Abstract
Differentiation of intestinal epithelial cells is accompanied by alterations in levels of expression of many genes, including those involved in nutrient uptake. Effects of differentiation of intestinal epithelial cells on the physiological and molecular parameters of the intestinal folate uptake process are not well characterized. To address this issue, we used two models, Caco-2 cells and native mouse intestine. Studies with Caco-2 cells showed a significant increase in the initial rate of carrier-mediated folic acid uptake during differentiation (i.e., as the cells transitioned from preconfluent to confluent and then to postconfluent stages). This increase was associated with an increase in the level of expression of the human reduced folate carrier (hRFC) and the human proton-coupled folate transporter (hPCFT) both at the protein and mRNA levels with differentiation; it was also associated with a significant increase in activity of the hRFC and hPCFT promoters. Studies with native mouse intestine showed a significantly higher folate uptake in villus compared with crypt cells, which was again associated with a significantly higher level of expression of the mouse RFC and PCFT at the protein and mRNA levels. Together, these studies demonstrate that the intestinal folate uptake process undergoes differentiation-dependent regulation and that this regulation is mediated via changes in the level of expression of both the RFC and PCFT. In addition, the studies suggest the possible involvement (at least in part) of a transcriptional mechanism(s) in this type of regulation of the intestinal folate uptake process.
Keywords: transport, epithelial transport, reduced folate carrier, proton-coupled folate transporter
folate is essential for normal cellular proliferation, growth, and functions. The coenzyme derivatives of folic acid are required for the synthesis of precursors of DNA and RNA and the metabolism of several amino acids including homocysteine (6, 21, 26, 42). An adequate supply of folate is therefore necessary for normal human health and well-being. This is demonstrated by the variety of clinical abnormalities that result from folate deficiency (e.g., megaloblastic anemia, growth retardation, and neurological disorders) (7, 15, 17, 26) and by the health benefits attained by optimizing folate body homeostasis [e.g., prevention of neural tube defects and omphalocele and reduction in the risk of cardiovascular disease, colorectal cancer, stroke, and Alzheimer disease (3, 8, 9, 13, 18, 24, 41)]. Folate deficiency is prevalent in underdeveloped countries, and in the Western Hemisphere it often occurs as a result of impairment in intestinal absorption due to intestinal diseases (e.g., inflammatory bowl disease and celiac disease), drug interactions, and chronic alcohol intake (11, 19, 20, 28, 50). Folate deficiency has also been reported recently in hereditary folate malabsorption syndrome, a condition believed to be due to a defect(s) in the intestinal folate absorption process (35, 49).
Mammals are not capable of de novo synthesis of folate and thus must obtain the vitamin from exogenous sources via absorption in the intestine. Therefore, the intestine plays a central role in the regulation of normal body folate homeostasis. Studies from our laboratory and others have shown that the intestinal folate uptake process occurs via an acidic pH-dependent and specific carrier-mediated process. The molecular identity of the systems involved in the intestinal uptake process has also been reported following the cloning of the folate transporters [reduced folate carrier (RFC) and proton coupled folate transporter (PCFT)] from a variety of human and mouse tissues (14, 31, 33, 35, 36, 40). Both RFC and PCFT (the products of the SLC19A1 and SLC46A1 genes, respectively) are expressed in human and mouse intestinal epithelial cells and are involved in folate absorption (33, 35, 36, 39). Previous studies have identified and characterized the 5′-regulatory region of the human SLC19A1 gene in intestinal epithelial cells, demonstrating that the intestinal folate uptake process is regulated (at least in part) by transcriptional regulatory mechanisms during folate deficiency and developmental maturation (4, 38, 43, 47).
The normal function of intestinal epithelium depends on proper differentiation (maturation) of epithelial cells as they move from the crypt region to the villus tip along the crypt-villus axis. This differentiation event is associated with changes in the level of expression of many genes including those involved in nutrient transport (1, 10, 12, 16, 27, 29, 30, 32, 45, 48). Upregulation has been observed for genes involved in the uptake of thiamine, ascorbic acid, and iron, whereas a decrease in the level of expression of the gene involved in glutamine uptake was reported (5, 12, 29, 32). Little is currently known about the possible differentiation-dependent regulation of the intestinal folate uptake process and the mechanisms involved in any such regulation (10, 25, 39). In the present study we used the cultured human intestinal epithelial Caco-2 cells, and native mouse crypt and villus cells as models to address these issues. Caco-2 cells were chosen because they differentiate spontaneously in culture upon reaching confluence to become enterocyte-like cells and have been widely used as a model in such types of investigations (5, 12, 29, 32, 44, 45, 48). The results showed that the intestinal folate uptake process does indeed undergo clear differentiation-dependent regulation and that this regulation is mediated, at least in part, via transcriptional mechanism(s).
MATERIALS AND METHODS
Materials.
[3H]folic acid (specific activity >20 Ci/mmol; radiochemical purity >98.0%) was obtained from Moravek Biochemicals (Brea, CA). Lipofectamine 2000 and TRIzol reagent were purchased from Life Technologies (Rockville, MD). DNA oligonucleotide primers were from Sigma Genosys (The Woodlands, TX). Laboratory chemicals, reagents, enzymes, and kits used in the present study were of analytical/molecular biology grade and were obtained from commercial vendors.
Cell culture and uptake assay.
The human-derived intestinal epithelial Caco-2 cells (passages 20 to 33; American Type Culture Collection, Manassas, VA; these cells were derived from colorectal adenocarcinoma obtained from a 72-year-old Caucasian male) were grown in Dulbecco's modified Eagle's medium supplemented with 10% (vol/vol) fetal bovine serum, glutamine (0.29 g/l), sodium bicarbonate (2.2 g/l), penicillin (100,000 U/l), and streptomycin (10 mg/l). Caco-2 cells were plated at a density of 2 × 105 cells/well onto collagen-coated 12-well plates. Uptake studies were performed on preconfluent (1 day after seeding), confluent (3 days after seeding when cells were visibly confluent), and postconfluent (3 days after confluence) monolayers of Caco-2 cells. [3H]-folic acid uptake was measured at 37°C in Krebs-Ringer buffer as described previously (43).
Isolation of mouse villus and crypt intestinal epithelial cells and uptake studies.
Mouse villus and crypt intestinal epithelial cells were isolated as described by us previously (32, 39, 46) using a well-established fractionation procedure (34). Briefly, we collected 10 consecutive fractions with most of the villus tip cells collected in fractions 1 and 2, while fractions 9 and 10 represented mostly crypt cells. We have previously established the relative purity of these fractions by means of marker enzymes [alkaline phosphatase and thymidine kinase for villus and crypt epithelial cells, respectively (32)]. [3H]folic acid uptake by villus and crypt cells was measured as described previously (10, 32) using an established rapid-filtration technique (22) at 37°C in Krebs-Ringer buffer at pH 5.5. [3H]folic acid and/or unlabeled folic acid was added to the incubation buffer at the onset of incubation, and uptake was performed during the initial linear period of uptake assay (data not shown).
Quantitative real-time PCR.
Quantitative real-time PCR (qPCR) was performed using the Bio-Rad iCycler (Hercules, CA) and a Qiagen Quantitect SYBR green PCR kit (Valencia, CA). The DNase-treated total RNA was prepared using TRIzol (Invitrogen, CA) from preconfluent, confluent, and postconfluent Caco-2 cells and from mouse jejunum villus and crypt cells. Total RNA (5 μg) was reverse transcribed with oligo(dT) primers using the Superscript II kit (Life Technologies, Rockville, MD). Combinations of gene-specific primers corresponding to the PCR targets were synthesized using sequences given by the vendor (Bio-Rad) and are shown in Table 1. The conditions for qPCR consisted of a 15-s 95°C melt followed by 40 cycles of 95°C melt for 30 s, 58°C annealing for 30 s, and 72°C extension and data collection for 1 min. Melt curve analysis was performed for the generation of standard curves, and negative controls without RT were used with every reaction. To determine the relative level of expression of the human RFC (hRFC) and human PCFT (hPCFT) in Caco-2 cells at different differentiation stages and that of the mouse RFC (mRFC) and mouse PCFT (mPCFT) in mouse intestinal villus/crypt cells, we used a calculation method provided by the iCycler manufacturer (Bio-Rad) as described previously (37).
Table 1.
Gene-specific primers used for real-time PCR analysis of hRFC, hPCFT, mRFC, mPCFT, and β-actin in Caco-2 cells and mice
| Gene | Forward and Reverse Primers (5′-3′) | Fragment Size, bp |
|---|---|---|
| hRFC | CACCGACTAQCCTGCGCTACA | 130 |
| GCCATGGTGACGCTGTAGAA | ||
| hPCFT | ATGCAGCTTTCTGCTTTGGT | 100 |
| GGAGCCACATAGAGCTGGAC | ||
| mRFC | CGCATGCTAAGTGAACTGGTG | 130 |
| TCAGTGCTTCTCCACAGGACAT | ||
| mPCFT | CTCATGTTCACAGGGTACGGATT | 115 |
| ACAGCAGAGAACAGAGCACCCT | ||
| β-actin | CATCCTGCGTCTGGACCT | 116 |
| TAATGTCACGCACGATTTCC |
hRFC, human reduced folate carrier; hPCFT, human proton-coupled folate transporter; mRFC, mouse RFC; mPCFT, mouse PCFT.
Western blot analysis.
Western blot analysis was performed as described previously (38, 43) using polyclonal antibodies specifically designed for either hRFC, mRFC (Alpha Diagnostics, San Antonio, TX) or hPCFT/mPCFT (Abcam, Cambridge, MA) proteins. The membranous fractions were isolated from preconfluent, confluent, and postconfluent Caco-2 cells and villus/crypt cells using a previously described protocol (2, 32, 43). Proteins (150 μg) were resolved on SDS-PAGE and subjected to Western blot analysis as described previously (43). Specific band densities were determined using the Eagle Eye II system (Stratagene, La Jolla, CA). Western blot analysis for β-actin was performed as a loading control as described previously (2).
Generation of hPCFT full-length promoter constructs.
The hPCFT promoter was cloned from 0.1 μg human genomic DNA (Clontech, Mountain View, CA) and specific primers designed to amplify a ∼2-kb fragment of DNA including up to the reported initiator ATG (Table 2). Sequences were obtained using the National Center for Biotechnology Information Entrez nucleotide database (accession no. NM 080669.2). Amplification was performed using 95°C melt for 5 min, followed by 35 cycles of 95°C melt for 30 s, 68°C annealing for 30 s, 72°C extension for 3 min, with a final 15-min extension at 72°C. Products were isolated and purified on a 0.7% agarose gel and using a Gene Clean II kit. The PCR product was subcloned into the TA vector (Promega, Madison, WI). The sequence was confirmed by Laragen (Los Angeles, CA) and submitted to GenBank (accession no. 1022187). A ∼1.4-kb fragment, excised using NheI and XhoI, was subcloned into the promoterless pGL3-Basic vector (Promega) cut with Xba I and XhoI.
Table 2.
Sequence and combination of primers used for construction of the hPCFT full-length promoter construct by PCR
| Gene | Forward and Reverse Primers (5′-3′) | Fragment Size, bp |
|---|---|---|
| hPCFT | TTGGTACCCATTCCTGATGAGGGACC | 2022 |
| TTGAGCTCGGCGGAGCTGTCGCCAGG |
Restriction sites for KpnI (bold italic text) and XhoI (underlined text) for hPCFT primers to assist cloning into pGL3-Basic vector.
The hRFC promoter [i.e., promoter B (pB), the predominant promoter in the intestine (2, 40, 43)] used in these investigations, fused with the luciferase reporter gene, hRFC-pB-luciferase, was obtained from Dr. Larry H. Matherly of Wayne State University School of Medicine, Detroit, MI (47).
Transfection and reporter gene assay.
The hRFC and hPCFT promoter-luciferase reporter constructs were transfected into Caco-2 cells grown in 12-well plates at less than 50% confluence with 2 μg of each full-length construct and cotransfected with 100 ng of the transfection control plasmid Renilla luciferase-thymidine kinase (Promega). Transfection was performed with Lipofectamine 2000 reagent (Life Technologies) according to the manufacturer's instructions. Cells were then lysed the next day after transfection (preconfluence), at 2–3 days after transfection (confluence), or at 3 days after reaching confluence (postconfluence), and Renilla-normalized firefly luciferase activity was measured by using the Dual Luciferase Assay kit (Promega) and a Turner Design 20/20 luminometer (Sunnyvale, CA) (32, 43). Data are presented as means ± SE of at least three independent experiments and given as fold over pGL3-Basic expression set arbitrarily at one.
Immunofluorescence.
Wild-type mice were killed by CO2 inhalation followed by cervical dislocation, the abdomen was opened, and the intestine was removed and washed with PBS. The jejunum segment was immediately fixed in formalin or snap frozen, and thin sections (8 μm) were prepared using a microtome and placed on microscope slides (23). Sections were blocked for 30 min with normal goat serum. Slides containing tissue sections were then incubated with primary anti-RFC or PCFT (1:100 dilution) antibodies for 1 h at room temperature. The tissue was washed with PBS and then incubated with a fluorescein anti-rabbit IgG (H+L) secondary antibody (1:200 dilutions) (Vector Laboratories, Burlingame, CA). Tissue samples were washed with PBS and mounted with Prolong Gold antifade reagent (Molecular Probes, Eugene, OR). Samples were imaged using a Nikon upright fluorescence microscope. All animals received humane care in compliance with the American Association for Accreditation of Laboratory Animal Care, and the study was conducted according to protocols approved by the Veterans Affairs Medical Center-Long Beach Subcommittee of Animal Studies.
Data presentation and statistical analysis.
In the current study, transport assay data are presented as means ± SE of multiple separate uptake determinations and are expressed as fmol·mg protein−1·unit time−1. One-way ANOVA and/or Student's t-test was used for statistical analysis. P < 0.05 was considered statistically significant. Uptake of folic acid by the carrier-mediated system was determined by subtracting uptake by passive diffusion (represented by residual uptake of [3H]folic acid in the presence of a high pharmacological concentration of unlabeled folic acid; 1 mM) from total [3H]folic acid uptake.
RESULTS
Effect of differentiation on physiological and molecular parameters of folic acid uptake by Caco-2 cells.
We investigated the effect of differentiation on the initial rate of carrier-mediated [3H]folic acid (9 nM) uptake by Caco-2 cells. The results showed a progressive and significant increase in carrier-mediated folic acid uptake with differentiation, i.e., as the cells transitioned from preconfluent to (P < 0.01) confluent and then to (P < 0.05) postconfluent stages (Fig. 1). We also examined the effect of differentiation on the protein level of hRFC and hPCFT, the two folate transporters that are expressed in human intestinal epithelial cells and participate in folate uptake (33, 35). This was achieved by means of Western blot analysis using membranous fractions isolated from preconfluent, confluent, and postconfluent Caco-2 cells and specific polyclonal antibodies against the hRFC and the hPCFT proteins. The results (Fig. 2, A and D) showed a significant (P < 0.05) and progressive increase in the level of expression of the hRFC and the hPCFT proteins in Caco-2 cells with differentiation (data were normalized relative to β-actin). Specificities (in arbitrary units) for hRFC were 6.2 ± 1.4, 16.2 ± 2.9, and 27.8 ± 2.9 and for hPCFT were 3.9 ± 0.7, 8 ± 1.1, and 16.8 ± 2.2 in preconfluent, confluent, and postconfluent Caco-2 cells, respectively.
Fig. 1.
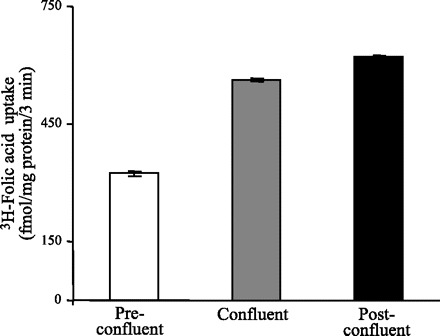
Uptake of folic acid by preconfluent, confluent, and postconfluent Caco-2 cells. Initial rate of carrier-mediated uptake of [3H]folic acid (9 nM) by preconfluent, confluent, and postconfluent Caco-2 cells. Incubation was performed at 37°C in Krebs-Ringer buffer (pH 5.5). Folic acid uptake was determined as described in materials and methods. Data represent means ± SE of at least 3 independent uptake experiments.
Fig. 2.
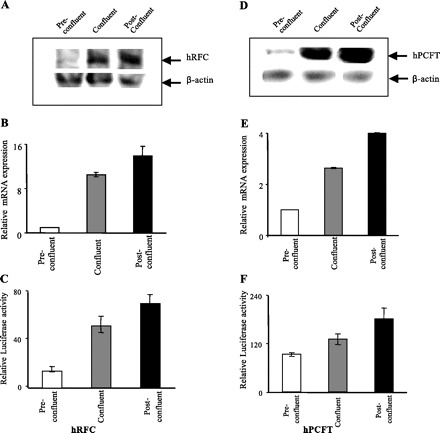
Level of expression of human reduced folate carrier (hRFC) and human proton-coupled folate transporter (hPCFT) protein and mRNA as well as activity of the SLC19A1 and SLC46A1 promoters in preconfluent, confluent, and postconfluent Caco-2 cells. A and D: Western blot analysis was performed using membranous fraction (150 μg) isolated from preconfluent, confluent, and postconfluent Caco-2 cells and specific anti-hRFC and anti-hPCFT polyclonal antibodies. Using an enhanced chemiluminescence kit (Amersham, Arlington Heights, IL), immunoreactive bands were detected as described in materials and methods. B and E: quantitative real-time PCR was performed using hRFC (B) and hPCFT (E) gene-specific primers and total RNA (5 μg) from preconfluent, confluent, and postconfluent Caco-2 cells. Real-time PCR was performed as described in materials and methods. Data represent means ± SE of at least 3 independent sets of experiments and were normalized relative to β-actin and calculated using a relative relationship method supplied by the iCycler manufacturer (Bio-Rad, Hercules, CA). Note that the level of expression of hRFC and hPCFT in preconfluent cells was set at 1 for each figure and the expression during differentiation is in relation to that level; therefore the results are not representative of the levels of hRFC compared with hPCFT (hPCFT mRNA expression is ∼3-fold higher than hRFC at all 3 stages of differentiation). C and F: activity of full-length hRFC promoter B (C) and hPCFT promoters (F) in pGL3-Basic was determined following transient expression in preconfluent, confluent, and postconfluent Caco-2 cells. Luciferase activity was determined and normalized relative to the activity of simultaneously expressed Renilla luciferase as described in materials and methods. The results are expressed relative to the PGL3-Basic vector set at 1. Data represent means ± SE of at least 3 independent sets of determinations.
To determine whether differentiation also affects the mRNA levels of the hRFC and hPCFT, we performed qPCR. The results showed a significant increase in mRNA levels of both folate transporters during transition of the Caco-2 cells from preconfluent to confluent (P < 0.01) then to postconfluent (P < 0.05) stages (Fig. 2, B and E).
The above described data suggest the possible involvement of a transcriptional regulatory mechanism(s) in the differentiation-dependent upregulation of carrier-mediated folate uptake in Caco-2 cells. To test for this possibility, we directly examined and compared the activity of the hRFC and hPCFT promoter-luciferase constructs during differentiation following their transient transfection into Caco-2 cells. Transfection was performed as described previously (32, 43), and promoter activity was assayed in preconfluent, confluent, or postconfluent Caco-2 cells. For SLC19A1 (the gene that encodes the hRFC), we used full-length promoter B (hRFC pB) since this promoter is responsible for driving the expression of hRFC variant I, the predominant variant in the intestine (2, 40, 43). For SLC46A1 (the gene that encodes the hPCFT), we cloned and sequenced a 1.4-kb fragment of the 5′-regulatory region of this gene that is proximal to the ATG start site (see materials and methods). Computer analysis (TRANSFAC program; MatInspector; Genomatix) of this region suggested that it contains the promoter region with a typical TATA box and guanine- and cytosine-rich sites (indeed the cloned genomic fragment demonstrated marked promoter activity when transfected into confluent Caco-2 cells). The results showed a significant increase in the activity of hRFC and hPCFT promoters during transition of the Caco-2 cells from preconfluent to confluent (P < 0.01) then to postconfluent (P < 0.05) stages (Fig. 2, C and F).
Physiological and molecular parameters of the folic acid uptake process in native mouse intestinal crypt and villus epithelial cells.
To validate our results with the cultured intestinal epithelial cell line described above and to establish the physiological relevance of these findings, we extended our investigations into the native mouse intestine. For this we used freshly isolated mouse intestinal crypt and villus epithelial cells [isolated by an established procedure (22, 32, 39, 46)] and studies that compared the initial rate of folic acid (9 nM) uptake. The results (Fig. 3) showed a significantly (P < 0.05; ∼2-fold) higher carrier-mediated folic acid uptake in the differentiated/mature cells of the intestinal villus tip compared with those of the undifferentiated/immature crypt cells.
Fig. 3.
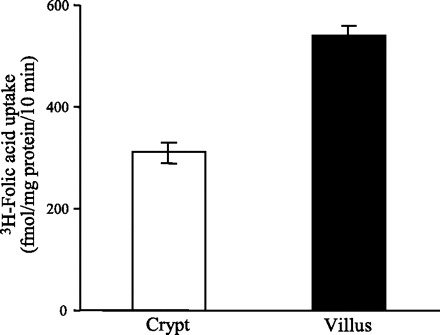
Carrier-mediated folic acid uptake by native mouse jejunum crypt and villus epithelial cells. Initial rate (10 min; data not shown) of carrier-mediated uptake of [3H]folic acid (9 nM) by crypt and villus intestinal epithelial cells isolated from jejunum of wild-type mice was examined in Krebs-Ringer buffer (pH 5.5). Data represent means ± SE of at least 3 independent uptake determinations performed on cells isolated from different mice.
We also examined and compared the levels of mouse RFC and PCFT proteins in native mouse intestinal crypt and villus epithelial cells by means of Western blot analysis and immunofluorescence analysis [mouse intestine, like human intestine, expresses both RFC and PCFT systems (14, 31, 33, 35, 36)]. Specific polyclonal antibodies against the mouse RFC and PCFT proteins were used in these studies. Results of the Western blot analysis (Fig. 4, A and C) showed the level of mRFC and mPCFT proteins to be significantly (P < 0.05 for both) higher in the differentiated cells of the villus tip compared with those of the undifferentiated crypt (data were normalized relative to β-actin). Specific band densities (in arbitrary units) for mRFC were 4.5 ± 0.8 and 12.5 ± 2.9 and for mPCFT were 3.5 ± 1.1 and 11.3 ± 1.7 in intestinal crypt and villus epithelial cells, respectively. Intestinal sections for immunofluorescence analysis were prepared from mouse jejunum, with the results confirming the findings of the Western blot analysis by showing the level of expression of the mouse RFC and PCFT proteins to be more intense in the intestinal villus region compared with the crypt region (Fig. 5, A and B) [no staining was detected when the sections were incubated with secondary antibody alone (Fig. 5C)].
Fig. 4.
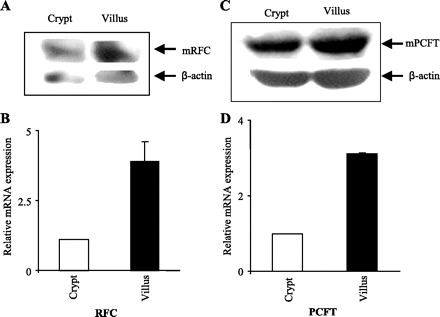
Levels of expression of mouse RFC (mRFC) and mouse PCFT (mPCFT) protein and mRNA in native mouse jejunum crypt and villus epithelial cells. A and C: Western blot analysis was performed using membranous fractions (150 μg) separated from crypt and villus of mouse jejunum and hybridized with polyclonal antibodies specific for mRFC and mPCFT as described in materials and methods. Immunodetection was performed with an enhanced chemiluminescence detection system (Amersham). Images and data shown are representative of 3 separate sets of experiments on cells isolated from at least 4–6 mice. B and D: quantitative real-time PCR was performed using mRFC (B) and mPCFT (D) gene-specific primers and total RNA from crypt and villus wild-type mouse intestinal epithelial cells. Primers (Table 1) and PCR conditions were used as previously described (32, 37). Data represent means ± SE of at least 3–8 separate determinations on cells isolated from 3–6 wild-type mice and were normalized relative to β-actin and calculated using a relative relationship method provided by a commercial vendor (Bio-Rad, Hercules, CA). Note that the level of expression of mRFC and mPCFT in crypt cells was set at 1 for each figure and the expression in villus is in relation to that level; therefore the results are not representative of the levels of mRFC compared with mPCFT (mPCFT mRNA expression is ∼3-fold higher than mRFC at both crypt and villus stages).
Fig. 5.
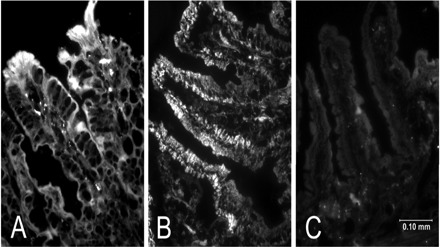
Immunofluorescence analysis of mRFC and mPCFT proteins in crypt and villus mouse jejunum sections. A and B: immunofluorescence analysis was performed using mouse jejunum sections (8 μm) and polyclonal antibodies specific for mRFC and mPCFT, respectively, as described in materials and methods (23). C: jejunum section was incubated only with secondary antibody. Data and images shown are representative of 3–4 separate sets of tissues from different mice.
To determine whether the levels of expression of RFC and PCFT messages in native mouse intestinal crypt and villus epithelial cells parallel the changes in functional and protein level data, we performed qPCR analysis using gene-specific primers and RNA isolated from these two cell populations. The results (Fig. 4, B and D) showed significantly (P < 0.01) higher mRFC and mPCFT mRNA levels in intestinal villus compared with crypt epithelial cells.
DISCUSSION
Intestinal differentiation is associated with changes in the levels of expression of a variety of genes including those involved in nutrient absorption (16, 29, 32). Our aim in the present study was to investigate whether the intestinal folate uptake process undergoes differentiation-dependent regulation, and if so, to develop an understanding of the mechanism(s) involved in this type of regulation. Such investigations are of physiological importance since interference with normal intestinal differentiation processes may lead to impairment in the normal function of this vital organ. Also, results of such investigations may assist in the designing of effective strategies to enhance speedy recovery of intestinal damage that occurs under certain disease conditions and may prove insightful in understanding intestinal cancer biology. This is especially relevant in this case, since folate is essential for normal synthesis of DNA and RNA, and its derivative methotrexate is a widely used anti-tumor agent.
We used the human cultured intestinal epithelial Caco-2 cells in our investigations. These cells differentiate spontaneously in culture upon reaching confluence and have been widely used in such types of investigations (5, 12, 29, 32, 44, 45, 48). We complemented our investigations with the cultured Caco-2 cell line with studies using native mouse intestine. Our results with Caco-2 cells showed that differentiation, i.e., transitioning of the cells from preconfluent to confluent and then to postconfluent stages, is associated with a significant increase in carrier-mediated folate uptake. This increase was associated with an increase in protein and mRNA levels of the two human folate transporter systems that are expressed and operate in the intestine, i.e., hRFC and hPCFT (33, 35). The latter conclusion was based on findings with Western blot and qPCR analysis, respectively. These findings suggest the possible involvement of a transcriptional mechanism(s) in the differentiation-dependent regulation of the intestinal folate uptake process. To test for the latter possibility, we first cloned the SLC46A1 promoter and fused it to the luciferase reporter gene. We then tested the activity of this promoter and that of the SLC19A1 (hRFC) promoter in preconfluent, confluent, and postconfluent Caco-2 cells. The results showed activity of the hRFC and the hPCFT promoters to significantly increase as a function of cell differentiation (as the cells transition from preconfluent to confluent and then to postconfluent stages). These findings support the possibility that the differentiation-dependent regulation of intestinal folate uptake process is mediated, at least in part, via a transcriptional mechanism(s). Obviously, this does not exclude the possibility that part of the effect is also mediated via changes in hRFC and hPCFT RNA stability; the latter issue is in need of further investigation. These results taken with data published previously on the other two members of this SLC19A family of transporters, i.e., the thiamine transporters 1 and 2 (SLC19A2 and SLC19A3, respectively), which were also upregulated by transcriptional mechanisms during differentiation, underscore commonalities shared between members of this family (32). Further studies are needed to identify the cis-regulatory element(s) in the SLC19A1 and SLC46A1 promoters and the trans-acting factor(s) that mediate these effects.
The studies with native mouse intestine were performed to complement the findings with the cultured Caco-2 cell line so as to support the physiological relevance of the findings with the cell line system. Carrier-mediated folate uptake was found to be significantly higher in the mature differentiated cells of the mouse intestinal villus tip compared with those of the undifferentiated crypt. The higher uptake in villus compared with crypt cells was associated with a significantly higher protein level of the two mouse folate transporters, i.e., the mRFC and mPCFT (products of the slc19a1 and slc46a1 genes, orthologs of the human SLC19A1 and SLC46A1, respectively) (14, 36, 39) in the former compared with the latter cells. This was demonstrated by means of Western blot analysis and by immunofluorescence analysis. Furthermore, the level of the mRFC and mPCFT message was also found to be higher in villus compared with crypt epithelial cells by means of qPCR analysis. These findings are similar to those seen with the human intestinal epithelial Caco-2 cells and thus establish the physiological relevance to the findings with the cultured cell line. These data also suggest the possible involvement of a transcriptional mechanism(s) in mediating the differentiation-dependent regulation of mouse intestinal folate uptake process.
In summary, the results of these investigations clearly demonstrate that differentiation of intestinal epithelial cells is associated with an upregulation in carrier-mediated folate uptake. The results also show that this upregulation is associated with an increase in the protein and mRNA levels of the folate transporters RFC and PCFT as well as in the activity of their promoters. The latter suggests the possible involvement of transcriptional mechanism(s) in this type of regulation in intestinal folate uptake.
GRANTS
This study in our laboratory is supported by the Department of Veterans Affairs and by National Institutes of Health Grants DK-75348 (to H. M. Said), DK-71538 (to V. S. Subramanian), and DK-73032 (to J. C. Reidling).
Acknowledgments
We thank Neil Hoa for technical assistance in immunohistochemistry.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Anderle P, Rakhmanova V, Woodford K, Zerangue N, Sadee W. Messenger RNA expression of transporter and ion channel genes in undifferentiated and differentiated Caco-2 cells compared to human intestine. Pharm Res 20: 3–15, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Ashokkumar B, Mohammed ZM, Vaziri ND, Said HM. Effect of folate oversupplementation on folate uptake by human intestinal and renal epithelial cells. Am J Clin Nutr 86: 159–166, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Bailey LB, Rampersaud GC, Kauwell GPA. Folic acid supplements and fortification affect the risk for neural tube defects, vascular disease and cancer: evolving science. J Nutr 133: 1961–1968, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Balamurugan K, Said HM. Ontogenic regulation of folate transport across rat jejunal brush-border membrane. Am J Physiol Gastrointest Liver Physiol 285: G1068–G1073, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Bedrine-Ferran H, Meur NL, Gicquel I, Cunff ML, Soriano N, Guisle I, Mottier S, Monnier A, Teusan R, Fergelot P, Gall JYL, Leger J, Mosser J. Transcriptome variations in human Caco-2 cells: a model for enterocyte differentiation and its link to iron absorption. Genomics 83: 772–789, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Blakley RL, Benkovic SJ. Folates and Peterines. Chemistry and Biochemistry of Folate. New York: Wiley and Sons, 1985, vol. 3 [Google Scholar]
- 7.Blakley RL, Whithead VA. Folates and Peterines. Nutritional, Pharmacological, and Physiological Aspects. New York: Wiley and Sons, 1986, vol. 1 [Google Scholar]
- 8.Botto LD, Mulinare J, Erickson JD. Occurrence of omphalocele in relation to maternal multivitamin use: a population-based study. Pediatrics 109: 904–908, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Butterworth CE, Bendich A. Folic acid and the prevention of birth defects. Annu Rev Nutr 16: 73–97, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Chiao JH, Roy K, Tolner B, Yang CH, Sirotnak FM. RFC-1 gene expression regulates folate absorption in mouse small intestine. J Biol Chem 272: 11165–11170, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Corcinero JJ, Reisenauer AM, Halsted CH. Jejunal perfusion of simple and conjugated folates in tropical sprue. J Clin Invest 58: 298–305, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa C, Huneau JF, Tome D. Characteristics of l-glutamine transport during Caco-2 cell differentiation. Biochim Biophys Acta 1509: 95–192, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Czeizel AE. Primary prevention of neural-tube defects and some other major congenital abnormalities: recommendations for the appropriate use of folic acid during pregnancy. Paediatr Drugs 2: 437–449, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Dixon KH, Lanpher BC, Chiu J, Kelly K, Cowan KH. A novel cDNA restores reduced folate carrier activity and methotrexate sensitivity to transport deficient cells. J Biol Chem 269: 17–20, 1994 [PubMed] [Google Scholar]
- 15.Elsborg L, Lyngbye J, Ryttig K. Folic Acid and Folic Acid Deficiency. Copenhagen, Denmark: Ferrosan, 1981
- 16.Fan MZ, Matthews JC, Etienne NMP, Stoll B, Lackeyram D, Burrin DG. Expression of apical membrane l-glutamate transporters in neonatal porcine epithelial cells along the small intestinal crypt-villus axis. Am J Physiol Gastrointest Liver Physiol 287: G385–G398, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Geller J, Kronn D, Jayabose S, Sandoval C. Hereditary folate malabsorption: family report and review of the literature. Medicine 81: 51–68, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Glynn SA, Albanes D, Pietinen P, Brown CC, Rautalahti M, Tangrea JA, Gunter EW, Barrett MJ, Virtamo J, Taylor PR. Colorectal cancer and folate status: a nested case-control study among male smokers. Cancer Epidemiol Biomarkers Prev 5: 487–494, 1996 [PubMed] [Google Scholar]
- 19.Halsted CH, Gandi G, Tamura T. Sulfasalazine inhibits the absorption of folates in ulcerative colitis. N Engl J Med 305: 1513–1517, 1961 [DOI] [PubMed] [Google Scholar]
- 20.Halsted CH, Reisenauer AM, Romero JJ, Cantor DS, Ruebner B. Jejunal perfusion of simple and conjugated folates in celiac sprue. J Clin Invest 59: 933–940, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herbert V. Folic Acid in Modern Nutrition in Health and Disease (9th ed.), edited by Shills ME, Olson JA, Shike M, Ross AH. London, UK: Lippincott, Williams & Wilkins, 1999, p. 433–446.
- 22.Hopfer U, Nelson K, Perrotto J, Isselbacher KJ. Glucose transport in isolated brush border membrane from rat small intestine. J Biol Chem 248: 25–32, 1973 [PubMed] [Google Scholar]
- 23.Jeffes EWB, Zhang JG, Hoa N, Petkar A, Delgado C, Chong S, Obenaus A, Khalaghizadeh S, Khomenko T, Knight BA, Alipanah R, Nguyen TV, Shah C, Vohra S, Zhuang JL, Liu J, Wepsic HT, Jadus MR. Antiangiogenic drugs synergize with a membrane macrophage colony-stimulating factor-based tumor vaccine to therapeutically treat rats with an established malignant intracranial glioma. J Immunol 174: 2533–2543, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Lashner BA, Provencher KS, Seidner DL, Knesebeck A, Brzezinski A. The effect of folate supplementation on the risk of cancer or dysplasia in ulcerative colitis. Gastroenterology 112: 29–32, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Latunde-Dada GO, Takeuchi K, Simpson RJ, McKie AT. Haem carrier protein 1 (HCP1): expression and functional studies in cultured cells. FEBS Lett 580: 6865–6870, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Lucock M. Folic acid: nutritional biochemistry, molecular biology, and role in disease processes. Mol Genet Metab 71: 121–138, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Mariadason JM, Nicolas C, L'Italien KE, Zhuang M, Smartt HJM, Heerdt BG, Yang W, Corner GA, Wilson AJ, Klampfer L, Arango D, Augenlicht LH. Gene expression profiling of intestinal epithelial cell maturation along the crypt-villus axis. Gastroenterology 128: 1081–1088, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Mason JB, Rosenberg IH. Intestinal absorption of folate. In: Physiology of the Gastrointestinal Tract, edited by Johnson LR. New York: Raven, 1994
- 29.Maulen NP, Henriquez EA, Kempe S, Carcamo JG, Schmid-Kotsa A, Bachem Grunert A, Bustamante ME, Nualart F, Vera JC. Up-regulation and polarized expression of the sodium-ascorbic acid transporter SVCT1 in post-confluent differentiated Caco-2 cells. J Biol Chem 278: 9035–9041, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Mordrelle A, Jullian E, Costa C, Cormet-Boyaka E, Benamouzig R, Tome D, Huneau JF. EAAT1 is involved in transport of l-glutamate during differentiation of the Caco-2 cell line. Am J Physiol Gastrointest Liver Physiol 279: G366–G373, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Moscow JA, Gong M, He R, Sgagias MK, Dixon KH, Anzick SL, Meltzer PS, Cowen KH. Isolation of a gene encoding a human reduced folate carrier (RFC1) and analysis of its expression in transport-deficient, methotrexate-resistant human breast cancer cells. Cancer Res 55: 3790–3794, 1995 [PubMed] [Google Scholar]
- 32.Nabokina SM, Reidling JC, Said HM. Differentiation-dependent up-regulation of intestinal thiamin uptake: cellular and molecular mechanisms. J Biol Chem 280: 32676–32682, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Nguyen TT, Dyer DL, Dunning DD, Rubin SA, Grant K, Said HM. Human intestinal folate transport: cloning, expression and distribution of complementary RNA. Gastroenterology 1112: 783–791, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Pinkus LM. Separation and use of enterocytes. Methods Enzymol 77: 154–162, 1981 [DOI] [PubMed] [Google Scholar]
- 35.Qui A, Min SH, Jansen M, Malhotra U, Tsai E, Sandoval C, Zhao R, Akabas MH, Goldman ID. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell 127: 917–928, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Qui A, Min SH, Jansen M, Malhotra U, Tsai E, Cabelof DC, Matherly LH, Zhao R, Akabas MH, Goldman ID. Rodent intestinal folate transporters (SLC46A1): secondary structure, functional properties, and response to dietary folate restriction. Am J Physiol Cell Physiol 293: C1669–C1678, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Reidling JC, Nabokina SM, Balamurugan K, Said HM. Developmental maturation of intestinal and renal thiamin uptake: studies in wild-type and transgenic mice carrying human THTR-1 and 2 promoters. J Cell Physiol 206: 371–377, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Said HM, Chatterjee N, Haq RU, Subramanian VS, Ortiz A, Matherly LH, Sirotnak FM, Halsted C, Rubin SA. Adaptive regulation of intestinal folate uptake: effect of dietary folate deficiency. Am J Physiol Cell Physiol 279: C1889–C1895, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Said HM, Nguyen TT, Dyer DL, Cowan KH, Rubin SA. Intestinal folate transport: identification of a cDNA involved in folate transport and the functional expression and distribution of its mRNA. Biochim Biophys Acta 1281: 164–172, 1996 [DOI] [PubMed] [Google Scholar]
- 40.Sirotnak FM, Tolner B. Carrier-mediated transport of folates in mammalian cells. Ann Rev Nutr 19: 91–122, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Sommer BR, Hoff AL, Costa M. Folic acid supplementation in dementia: a preliminary report. J Geriatr Psychiatry Neurol 16: 156–159, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Stanger O. Physiology of folic acid in health and disease. Curr Drug Metab 3: 211–223, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Subramanian VS, Chatterjee N, Said HM. Folate uptake in the human intestine: promoter activity and effect of folate deficiency. J Cell Physiol 196: 403–408, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Vachon PH, Beaulieu JF. Transient mosaic patterns of morphological and functional differentiation in the Caco-2 cell line. Gastroenterology 103: 414–423, 1992 [DOI] [PubMed] [Google Scholar]
- 45.Wang L, Klopot A, Freund JN, Dowling LN, Krasinski SD, Fleet JC. Control of differentiation-induced calbindin-D9k gene expression in Caco-2 cells by cdx-2 and HNF-1α. Am J Physiol Gastrointest Liver Physiol 287: G943–G953, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiser MM. Intestinal epithelial cell surface membrane glycoprotein synthesis. J Biol Chem 248: 2536–2541, 1973 [PubMed] [Google Scholar]
- 47.Whetstine JR, Matherly LH. The basal promoters for the human reduced folate carrier gene are regulated by a GC-box and a cAMP-response element/AP-1 like element: basis for tissue-specific gene expression. J Biol Chem 276: 6350–6358, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Wong WK, Chen K, Shih JC. Decreased methylation and transcription repressor Sp3 up-regulated human monoamine oxidase (MAO) B expression during Caco-2 differentiation. J Biol Chem 278: 36227–36235, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Zhao R, Min SH, Qiu A, Sakaris A, Goldberg GL, Sandoval C, Malatack JJ, Rosenblatt DS, Goldman ID. The spectrum of mutations in the PCFT gene, coding for an intestinal folate transporter, that are the basis for hereditary folate malabsorption. Blood 110: 1147–1152, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zimmerman J, Selhub J, Rosenberg IH. Competitive inhibition of folic acid absorption in rat jejunum by triamterene. J Lab Clin Med 108: 272–276, 1986 [PubMed] [Google Scholar]


