in submembranous stiffness were inhibited by agents that inhibit actin polymerization but not by ML-7, and they concluded that the increased stiffness induced by agonist stimulation reflected dynamic changes in the submembranous actin cytoskeleton that were independent of the activation of myosin.
Taken in sum, this body of studies provides compelling evidence that agonist-induced actin polymerization does not regulate processes involved in activating the contractile apparatus, and that actin polymerization regulates tension development by a cellular process that is distinct from and independent of cross-bridge cycling. The collective evidence suggests a model for smooth muscle contraction in which a contractile stimulus activates independent but parallel signaling pathways that regulate the processes of actin polymerization and contractile protein activation, both of which are essential to the process of shortening and tension development in smooth muscle tissues (Fig. 1).
Fig. 1.
Signals activated by integrin receptors and G protein-coupled receptors (GPCR) collaborate to regulate actin cytoskeletal remodeling and activation of the actomyosin system in smooth muscle. These two processes can be activated independently of one another, but the development of contractile tension requires the activation of both the actomyosin system and actin polymerization.
The observation that only a small amount of actin undergoes polymerization during smooth muscle contraction suggests that this labile pool of actin serves a specialized function that is distinct from that of the “thin filament” actin that interacts with myosin to regulate cross-bridge cycling. However, the nature of the pool of actin that undergoes polymerization and its function during the contractile process remain to be established. Much of the existing evidence points to a model in which actin polymerization occurs in a submembranous area of the smooth muscle cell (see Fig. 4). The formation of a network of submembranous actin in smooth muscle cells may function to enhance membrane rigidity and to connect the contractile and cytoskeletal filament lattice to the membrane to transmit the tension generated by cross-bridge cycling (60, 142, 143). Submembranous actin polymerization may also enable the cell to adapt its shape and stiffness and contractility to external and internal mechanical forces imposed on it, and it may occur in regions within smooth muscle cells in which the membrane tension is greatest.
Fig. 4.
Integrated model for function of cytoskeletal dynamics in smooth muscle contraction. The activation of the smooth muscle cell stimulates the assembly of macromolecular protein complexes at cell membrane/ECM adhesion junctions that regulate the formation of a subcortical network of actin filaments and fortify connections between the actin filaments and integrin proteins. The formation of a subcortical actin filament network strengthens the membrane for the transmission of force generated by the actomyosin system and enables adaptation of the smooth muscle cell to external forces.
Activation of the Arp2/3 Complex by WASp Family Proteins Regulates Actin Polymerization During Smooth Muscle Contraction
How are signals from contractile stimuli transduced to regulate actin polymerization during the contraction of smooth muscle cells? Models for the regulation of actin dynamics in migrating cells can provide a template for a mechanistic model for the regulation of actin polymerization in smooth muscle tissues during contraction (55, 79, 102). During cell migration, mechanical tension at sites of membrane extension at the leading edge of the cell induces integrin clustering and activation, the recruitment of cytoskeletal adhesion and signaling proteins to sites of cell adhesion, and the polymerization of actin filaments, resulting in extension of the cell membrane and cell crawling (39, 86, 111).
The spontaneous initiation of actin filament assembly by the nucleation of new actin filaments is not favored kinetically; actin nucleation factors are required to promote the initiation of actin filament assembly. Currently, three classes of actin nucleating proteins have been identified: the Arp2/3 complex, spire, and formins, each of which promotes actin filament nucleation by a distinct mechanism (57, 121). The Arp2/3 complex was the first actin assembly factor to be identified, and, to date, it is the only one that has been documented to play a role in the regulation of actin polymerization in smooth muscle. The potential role of other actin assembly factors has not been evaluated; the evaluation of their function may also define a role for them in the regulation of actin dynamics and contractility in smooth muscle.
The central role of the Arp2/3 complex in the regulation of actin polymerization and the organization of actin filament networks at the cell membrane of migrating cells is well established (57, 82, 88, 97, 102, 121). The Arp2/3 complex consists of seven strongly associated protein subunits that include Arp2 and Arp3 (actin-related proteins), which form a template for the formation of new actin filaments (82, 102). The Arp2/3 complex requires activation by one of several nucleation promoting factors, which in mammalian cells include the WASp and Scar/WAVE families of proteins. The WASp proteins are key activators of the Arp2/3 complex, and they regulate actin polymerization that occurs at the leading edge of the membrane in response to signals from adhesion proteins. In motile cells, a stimulus for motility causes the activation of the small GTPase, cdc42, which catalyzes the recruitment of a WASP family protein to the membrane and its activation, resulting in the binding of the Arp2/3 complex to WASp and Arp2/3 complex activation (34, 79, 106).
There is currently very limited information regarding the potential roles of actin nucleation promoting factors in smooth muscle contraction; however, a role for the WASp family protein, N-WASp, in the regulation of actin polymerization during the contraction of tracheal smooth muscle tissues has been documented (143). Studies using fluorescence imaging and coimmunoprecipitation analysis have shown that contractile stimulation with acetylcholine initiates the recruitment of N-WASp to the cortex of freshly dissociated tracheal muscle cells and tracheal smooth muscle tissues, where it associates with the Arp2/3 complex (143). The importance of Arp2/3 activation in the regulation of actin polymerization and tension development in intact tracheal smooth muscle tissues was demonstrated using a dominant-negative COOH-terminal peptide of N-WASp to inhibit activation of the Arp2/3 complex (the N-WASp CA domain) (143). The N-WASp CA domain completes with full-length N-WASp for binding to the Arp2/3 complex and thereby inhibits its activation (66). When the N-WASp CA domain was introduced into or expressed in tracheal smooth muscle tissues, it inhibited actin polymerization and depressed tension generation in response to a contractile stimulus (acetylcholine), indicating that activation of the Arp2/3 complex is critical for stimulus-induced actin polymerization and tension development (143). There is also evidence that N-WASp is activated during the contraction of rat mesenteric arteries (7). It is likely that other classes of actin filament nucleating factors (e.g., formins, spire proteins) and nucleation promoting factors (e.g., Scar/WAVE proteins) play important roles in regulating the contractility and physiological properties of smooth muscle tissues; however, to date, there is no information regarding their physiological function in smooth muscle.
Contractile Stimulation Catalyzes the Assembly of Macromolecular Adhesion Complexes at Cytoskeletal/Cell Matrix Junctions in Smooth Muscle Tissues
In motile cells, mechanical tension at sites of cell adhesion stimulates the assembly of macromolecular protein complexes at the membrane junctions between the extracellular matrix and the actin cytoskeleton (18, 37, 53, 111). Proteins bound within these adhesion complexes are in a constant state of dynamic exchange with their cytoplasmic pools, where they may exist in an inactive state. These adhesion complexes consist of dozens of structural and signaling proteins that link actin and other cytoskeletal filaments to the cytoplasmic domains of heterodimeric transmembrane integrin proteins, which are specific ligands for extracellular matrix proteins. They orchestrate the array of cytoskeletal processes that result in movement of the crawling cell, including the polymerization of new actin filaments that causes protrusion of the membrane (8, 39, 110, 135). There is evidence that a similar process of adhesion complex assembly also occurs in response to the contractile stimulation of smooth muscle tissues, and that the formation of this complex is essential for the regulation of stimulus-induced actin polymerization and tension generation.
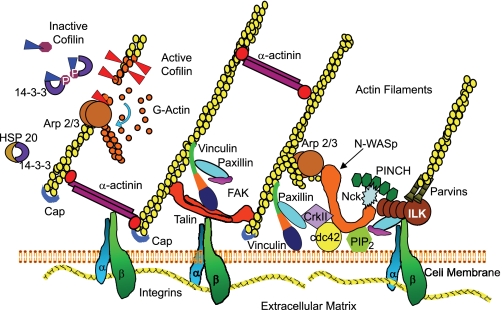
In smooth muscle tissues, macromolecular adhesion junctions, often referred to as “membrane-associated dense bodies,” form on the intracellular side of the plasma membrane at the junctions between actin filaments and the extracellular matrix (45, 114, 142) (Fig. 2). At these junctions, actin filaments are connected to integrin proteins via “linker” proteins within the adhesion complexes such as α-actinin, talin, and filamin, all of which form homodimers that can both cross-link actin filaments and bind to the β-subunit of integrin heterodimers (36, 96).
Fig. 2.
Molecular organization of integrin/cytoskeletal adhesion junctions in smooth muscle. Actin filaments are linked to integrin proteins via actin cross-linking proteins that bind to the cytoplasmic tails of integrin proteins. Scaffolding proteins regulate the assembly of protein complexes at adhesion junctions in response to contractile stimulation. Proteins that assemble into macromolecular complexes at adhesion junctions regulate actin polymerization. HSP, heat shock protein; ILK, integrin-linked kinase; FAK, focal adhesion kinase; N-WASp, neuronal Wiskott-Aldrich syndrome protein.
There is accumulating evidence that the adhesion complexes of smooth muscle are not static structures. Studies in airway and vascular smooth muscle tissues and in isolated smooth muscle cells show that the localization of cytoskeletal proteins to adhesion complexes and to the cell cortex is dynamically regulated during contractile stimulation (51, 74, 93, 95, 99, 100, 143–145). Diverse approaches including coimmunoprecipitation, cell fractionation, immunofluorescence analysis, and cellular imaging have demonstrated that contractile stimulation initiates the recruitment of both structural and signaling proteins to the smooth muscle cell cortex, and that it stimulates the association of adhesion proteins with β-integrins (51, 74, 93, 95, 99, 100, 143–145). The imposition of mechanical tension or the twisting of integrin proteins bound to magnetic beads on the surface of isolated smooth muscle cells also stimulates the recruitment of adhesion complex proteins to sites of membrane tension (40, 41, 117).
Digital video-imaging has been used to monitor the effects of contractile stimulation on the localization of a number of proteins that are components of adhesion complexes in living smooth muscle cells in vivo (142, 144, 145). In these studies, green fluorescent protein (GFP) fusion proteins for adhesion complex proteins were expressed in intact tracheal smooth muscle tissues, and smooth muscle cells were then enzymatically dissociated from the tissues and studied immediately. Thus, the isolated cells retained the full contractile phenotype and spindle-shaped morphology typical of a smooth muscle cell. The movement of GFP fusion proteins was observed by confocal microscopy during contraction of the live smooth muscle cells. In these studies, the administration of a contractile agonist resulted in the rapid translocation of the proteins to the membrane, within seconds of the administration of the stimulus.
G Protein-Coupled Receptors Collaborate with Adhesion Complex Proteins to Initiate Signals for Actin Polymerization During Smooth Muscle Contraction
The assembly of macromolecular complexes at membrane adhesion sites during the contractile stimulation of smooth muscle tissues is necessary for the initiation of actin filament polymerization and to regulate the formation of the adhesion complexes that link actin filaments to the extracellular matrix (Fig. 3). (142). Proteins within adhesion complexes may also modulate signaling pathways that regulate intracellular Ca2+ and myosin light chain phosphorylation in smooth muscle tissues (38, 80).
Fig. 3.
Signaling pathway for the regulation of actin polymerization in smooth muscle. ECM, extracellular matrix.
In tracheal smooth muscle, the activation of the actin nucleation initiating factor, N-WASp, and the subsequent polymerization of actin depend on a cascade of protein interactions within adhesion complexes that are initiated by stimulation with a contractile agonist. In tracheal smooth muscle, a stable trimeric protein complex consisting of integrin-linked kinase (ILK), a β-integrin binding scaffolding protein and protein kinase, and its binding partners PINCH, a LIM-only domain protein, and α-parvin, an actin-binding protein (ILK/PINCH/α-parvin complex) (146) is rapidly recruited to integrin adhesion sites in response to a contractile stimulus, where it couples to β-integrin proteins and forms a scaffold for the recruitment of other structural and signaling proteins that are required for processes of cytoskeletal reorganization and actin polymerization (144). If the recruitment of the ILK/PINCH/α-parvin complex to the membrane is prevented by expressing an inhibitory PINCH fragment in the smooth muscle tissues, the activation of N-WASp, actin polymerization, and tension development are markedly suppressed.
In tracheal smooth muscle, the cytoskeletal proteins paxillin and vinculin are also critical for the initiation of actin polymerization during the contractile activation of smooth muscle. Paxillin and vinculin bind together in the cytosol (132, 136) and are also recruited to membrane adhesion sites in response to contractile stimulation, where they associate with the ILK/PINCH/α-parvin complex (95, 144). Paxillin is a scaffolding protein that has been shown to undergo tyrosine phosphorylation in response to contractile stimulation in many smooth muscle cell and tissue types (19, 71, 78, 93, 101, 104, 116, 122, 124, 125, 129, 134, 139). The tyrosine phosphorylation of paxillin at two sites, tyrosine 31 and 118, enables it to couple to the SH2/SH3 adaptor protein CrkII, which binds to N-WASp and contributes to N-WASp and Arp2/3 activation (126–128, 143). In airway smooth muscle, the phosphorylation of paxillin increases rapidly after contractile stimulation, coincident with the development of contractile tension (134). The rapid activation of paxillin is consistent with its role as an upstream regulator of actin polymerization. The expression of nonphosphorylatable paxillin mutants in tracheal smooth muscle tissues prevents the binding of paxillin to CrkII and inhibits actin polymerization in response to a contractile stimulus (126).
In tracheal muscle, the activation of N-WASp and actin polymerization in response to contractile stimulation also requires the concurrent activation of the small GTPase, cdc42(128). Agonist-activated paxillin tyrosine phosphorylation mediates the formation of a complex that includes CrkII, Cdc42, and N-WASp, which may facilitate the activation of Cdc42 by an unknown guanine nucleotide exchange factor (GEF) and the consequent activation of N-WASp(127). The widespread observations of paxillin phosphorylation in response to contractile stimulation in diverse types of smooth muscle tissues and cells suggest a ubiquitous role for this protein as an upstream regulator of actin polymerization during smooth muscle contraction.
Actin polymerization is mechanosensitive in some smooth muscle tissues, and the mechanotransduction process may be mediated by proteins that localize to adhesion sites (40, 41, 64, 84, 116, 117, 122, 125, 140, 141). In airway smooth muscle, the degree of tension depression caused by the inhibition of actin polymerization depends on the mechanical strain on the muscle, and actin polymerization may be important in regulating the length-sensitivity of tension development (84, 140). Mechanical torque applied to integrin-bound magnetic beads also stimulates the formation of submembranous actin structures in airway muscle cells (40, 41). In both airway muscle cells and tissues, the degree of paxillin tyrosine phosphorylation is mechanosensitive during contractile stimulation, suggesting that paxillin may play a critical role in mechanotransduction at membrane adhesion sites (116, 122, 124, 125). Modulation of the phosphorylation of paxillin in response to mechanical stimuli in smooth muscle tissues could provide a mechanism for the mechanosensitive regulation of N-WASp-mediated actin polymerization, and thereby provide a molecular pathway by which cytoskeletal organization and actin filament structure are modulated to facilitate the adaptation of smooth muscle cell shape and stiffness to their environment (Fig. 3).
The molecular mechanisms by which G protein-coupled receptor activation links to signaling events that regulate actin polymerization in smooth muscle have not been delineated. Members of the family of small GTPases, Rho, Rac, and Cdc42, are widely recognized as key regulators of the actin cytoskeleton in diverse nonmuscle cells (8, 21, 73, 89), and they can couple the activation of G protein-coupled receptors to cytoskeletal functions (105). In smooth muscle, the role of RhoA GTPases in regulating contractile force and myosin light chain phosphorylation through an inhibitory effect on myosin light chain phosphatase is well established (118). Hirshman and colleagues (67, 68) demonstrated that G protein subunits activated by acetylcholine in airway smooth muscle cells regulate actin polymerization via the activation of RhoA. Welsh and colleagues reached similar conclusions from studies of isolated cerebral arteries, in which they found that the principal role of RhoA activation in agonist-induced constriction was to facilitate the formation of F-actin rather than to regulate myosin light chain phosphorylation (33). However, the downstream molecular pathways by which RhoA regulates actin polymerization and remodeling in response to agonist stimulation in smooth muscle tissues have not been determined.
Contractile Stimulation Regulates the Formation of Connections Between Actin Filaments and the Extracellular Matrix
The contractile stimulation of smooth muscle cells and tissues also catalyzes the recruitment of structural proteins that connect actin filaments and transmembrane integrin proteins to the adhesion junctions of smooth muscle cells. α-Actinin, an actin cross-linking protein that also binds to integrin proteins, is rapidly recruited to the integrin adhesion complexes of tracheal smooth muscle cells and tissues in response to a contractile stimulus (145). Similar observations have been made in cells from an aortic smooth muscle cell line (51). In the tracheal tissues, the recruitment of α-actinin is required for tension development but not for actin polymerization or myosin light chain phosphorylation, suggesting a distinct role for α-actinin in smooth muscle contraction. Studies of migrating cells have shown that α-actinin reinforces integrin-cytoskeletal connections to enable them to withstand the force transmitted to the extracellular matrix (9, 14, 133). α-Actinin may serve a similar function during smooth muscle contraction: its recruitment to the cell cortex and to integrin adhesion junctions may serve to strengthen connections between the cytoskeleton and extracellular matrix for the transmission of force generated by actomyosin cycling during contraction. α-Actinin may also connect and support newly formed subcortical actin filaments within the muscle cells and link them to integrin proteins within adhesion junctions.
The contractile stimulation of smooth muscle may also regulate connections between actin filaments and membrane adhesion junctions by activating the adhesion complex protein vinculin. Vinculin binds to both filamentous actin and talin, which is another integrin binding protein that also cross-links actin. The conformation of vinculin can be reversibly regulated between an open state in which it can bind to actin filaments as well as to talin and α-actinin, and a closed conformation in which it does not bind to either of these proteins, thus enabling it to reversibly form connections between actin filaments and membrane adhesion sites (24, 29). Vinculin binds to paxillin and is recruited with paxillin to the adhesion complexes of tracheal smooth muscle cells during contractile stimulation (95). The expression of mutant vinculin peptides that inhibit the activation of endogenous vinculin prevents the stimulus-induced contraction of tracheal smooth muscle (69). The controlled formation and dissolution of actin filament/adhesion complex linkages by α-actinin, vinculin, and other proteins may be an important mechanism for regulating the cytoskeletal organization of the smooth muscle cell and enable the transmission of contractile tension (61, 62).
The formation of macromolecular complexes at the leading edge of crawling or spreading cells depends on a carefully orchestrated process involving the stepwise assembly of an adhesion complex, and the assembly of this adhesion complex is required for the initiation of localized actin filament polymerization and dissolution. The cytoskeletal processes that occur in smooth muscle during contractile activation may serve parallel functions to some of the processes that occur during cell migration: mechanical force generated either internally by the contractile apparatus or externally by strain imposed on the cell may stimulate the assembly of adhesion complexes that catalyze the formation of a cortical network of actin filaments, anchor the actin filament network to integrin proteins at adhesion sites, and strengthen the network of newly formed actin filaments. The formation of this cortical cytoskeletal structure might serve to form a more rigid submembranous structure that is capable of transmitting the force generated by the activation of contractile proteins to the extracellular matrix filaments and across the tissue (Fig. 4).
Actin Depolymerizing Proteins (ADF/Cofilin) in the Regulation of Actin Dynamics in Smooth Muscle Tissues
Studies of actin dynamics in vitro and in cells have shown that the polymerization of actin is regulated by proteins that control the availability of a pool of monomeric (G) actin for incorporation into actin filaments, and the availability of free “barbed” or “plus” ends of actin filaments that can undergo polymerization (22, 77). The family of ADF (actin depolymerization factor)/cofilin proteins can regulate both of these processes. These proteins have therefore been termed “actin-dynamizing” proteins because of their critical role in regulating the actin filament remodeling that enables the rapid adaptation of the actin cytoskeleton to localized cellular functions (11, 23, 30).
The important role of ADF/cofilin proteins in cell migration is well established (10, 23, 30). During cell migration, new actin filaments are generated at the leading edge of the cell, and actin filaments at the rear of the actin network are disassembled. Cofilin plays an essential role in both the actin polymerization and depolymerization processes during cell movement: It binds to actin filaments and severs them, thereby promoting actin disassembly and the formation of new barbed ends, which enables the nucleation of new actin filaments by the Arp2/3 complex. Cofilin also contributes to actin filament assembly by replenishing the actin monomer pool required for polymerization (11, 23, 75, 98).
Cofilin activity is regulated through phosphorylation and dephosphorylation of its NH2-terminal Ser3. Serine 3 phosphorylation abolishes the ability of cofilin to bind to F-actin and thus inhibits its severing function (2, 10, 83, 87). The dephosphorylation of cofilin is mediated by the cofilin-specific phosphatases slingshot and chronophin (70). Phosphorylated cofilin binds to the intracellular scaffolding protein 14-3-3 (56), which acts to sequester it. Displacement from 14-3-3 can result in the activation of cofilin by cofilin-specific phosphatases. Cofilin is abundantly expressed in vascular smooth muscle cells and tissues, where it has been implicated in the regulation of vascular smooth muscle cell migration (109). It also plays a role in regulating the expression of smooth muscle differentiation markers in vascular tissues (3, 64).
Recent evidence suggests that cofilin also plays a critical role in regulating actin dynamics during the contraction and relaxation of smooth muscle tissues. In tracheal smooth muscle tissues, stimulation of the tissues with acetylcholine causes the dephosphorylation and activation of cofilin (147). Cofilin dephosphorylation can be inhibited by expression of dominant-negative inactive cofilin mutant, cofilin S3E (83). When cofilin S3E is expressed in tracheal muscle tissues, it inhibits cofilin dephosphorylation and prevents the increase in actin polymerization stimulated by acetylcholine (147). These observations are consistent with the postulated role of cofilin in regulating the size of the pool of G-actin available for actin polymerization during contraction. In cultured human airway smooth muscle cells, cofilin is activated (dephosphorylated) in response to forskolin and isoproterenol, suggesting a possible role in regulating actin dynamics during the relaxation of smooth muscle (76).
Cofilin activity may also be indirectly modulated by small heat shock proteins, which have been proposed to regulate smooth muscle contractility by affecting actin dynamics. The small heat shock proteins HSP20 and HSP27 are chaperone proteins that are highly expressed in smooth muscle tissues. There is evidence implicating both proteins in the modulation of vascular, airway, and visceral smooth muscle contractility and tone (108). HSP20 phosphorylation at serine 16 can be catalyzed by the cAMP-dependent kinase, PKA (13). Phosphopeptide analogs of HSP20 induce the relaxation of several different smooth muscle tissues and cell types (46, 49, 76, 103, 137). In vitro, phosphorylated HSP20 interacts directly with 14-3-3, the chaperone protein that sequesters cofilin (26). Brophy and colleagues have proposed that HSP20 mediates smooth muscle relaxation in response to cyclic nucleotide-dependent pathways by competing with phosphocofilin for binding to the chaperone protein, 14-3-3, leading to an increase in the amount of activated cofilin (46, 76). They demonstrated that activation of the cAMP-dependent pathway by isoproterenol or forskolin in tracheal smooth muscle cells leads to the phosphorylation of HSP20 as well as the dephosphorylation and activation of cofilin (76).
A number of lines of evidence also implicate HSP27 in the regulation of smooth muscle contractility and actin dynamics: Antibodies to HSP27 inhibit the contraction of intestinal smooth muscle cells (15), and the activation of the p38 MAP kinase pathway that leads to HSP27 phosphorylation is necessary for the migration of tracheal smooth muscle cells (63). The overexpression of phosphorylated HSP27 in cultured airway smooth muscle cells increases cytoskeletal stability as determined from the motion of integrin-bound beads on the cell surface, suggesting an increase in actin polymerization and cytoskeletal rigidity (5). More studies will be needed to establish the functional roles of heat shock proteins in the regulation of smooth muscle contractility; however, these observations suggest novel mechanisms by which muscle properties may be regulated through effects on actin dynamics.
Observations that the phosphorylation of cofilin is sensitive to modulation by diverse signaling pathways that affect smooth muscle contractility suggest that it may be a potent regulator of smooth muscle actin dynamics and thus act to regulate muscle contractility and tension. However, most isoforms of the actin-filament binding protein tropomyosin compete with cofilin for binding to actin filaments, and thereby protect them from the severing action of cofilin (16, 20, 31, 42, 94). Most of the filamentous actin that interacts with myosin in smooth muscle is believed to be bound to tropomyosin, and therefore it should be resistant to cofilin activity, which would be consistent with evidence that most of the actin remodeling during contraction and relaxation of smooth muscle is restricted to a small pool of actin that is distinct from the actin that participates in contraction.
A Functional Role for Cytoskeletal Dynamics During Smooth Muscle Contraction
Mechanistic models for the regulation of mechanical behavior of smooth muscle have traditionally centered primarily on the processes involved in regulating the actomyosin cross-bridge interaction. While these processes are undoubtedly central to the development of contractile tension, it is becoming increasingly evident that the myosin activation and cross-bridge cycling are but one component of a complex and integrated series of cytoskeletal events that regulate tension development and the mechanical properties of a smooth muscle cell during contractile activation. There is compelling evidence that an array of cytoskeletal processes are concurrently activated by contractile stimulation and that these processes are also essential for active tension development. Although the specific cellular functions of these cytoskeletal processes in the development of mechanical tension during smooth muscle contraction remain to be fully elucidated, existing observations from studies of smooth muscle as well as evidence for the functions of these cytoskeletal proteins and processes in other cell types suggest a mechanistic paradigm for their functional role in contraction and tension development in smooth muscle (Figs. 3 and 4).
This paradigm can be summarized as follows: Contractile and mechanical stimuli activate local transduction processes at extracellular matrix/cytoskeletal junctions that catalyze the recruitment of cytoskeletal proteins and their regulated assembly into a macromolecular adhesion complex at cytoskeletal/extracellular matrix junctions. Adhesion complex proteins catalyze the polymerization of new actin filaments at the cell cortex, mediate the reorganization of connections between actin filaments and the cell membrane, and stabilize the actin cytoskeletal network. The formation of this cortical actin/cytoskeletal network provides a rigid structure for the transmission of the tension generated by actomyosin cross-bridge cycling to the outside of the cell, and for the transmission of force throughout the smooth muscle tissue. Actin polymerization and remodeling may be locally regulated in response to tension or mechanical strain at discrete points along the cell membrane and may thereby strengthen tension transmission to the exterior of the cell at sites of membrane stress or strain. Local mechanotransduction events at adhesion sites may also modulate the cytoskeletal events that enable the cell to adapt and remodel its cytoskeletal structure and organization to conform to forces generated by external and internal physiological processes. This structural plasticity may enable smooth muscle cells to conform to forces generated by external and internal physiological events, thus providing for the mechanism for the mechanical adaptation of the smooth muscle of hollow organs.
This model for the regulation of smooth muscle function can provide novel perspectives to explain the normal physiological behavior of smooth muscle tissues. The malleability of some smooth muscle tissues and their ability to adapt to environmental influences may be a function of the dynamic nature of their cytoskeletal structure. The ability of airway smooth muscle to alter its stiffness and contractility in response to mechanical oscillation and stretch has been widely observed and described, and it is considered to be critically important for the regulation of normal airway responsiveness (4, 50, 54, 59, 60, 62, 113). Such adaptive properties have also been demonstrated in other visceral smooth muscles (58, 85, 119) and may be inherent to smooth muscle tissues within organs that must adapt to large changes in volume.
Actin polymerization may also occur at other sites within smooth muscle cells and may play other functional roles in some smooth muscle tissues. For example, Seow and colleagues have reported that contractile stimulation results in an increase in the density of cytosolic actin filaments around cytosolic dense bodies in electron microscopic sections of tracheal muscle tissues (65). Evidence from several laboratories suggests that actin polymerization underlies the myogenic response in small arteries (27, 28, 48). Actin polymerization in these tissues may occur at different cytosolic sites and be governed by different molecular processes.
Much more work will be needed to fully delineate the molecular processes involved in cytoskeletal dynamics in diverse smooth muscle tissues and physiological conditions, and to evaluate the functional roles of the cytoskeletal processes in the regulation of smooth muscle contractility and mechanical tension. Existing research on smooth muscle has barely begun to elucidate the complex processes that may contribute to the regulation of cytoskeletal dynamics in these tissues. The cytoskeletal processes and regulatory pathways may differ very significantly among smooth muscle tissues that serve widely differing physiological functions.
Conclusions and Future Directions
A growing body of data supports a view of the actin cytoskeleton of smooth muscle cells as a dynamic structure that plays an integral role in regulating the development of mechanical tension and the material properties of smooth muscle tissues. The increase in the proportion of filamentous actin that occurs in response to the stimulation of smooth muscle cells and the essential role of stimulus-induced actin polymerization and cytoskeletal dynamics in the generation of mechanical tension have been convincingly documented in many smooth muscle tissues and cells using a wide variety of experimental approaches. There is also extensive evidence that the functional role of actin polymerization during contraction is distinct and separately regulated from the actomyosin cross-bridge cycling process. However, much more data will be needed to establish the molecular basis for the regulation of actin polymerization and its functional roles in diverse types of smooth muscle cells and tissues. Existing evidence, which has been obtained largely from studies of airway smooth muscle, supports a model in which contractile stimulation initiates a signaling pathway mediated by proteins within cytoskeletal/extracellular matrix adhesion complexes at the membrane, and that proteins within this complex orchestrate the polymerization and organization of a network of actin filaments below the plasma membrane. This cytoskeletal network may serve to strengthen the membrane for the transmission of force generated by the contractile apparatus. The dynamic reorganization of this cytoskeletal network may also form the basis for the ability of smooth muscle cells to adapt and accommodate to their external environment.
There is compelling evidence that actin polymerization plays a role in the regulation of the contractile behavior of many vascular and visceral smooth muscle tissues; however, it may serve different physiological functions in different smooth muscle tissue types. The importance of the actin polymerization process for tension development and shortening may also vary among smooth muscle tissues that serve different physiological functions. It is possible that actin cytoskeletal dynamics plays a more prominent role in smooth muscle tissues that are required to undergo large shape changes or volume accommodations under physiological conditions. The signaling pathways that couple actin polymerization and cytoskeletal dynamics to receptor stimulation, the molecular processes that regulate the assembly of actin filament networks, and the nature and organization of dynamic actin structures may also differ among different smooth muscle tissue types. The molecular processes that regulate the assembly of actin filaments in smooth muscle tissues and the nature of the actin filament networks that are formed during contractile activation are areas for which there is currently very limited information. Better understanding of the mechanisms for these dynamic cytoskeletal processes and their physiological functions in diverse smooth muscle types may provide important insights into the physiological regulation of the properties of smooth muscle tissues and organs and may lead to novel targets for the development of new therapeutic agents.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-29289 and HL-074099, the American Heart Association, and the American Lung Association.
REFERENCES
- 1.Adler KB, Krill J, Alberghini TV, Evans JN. Effect of cytochalasin D on smooth muscle contraction. Cell Motil 3: 545–551, 1983. [DOI] [PubMed] [Google Scholar]
- 2.Agnew BJ, Minamide LS, Bamburg JR. Reactivation of phosphorylated actin depolymerizing factor and identification of the regulatory site. J Biol Chem 270: 17582–17587, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Albinsson S, Nordstrom I, Hellstrand P. Stretch of the vascular wall induces smooth muscle differentiation by promoting actin polymerization. J Biol Chem 279: 34849–34855, 2004. [DOI] [PubMed] [Google Scholar]
- 4.An SS, Bai TR, Bates JH, Black JL, Brown RH, Brusasco V, Chitano P, Deng L, Dowell M, Eidelman DH, Fabry B, Fairbank NJ, Ford LE, Fredberg JJ, Gerthoffer WT, Gilbert SH, Gosens R, Gunst SJ, Halayko AJ, Ingram RH, Irvin CG, James AL, Janssen LJ, King GG, Knight DA, Lauzon AM, Lakser OJ, Ludwig MS, Lutchen KR, Maksym GN, Martin JG, Mauad T, McParland BE, Mijailovich SM, Mitchell HW, Mitchell RW, Mitzner W, Murphy TM, Pare PD, Pellegrino R, Sanderson MJ, Schellenberg RR, Seow CY, Silveira PS, Smith PG, Solway J, Stephens NL, Sterk PJ, Stewart AG, Tang DD, Tepper RS, Tran T, Wang L. Airway smooth muscle dynamics: a common pathway of airway obstruction in asthma. Eur Respir J 29: 834–860, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.An SS, Fabry B, Mellema M, Bursac P, Gerthoffer WT, Kayyali US, Gaestel M, Shore SA, Fredberg JJ. Role of heat shock protein 27 in cytoskeletal remodeling of the airway smooth muscle cell. J Appl Physiol 96: 1701–1713, 2004. [DOI] [PubMed] [Google Scholar]
- 6.An SS, Laudadio RE, Lai J, Rogers RA, Fredberg JJ. Stiffness changes in cultured airway smooth muscle cells. Am J Physiol Cell Physiol 283: C792–C801, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Anfinogenova Y, Wang R, Li QF, Spinelli AM, Tang DD. Abl silencing inhibits CAS-mediated process and constriction in resistance arteries. Circ Res 101: 420–428, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arthur WT, Noren NK, Burridge K. Regulation of Rho family GTPases by cell-cell and cell-matrix adhesion. Biol Res 35: 239–246, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, Geiger B. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol 3: 466–472, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Bamburg JR Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol 15: 185–230, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Bamburg JR, McGough A, Ono S. Putting a new twist on actin: ADF/cofilins modulate actin dynamics. Trends Cell Biol 9: 364–370, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Barany M, Barron JT, Gu L, Barany K. Exchange of the actin-bound nucleotide in intact arterial smooth muscle. J Biol Chem 276: 48398–48403, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Beall A, Bagwell D, Woodrum D, Stoming TA, Kato K, Suzuki A, Rasmussen H, Brophy CM. The small heat shock-related protein, HSP20, is phosphorylated on serine 16 during cyclic nucleotide-dependent relaxation. J Biol Chem 274: 11344–11351, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol 19: 677–695, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Bitar KN, Kaminski MS, Hailat N, Cease KB, Strahler JR. Hsp27 is a mediator of sustained smooth muscle contraction in response to bombesin. Biochem Biophys Res Commun 181: 1192–1200, 1991. [DOI] [PubMed] [Google Scholar]
- 16.Blanchoin L, Pollard TD, Hitchcock-DeGregori SE. Inhibition of the Arp2/3 complex-nucleated actin polymerization and branch formation by tropomyosin. Curr Biol 11: 1300–1304, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Braet F, De Zanger R, Jans D, Spector I, Wisse E. Microfilament-disrupting agent latrunculin A induces and increased number of fenestrae in rat liver sinusoidal endothelial cells: comparison with cytochalasin B. Hepatology 24: 627–635, 1996. [DOI] [PubMed] [Google Scholar]
- 18.Brakebusch C, Fassler R. The integrin-actin connection, an eternal love affair. EMBO J 22: 2324–2333, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown MC, Turner CE. Characterization of paxillin LIM domain-associated serine threonine kinases: activation by angiotensin II in vascular smooth muscle cells. J Cell Biochem 76: 99–108, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Burgstaller G, Gimona M. Actin cytoskeleton remodelling via local inhibition of contractility at discrete microdomains. J Cell Sci 117: 223–231, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell 116: 167–179, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Carlier MF, Wiesner S, Le Clainche C, Pantaloni D. Actin-based motility as a self-organized system: mechanism and reconstitution in vitro. C R Biol 326: 161–170, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Carlier MF, Laurent V, Santolini J, Melki R, Didry D, Xia GX, Hong Y, Chua NH, Pantaloni D. Actin depolymerizing factor (ADF/cofilin) enhances the rate of filament turnover: implication in actin-based motility. J Cell Biol 136: 1307–1322, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen H, Choudhury DM, Craig SW. Coincidence of actin filaments and talin is required to activate vinculin. J Biol Chem 281: 40389–40398, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Pavlish K, Zhang HY, Benoit JN. Effects of chronic portal hypertension on agonist-induced actin polymerization in small mesenteric arteries. Am J Physiol Heart Circ Physiol 290: H1915–H1921, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Chernik IS, Seit-Nebi AS, Marston SB, Gusev NB. Small heat shock protein Hsp20 (HspB6) as a partner of 14-3-3gamma. Mol Cell Biochem 295: 9–17, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Cipolla MJ, Gokina NI, Osol G. Pressure-induced actin polymerization in vascular smooth muscle as a mechanism underlying myogenic behavior. FASEB J 16: 72–76, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Cipolla MJ, Osol G. Vascular smooth muscle actin cytoskeleton in cerebral artery forced dilatation. Stroke 29: 1223–1228, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Cohen DM, Kutscher B, Chen H, Murphy DB, Craig SW. A conformational switch in vinculin drives formation and dynamics of a talin-vinculin complex at focal adhesions. J Biol Chem 281: 16006–16015, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Condeelis J How is actin polymerization nucleated in vivo? Trends Cell Biol 11: 288–293, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Cooper JA Actin dynamics: tropomyosin provides stability. Curr Biol 12: R523–R525, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Cooper JA Effects of cytochalasin and phalloidin on actin. J Cell Biol 105: 1473–1478, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corteling RL, Brett SE, Yin H, Zheng XL, Walsh MP, Welsh DG. The functional consequence of RhoA knockdown by RNA interference in rat cerebral arteries. Am J Physiol Heart Circ Physiol 293: H440–H447, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Cory GO, Ridley AJ. Cell motility: braking WAVEs. Nature 418: 732–733, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Coue M, Brenner SL, Spector I, Korn ED. Inhibition of actin polymerization by latrunculin A. FEBS Lett 213: 316–318, 1987. [DOI] [PubMed] [Google Scholar]
- 36.Critchley DR Focal adhesions - the cytoskeletal connection. Curr Opin Cell Biol 12: 133–139, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Critchley DR, Holt MR, Barry ST, Priddle H, Hemmings L, Norman J. Integrin-mediated cell adhesion: the cytoskeletal connection. Biochem Soc Symp 65: 79–99, 1999. [PubMed] [Google Scholar]
- 38.Davis MJ, Wu X, Nurkiewicz TR, Kawasaki J, Davis GE, Hill MA, Meininger GA. Integrins and mechanotransduction of the vascular myogenic response. Am J Physiol Heart Circ Physiol 280: H1427–H1433, 2001. [DOI] [PubMed] [Google Scholar]
- 39.DeMali KA, Wennerberg K, Burridge K. Integrin signaling to the actin cytoskeleton. Curr Opin Cell Biol 15: 572–582, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Deng L, Fairbank NJ, Cole DJ, Fredberg JJ, Maksym GN. Airway smooth muscle tone modulates mechanically induced cytoskeletal stiffening and remodeling. J Appl Physiol 99: 634–641, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Deng L, Fairbank NJ, Fabry B, Smith PG, Maksym GN. Localized mechanical stress induces time-dependent actin cytoskeletal remodeling and stiffening in cultured airway smooth muscle cells. Am J Physiol Cell Physiol 287: C440–C448, 2004. [DOI] [PubMed] [Google Scholar]
- 42.DesMarais V, Ichetovkin I, Condeelis J, Hitchcock-DeGregori SE. Spatial regulation of actin dynamics: a tropomyosin-free, actin-rich compartment at the leading edge. J Cell Sci 115: 4649–4660, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Dowell ML, Lakser OJ, Gerthoffer WT, Fredberg JJ, Stelmack GL, Halayko AJ, Solway J, Mitchell RW. Latrunculin B increases force fluctuation-induced relengthening of ACh-contracted, isotonically shortened canine tracheal smooth muscle. J Appl Physiol 98: 489–497, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Draeger A, Amos WB, Ikebe M, Small JV. The cytoskeletal and contractile apparatus of smooth muscle: contraction bands and segmentation of the contractile elements. J Cell Biol 111: 2463–2473, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Draeger A, Stelzer EH, Herzog M, Small JV. Unique geometry of actin-membrane anchorage sites in avian gizzard smooth muscle cells. J Cell Sci 94: 703–711, 1989. [DOI] [PubMed] [Google Scholar]
- 46.Dreiza CM, Brophy CM, Komalavilas P, Furnish EJ, Joshi L, Pallero MA, Murphy-Ullrich JE, von Rechenberg M, Ho YS, Richardson B, Xu N, Zhen Y, Peltier JM, Panitch A. Transducible heat shock protein 20 (HSP20) phosphopeptide alters cytoskeletal dynamics. FASEB J 19: 261–263, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Drew JS, Moos C, Murphy RA. Localization of isoactins in isolated smooth muscle thin filaments by double gold immunolabeling. Am J Physiol Cell Physiol 260: C1332–C1340, 1991. [DOI] [PubMed] [Google Scholar]
- 48.Flavahan NA, Bailey SR, Flavahan WA, Mitra S, Flavahan S. Imaging remodeling of the actin cytoskeleton in vascular smooth muscle cells after mechanosensitive arteriolar constriction. Am J Physiol Heart Circ Physiol 288: H660–H669, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Flynn CR, Komalavilas P, Tessier D, Thresher J, Niederkofler EE, Dreiza CM, Nelson RW, Panitch A, Joshi L, Brophy CM. Transduction of biologically active motifs of the small heat shock-related protein HSP20 leads to relaxation of vascular smooth muscle. FASEB J 17: 1358–1360, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Fredberg JJ, Inouye D, Miller B, Nathan M, Jafari S, Raboudi SH, Butler JP, Shore SA. Airway smooth muscle, tidal stretches, and dynamically determined contractile states. Am J Respir Crit Care Med 156: 1752–1759, 1997. [DOI] [PubMed] [Google Scholar]
- 51.Fultz ME, Li C, Geng W, Wright GL. Remodeling of the actin cytoskeleton in the contracting A7r5 smooth muscle cell. J Muscle Res Cell Motil 21: 775–787, 2000. [DOI] [PubMed] [Google Scholar]
- 52.Furst DO, Cross RA, De Mey J, Small JV. Caldesmon is an elongated, flexible molecule localized in the actomyosin domains of smooth muscle. EMBO J 5: 251–257, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galbraith CG, Yamada KM, Sheetz MP. The relationship between force and focal complex development. J Cell Biol 159: 695–705, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerthoffer WT, Gunst SJ. Invited review: focal adhesion and small heat shock proteins in the regulation of actin remodeling and contractility in smooth muscle. J Appl Physiol 91: 963–972, 2001. [DOI] [PubMed] [Google Scholar]
- 55.Gerthoffer WT Migration of airway smooth muscle cells. Proc Am Thorac Soc 5: 97–105, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gohla A, Bokoch GM. 14-3-3 regulates actin dynamics by stabilizing phosphorylated cofilin. Curr Biol 12: 1704–1710, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Goley ED, Welch MD. The ARP2/3 complex: an actin nucleator comes of age. Nat Rev Mol Cell Biol 7: 713–726, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Gregersen H, Emery JL, McCulloch AD. History-dependent mechanical behavior of guinea-pig small intestine. Ann Biomed Eng 26: 850–858, 1998. [DOI] [PubMed] [Google Scholar]
- 59.Gunst SJ Role of airway smooth muscle mechanical properties in the regulation of airway caliber. In: Mechanics of Breathing: Pathophysiology, Diagnosis and Treatment, edited by Alverti A. Milan, Italy: Springer-Verlag Italia, 2002, p. 34–44.
- 60.Gunst SJ, Fredberg JJ. The first three minutes: smooth muscle contraction, cytoskeletal events, and soft glasses. J Appl Physiol 95: 413–425, 2003. [DOI] [PubMed] [Google Scholar]
- 61.Gunst SJ, Meiss RA, Wu MF, Rowe M. Mechanisms for the mechanical plasticity of tracheal smooth muscle. Am J Physiol Cell Physiol 268: C1267–C1276, 1995. [DOI] [PubMed] [Google Scholar]
- 62.Gunst SJ, Tang DD, Opazo SA. Cytoskeletal remodeling of the airway smooth muscle cell: a mechanism for adaptation to mechanical forces in the lung. Respir Physiol Neurobiol 137: 151–168, 2003. [DOI] [PubMed] [Google Scholar]
- 63.Hedges JC, Dechert MA, Yamboliev IA, Martin JL, Hickey E, Weber LA, Gerthoffer WT. A role for p38(MAPK)/HSP27 pathway in smooth muscle cell migration. J Biol Chem 274: 24211–24219, 1999. [DOI] [PubMed] [Google Scholar]
- 64.Hellstrand P, Albinsson S. Stretch-dependent growth and differentiation in vascular smooth muscle: role of the actin cytoskeleton. Can J Physiol Pharmacol 83: 869–875, 2005. [DOI] [PubMed] [Google Scholar]
- 65.Herrera AM, Martinez EC, Seow CY. Electron microscopic study of actin polymerization in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 286: L1161–L1168, 2004. [DOI] [PubMed] [Google Scholar]
- 66.Higgs HN, Pollard TD. Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins. Annu Rev Biochem 70: 649–676, 2001. [DOI] [PubMed] [Google Scholar]
- 67.Hirshman CA, Emala CW. Actin reorganization in airway smooth muscle cells involves Gq and Gi-2 activation of Rho. Am J Physiol Lung Cell Mol Physiol 277: L653–L661, 1999. [DOI] [PubMed] [Google Scholar]
- 68.Hirshman CA, Togashi H, Shao D, Emala CW. Gαi-2 is required for carbachol-induced stress fiber formation in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 275: L911–L916, 1998. [DOI] [PubMed] [Google Scholar]
- 69.Huang Y, Craig SW, Gunst SJ. Activation of vinculin induced by contractile stimulation regulates tension development in airway smooth muscle (ASM) (Abstract). Am J Respir Crit Care Med 177: A594, 2008. [Google Scholar]
- 70.Huang TY, DerMardirossian C, Bokoch GM. Cofilin phosphatases and regulation of actin dynamics. Curr Opin Cell Biol 18: 26–31, 2006. [DOI] [PubMed] [Google Scholar]
- 71.Ishida T, Ishida M, Suero J, Takahashi M, Berk BC. Agonist-stimulated cytoskeletal reorganization and signal transduction at focal adhesions in vascular smooth muscle cells require c-Src. J Clin Invest 103: 789–797, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jones KA, Perkins WJ, Lorenz RR, Prakash YS, Sieck GC, Warner DO. F-actin stabilization increases tension cost during contraction of permeabilized airway smooth muscle in dogs. J Physiol 519: 527–538, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaibuchi K, Kuroda S, Amano M. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu Rev Biochem 68: 459–486, 1999. [DOI] [PubMed] [Google Scholar]
- 74.Kim HR, Hoque M, Hai CM. Cholinergic receptor-mediated differential cytoskeletal recruitment of actin- and integrin-binding proteins in intact airway smooth muscle. Am J Physiol Cell Physiol 287: C1375–C1383, 2004. [DOI] [PubMed] [Google Scholar]
- 75.Kiuchi T, Ohashi K, Kurita S, Mizuno K. Cofilin promotes stimulus-induced lamellipodium formation by generating an abundant supply of actin monomers. J Cell Biol 177: 465–476, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Komalavilas P, Penn RB, Flynn CR, Thresher J, Lopes LB, Furnish EJ, Guo M, Pallero MA, Murphy-Ullrich JE, Brophy CM. The small heat shock-related protein, HSP20, is a cAMP-dependent protein kinase substrate that is involved in airway smooth muscle relaxation. Am J Physiol Lung Cell Mol Physiol 294: L69–L78, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kuhn TB, Meberg PJ, Brown MD, Bernstein BW, Minamide LS, Jensen JR, Okada K, Soda EA, Bamburg JR. Regulating actin dynamics in neuronal growth cones by ADF/cofilin and rho family GTPases. J Neurobiol 44: 126–144, 2000. [PubMed] [Google Scholar]
- 78.Leduc I, Meloche S. Angiotensin II stimulates tyrosine phosphorylation of the focal adhesion-associated protein paxillin in aortic smooth muscle cells. J Biol Chem 270: 4401–4404, 1995. [DOI] [PubMed] [Google Scholar]
- 79.Machesky LM, Insall RH. Signaling to actin dynamics. J Cell Biol 146: 267–272, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martinez-Lemus LA, Wu X, Wilson E, Hill MA, Davis GE, Davis MJ, Meininger GA. Integrins as unique receptors for vascular control. J Vasc Res 40: 211–233, 2003. [DOI] [PubMed] [Google Scholar]
- 81.Mauss S, Koch G, Kreye VA, Aktories K. Inhibition of the contraction of the isolated longitudinal muscle of the guinea-pig ileum by botulinum C2 toxin: evidence for a role of G/F-actin transition in smooth muscle contraction. Naunyn Schmiedebergs Arch Pharmacol 340: 345–351, 1989. [DOI] [PubMed] [Google Scholar]
- 82.May RC The Arp2/3 complex: a central regulator of the actin cytoskeleton. Cell Mol Life Sci 58: 1607–1626, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meberg PJ, Ono S, Minamide LS, Takahashi M, Bamburg JR. Actin depolymerizing factor and cofilin phosphorylation dynamics: response to signals that regulate neurite extension. Cell Motil Cytoskeleton 39: 172–190, 1998. [DOI] [PubMed] [Google Scholar]
- 84.Mehta D, Gunst SJ. Actin polymerization stimulated by contractile activation regulates force development in canine tracheal smooth muscle. J Physiol 519: 829–840, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meiss RA Persistent mechanical effects of decreasing length during isometric contraction of ovarian ligament smooth muscle. J Muscle Res Cell Motil 14: 205–218, 1993. [DOI] [PubMed] [Google Scholar]
- 86.Moissoglu K, Schwartz MA. Integrin signalling in directed cell migration. Biol Cell 98: 547–555, 2006. [DOI] [PubMed] [Google Scholar]
- 87.Moriyama K, Iida K, Yahara I. Phosphorylation of Ser-3 of cofilin regulates its essential function on actin. Genes Cells 1: 73–86, 1996. [DOI] [PubMed] [Google Scholar]
- 88.Mullins RD, Heuser JA, Pollard TD. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc Natl Acad Sci USA 95: 6181–6186, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol 144: 1235–1244, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.North AJ, Gimona M, Lando Z, Small JV. Actin isoform compartments in chicken gizzard smooth muscle cells. J Cell Sci 107: 445–455, 1994. [DOI] [PubMed] [Google Scholar]
- 91.Obara K, Yabu H. Effect of cytochalasin B on intestinal smooth muscle cells. Eur J Pharmacol 255: 139–147, 1994. [DOI] [PubMed] [Google Scholar]
- 92.Ogden K, Thompson JM, Hickner Z, Huang T, Tang DD, Watts SW. A new signaling paradigm for serotonin: use of Crk-associated substrate in arterial contraction. Am J Physiol Heart Circ Physiol 291: H2857–H2863, 2006. [DOI] [PubMed] [Google Scholar]
- 93.Ohanian V, Gatfield K, Ohanian J. Role of the actin cytoskeleton in G-protein-coupled receptor activation of PYK2 and paxillin in vascular smooth muscle. Hypertension 46: 93–99, 2005. [DOI] [PubMed] [Google Scholar]
- 94.Ono S, Ono K. Tropomyosin inhibits ADF/cofilin-dependent actin filament dynamics. J Cell Biol 156: 1065–1076, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Opazo SA, Zhang W, Wu Y, Turner CE, Tang DD, Gunst SJ. Tension development during contractile stimulation of smooth muscle requires recruitment of paxillin and vinculin to the membrane. Am J Physiol Cell Physiol 286: C433–C447, 2004. [DOI] [PubMed] [Google Scholar]
- 96.Otey CA, Carpen O. Alpha-actinin revisited: a fresh look at an old player. Cell Motil Cytoskeleton 58: 104–111, 2004. [DOI] [PubMed] [Google Scholar]
- 97.Panchal SC, Kaiser DA, Torres E, Pollard TD, Rosen MK. A conserved amphipathic helix in WASP/Scar proteins is essential for activation of Arp2/3 complex. Nat Struct Biol 10: 591–598, 2003. [DOI] [PubMed] [Google Scholar]
- 98.Pantaloni D, Le Clainche C, Carlier MF. Mechanism of actin-based motility. Science 292: 1502–1506, 2001. [DOI] [PubMed] [Google Scholar]
- 99.Parker CA, Takahashi K, Tao T, Morgan KG. Agonist-induced redistribution of calponin in contractile vascular smooth muscle cells. Am J Physiol Cell Physiol 267: C1262–C1270, 1994. [DOI] [PubMed] [Google Scholar]
- 100.Parker CA, Takahashi K, Tang JX, Tao T, Morgan KG. Cytoskeletal targeting of calponin in differentiated, contractile smooth muscle cells of the ferret. J Physiol 508: 187–198, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pavalko FM, Adam LP, Wu MF, Walker TL, Gunst SJ. Phosphorylation of dense-plaque proteins talin and paxillin during tracheal smooth muscle contraction. Am J Physiol Cell Physiol 268: C563–C571, 1995. [DOI] [PubMed] [Google Scholar]
- 102.Pollard TD, Blanchoin L, Mullins RD. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu Rev Biophys Biomol Struct 29: 545–576, 2000. [DOI] [PubMed] [Google Scholar]
- 103.Rembold CM, Foster DB, Strauss JD, Wingard CJ, Eyk JE. cGMP-mediated phosphorylation of heat shock protein 20 may cause smooth muscle relaxation without myosin light chain dephosphorylation in swine carotid artery. J Physiol 524: 865–878, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rembold CM, Tejani AD, Ripley ML, Han S. Paxillin phosphorylation, actin polymerization, noise temperature, and the sustained phase of swine carotid artery contraction. Am J Physiol Cell Physiol 293: C993–C1002, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ridley AJ Rho family proteins and regulation of the actin cytoskeleton. Prog Mol Subcell Biol 22: 1–22, 1999. [DOI] [PubMed] [Google Scholar]
- 106.Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, Kirschner MW. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 97: 221–231, 1999. [DOI] [PubMed] [Google Scholar]
- 107.Saito SY, Hori M, Ozaki H, Karaki H. Cytochalasin D inhibits smooth muscle contraction by directly inhibiting contractile apparatus. J Smooth Muscle Res 32: 51–60, 1996. [DOI] [PubMed] [Google Scholar]
- 108.Salinthone S, Tyagi M, Gerthoffer WT. Small heat shock proteins in smooth muscle. Pharmacol Ther 119: 44–54, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.San Martin A, Lee MY, Williams HC, Mizuno K, Lassegue B, Griendling KK. Dual regulation of cofilin activity by LIM kinase and Slingshot-1L phosphatase controls platelet-derived growth factor-induced migration of human aortic smooth muscle cells. Circ Res 102: 432–438, 2008. [DOI] [PubMed] [Google Scholar]
- 110.Sastry SK, Burridge K. Focal adhesions: a nexus for intracellular signaling and cytoskeletal dynamics. Exp Cell Res 261: 25–36, 2000. [DOI] [PubMed] [Google Scholar]
- 111.Schmidt CE, Horwitz AF, Lauffenburger DA, Sheetz MP. Integrin-cytoskeletal interactions in migrating fibroblasts are dynamic, asymmetric, and regulated. J Cell Biol 123: 977–991, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shaw L, Ahmed S, Austin C, Taggart MJ. Inhibitors of actin filament polymerisation attenuate force but not global intracellular calcium in isolated pressurised resistance arteries. J Vasc Res 40: 1–10, 2003. [DOI] [PubMed] [Google Scholar]
- 113.Skloot G, Permutt S, Togias A. Airway hyperresponsiveness in asthma: a problem of limited smooth muscle relaxation with inspiration. J Clin Invest 96: 2393–2403, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Small JV Geometry of actin-membrane attachments in the smooth muscle cell: the localisations of vinculin and alpha-actinin. EMBO J 4: 45–49, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Smith BA, Tolloczko B, Martin JG, Grutter P. Probing the viscoelastic behavior of cultured airway smooth muscle cells with atomic force microscopy: stiffening induced by contractile agonist. Biophys J 88: 2994–3007, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Smith PG, Garcia R, Kogerman L. Mechanical strain increases protein tyrosine phosphorylation in airway smooth muscle cells. Exp Cell Res 239: 353–360, 1998. [DOI] [PubMed] [Google Scholar]
- 117.Smith PG, Garcia R, Kogerman L. Strain reorganizes focal adhesions and cytoskeleton in cultured airway smooth muscle cells. Exp Cell Res 232: 127–136, 1997. [DOI] [PubMed] [Google Scholar]
- 118.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003. [DOI] [PubMed] [Google Scholar]
- 119.Speich JE, Borgsmiller L, Call C, Mohr R, Ratz PH. ROK-induced cross-link formation stiffens passive muscle: reversible strain-induced stress softening in rabbit detrusor. Am J Physiol Cell Physiol 289: C12–C21, 2005. [DOI] [PubMed] [Google Scholar]
- 120.Srinivasan R, Forman S, Quinlan RA, Ohanian J, Ohanian V. Regulation of contractility by Hsp27 and Hic-5 in rat mesenteric small arteries. Am J Physiol Heart Circ Physiol 294: H961–H969, 2008. [DOI] [PubMed] [Google Scholar]
- 121.Stradal TE, Scita G. Protein complexes regulating Arp2/3-mediated actin assembly. Curr Opin Cell Biol 18: 4–10, 2006. [DOI] [PubMed] [Google Scholar]
- 122.Sul D, Baron CB, Broome R, Coburn RF. Smooth muscle length-dependent PI(4,5)P2 synthesis and paxillin tyrosine phosphorylation. Am J Physiol Cell Physiol 281: C300–C310, 2001. [DOI] [PubMed] [Google Scholar]
- 123.Tang DD, Gunst SJ. Dominant negative Cdc42 mutant inhibits contractile force development and the association of N-WASp with Arp2/3 complex during stimulation of smooth muscle tissues. Am J Respir Crit Care Med 169: 2004.
- 124.Tang DD, Gunst SJ. Roles of focal adhesion kinase and paxillin in the mechanosensitive regulation of myosin phosphorylation in smooth muscle. J Appl Physiol 91: 1452–1459, 2001. [DOI] [PubMed] [Google Scholar]
- 125.Tang DD, Mehta D, Gunst SJ. Mechanosensitive tyrosine phosphorylation of paxillin and focal adhesion kinase in tracheal smooth muscle. Am J Physiol Cell Physiol 276: C250–C258, 1999. [DOI] [PubMed] [Google Scholar]
- 126.Tang DD, Turner CE, Gunst SJ. Expression of non-phosphorylatable paxillin mutants in canine tracheal smooth muscle inhibits tension development. J Physiol 553: 21–35, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tang DD, Zhang W, Gunst SJ. The adapter protein CrkII regulates neuronal Wiskott-Aldrich syndrome protein, actin polymerization, and tension development during contractile stimulation of smooth muscle. J Biol Chem 280: 23380–23389, 2005. [DOI] [PubMed] [Google Scholar]
- 128.Tang DD, Gunst SJ. The small GTPase Cdc42 regulates actin polymerization and tension development during contractile stimulation of smooth muscle. J Biol Chem 279: 51722–51728, 2004. [DOI] [PubMed] [Google Scholar]
- 129.Tang DD, Wu MF, Opazo Saez AM, Gunst SJ. The focal adhesion protein paxillin regulates contraction in canine tracheal smooth muscle. J Physiol 542: 501–513, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Togashi H, Emala CW, Hall IP, Hirshman CA. Carbachol-induced actin reorganization involves Gi activation of Rho in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 274: L803–L809, 1998. [DOI] [PubMed] [Google Scholar]
- 131.Tseng S, Kim R, Kim T, Morgan KG, Hai CM. F-actin disruption attenuates agonist-induced [Ca2+], myosin phosphorylation, and force in smooth muscle. Am J Physiol Cell Physiol 272: C1960–C1967, 1997. [DOI] [PubMed] [Google Scholar]
- 132.Turner CE, Glenney JR Jr, Burridge K. Paxillin: a new vinculin-binding protein present in focal adhesions. J Cell Biol 111: 1059–1068, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Von Wichert G, Haimovich B, Feng GS, Sheetz MP. Force-dependent integrin-cytoskeleton linkage formation requires downregulation of focal complex dynamics by Shp2. EMBO J 22: 5023–5035, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang Z, Pavalko FM, Gunst SJ. Tyrosine phosphorylation of the dense plaque protein paxillin is regulated during smooth muscle contraction. Am J Physiol Cell Physiol 271: C1594–C1602, 1996. [DOI] [PubMed] [Google Scholar]
- 135.Wiesner S, Legate KR, Fassler R. Integrin-actin interactions. Cell Mol Life Sci 62: 1081–1099, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wood CK, Turner CE, Jackson P, Critchley DR. Characterisation of the paxillin-binding site and the C-terminal focal adhesion targeting sequence in vinculin. J Cell Sci 107: 709–717, 1994. [PubMed] [Google Scholar]
- 137.Woodrum D, Pipkin W, Tessier D, Komalavilas P, Brophy CM. Phosphorylation of the heat shock-related protein, HSP20, mediates cyclic nucleotide-dependent relaxation. J Vasc Surg 37: 874–881, 2003. [DOI] [PubMed] [Google Scholar]
- 138.Wright G, Hurn E. Cytochalasin inhibition of slow tension increase in rat aortic rings. Am J Physiol Heart Circ Physiol 267: H1437–H1446, 1994. [DOI] [PubMed] [Google Scholar]
- 139.Wu X, Davis GE, Meininger GA, Wilson E, Davis MJ. Regulation of the L-type calcium channel by α5β1 integrin requires signaling between focal adhesion proteins. J Biol Chem 276: 30285–30292, 2001. [DOI] [PubMed] [Google Scholar]
- 140.Youn T, Kim SA, Hai CM. Length-dependent modulation of smooth muscle activation: effects of agonist, cytochalasin, and temperature. Am J Physiol Cell Physiol 274: C1601–C1607, 1998. [DOI] [PubMed] [Google Scholar]
- 141.Zeidan A, Nordstrom I, Albinsson S, Malmqvist U, Sward K, Hellstrand P. Stretch-induced contractile differentiation of vascular smooth muscle: sensitivity to actin polymerization inhibitors. Am J Physiol Cell Physiol 284: C1387–C1396, 2003. [DOI] [PubMed] [Google Scholar]
- 142.Zhang W, Gunst SJ. Interactions of airway smooth muscle cells with their tissue matrix: implications for contraction. Proc Am Thorac Soc 5: 32–39, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang W, Wu Y, Du L, Tang DD, Gunst SJ. Activation of the Arp2/3 complex by N-WASp is required for actin polymerization and contraction in smooth muscle. Am J Physiol Cell Physiol 288: C1145–C1160, 2005. [DOI] [PubMed] [Google Scholar]
- 144.Zhang W, Wu Y, Wu C, Gunst SJ. Integrin-linked kinase (ILK) regulates N-WASp-mediated actin polymerization and tension development in tracheal smooth muscle. J Biol Chem 282: 34568–34580, 2007. [DOI] [PubMed] [Google Scholar]
- 145.Zhang WW, Gunst SJ. Dynamic association between alpha-actinin and beta-integrin regulates contraction of canine tracheal smooth muscle. J Physiol 572: 659–676, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang YJ, Chen K, Tu YZ, Velyvis A, Yang YW, Qin J, Wu CY. Assembly of the PINCH-ILK-CH-ILKBP complex precedes and is essential for localization of each component to cell-matrix adhesion sites. J Cell Sci 115: 4777–4786, 2002. [DOI] [PubMed] [Google Scholar]
- 147.Zhao R, Du L, Bamburg J, Gunst SJ. Cofilin phosphorylation regulates actin dynamics during contraction of canine tracheal smooth muscle (Abstract). Proc Am Thorac Soc 2007 4: A313, 2007. [Google Scholar]