Abstract
Glucose transport is a highly regulated process and is dependent on a variety of signaling events. Glycogen synthase kinase-3 (GSK-3) has been implicated in various aspects of the regulation of glucose transport, but the mechanisms by which GSK-3 activity affects glucose uptake have not been well defined. We report that basal glycogen synthase kinase-3 (GSK-3) activity regulates glucose transport in several cell types. Chronic inhibition of basal GSK-3 activity (8–24 h) in several cell types, including vascular smooth muscle cells, resulted in an approximately twofold increase in glucose uptake due to a similar increase in protein expression of the facilitative glucose transporter 1 (GLUT1). Conversely, expression of a constitutively active form of GSK-3β resulted in at least a twofold decrease in GLUT1 expression and glucose uptake. Since GSK-3 can inhibit mammalian target of rapamycin (mTOR) signaling via phosphorylation of the tuberous sclerosis complex subunit 2 (TSC2) tumor suppressor, we investigated whether chronic GSK-3 effects on glucose uptake and GLUT1 expression depended on TSC2 phosphorylation and TSC inhibition of mTOR. We found that absence of functional TSC2 resulted in a 1.5-to 3-fold increase in glucose uptake and GLUT1 expression in multiple cell types. These increases in glucose uptake and GLUT1 levels were prevented by inhibition of mTOR with rapamycin. GSK-3 inhibition had no effect on glucose uptake or GLUT1 expression in TSC2 mutant cells, indicating that GSK-3 effects on GLUT1 and glucose uptake were mediated by a TSC2/mTOR-dependent pathway. The effect of GSK-3 inhibition on GLUT1 expression and glucose uptake was restored in TSC2 mutant cells by transfection of a wild-type TSC2 vector, but not by a TSC2 construct with mutated GSK-3 phosphorylation sites. Thus, TSC2 and rapamycin-sensitive mTOR function downstream of GSK-3 to modulate effects of GSK-3 on glucose uptake and GLUT1 expression. GSK-3 therefore suppresses glucose uptake via TSC2 and mTOR and may serve to match energy substrate utilization to cellular growth.
Keywords: metabolism, cell signaling, S6 kinase, mammalian target of rapamycin, glycogen synthetase kinase, tuberous sclerosis complex
the movement of glucose in and out of cells is mediated by facilitative glucose transporters (GLUTs) in almost all mammalian cells. There are at least 13 glucose transporter isoforms differentially expressed in mammalian tissues (26), eight of which have been found to participate in glucose uptake. The first member of this family, GLUT1, is expressed in most cells and plays a role in basal glucose uptake in many tissues (19), but it may be regulated by insulin and other hormonal factors (16, 27). Glucose transporter expression and localization is regulated in a variety of ways depending on the cell type and the stimulus involved. The signaling pathways involved in acute insulin-stimulated GLUT4 translocation have been the subject of many studies over the past two decades. However, relatively few studies have examined the signaling systems involved in long-term regulation of GLUT1 mediated glucose uptake in noninsulin responsive tissues (4, 5, 11, 18, 36), and none of these previous reports have investigated the role of a signaling pathway that is an important regulator of cellular growth and metabolism, namely, the pathway that includes glycogen synthase kinase-3 (GSK-3), tuberous sclerosis complex (TSC), and mammalian target of rapamycin (mTOR).
GSK-3 is an important signaling molecule that is ubiquitously expressed and has been implicated in the regulation of glucose transport and metabolism. Originally identified as a key regulator of glycogen synthase activity, GSK-3 is now known to function as an important signaling molecule in several pathways and is involved in regulating gene transcription, protein translation, and apoptosis, as well as hexose metabolism (12). Two distinct isoforms of GSK-3 (α/β-isoforms) that are products of two independent genes (10) have been identified. GSK-3 is known to function downstream of phosphatidylinositol 3-kinase (PI3K) and Akt (41). There is a paucity of reports about regulation of GLUT1 mediated glucose uptake by GSK-3, and the few studies that have been performed have produced different findings in different cell systems (37, 42).
Recently, the TSC/mTOR signaling pathway has been shown to be downstream of GSK-3 (23). The TSC comprises two subunits, TSC1 and TSC2. Normal copies of each protein are needed for proper function of the complex (29), and mutations in either of the genes encoding the proteins have been found to cause tuberous sclerosis (17, 43). Various studies have implicated TSC in several different cellular functions, the most important of which seems to be growth regulation (24). Studies in Drosophila melanogaster have shown that mutations in the dTSC1 and dTSC2 genes lead to increased cell size. Mutations in TSC genes result in the constitutive activation of S6 kinase (S6K), which subsequently enhances protein translation (1, 6–9, 13, 14, 20, 22). The carboxyl terminus of TSC2 displays GTPase-activating protein (GAP) activity and has been shown to interact with the GTPase Rheb directly (21, 30, 31). Inactivation of TSC2 represses GAP activity and allows Rheb-GTP to accumulate. Rheb-GTP activates the protein kinase activity of mTOR, which in turn phosphorylates and activates S6K, leading to enhanced translation (21, 30, 31). Conversely, active TSC2 reduces Rheb-GTP accumulation (21, 30, 31) and reduces downstream mTOR and S6K activation.
Since the tuberous sclerosis complex (TSC) is implicated in both the insulin signaling pathway as a substrate for Akt and in the growth regulation pathway as an upstream regulator of mTOR and S6K, we hypothesized that GSK-3 acts via TSC2, as a negative growth regulator, to reduce glucose uptake by suppressing expression of GLUT1. In this report we present data showing that GSK-3 is a negative regulator of basal glucose uptake and GLUT1 expression and that GSK-3 exerts its inhibitory effects through a TSC2- and mTOR-dependent pathway.
MATERIALS AND METHODS
Materials and cell lines.
Rat LEF cell lines were derived from spontaneous renal tubular tumors in Long Evans Eker rats (17). Cells from these rats have an inactivating germline mutation in the TSC2 gene (28, 45). The embryonic fibroblast cells, EEF4 (EEF126-4) and EEF8 (EEF126-8), were derived from passages 12 to 14 of primary explants of embryos from a single heterozygous mating of Eker rats (25, 43). The retrovirus constructs containing TSC2 and enhanced green fluorescent protein (EGFP) genes were previously reported (22). TSC2-3A is a TSC2 mutant in which three of the four putative GSK-3 activating phosphorylation sites, S1337, S1341, and S1345, have been changed to alanines (23). The A7r5 rat vascular smooth muscle cell line was obtained from the American Type Culture Collection (CRL-1444). The GLUT1 antibody was a gift from Dr. Christin Carter-Su (University of Michigan). The anti-β-tubulin antibody was obtained from Upstate Biotechnology (Lake Placid, NY). Polyclonal antibodies for phosphorylated GSK-3α/βSer21/9, and total Akt were obtained from Cell Signaling Technology (Beverly, MA). Rapamycin, a mTOR inhibitor, was obtained from Sigma (St. Louis, MO). The GSK-3 inhibitor, SB-216763, was from BIOMOL International (Plymouth Meeting, PA). The GSK-3β inhibitor, Glycogen Synthase Kinase-3β Inhibitor II, was obtained from Calbiochem (San Diego, CA).
[3H]-2-deoxy-glucose (2-DOG) and [3H]-3-O-methyl-glucose (3-OMG) uptakes.
EEF or LEF cells were plated in six-well plates in Dulbecco's modified Eagle's medium (DMEM)/F12 medium with 10% fetal calf serum (FCS). A7r5 cells were plated on 60-mm plates in DMEM with 10% FCS. Cells were grown to ∼80% confluence before treatment and 2-DOG uptake analysis. 2-DOG uptakes were performed as previously described (32, 34). Slight protocol modifications were made in the 2-DOG protocol to accommodate rapid equilibration when using 3-OMG. Briefly, plates were washed once with Krebs-Ringer phosphate buffer (KRP) (in mmol/l: 128 NaCl, 5.2 KCl, 1.3 CaCl2, 2.6 MgSO4, and 10 Na2HPO4) and were then incubated with KRP buffer supplemented with 1% BSA for 10 min to 1 h at 37°C. Drugs were added to the KRP buffer during the 1-h incubation period for various time points as noted. The KRP buffer was removed and replaced with 0.1 mM unlabeled 2-DOG (Sigma) and 0.5 μCi/ml [3H]-2-DOG (Perkin Elmer, Waltham, MA) or [3H]-3-OMG (Sigma Aldrich) in KRP buffer + 1% (wt/vol) BSA ± 20 nM cytochalasin B, an inhibitor of glucose transport, at 37°C for 5 min, or room temperature for 6 s, respectively. We have found that 2-DOG uptake is linear for at least 10 min in EEF, LEF (data not shown), and A7r5 cell lines (32). The plates were subsequently washed twice for 5 min each time with cold KRP solution containing 200 μM phloretin to quench 2-DOG uptake. The samples were then lysed in buffer (EEF and LEF cell lines in 10 mM Tris·HCl, pH 7.0, 150 mM NaCl, 1% Triton X-100, and 1% SDS, and the A7r5 cell line in 0.1% SDS). A portion of each sample was used for determination of protein concentration by a bicinchoninic acid assay (Pierce, Rockford, IL), and the rest was used for scintillation counting. 2-DOG uptake was expressed as nmol·min−1·mg protein−1 after correction for nonspecific uptake in the presence of cytochalasin B.
Infection studies.
Retroviral infections were performed as previously described (22, 23). Human embryonic kidney (HEK)-293 cells at ∼50–80% confluence were transfected with a retroviral vector pPGS-CMV-CITE-Neo expressing either wild-type TSC2, mutant TSC2, or EGFP genes. Forty-eight hours after transfection, the media of transfected cells were harvested and passed through a 0.45-μM filter. Polybrene (5 μg/ml) was added to the viral solution before the mixture was added to the medium of freshly split EEF8 cells. The infection was repeated two more times at 12 and 24 h. Cells were ready to assay 16 to 24 h after the final infection.
A nonphosphorylatable GSK-3β adenoviral construct (GSK-3βS9A) was a gift from Dr. Morris Birnbaum (University of Pennsylvania School of Medicine). A7r5 cells were cultured at a density of 2 × 104 cells per dish in OptiMEM media for 2 h at 37°C, and a multiplicity of infection (MOI) of 1,000 was used to infect the cells. After a 2-h incubation period, OptiMEM media was replaced with DMEM + 10% FCS, and the cells were returned to 37°C for varying time points.
Immunoblotting.
Whole cell lysates were separated by SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked with TBS-Tween 20 (TBS-T) and 5% milk for 1 h and placed in primary antibody in TBS-T 5% milk overnight at 4°C (32). After three 10-min washes in TBS-T, the appropriate horseradish peroxidase-linked secondary antibody was then added in TBS-T 5% milk and incubated at room temperature for 1 h. Membranes were then washed three times (10 min each) in TBS-T, subjected to an enhanced chemiluminescence reaction (ECL), and exposed to autoradiography film. After autoradiography, the films were scanned and quantified using NIH ImageJ software.
Animals.
Adult male Sprague-Dawley rats were obtained from Harlan (Indianapolis, IN). Aortic lysates were prepared similarly as described previously (2).
Statistical analysis.
Data are expressed as means ± SE and were analyzed using one-way analysis of variance, followed by Newman-Keuls post hoc analysis or by Student's t-test. Data were considered significant at P < 0.05.
RESULTS
GSK-3 regulates acute and long-term GLUT1 expression and 2-DOG uptake.
Acute and 24-h inhibition of GSK-3 with either LiCl (20 mM) or SB-216763 (10 μM) in A7r5 cells resulted in a progressive increase in GLUT1 expression and 2-DOG uptake (Fig. 1). Similarly, GSK-3 inhibition for 24 or 48 h in rat aortic explants resulted in increased GLUT1 protein expression (Fig. 2). Moreover, adenoviral infection of A7r5 cells with a constitutively active form of GSK-3β (GSK-3βS9A) for 24 h resulted in a decrease in GLUT1 expression and 2-DOG uptake compared with values in cells infected with the vector control adenovirus (Fig. 3).
Fig. 1.
Acute inhibition (30 min) of glycogen synthase kinase-3 (GSK-3) activity with lithium chloride (20 mM) or SB-216763 (10 μM) caused increased [3H]-2-deoxy-glucose (2-DOG) uptake in A7r5 cells (*P < 0.05 vs. control) (A). More chronic GSK-3 inhibition (24 h) induced glucose transporter type 1 (GLUT1) protein expression (n = 6, *P < 0.05 vs. control) (B) as well as 2-DOG uptake (n = 8, **P < 0.01) (C).
Fig. 2.
GSK-3 inhibition with 20 mM lithium chloride (A) or 10 μM SB-216763 (B) in rat aortic explants resulted in increased GLUT1 protein expression. SMA, smooth muscle actin.
Fig. 3.
Adenoviral infection with a constitutively active GSK-3β for 48 h decreased GLUT1 expression (A) and 2-DOG uptake (B) in vascular smooth muscle cells (n = 7 each, *P < 0.05; **P < 0.01 vs. control).
Inactivation of the TSC2 gene results in increased 2-DOG uptake, GLUT1 expression, and S6K phosphorylation.
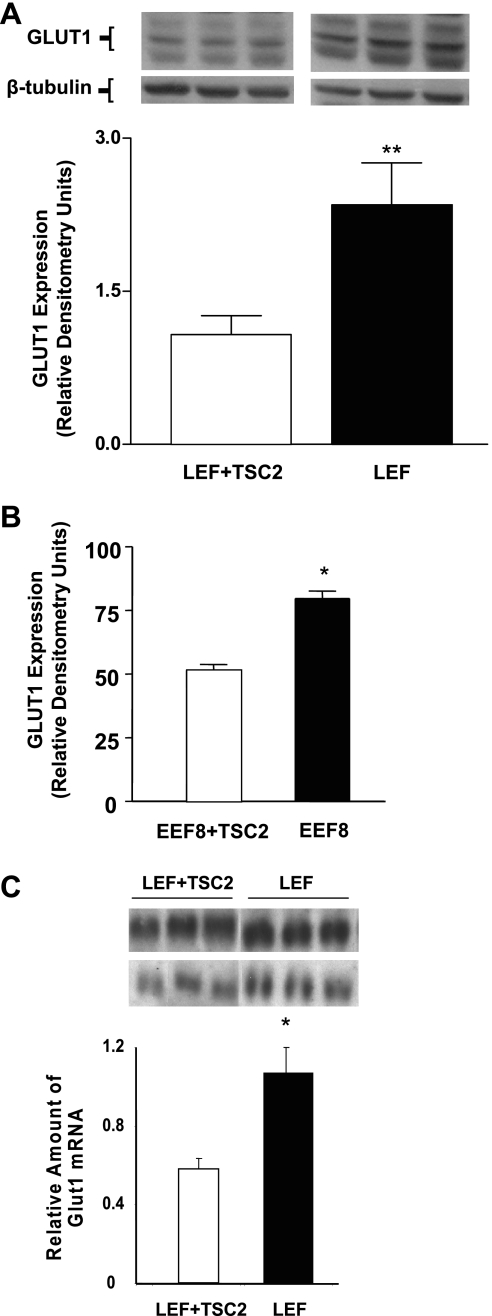
Glucose uptake in TSC2 mutant cell lines was substantially higher than in the respective control cells (Fig. 4A). Conversely, 2-DOG uptake was reduced in mutant cell lines acutely infected with a wild-type TSC2 expressing retrovirus compared with those infected with a control virus, consistent with the observation that wild-type TSC2 constitutively inhibits glucose uptake (Fig. 4B). To determine whether the increased rate of 2-DOG uptake corresponded to glucose transporter expression, we examined the levels of GLUT1 in TSC2 mutant cells. Introduction of the wild-type retroviral TSC2 vector into both TSC2 mutant cell lines caused an approximately twofold reduction in GLUT1 protein expression (Fig. 5, A and B) and a similar reduction in GLUT1 mRNA levels in the LEF+TSC2 cells (Fig. 5C). Expression of functional TSC2 in LEF cells also led to reduced phosphorylation on threonine 389 of S6K (Fig. 6).
Fig. 4.
2-DOG uptake was increased in embryonic fibroblast cells (EEF8) and renal cells (LEF) lacking functional tuberous sclerosis complex subunit 2 (TSC2) compared with wild-type cells (EEF4) or TSC2 mutant cells in which a wild-type TSC2 construct was stably expressed (LEF+TSC2) (n = 12 each, *P < 0.05) (A). Similarly, 2-DOG uptake was increased in TSC2 mutant cells acutely infected with a retrovirus expressing wild-type TSC2 (EEF8+TSC2; n = 14) vs. the same cells infected with a control vector (EEF8; n = 11) for 24 h (*P < 0.05 vs. TSC2-infected cells) (B).
Fig. 5.
GLUT1 protein expression was increased in the absence of functional TSC2 in LEF cells (n = 12, **P < 0.01) (A) and in EEF8 cells (n = 3, *P < 0.05) (B) when compared with cells with stable (LEF) or transient (EEF8) expression of a wild-type TSC2 construct. GLUT1 RNA expression was also increased in cells that do not express functional TSC2 (n = 6, *P < 0.05 vs. LEF+TSC2) (C).
Fig. 6.
LEF cells that lack functional TSC2 exhibit increased S6 kinase (SK6) phosphorylation on mammalian target of rapamycin (mTOR) C1-specific residue, T389 (n = 8, *P < 0.05 vs. LEF+TSC2).
GSK-3 regulates glucose uptake in TSC2-positive cells.
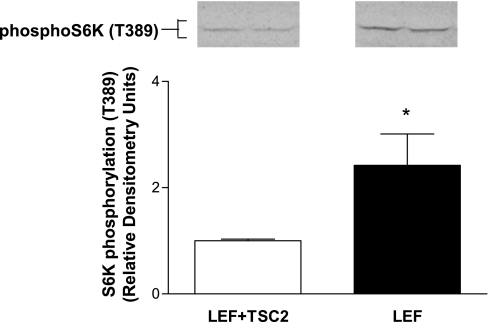
GSK-3 phosphorylates TSC2 to enhance TSC complex stability and hence TSC inhibition of mTOR (23). Therefore, we assessed whether GSK-3 is upstream of TSC2 in the regulation of glucose uptake by testing the effect of a GSK-3 inhibitor on LEF cells lacking functional TSC2. Uptakes of 3-OMG also were performed in these cells to ensure that the effects of GSK-3 and TSC were on GLUT1-mediated transport and not on hexokinase activity, since 2-DOG is phosphorylated by hexokinases. Treatment of TSC2 mutant LEF cells with the GSK-3 inhibitor SB-216763 (10 μM) for 24 h did not affect GLUT1 expression or either 2-DOG or 3-OMG uptake. However, SB-216763 treatment of LEF+TSC2 cells led to a significant increase in GLUT1 protein and glucose uptake (Fig. 7). To confirm that GSK-3 is upstream of TSC2, TSC2 mutant (EEF8) cells were infected with a wild-type TSC2 construct or a TSC2 construct mutated at three GSK-3 phosphorylation sites (23). 2-DOG uptake was reduced in cells infected with the wild-type, but not with the mutant, TSC2 construct (Fig. 8). Together, these data indicate that GSK-3 phosphorylation of TSC2 plays a critical role in stimulating TSC2-mediated suppression of glucose uptake and that essentially all of the GSK-3 effect on glucose uptake is mediated via TSC2 in this cell system. Despite the role of GSK-3 in glucose uptake in these cells and the potential role of GSK-3 in mediating glucose uptake in response to insulin signaling (16), insulin (100 nM) failed to increase glucose uptake at any time point between 15 min and 2 h in either TSC2-positive or TSC2 mutant cells (data not shown).
Fig. 7.
GSK-3 inhibition (24 h) with SB-216763 (10 μM) increased GLUT1 expression (n = 9, **P < 0.01 vs. LEF+TSC2 control) (A), increased 2-DOG uptake (n = 12 *P < 0.05 vs. LEF+TSC2 control) (B), and increased [3H]-3-O-methyl-glucose uptake (n = 8, **P < 0.01, *P < 0.05 vs. LEF+TSC2 control) (C) only in the presence of functional TSC2.
Fig. 8.
Wild-type, but not mutant, TSC2 expression resulted in decreased 2-DOG uptake in EEF8 cells infected (24 h) with either a wild-type TSC2 construct (TSC2), empty control vector (Empty), or a TSC2 construct mutated at three GSK-3 phosphorylation sites (TSC2–3A). GSK-3 inhibition (10 nM Glycogen Synthase Kinase-3β Inhibitor II, 8 h) abrogated the wild-type TSC2 effect (n = 5, *P < 0.05, vs. TSC2 control).
Glucose uptake in the absence of TSC2 is partly mediated by a rapamycin-sensitive mTOR-dependent pathway.
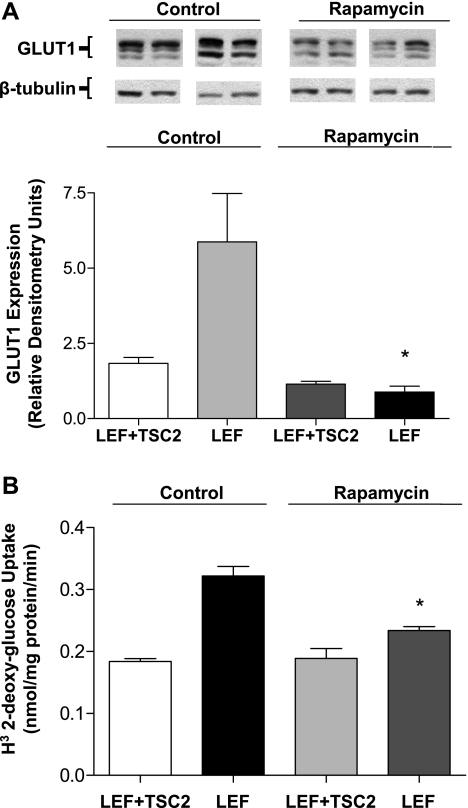
TSC inhibits mTOR through the small G protein Rheb (21, 30, 31). Inactivation of TSC or deletion of TSC2 is associated with an increase in mTOR signaling (21, 30, 31). To determine whether mTOR activation is required for the enhanced GLUT1 expression and glucose uptake seen in TSC2 mutant cells, we treated cells with rapamycin to inhibit mTOR signaling. Exposure to rapamycin was restricted to 8 h instead of 24 h to eliminate possible effects on mTORC2 activity or other nonspecific effects. After treatment with 20 nM rapamycin for 8 h, GLUT1 protein expression was significantly reduced compared with that in vehicle-treated cells, but it was only slightly and statistically insignificantly reduced in TSC2-positive cells (Fig. 9A). The effects on 2-DOG uptake paralleled the changes in GLUT1, with rapamycin reducing uptake only in the TSC2-positive cells (Fig. 9B).
Fig. 9.
Rapamycin treatment (20 nM, 8 h) resulted in reduced GLUT1 expression (n = 3, *P < 0.05, vs. LEF control) (A) and 2-DOG uptake (n = 12, *P < 0.05 vs. LEF control) (B) only in cells lacking functional TSC2.
DISCUSSION
In the current report, GSK-3 was found to reduce glucose uptake by suppressing GLUT1 expression. We also found that chronic GSK-3 inhibition enhanced glucose uptake and GLUT1 expression in TSC2-expressing cells but not in cells lacking functional TSC2. In addition, mutation of three GSK-3 phosphorylation sites on TSC2 abrogated the effect of GSK-3 on glucose uptake. Finally, the increase in GLUT1 expression found in TSC2 mutant cells was prevented by the mTOR inhibitor rapamycin. Thus the effects of GSK-3 on glucose uptake and GLUT1 were dependent on TSC2, whereas TSC2 effects on GLUT1 were mediated by mTOR. The chronic changes in glucose uptake and GLUT1 expression were independent of acute insulin signaling in the cells we studied.
In contrast to the literature on participation of GSK-3 in insulin signaling, there have been few studies on the role of GSK-3 in the regulation of glucose uptake in noninsulin-stimulated conditions or in cell types in which glucose uptake is not insulin sensitive. In one of the few reports, Nikoulina et al. (37) found that inhibition of GSK-3 for 4 days increased both basal and insulin-stimulated glucose uptake in skeletal muscle without changing expression of either GLUT1 or GLUT4. Similarly, Bentley et al. (4) showed that IL-3 stimulated translocation of GLUT1 to the cell surface of mast cells, and Wieman et al. (42) found that this IL-3 effect on GLUT1 translocation was via a PI3K/Akt/GSK-3-dependent mechanism that was not mTOR dependent. Interestingly, however, these latter investigators found that glucose uptake was reduced by mTOR inhibition without changes in GLUT1 cell surface levels, suggesting that mTOR enhanced the intrinsic activity of GLUT1 transporters (42). Our results, in different cells, under different conditions, and with different time points, contrast with those of these previous studies. Our findings strongly support participation of the GSK-3/TSC2/mTOR pathway in the chronic regulation of basal GLUT1 and glucose uptake in several cell types. We did not directly examine GSK-3/TSC2/mTOR effects on cell surface GLUT1 expression, yet the observed effects on total GLUT1 expression were equal to or greater than the effects on glucose uptake, suggesting that regulation of translocation, if present, was modest. Nonetheless, this aspect of GLUT1 regulation was not monitored in our studies and would need to be confirmed with cell surface localization studies. By comparison, the study of Wieman et al. (42) evaluated a FLAG-tagged transfected GLUT1, and it did not consider GSK-3 or mTOR effects on total cellular GLUT1 expression.
Previous studies on GLUT1 regulation have included evidence that cytokines, such as IL-3, can stimulate GLUT1-mediated glucose transport in mast cells via a PI3K-dependent mechanism (4). In addition, a number of investigators have shown that cellular metabolism and ATP availability play a significant role in regulating GLUT1 expression and function through both direct and indirect mechanisms. Another report suggests that direct interaction of ATP with GLUT1-specific peptide sequences can lead to conformational changes that modulate transport of glucose and inhibit degradation of GLUT1 (18, 35, 36). Moreover, studies in skeletal muscle have shown that expression of a constitutively active form of AMP kinase (AMPK) results in increased GLUT1 and GLUT4 protein levels (11). Direct regulation of GLUT1 by availability of energy substrates has been reported by multiple groups. Blodgett et al. (5) have identified GLUT1 domains that undergo conformational changes that may prolong protein longevity and alter glucose transport after direct interaction with ATP. Other investigators have demonstrated that energy depletion and osmotic stress activate AMPK, which, in turn, leads to concordant increases in glucose transport and GLUT1 expression (3, 11). Further experimentation will be necessary to ascertain whether AMPK participates in GSK-3/TSC2 regulation of GLUT1.
mTOR functions in one of two protein complexes, the rapamycin-sensitive mTOR complex 1 (mTORC1) and the rapamycin insensitive mTOR complex 2 (mTORC2) (20, 39, 40). mTORC1 consists of mTOR, raptor, and mLST8, whereas mTORC2 consists of mTOR, rictor, mLST8, and Sin-1 (20, 40). Although mTORC2 has been identified as PDK2, responsible for activation of Akt by phosphorylation at serine 473 (39, 40), whether mTORC2 plays a significant role in regulation of expression and function of glucose transporters is not known. It appears that TSC2 effects on GLUT1 expression and glucose uptake are dependent on inhibition of mTORC1 since the mTORC1 inhibitor rapamycin reversed the effects of TSC2 mutation or abrogation. Conversely, rapamycin had no effect on GLUT1 expression or glucose uptake in cells lacking functional TSC2.
It appears that the chronic regulatory effects of GSK-3 on GLUT1 expression and glucose uptake are mediated via TSC2. Indeed, the effects of GSK-3 were entirely abrogated in the rat fibroblast system in the absence of TSC2 as well as when a TSC2 molecule with mutations at three of the four GSK-3 phosphorylation sites was expressed. Although, to our knowledge, this is the first report to show GSK-3 signaling via TSC2 and mTOR effects on GLUT1 gene expression and glucose uptake, Kaelin's group has reported that hypoxia-inducible factor (HIF)-1α levels were enhanced through an mTOR-dependent mechanism in cells derived from TSC2−/− mouse embryo fibroblasts (6). HIF-1α enhanced the transcription of a number of genes, including GLUT1. Although we did not measure HIF-1α levels in these studies, it seems unlikely that it played a role in GLUT1 expression in the cell systems in our report because there was no stimulus for an increase in HIF-1α levels.
GSK-3/TSC2/mTOR regulation of glucose transport likely serves to couple cellular growth with substrate uptake. Cell growth induced by growth factors is mediated in part via activation of the PI3K/Akt pathway. Akt in turn phosphorylates and inactivates both GSK-3β and TSC2. As we have shown in the present study, this inactivation of GSK-3β and TSC2 results in increased GLUT1 levels and in enhanced glucose uptake through these high-affinity transporters. Conversely, when the PI3K/Akt pathway is inactivated on withdrawal of growth factors, GSK-3 and TSC2 are activated resulting in TSC2-mediated inhibition of Rheb, which, in turn, inactivates mTOR, leading to reduced GLUT1 levels as well as reduced glucose uptake. This regulatory process would help match fuel supply to metabolic requirements and would prevent increases in intracellular glucose concentration that could result in glucose toxicity. However, there are hints that this regulatory pathway could be active in a less homeostatic relationship. Both enhanced mTOR activity (38, 44) and increased GLUT1 expression (15) have been implicated in the pathogenesis of diabetic complications, especially nephropathy. Since upregulation of mTOR leads to enhanced GLUT1 expression, it is possible that mTOR plays a pathogenic role by stimulating GLUT1 expression and glucose uptake into susceptible glomerular cells in the kidney. Further experimentation will be needed to test this possibility.
The data herein suggest that TSC2 is an important negative regulator of GLUT1 expression and glucose uptake and is likely to be active in many cell types. Inactivating mutations in TSC2 are likely to lead to enhanced GLUT1 expression and basal glucose uptake in a noninsulin-sensitive manner. Since GLUT1 expression is enhanced in many neoplasms and can lead to increased growth and reduced apoptosis (33), one may speculate that inactivation of TSC2 may contribute to abnormal cell growth and hamartomas via this mechanism.
GRANTS
This work was supported by National Institutes of Health Grants RO1-HL-60156 and HL-65567 (to F. C. Brosius), an American Heart Association fellowship award 0215178Z (to R. D. Loberg), and the University of Michigan Systems and Integrative Biology Training Grant (to C. L. Buller).
Acknowledgments
This work was supported by the University of Michigan Morphology and Image Analysis Core (MIAC) and the Michigan Diabetes Research and Training Core (MDRTC).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Asnaghi L, Bruno P, Priulla M, Nicolin A. mTOR: a protein kinase switching between life and death. Pharmacol Res 50: 545–549, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Atkins KB, Johns D, Watts S, Webb CR, Brosius FC. Decreased vascular glucose transporter expression and glucose uptake in DOCA-salt hypertension. J Hypertens 19: 1581–1587, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Barnes K, Ingram JC, Porras OH, Barros LF, Hudson ER, Fryer L, Foufelle F, Carling D, Hardie DG, Baldwin SA. Activation of GLUT1 by metabolic and osmotic stress: potential involvement of AMP-activated protein kinase (AMPK). J Cell Sci 115: 2433–2442, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Bentley J, Itchayanan D, Barnes K, McIntosh E, Tang X, Downes CP, Holman GD, Whetton AD, Owen-Lynch PJ, Baldwin SA. Interleukin-3-mediated cell survival signals include phosphatidylinositol 3-kinase-dependent translocation of the glucose transporter GLUT1 to the cell surface. J Biol Chem 278: 39337–39348, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Blodgett DM, DeZutter JK, Levine KB, Karim P, Carruthers A. Structural basis of GLUT1 inhibition by cytoplasmic ATP. J Gen Physiol 130: 157–168, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brugarolas JB, Vasquez F, Reddy A, Sellers WR, Kaelin WG. TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell 4: 147–158, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Carter AJ TOR of the cell cycle: are there important implications for diabetics in the era of the drug-eluting stent? Catheter Cardiovasc Interv 61: 233–236, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Choo AY, Roux PP, Blenis J. Mind the GAP: Wnt steps onto the mTORC1 train. Cell 126: 834–836, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Dennis PB, Jaeschke A, Saitoh M, Fowler B, Kozma SC, Thomas G. Mammalian TOR: a homeostatic ATP sensor. Science 1102–1105, 2001. [DOI] [PubMed]
- 10.Frame S, Cohen P, Biondi RM. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol Cell 7: 1321–1327, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Fryer L, Foufelle F, Barnes K, Baldwin SA, Woods A, Carling D. Characterization of the role of AMP-activated protein kinase in the stimulation of glucose transport in skeletal muscle cells. Biochem J 363: 167–174, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol 65: 391–426, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110: 177–189, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Harris TE, Lawrence JC. TOR signaling. Sci STKE 2003: re15, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Heilig CW, Brosius FC, Cunningham C. Role for GLUT1 in diabetic glomerulosclerosis. Expert Rev Mol Med 8: 1–18, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Henriksen EJ, Kinnick TR, Teachey MK, O'Keefe MP, Ring D, Johnson KW, Harrison SD. Modulation of muscle insulin resistance by selective inhibition of GSK-3 in Zucker diabetic fatty rats. Am J Physiol Endocrinol Metab 284: E892–E900, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Hino O, Klein-Szanto AJ, Freed JJ, Testa JR, Brown DQ, Vilensky M, Yeung RS, Tartof KD, Knudson AG. Spontaneous and radiation-induced renal tumors in the Eker rat model of dominantly inherited cancer. Proc Natl Acad Sci USA 90: 327–331, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holyoake J, Caulfeild V, Baldwin SA, Sansom M. Modeling, docking, and simulation of the major facilitator superfamily. Biophys J 91: L84–L86, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hruz PW, Mueckler MM. Structural analysis of the GLUT1 facilitative glucose transporter (review). Mol Membr Biol 18: 183–193, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Inoki K, Guan KL. Complexity of the TOR signaling network. TRENDS Cell Biol 1–7, 2006. [DOI] [PubMed]
- 21.Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev 1829–1834, 2003. [DOI] [PMC free article] [PubMed]
- 22.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signaling. Nat Cell Biol 4: 648–657, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126: 955–968, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115: 577–590, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Jin F, Wienecke R, Xiao GH, Maize JC, DeClue JE, Yeung RS. Suppression of tumorigenicity by the wild-type tuberous sclerosis 2 (Tsc2) gene and its C-terminal region. Proc Natl Acad Sci USA 93: 9154–9159, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joost HG, Bell GI, Best JD, Birnbaum MJ, Charron MJ, Chen YT, Doege H, James DE, Lodish HF, Moley KH, Moley JF, Mueckler M, Rogers S, Schurmann A, Seino S, Thorens B. Nomenclature of the GLUT/SLC2A family of sugar/polyol transport facilitators. Am J Physiol Endocrinol Metab 282: E974–E976, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Kandror KV A long search for Glut4 activation. Sci STKE 2003: pe5, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi T, Hirayama Y, Kobayashi E, Kubo Y, Hino O. A germline insertion in the tuberous sclerosis (Tsc2) gene gives rise to the Eker rat model of dominantly inherited cancer. Nature Genetics 70–74, 1995. [DOI] [PubMed]
- 29.Krymskaya VP Tumour suppressors hamartin and tuberin: intracellular signalling. Cell Signal 15: 729–739, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Corradetti MN, Inoki K, Guan KL. TSC2: filling the GAP in the mTOR signaling pathway. Trends Biochem Sci 29: 32–38, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Inoki K, Guan KL. Biochemical and functional characterizations of small GTPase Rheb and TSC2 GAP activity. Mol Cell Biol 24: 7965–7975, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loberg RD, Vesely E, Brosius FC. Enhanced glycogen synthase kinase-3 beta activity mediates hypoxia-induced apoptosis of vascular smooth muscle cells and is prevented by glucose transport and metabolism. J Biol Chem 277: 41667–41673, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins and cancer. J Cell Physiol 202: 654–662, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Marcus RG, England R, Nguyen K, Charron MJ, Briggs JP, Brosius FC. Altered renal expression of the insulin-responsive glucose transporter GLUT4 in experimental diabetes mellitus. Am J Physiol Renal Fluid Electrolyte Physiol 267: F816–F824, 1994. [DOI] [PubMed] [Google Scholar]
- 35.Mueckler M, Makepeace C. Analysis of transmembrane segment 8 of the GLUT1 glucose transporter by cysteine-scanning mutagenesis and substituted cysteine accessibility. J Biol Chem 279: 10494–10499, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Mueckler M, Makepeace C. Transmembrane segment 6 of the Glut1 glucose transporter is an outer helix and contains amino acid side chains essential for transport activity. J Biol Chem 283: 11550–11555, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nikoulina SE, Ciaraldi TP, Mudaliar S, Carter L, Johnson K, Henry RR. Inhibition of glycogen synthase kinase 3 improves insulin action and glucose metabolism in human skeletal muscle. Diabetes 51: 2190–2198, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Sakaguchi M, Isono M, Ishiki K, Sugimoto T, Koya D, Kashiwagi A. Inhibition of mTOR signaling with rapamycin attenuates renal hypertrophy in early diabetic mice. Biochem Biophys Res Commun 340: 296–301, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol 14: 1296–1302, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the Rictor-mTOR complex. Science 307: 1098–1101, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Tong H, Imahashi K, Steenbergen C, Murphy E. Phosphorylation of glycogen synthase kinase-3beta during preconditioning through a phosphatidylinositol-3-kinase–dependent pathway is cardioprotective. Circ Res 90: 377–379, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Wieman HL, Wofford JA, Rathmell JC. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell 18: 1437–1446, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao GH, Shoarinejad F, Jin F, Golemis EA, Yeung RS. The tuberous sclerosis 2 gene product, tuberin, functions as a Rab5 GTPase activating protein (GAP) in modulating endocytosis. J Biol Chem 272: 6097–6100, 1997. [DOI] [PubMed] [Google Scholar]
- 44.Yang Y, Wang J, Qin L, Shou Z, Zhao J, Wang H, Chen Y, Chen J. Rapamycin prevents early steps of the development of diabetic nephropathy. Am J Nephrol 27: 495–502, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Yeung RS, Xiao GH, Jin F, Lee WC, Testa JR, Knudson AG. Predisposition to renal carcinoma in the Eker rat is determined by germ-line mutation of the tuberous sclerosis 2 (TSC2) gene. Proc Natl Acad Sci USA 91: 11413–11416, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]