Abstract
The selectivity of sensory neurons for stimuli is often shaped by a balance between excitatory and inhibitory inputs, making this balance an effective target for regulation. In the inferior colliculus (IC), an auditory midbrain nucleus, the amplitude and selectivity of frequency response curves are altered by the neuromodulator serotonin, but the changes in excitatory-inhibitory balance that mediate this plasticity are not well understood. Previous findings suggest that the presynaptic 5-HT1B receptor may act to decrease the release of GABA onto IC neurons. Here, in vivo extracellular recording and iontophoresis of the selective 5-HT1B agonist CP93129 were used to characterize inhibition within and surrounding frequency response curves using two-tone protocols to indirectly measure inhibition as a decrease in spikes relative to an excitatory tone alone. The 5-HT1B agonist attenuated such two-tone spike reduction in a varied pattern among neurons, suggesting that the function of 5-HT1B modulation also varies. The hypothesis that the 5-HT1B receptor reduces inhibition was tested by comparing the effects of CP93129 and the GABAA antagonists bicuculline and gabazine in the same neurons. The effects of GABAA antagonists on spike count, tuning bandwidth, two-tone ratio, and temporal response characteristics mimicked those of CP93129 across the neuron population. GABAA antagonists also blocked or reduced the facilitation of evoked responses by CP93129. These results are all consistent with the reduction of GABAA-mediated inhibition by 5-HT1B receptors in the IC, resulting in an increase in the level of evoked responses in some neurons, and a decrease in spectral selectivity in others.
INTRODUCTION
Neuromodulatory signals such as serotonin are broadly released in the brain in response to changes in internal state (Trulson and Jacobs 1979, 1981) but transform the response properties of sensory neurons in highly specific ways (Hurley et al. 2004; Mooney et al. 1996; Xiang and Prince 2003). This specificity is achieved through the targeting of classes of neurons by a variety of receptor types that may alter intrinsic neuron properties or target presynaptic neurons and alter the release of neurotransmitter (Hoyer et al. 1994, 2002). One consequence of these effects is that serotonin can alter the balance of different types of inputs within sensory regions. For example, serotonin may alter the balance between ascending versus descending projections (Mooney et al. 1996) or the balance between within- versus between-layer communication in the cortex (Xiang and Prince 2003).
In the inferior colliculus (IC), an auditory midbrain nucleus, serotonin modifies a number of response properties, including both spectral and temporal aspects of evoked auditory responses (Hurley and Pollak 2005; Hurley et al. 2002). One of the properties of IC neurons that has been the best studied in terms of its modulation by serotonin is frequency tuning, which can be expanded or narrowed by serotonin (Hurley and Pollak 2001). Frequency response curves in the IC are often shaped by inhibition, which may narrow the range of frequencies and intensities that evoke action potentials and decrease the level of responses within the excitatory curve itself (Fuzessery and Hall 1996; Hall 1999; Lu and Jen 2001; Palombi and Caspary 1996; Suga 1965; Yang et al. 1992). In addition to regions near the excitatory frequency response curve, tones at frequencies that are distant from the excitatory curve by an octave or more also decrease or facilitate excitatory responses (Mittman and Wenstrup 1995). These phenomena are simply inherited from lower auditory nuclei for many neurons (Portfors and Wenstrup 2001) but are influenced by the convergence of excitatory and inhibitory inputs within the IC for other neurons (Sanchez et al. 2008). The effect of any serotonergic mechanism that regulated excitatory-inhibitory balance would therefore be likely to affect not only frequency tuning but also broader spectral sensitivity in the IC.
Of the multiple types of serotonin receptor present in the IC (Cornea-Hebert 1999; Harlan et al. 2000; Heidmann et al. 1998; Morales et al. 1998; Peruzzi and Dut 2004; Thompson et al. 1994; To et al. 1995; Vilaró et al. 2005), the 5-HT1B receptor has the potential to regulate the excitatory-inhibitory balance that shapes spectral sensitivity in a highly targeted way. This is because the 5-HT1B receptor is localized near presynaptic terminals and in axons and decreases the release of a variety of neurotransmitters (Bennett-Clarke et al. 1993; Chadha et al. 2000; Hwang and Dun 1999; Johnson et al. 1992; Matsuoka et al. 2004; Sari 2004; Stanford and Lacey 1996). Co-localization of the 5-HT1B receptor with a subset of GABAergic neurons in the IC suggests that this receptor could diminish intrinsic inhibitory inputs, although the 5-HT1B receptor is also observed in the cell bodies of non-GABAergic neurons (Peruzzi and Dut 2004). Furthermore, local activation of this receptor in the IC usually increases stimulus-evoked responses and in some neurons also increases the bandwidth of excitatory responses (Hurley 2006). The similarity of these effects to the effects of drugs that antagonize inhibitory transmission in the IC, particularly GABAergic transmission through GABAA receptors (Fuzessery and Hall 1996; Hall 1999; LeBeau et al. 2001; Lu and Jen 2001; Palombi and Caspary 1996; Suga 1965; Yang et al. 1992) suggests a testable hypothesis that 5-HT1B activation decreases inhibition of IC neurons.
The goals of this study were to better define both the functional consequences and mechanisms of 5-HT1B receptor activation in frequency tuning and spectral integration within the IC. To accomplish this, the selective 5-HT1B agonist CP93129 was iontophoretically applied to IC neurons in vivo, and its effects on excitatory-inhibitory balance were characterized across a broad spectral range. Excitatory-inhibitory balance was assessed with two-tone stimuli in which a fixed-frequency tone that evoked spikes was presented together with a second variable-frequency tone that could reduce the response to the first tone. Although such a reduction in response may have multiple sources along the auditory pathway, including mechanical properties of the cochlea (Pickles 1982; Ruggero et al. 1992), or intrinsic and synaptic properties of neurons in the IC or lower auditory nuclei, the local application of drugs allowed the local regulation of these two-tone interactions to be examined. To explore a possible link between the 5-HT1B receptor and GABAergic pathways, the effects of 5-HT1B activation were compared with the effects of GABAA receptor blockade in the same neurons, both in sequence and during co-application. If activation of the 5-HT1B receptor decreases the release of GABA that subsequently activates GABAA receptors, then its effects should be similar to those of GABAA antagonists and reducible by application of GABAA antagonists. Our results are consistent with the hypothesis that the 5-HT1B receptor reduces GABAergic inhibition as one mechanism of action, and that the result is a modulation of the spectral responsiveness of IC neurons.
METHODS
Experimental subjects and surgical techniques
Twenty-three male CBA/J mice of 3–8 wk of age were used in this study. Surgical procedures for mice were similar to those described previously for Mexican free-tailed bats (Hall and Hurley 2007). Mice were briefly anesthetized with isoflurane fumes, then injected intraperitoneally with 120 mg/kg ketamine and 5 mg/kg xylazine. Once surgical anesthesia was achieved, as assessed by lack of response to tail and toe pinch, mice were placed in a stereotaxic holder. Ear bars and a palate clamp were used to stabilize the head for surgery. Ear bars were custom made from Delrin and were blunted to preclude the possibility of puncturing the tympanum. A longitudinal incision was made on the top of the head, and the skin was retracted to the side. Two holes, each centered ∼1 mm lateral and 1 mm caudal to lambda, were made with a surgical drill above the two ICs, and the dura incised with a sharpened tungsten probe. After surgery, the ear bars were removed and the mice placed in a stereotaxic recording apparatus (Schuller et al. 1986) within a sound-attenuation chamber. The level of anesthesia was assessed by periodically observing the rate of breathing and the responsiveness to tail pinch and was maintained with a supplemental injection of either 24 mg/kg ketamine and 1 mg/kg xylazine (1/5 the surgical dose) or with 24 mg/kg ketamine alone when indicated. Recording sessions lasted for 6–9 h. At the conclusion of the experiment, mice were killed by exposure to isoflurane fumes. All procedures were approved by the Bloomington Animal Care and Use Committee and follow the Guidelines for the Care and Use of Laboratory Animals.
Extracellular recording of single neurons
A total of 126 single IC neurons were recorded through extracellular glass pipettes attached in a “piggy-back” configuration (Havey and Caspary 1980) to a tribarreled micropipette used for the iontophoresis of drugs. The single-barreled recording pipettes were filled with 1 M NaCl and had resistances of 8–20 MΩ. Pipettes were connected by a silver-silver chloride wire to a Dagan 2400 amplifier (Minneapolis, MN). Spikes were fed through a spike signal enhancer (FHC; Bowdoinham, ME) before being digitized through a data-acquisition processor board (Microstar; Bellevue, WA). Iontophoresis pipettes were broken to a tip diameter of 10–20 μm with the single-barreled recording pipette protruding 10–15 μm in front of the multibarreled pipette. Multibarreled electrodes were positioned above the IC under visual control through a dissecting microscope and lowered with a piezoelectric microdrive (Burleigh/EXFO inchworm, Mississauga, Ontario) until action potentials were observed. The top of the IC was defined as the point of initial contact of the recording electrode to ground.
Auditory stimuli
Auditory stimuli were created and data were collected with the custom software package Batlab (Dr. Donald Gans, Kent State University). Stimuli were regulated in intensity through a PA5 attenuator and were filtered through an FT-6 antialias filter (TDT; Alachua, FL). Stimuli were played through either an earphone biased with 200 V DC (Schuller 1997), positioned in the ear contralateral to the recording electrode, or a midline freefield speaker (Infinity Emit B, Harman International Industries; Woodbury, NY). The frequency response of the custom-made earphone was flat ±6 dB from 10 to 120 kHz with harmonic distortions ≥34 dB below the fundamental frequency. Calibration of the freefield speaker was accomplished by placing a measuring microphone (ACO Pacific PS9200 kit; Belmont, CA) in the position occupied by the mouse's head during experiments. The response of the speaker was flat within ±6 dB from 13 to 40 kHz, and harmonic distortions were 30–40 dB below the fundamental frequency across this range, which encompassed the characteristic frequencies of 68% of recorded neurons. The speaker produced a higher intensity of sound at lower frequencies.
Tone bursts of 20 ms in duration and 0.5-ms rise and fall times were used to measure frequency tuning and decreases in spikes in two-tone paradigms. Frequency tuning was measured by presenting tones across the frequency ranges of single neurons from 10 dB below threshold to 30–50 dB above threshold at characteristic frequency (CF). The frequency intervals of the presented tones varied from 1 to 5 kHz, depending on the bandwidth of the neuron recorded. Two-tone protocols were used to measure the decreases or increases of tone-evoked spikes by the presentation of a second tone. To generate two-tone stimuli, an excitatory probe tone was presented at a neuron's CF. The probe tone was set at 10–30 dB (mean ± SE) above the minimum threshold of a given neuron. To measure the frequency dependence of two-tone effects, a test tone was then varied in frequency and presented at the same onset time and for the same duration as the probe tone. Test frequencies ranging from 2 to ≤50–70 kHz were presented when a contact with a neuron was first established, and frequency regions evoking decreases or increases in spikes relative to the probe alone were assessed. Measurements of control and drug effects were then tailored to include these regions of interest. Because neurons were not sampled with uniform increments of test frequencies, regions that showed only mild effects of the test tone may have been missed. This focus on regions of interest was necessary for presenting test stimuli during multiple control and drug conditions while the neural recordings were maintained. Test tones were presented 10−20 dB (mean ± SE) above probe tones, to maximize inhibition. For a subset of 54 neurons, the time of onset of the probe tone was varied relative to that of a test tone at the frequency producing the maximal two-tone spike reduction, to generate delays between the two tones ranging from −20 ms (probe tone leading by 20 ms) to +40 ms (probe tone lagging by 40 ms).
Iontophoresis and drugs
Responses to stimuli were measured before, during, and after the iontophoresis of drugs through the multibarreled pipette of the piggy-back electrode. Two of the pipette barrels were filled with receptor agonists or antagonists, and one was filled with 1 M NaCl to serve as a sum channel, balancing the iontophoretic currents ejected through the other barrels. The barrels were connected by silver-silver chloride wire to iontophoresis pump modules (Dagan ION-100). The selective 5-HT1B agonist CP93129 dihydrochloride and the nonselective 5-HT antagonist methysergide maleate (both from Tocris Biosciences; Ellisville, MO) were used to activate and block the 5-HT1B receptor, respectively. Although multiple auditory response properties are altered by anesthetics, including ketamine/xylazine anesthesia (Astl et al. 1996), the effect of CP93129 itself is not statistically distinguishable in animals that are under maintenance ketamine/xylazine anesthesia versus awake (Hurley 2006). Either bicuculline methiodide (Sigma-Aldrich; St. Louis, MO) or SR95531 hydrobromide (gabazine; Tocris Bioscience) was used to block GABAA receptors. All drugs were dissolved at 10 mM in 200 mM NaCl, pH 4.5. This vehicle solution does not alter neural responses when iontophoresed alone (Hurley and Pollak 1999, 2001). Drugs were retained in the pipette with a negative current of 10–15 nA and ejected with positive current ranging from 10 to 80 nA. Drugs were ejected over a period of 5–10 min while their effects were monitored through the repetition of a small portion of the stimulus repertoire. When the spike counts stabilized, data were collected for the drug treatment. Similar procedures were followed for the collection of data for multiple drug and recovery conditions for each neuron.
Quantification and analysis
The effects of drugs on spike count, frequency bandwidth, and responses to two-tone stimuli were all measured. Spike counts were measured at CF, 10–30 dB above threshold. Spike counts during drug application were normalized to the spike counts before drug application and expressed as the proportional change in spike count [(drug − control)/control] or as the log value of the change [log(drug/control)]. Frequency response curves were generated by varying tones in frequency at a fixed intensity of 20–30 dB above the threshold at CF. Frequency bandwidth was measured as the difference between the low- and high-frequency borders of the response curves. Borders were determined by a linear interpolation between the frequencies evoking spike counts above and below the half-maximum value per 32 stimulus repetitions. Drug-evoked changes in bandwidth were expressed as the difference between drug and control values, in octaves. Decreases in spikes evoked in the two-tone protocol were measured as the two-tone ratio: the difference between the response evoked by the combination of the probe and test tones and the probe tone alone, normalized to the response evoked by the probe tone alone [(both tones − probe)/probe]. Negative two-tone ratio values indicate a reduction in spikes by the test tone relative to the probe tone alone, and positive values indicate an increase in spikes relative to the probe tone alone. The effects of drugs on the two-tone ratio were expressed as a simple difference (drug-control), so that positive values indicate that the test tone evoked a smaller decrease in spikes in the presence of a drug. None of the effects of CP93129 on these response characteristics was correlated with the age of individual mice (in days) at the time of experimentation (Pearson's correlations, P = 0.237 for spike count, P = 0.75 for bandwidth, and P = 0.91 for 2-tone ratio, n = 19 mice).
Spike data were exported from Batlab in ASCII format for statistical analysis. Differences in the effects of different drug treatments on the measurements above were assessed either with ANOVAs or t-test using Excel (Microsoft; Redmond, WA) or Statistica (StatSoft Inc; Tulsa, OK). Correlations among different drug treatments, or of a single drug treatment on different neural response properties, across the neuron population were assessed with Pearson's product-moment correlations.
RESULTS
To measure the responses of IC neurons to the activation of the 5-HT1B receptor in vivo, a total of 126 single neurons were recorded extracellularly through high-resistance glass micropipettes from the IC of 23 male CBA/J mice. The selective 5-HT1B agonist CP93129 was iontophoresed during the recording of all 126 neurons through a multibarreled pipette attached to the recording pipette in piggyback configuration (Havey and Caspary 1980). Responses of neurons to tone bursts were recorded before, during, and after the application of CP93129. Here we first present evidence that the specific patterns of effects of CP93129 on frequency tuning and two-tone interactions vary among IC neurons, but that these effects are similar to those evoked by a removal of inhibition. We further test whether the effects of CP93129 are consistent with a GABAergic mechanism.
CP93129 increases evoked responses
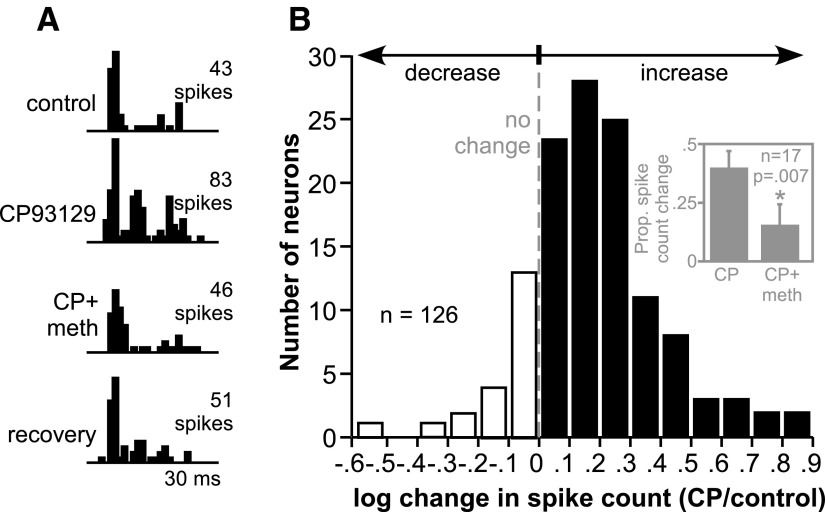
CP93129 usually increased spike counts evoked by the presentation of tone bursts. A typical effect of CP93129 is illustrated in Fig. 1A, the peristimulus time histograms of the response of a single IC neuron to a 20-ms tone burst at the neuron's CF of 29 kHz. The number of spikes fired by the neuron approximately doubled during the application of CP93129, largely through an increase in spikes in the latter part of the spike train. The co-application of methysergide, a nonselective 5-HT receptor antagonist, in conjunction with CP93129 (CP+meth), blocked the increase in spikes. After drug application was stopped, the neuron maintained close to the control level of response. This type of increase in sound-evoked responses was common among IC neurons as illustrated by the histogram in Fig. 1B, which shows the distribution of CP93129-evoked changes in the response to a tone at CF, 10–30 dB above threshold. Values are expressed as the log of proportional changes in spike count [log(CP/control)] due to an extensive diminishing “tail” of neurons with large increases in spike count. The dashed line represents the point of no change in spike count, filled bars are increases in spike count, and open bars are decreases in spike count. In all, 61% of neurons (77/126) increased their spike count by over 30%, a criterion used to assess serotonergic effects in previous studies (Hall and Hurley 2007; Hurley and Pollak 1999). In contrast, relatively few neurons responded to CP93129 by decreasing their evoked spikes by 30% or more (5/126).
FIG. 1.
Effect of the selective 5-HT1B agonist CP93129 on evoked responses. A: peristimulus time histograms (PSTHs) of the response of a single neuron to a 20-ms tone at its CF of 29 kHz presented at 50 dB SPL. Responses to the same tone are illustrated before the application of CP93129 (control), during CP93129 application, during the coapplication of CP93129 and the serotonergic antagonist methysergide (CP+meth), and several minutes after the application of all drugs was stopped (recovery). B: histogram of the changes in spike count induced by CP93129 in 126 inferior colliculus (IC) neurons. Values are the log of the ratio of spike counts in the presence of CP93129 and the control [log(CP/control)]. □, decreases in spike count (negative log values); ▪, increases in spike count (positive log values); - - -, no change in spike count. Inset: the average proportional changes in spike count in a group of 17 neurons to which CP93129 and methysergide were both applied. Methysergide significantly reduced the spike count facilitation evoked by CP93129 (P = 0.007, 2-tailed paired t-test). Error bars represent the standard error of the mean. CP, CP93129; meth, methysergide.
Methysergide was applied not only onto the neuron in Fig. 1A but also onto 16 additional neurons. Of these, 14 neurons significantly increased their average spike count in CP93129 (P < 0.05, 2-tailed unpaired t-test); methysergide significantly reduced this effect in 9 of the 14 neurons. Figure 1B, inset, shows the average proportional change in spike count evoked by CP93129 alone versus the change in spike count evoked by the concurrent application of CP93129 and methysergide, both relative to predrug values. CP93129 increased the spike count in this group of 17 neurons by 40.0% on average when applied alone, but only by 15.7% when co-applied with methysergide. This difference was significant (P = 0.007, 2-tailed paired t-test).
CP93129 expands frequency responses in some neurons
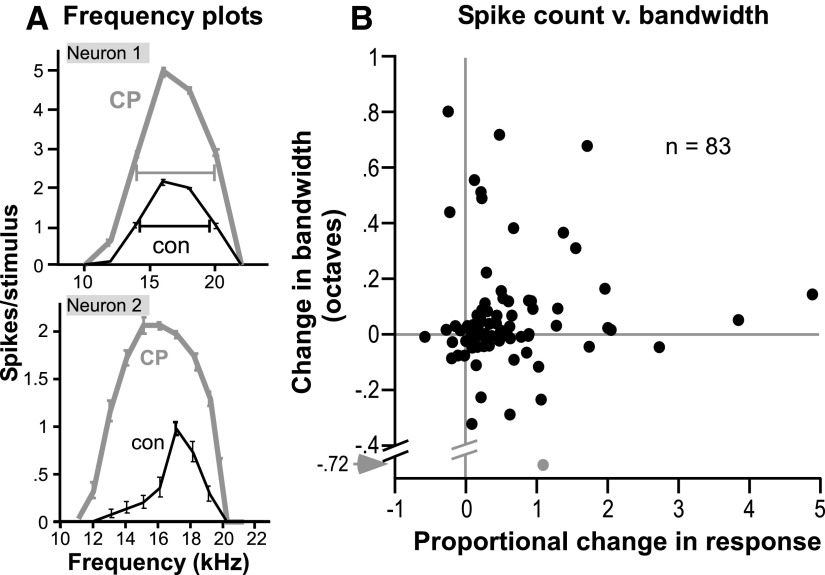
To determine how CP93129 alters frequency tuning in mice, we measured the effects of CP93129 on frequency response curve bandwidth for 83 neurons. For some neurons, CP93129 facilitated responses at all frequencies similarly without changing the range of frequencies that evoked a response. For other neurons, however, CP93129 not only facilitated spikes, but also expanded the excitatory frequency range. Both types of effects are illustrated in Fig. 2A, which consists of frequency response curves, or isointensity plots of mean spike count (±SE) versus frequency, for two different neurons. Neuron 1 had relatively broad tuning, with responses ranging from 12 to 24 kHz at 30 dB above the neuron's threshold at CF. CP93129 facilitated the spike count across this entire range. For neuron 2, CP93129 not only increased the spike count but also unmasked responses to tones that evoked little or no response in the control, changing the overall shape of the response curve. Neither neuron was held for long enough to obtain a recovery frequency response curve.
FIG. 2.
Effect of the selective 5-HT1B agonist CP93129 on frequency tuning. A: isointensity plots of average spike count per stimulus (±SE) vs. frequency for 2 individual neurons. For the upper neuron, tones were presented every 2 kHz from 10 to 22 kHz at 50 dB SPL. For the lower neuron, tones were presented every 1 kHz from 11–21 kHz at 40 dB SPL. B: scatterplot of CP93129-evoked changes in spike count vs. tuning bandwidth in a group of 83 neurons. Proportional changes in spike count are the difference between the drug and control counts, normalized to the control value [(CP93129-control)/control], and bandwidth is presented as the difference in octaves between drug and control bandwidths (CP93129-control). Gray lines mark no change along each axis. Gray point represents an extreme value.
The effects of CP93129 on frequency tuning were quantified by measuring the bandwidth of the frequency response curve as the difference between the frequencies marking the low and high borders of the curve at 10–30 dB above the neurons' thresholds at CF. Frequency response curve borders were calculated by a linear interpolation between the frequencies that evoked spike counts below and above the value of half of the maximum spike count. An example of this half-maximum bandwidth is shown for the upper neuron of Fig. 2A as bars stretching across the half-maximum value for the two tuning functions. Although CP93129 greatly facilitated the spike count for this neuron, its half-maximum bandwidth remained relatively unchanged. For the lower neuron, it is evident that the half-maximum bandwidth would increase in the presence of CP93129. Figure 2B compares proportional changes in spike count with changes in bandwidth in the set of 83 neurons for which frequency response curves were measured; the gray point represents an extreme value. Because CP93129-evoked changes in both spike count and bandwidth were usually positive, many data points are in the upper right quadrant of the plot. Within this quadrant, however, there is substantial spread in the data, emphasizing the variation in response patterns among neurons.
Effects of CP93129 are consistent with a decrease in inhibitory neurotransmission
Because inhibitory inputs cannot be measured directly through extracellular recording techniques, we performed two types of experiments to further test the hypothesis that CP93129 decreases inhibition within the IC. The first type of experiment was to visualize inhibition indirectly through a decrease in response to an excitatory tone evoked by a second tone, and to measure the effect of CP93129 on responses to these so-called two-tone stimuli. A second type of experiment was to apply CP93129 while simultaneously blocking GABAA receptors, and quantitatively compare the effects of the two drugs both in sequence and during co-application.
CP93129 has varied effects on two-tone spike reduction
Similar to the effects of CP93129 on frequency bandwidth, this drug had variable effects on responses to two-tone stimuli. Figure 3 illustrates two neurons in which CP93129 increased evoked responses but did not remove specific regions of two-tone spike reduction. Two-tone frequency response curves representing the response to the co-presented probe and test tones are plotted as mean spike count (±SE) versus the test frequency for responses to 32 repeated stimulus presentations. The excitatory probe frequency is indicated by an asterisk, and the responses evoked by the probe tone alone in the control and different drug treatments are indicated by horizontal lines.
FIG. 3.
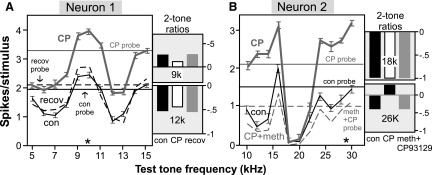
Effect of the selective 5-HT1B agonist CP93129 on 2-tone frequency interactions in two neurons that showed little change in tuning bandwidth. Data for both neurons is presented as the average spike count per stimulus (±SE) in response to the co-presentation of the 2 tones, plotted vs. the frequency of the variable test tone. *, frequency of the excitatory probe tone; —, the response to the probe tone alone at the control, drug application, and recovery. Plots of 2-tone ratio adjacent to the 2-tone plots were calculated by normalizing spike counts in response to both tone stimuli together relative to spike counts evoked by the excitatory probe tone alone ((both tones − probe tone)/ probe tone) for the individual test frequencies indicated. A: for neuron 1, the probe tone was presented at 10 kHz and 50 dB SPL and the test tone was presented every kHz from 5 to 15 kHz at 70 dB SPL. B: for neuron 2, the probe tone was presented at 29 kHz and 40 dB SPL, and the test tone was presented every 2 kHz from 10 to 30 kHz at 60 dB SPL.
Neuron 1 represents a relatively simple case. For this neuron, the probe was fixed at the neuron's CF of 10 kHz, and the test tone was varied in steps of 1 kHz from 5 to 15 kHz. In the control, regions in which the test tone decreased responses relative to the probe tone occurred both above and below the CF of 10 kHz. In the presence of CP93129, the level of the response increased proportionally across the entire range of test frequencies. This is illustrated by the plots of the two-tone ratio at 9 and 12 kHz. CP93129 did not change the two-tone ratio at 9 or 12 kHz despite the overall increase in evoked responses, thus maintaining the relative spectral selectivity of this neuron.
In contrast, the effect of CP93129 on the two-tone frequency response curve of neuron 2 varied with test frequency. For this neuron, the probe was fixed at the neuron's CF of 29 kHz, and the test tone was varied in steps of 2 kHz from 10 to 30 kHz. Test tones of 18 and 20 kHz produced a pronounced notch of spike reduction in the control. CP93129 decreased the two-tone ratio at frequencies above and below this notch. CP93129 did not reduce the notch at 18–20 kHz, however, and the two-tone ratio at 18 kHz did not vary much in any drug treatment. These effects of CP93129 resulted in a change of the spectral selectivity of the neuron with a greater contrast between the spike reduction at 18–20 kHz and the relative facilitation at other frequencies. The co-application of methysergide reversed this pattern of effects.
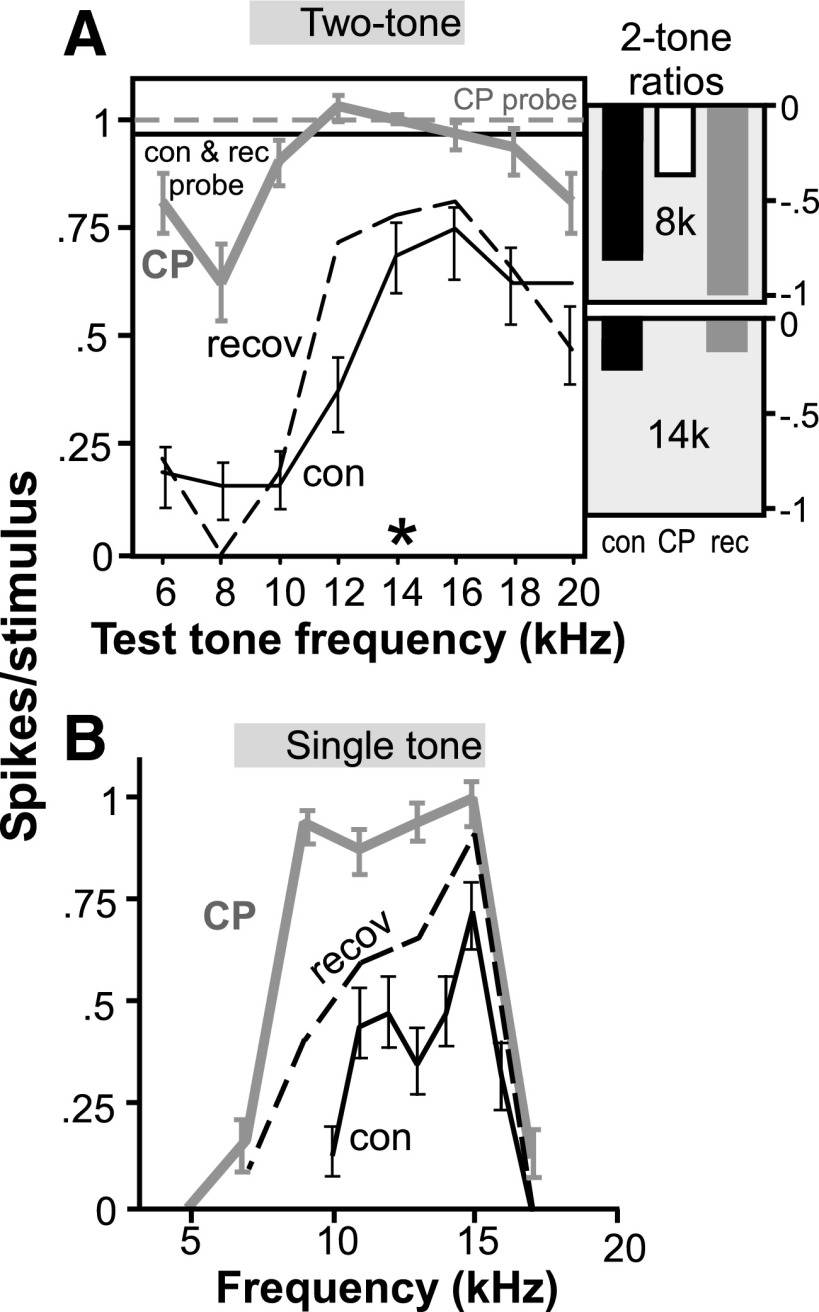
For some neurons, CP93129 did attenuate specific regions of two-tone spike reduction as demonstrated by the neuron in Fig. 4. For this neuron, CP93129 increased the spike count in the two-tone curve and decreased the spike reduction in a region ranging from 6 to 10 kHz (Fig. 4A). CP93129 also decreased the two-tone spike reduction at higher frequencies, but to a lesser extent, as seen by comparing the two-tone curve and two-tone ratios in the control versus CP93129 at 8 and 14 kHz. This correlated with the single-tone frequency response curve of the same neuron (Fig. 4B) in which tuning expanded into lower -frequency regions during CP93129 application. These findings suggest that CP93129 decreased the two-tone reduction of spikes in a frequency-specific manner for this neuron with a larger effect at frequencies lower than the control best frequency.
FIG. 4.
Effect of the selective 5-HT1B agonist CP93129 on 2-tone frequency interactions in a neuron that substantially changed in tuning bandwidth. A: 2-tone response curve for a single neuron in the control, CP93129, and recovery. The excitatory probe tone was presented at 14 kHz and 40 dB SPL, and the test tone was presented every 2 kHz from 6 to 20 kHz at 60 dB SPL. Conventions are as in Fig. 3. B: frequency response curve for the same neuron in the control, CP93129, and recovery. Tones were presented every kHz from 10 to 17 kHz in the control and every 2 kHz from 5 to 17 kHz in CP93129 and the recovery, at 60 dB SPL.
Effects of CP93129 vary across the neuron population
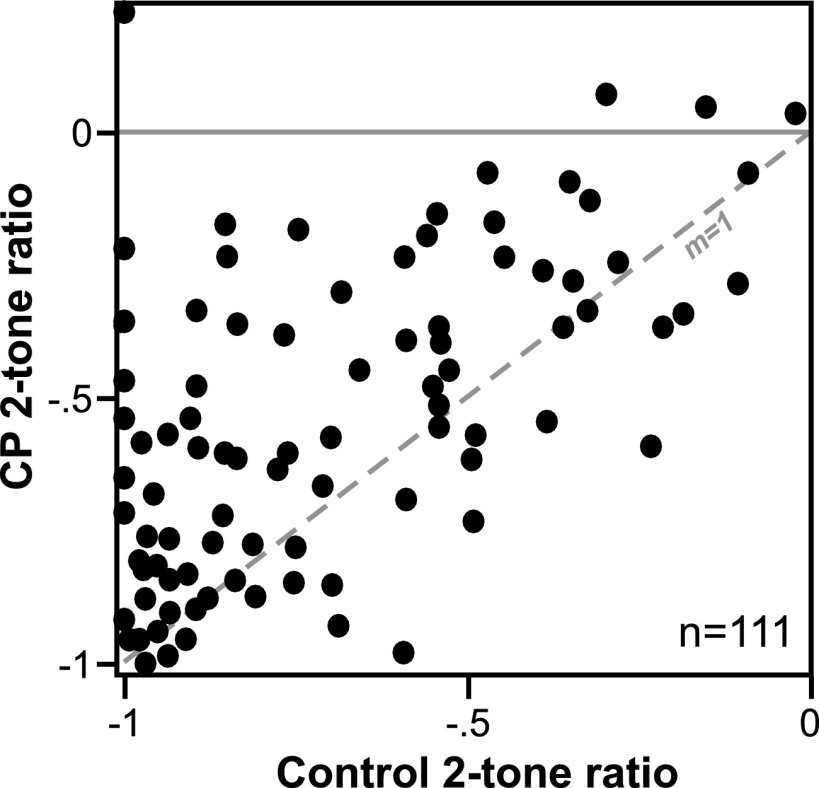
Figure 5, a plot of the two-tone ratio in the control versus during CP93129 application for 111 neurons that showed any amount of two-tone spike reduction, illustrates two key points of the effects of CP93129 across the neuron population. The first of these is that CP93129 usually decreased the two-tone ratio when it had an effect, consistent with a decrease in inhibition. This can be seen by the large number of neurons above the dashed line marking a slope of unity (m = 1). A second important point illustrated in this plot is that the effects of CP93129 varied substantially among neurons. Many neurons showed small or intermediate changes in the two-tone ratio, resulting in a relatively continuous distribution of shifts in the ratio. The effects of CP93129 across different response properties also varied. This was measured across the neuron population by performing a Pearson's correlation on the effects of CP93129 on two-tone ratio (drug-control), bandwidth of frequency response (difference in octaves), and spike count (drug-control)/control). The only significant correlation among these variables was between CP93129-evoked changes in spike count and two-tone ratio (P = 0.005), but the correlation itself was relatively low (r = 0.32).
FIG. 5.
Effect of the selective 5-HT1B agonist CP93129 on 2-tone effects in a group of 111 neurons. Scatterplot of 2-tone ratios, indicative of the amount of spike reduction caused by the test tone relative to the response to the probe tone alone, in the control (x axis) vs. during the application of CP93129 (y- axis). The dashed line with a slope of unity (m = 1) represents no change in 2-tone ratio. Many neurons exhibited decreased 2-tone ratios in the presence of CP93129.
CP93129 has qualitatively similar effects at different probe-test delays
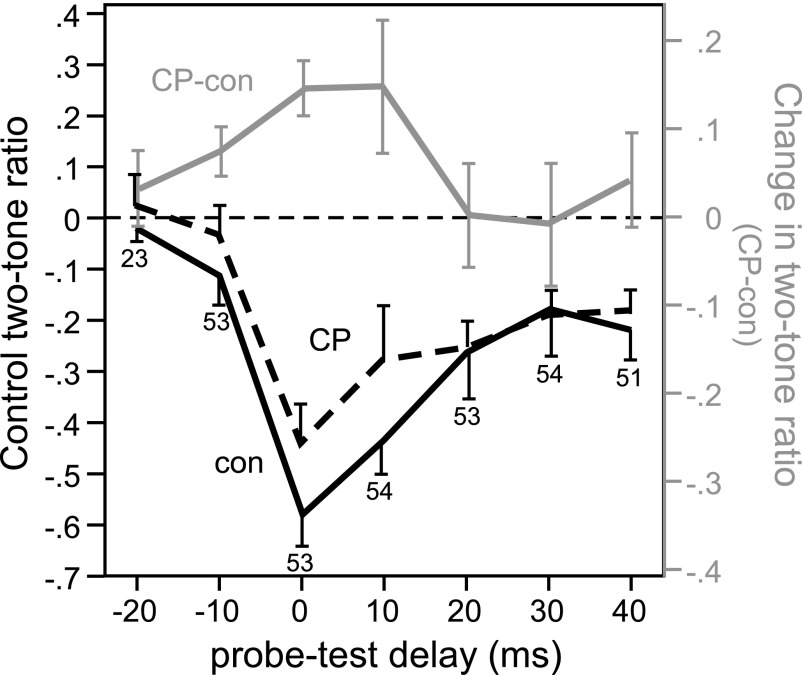
Figure 5 illustrates responses to two-tone stimuli in which the two tones were presented simultaneously and overlapped completely. IC neurons show a peak in two-tone reduction of spikes when tones are simultaneously presented (Nataraj and Wenstrup 2005). Two-tone suppression in the cochlea could make a relatively large contribution to the response to such simultaneously presented tones, as opposed to nonsimultaneously presented tones, because cochlear suppression has a rapid onset and decay relative to synaptic inhibition (Arthur et al. 1971; Suga et al. 1975). This raises the concern that events in the IC, and the effects of the local application of CP93129, could make a correspondingly weak contribution to the two-tone interactions we observed. To explore this issue, stimuli incorporating a variable delay between the probe and test tones, ranging from −20 ms (probe tone leading) to +40 ms (probe tone lagging), were presented to 54 neurons to determine whether the effect of CP93129 was weaker with simultaneous tone presentation. The average two-tone decrease in spikes in the control was greatest at a delay of 0 ms, when the 20-ms probe and test tones were presented simultaneously and overlapped completely, but was also apparent at other probe-test delays (Fig. 6, black line, mean − SE). CP93129 decreased the average two-tone ratio at probe-test delays at and around zero ms (Fig. 6, dashed black line, mean + SE), as indicated by positive values of the difference between the ratio in CP93129 and the control (Fig. 6, gray line, right ordinate, mean ± SE). This finding illustrates that the local application of CP93129 has similar effects when tones were simultaneously presented and when there was a delay in onset between tones.
FIG. 6.
Effects of CP93129 on 2-tone reduction of spikes at different probe-test delays. Two-tone ratios are plotted as a function of the delay between the onset of the probe and test tones, from −20 ms (probe tone leading by 20 ms) to +40 ms (probe tone lagging by 40 ms), in both the control (black line, left ordinate; mean − SE, n = 54 neurons) and in the presence of CP93129 (dashed black line, left ordinate; mean + SE). Test tones were presented at the frequency evoking peak reduction of spikes for each neuron. The difference in the 2-tone ratio between the control and drug application in the same set of neurons is plotted in parallel (gray line, right ordinate, mean ± SE, CP93129-control values). Positive values indicate a decrease in the 2-tone reduction of spikes. Numbers at particular probe-test delays indicate how many neurons were recorded at these delays.
CP93129 mimics GABAA antagonists
To test the predictions that GABAA antagonists should have effects similar to those of CP93129, and should also reduce the effects of CP93129, we applied CP93129 in conjunction with either bicuculline or gabazine in 37 neurons. Whenever neural recordings lasted long enough, neurons were tested with CP93129 and a GABAA antagonist alone as well as in combination.
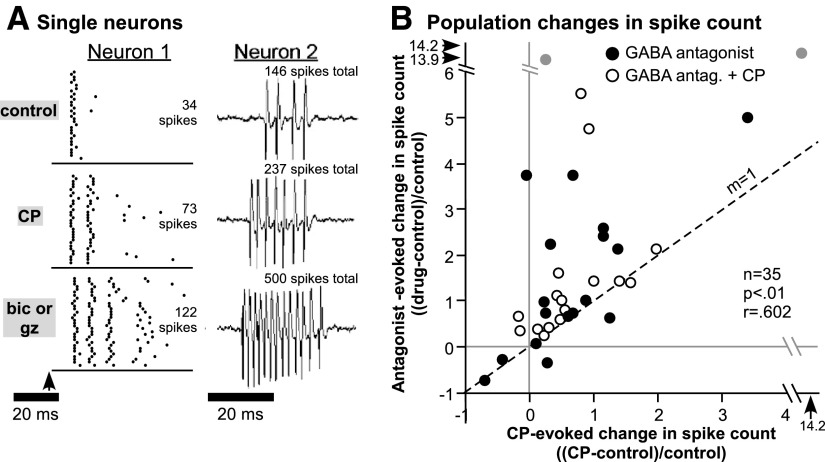
For most neurons, the effects of CP93129 and the GABAA antagonists were similar in direction and pattern, although the effects of GABAA antagonists were usually larger than those of CP93129. Examples of the effects of CP93129 and bicuculline or gabazine on the spike trains of single neurons are presented in Fig. 7A. For neuron 1, raster plots illustrate the separate applications of CP93129 and bicuculline. For this neuron, CP93129 approximately doubled and bicuculline approximately quadrupled the spike counts relative to the control value for 32 stimulus repetitions. Both CP93129 and bicuculline increased spikes not in the initial peak of the response but in the later part of the spike train. The 20-ms tone at 15 kHz used to evoke responses is represented by the bar below the raster plots; bicuculline increased the duration of the neuron's response beyond the duration of the stimulus. CP93129 and bicuculline also both maintained similar interspike intervals within the spike train. Thus CP93129 and bicuculline increased spikes in the same way but to a different degree. Neuron 2 shows interesting similarities to and differences from this pattern. The response of neuron 2 is plotted as a voltage trace to better illustrate the timing of action potentials, although spike counts also represent the summed response to 32 stimulus presentations for easier comparison to neuron 1. Similar to neuron 1, CP93129 increased the total spike count, and gabazine had an even larger effect. Unlike neuron 1, the increase in spike count evoked by both CP93129 and gabazine was accompanied not only by an increase in the duration of the spike train but also by a decrease in the first-spike latency and the interspike interval. Thus for both neurons 1 and 2, the effects of CP93129 mimicked the effects of GABAA antagonists not only on the spike count but on the timing of spikes within the spike train.
FIG. 7.
Comparison of the effects of CP93129 and GABAA antagonists on spike count. A: responses of single neurons. For neuron 1, responses to 32 stimulus repetitions in the control, during the application of CP93129 alone (CP), and during the application of bicuculline alone (bic or gz) are presented as raster plots. Spike count values represent summed responses to all 32 stimuli. The tone was presented at 15 kHz and 60 dB SPL. For neuron 2, responses to single stimuli in the control, during the application of CP93129 alone (CP), and during the application of gabazine alone (bic or gz) are presented as voltage traces, but spike counts represent responses to 32 stimulus repetitions, similar to neuron 1. The tone was presented at 36 kHz and 50 dB SPL. B: scatterplot comparing the effects of CP93129 vs. GABAA antagonists on spike count in a group of 35 neurons. Changes in spike count were normalized to control values [(CP − control)/control]. Filled circles, the effects of CP93129 vs. a GABAA antagonist alone; empty circles, the effects of CP93129 vs. a combination of CP93129 and a GABAA antagonist. Gray points, extreme values. The dashed line has a slope of 1, indicating an equal effect of CP93129 and GABAA antagonists on spike count. The correlation between the effects of CP93129 and GABAA antagonists is significant (Pearson's correlation, P < .001), but many neurons responded more strongly to GABAA antagonists than to CP93129. Bic, bicuculline; gz, gabazine.
Figure 7B illustrates changes in evoked spike counts in the presence of either CP93129 (x axis) or one of the GABAA antagonists (y axis) across a set of 35 neurons at CF, 20–30 dB above threshold at CF. Filled circles represent neurons for which responses to CP93129 and a GABAA antagonist alone were measured. For neurons not exposed to a GABAA antagonist alone, responses to the co-application of a GABAA antagonist and CP93129 are plotted (open circles). As seen for the individual neurons, the correlation between the effects of CP93129 and the GABAA antagonists was highly significant (P < 0.001, Pearson's correlation). Additionally, most data points fall above the dashed line marking a slope of 1 (labeled m = 1), indicating that spike counts were higher in the presence of a GABAA antagonist than in the presence of CP93129 alone.
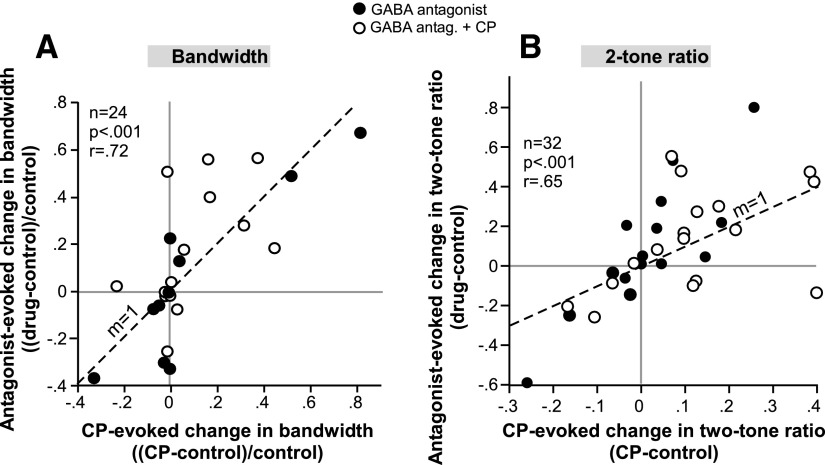
GABAA antagonists had effects similar to those of CP93129 not only on spike count but also on half-maximum bandwidth and peak spike reduction. These effects are summarized in the scatterplots of Fig. 8. Figure 8A plots the change in bandwidth evoked by CP93129 versus bicuculline or gabazine alone (closed circles) or by a combination of a GABAA antagonist and CP93129 (open circles). Across the population of 24 neurons for which bandwidth was measured in the presence of multiple drug combinations, the effects of CP93129 and GABAA antagonists were significantly correlated (P < 0.001, Pearson's correlation). Figure 8B shows the same relationship for drug-induced changes in the peak two-tone ratio. Similar to spike count and bandwidth, the effects of CP93129 and GABAA antagonists on peak spike reduction were significantly correlated (P < 0.001, Pearson's correlation).
FIG. 8.
Comparison of the effects of CP93129 and GABAA antagonists on frequency bandwidth (A) and peak 2-tone ratio (B). Conventions are as in Fig. 6. The correlation in both plots is significant (Pearson's correlation, P < 0.001).
Table 1 summarizes the similarities between the effects of CP93129 and GABAA antagonists on the evoked response properties illustrated in Figs. 7 and 8 and on three temporal response properties: the average first-spike latency, the average interval between the first and second spike (1st ISI) within a train, and the average interval between the second and third spike in a train (2nd ISI). Of these response properties, all show a significant correlation between the effects of CP93129 and the effects of a GABAA antagonist alone or a GABAA antagonist in the presence of CP93129 (Pearson's correlations).
TABLE 1.
Correlation between CP93129 and GABA antagonists
| Response Property | n | r Value | P Value |
|---|---|---|---|
| Spike count | 35 | 0.60 | <0.01 |
| 0.5 max bandwidth | 24 | 0.72 | <0.001 |
| Two-tone ratio | 32 | 0.65 | <0.001 |
| First-spike latency | 30 | 0.42 | 0.02 |
| First ISI | 30 | 0.76 | <0.001 |
| Second ISI | 22 | 0.90 | <0.001 |
Across-population correlation between the effects of CP93129 and GABAA antagonists on multiple response properties of inferior colliculus neurons. ISI, interspike interval.
Co-application of GABAA antagonist reduces response to CP93129
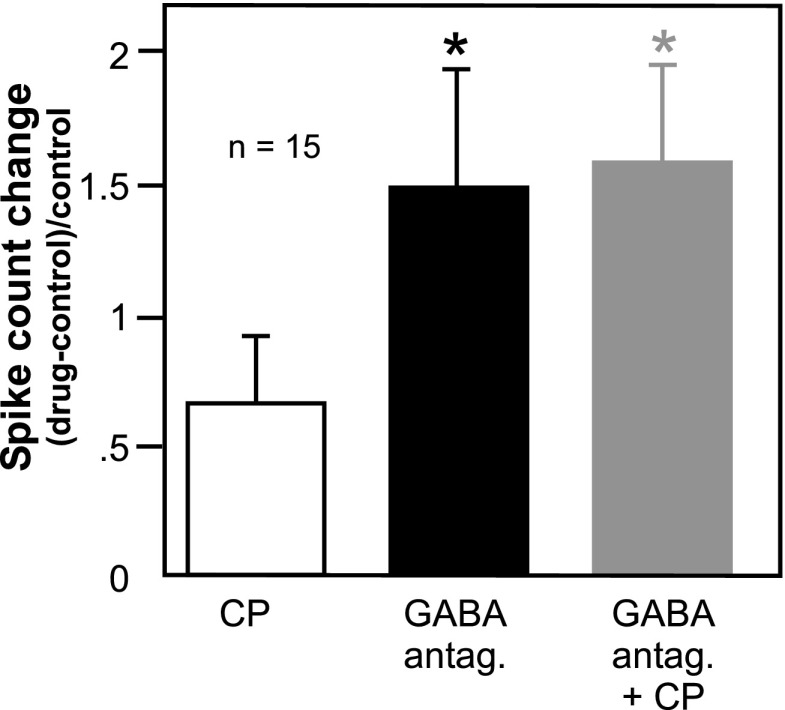
If CP93129 acts by reducing GABAergic transmission, a second prediction is that the presence of a GABAA antagonist should reduce the effect of CP93129. To test this prediction, we compared the effects of a GABAA antagonist and CP93129 alone to the effect of CP93129 in the presence of a GABAA antagonist. Relatively few neurons (n = 15) were held through all of these drug manipulations, and this analysis was carried out only for measurements of spike count because of the larger number of data points for this measurement relative to bandwidth and two-tone ratio. For this set of 15 neurons, the drug-evoked changes in spike count were measured at CF (bars in Fig. 9; asterisks denote significant difference from the CP93129-evoked change in spike count). The effects of a GABAA antagonist alone, or of the combination of a GABA antagonist and CP93129, were significantly greater than for CP93129 alone, as expected from the scatterplots of Figs. 7 and 8B. The addition of CP93129 to a GABAA antagonist did not lead to a further increase in spike count, however (repeated-measures ANOVA, df = 2, 28; P = 0.005 for the overall model; with least significant difference (LSD) post hoc tests P = 0.007 for CP93129 vs. a GABAA antagonist, P = 0.003 for CP93129 vs. the drug combination, P = 0.738 for a GABAA antagonist vs. the drug combination). In addition to this repeated-measures comparison, drug treatments were compared by examining subsets of neurons that were exposed to two of the three drug treatments. The similarities between treatments were then assessed with paired t-tests. This breakdown of drug treatments yielded 17 neurons that were exposed to both CP93129 and GABAA antagonists alone, 33 neurons exposed to CP93129 alone versus the drug combination, and 16 neurons exposed to GABAA antagonists alone versus the drug combination. The results for these pairs of drug treatments were similar to those for the smaller set of neurons. That is, the effect of CP93129 was significantly different from treatment with a GABAA antagonist (P = 0.013, 2-tailed paired t-test) and from treatment with the drug combination (P = 0.004, 2-tailed paired t-test), but the effects of the GABAA antagonist and the drug combination were similar (P = 0.402, 2-tailed paired t-test). Thus the effects of GABAA antagonists and CP93129 were strongly subadditive on average consistent with action at different points along the same inhibitory pathway.
FIG. 9.
Comparison of the effects of GABAA antagonists alone vs. GABAA antagonists and CP93129. Average changes in spike count at CF relative to the control are presented for a set of 15 neurons that were recorded for long enough for the sequential application of all 3 drug treatments. Spike count changes in the presence of a GABAA antagonist alone, or in the presence of a combination of a GABAA antagonist and CP93129, were each significantly greater than for CP93129, but not significantly different from each other (repeated-measures ANOVA, P = 0.005 overall, df = 2, 28; with post hoc LSD tests, P = 0.007 for CP93129 vs. GABAA antagonist alone, P = 0.003 for CP93129 vs. drug combination, P = 0.738 for GABAA antagonist alone vs. drug combination). *, significant difference from the CP93129-evoked change in spike count.
DISCUSSION
The data presented here support two main conclusions on the role of the 5-HT1B receptor in the IC. First, our findings are consistent with the 5-HT1B receptor acting to decrease the release of GABA. Second, activation of the 5-HT1B receptor causes changes in frequency response curves and on spectral integration that vary widely across the neuron population. In the following discussion, we summarize the logic supporting a GABAergic mechanism of action of the 5-HT1B receptor, consider alternate mechanisms of action, and describe functional implications of the regulation of frequency sensitivity by this receptor.
Evidence that the 5-HT1B receptor regulates inhibition
Activation of the 5-HT1B receptor decreases the release of a variety of neurotransmitters among different regions of the brain (Bennett-Clarke et al. 1993; Chadha et al. 2000; Hwang and Dun 1999; Johnson et al. 1992; Matsuoka et al. 2004; Stanford and Lacey 1996; Sari 2004). Although a definitive conclusion that 5-HT1B activation decreases GABA release would require measuring GABA release directly or monitoring synaptic activity in vitro, the in vivo results described here are all consistent with activation of the 5-HT1B receptor in the IC reducing GABAergic inhibition. This conclusion is based on four types of evidence resulting from selectively activating the 5-HT1B receptor. The first of these is that 5-HT1B activation caused an increase in sound-evoked responses and in some neurons an expansion in the excitatory frequency response curve. These phenomena have now been observed in both Mexican free-tailed bats (Hurley 2006) and in mice, suggesting that the role of the 5-HT1B receptor is similar in the IC of these divergent model animals. Second, 5-HT1B activation often attenuated and in some cases abolished the reduction of an excitatory response by a second stimulus. Third, 5-HT1B activation and GABAA blockade had remarkably similar effects both in single neurons and across populations of neurons. This was true not only for the direction and magnitude of the responses, but also for frequency response bandwidths, two-tone effects, and interspike intervals. Finally, blocking GABAA receptors reduced the facilitation of spike count by the 5-HT1B agonist, so that the effects of 5-HT1B activation and GABAA blockade were subadditive. An important feature of all of these effects of the 5-HT1B receptor is that they varied widely among neurons as do the effects of blocking GABAA receptors (Fuzessery and Hall 1996; Lu and Jen 2001; Palombi and Caspary 1996; Suga 1965; Yang et al. 1992). This implies that the functional consequences of receptor activation similarly vary among neurons.
In addition to the 5-HT1B receptor, several other types of neuromodulatory receptor are capable of altering GABAergic transmission in the IC (Ma et al. 2002; Tongjaroenbungam et al. 2004; Yigit et al. 2003), or have anatomical associations with GABAergic neurons (Kalyuzhny et al. 2000; Peruzzi and Dut 2004; Tongjaroenbuangam et al. 2006). These studies underscore that the 5-HT1B receptor is one of multiple neurochemical mechanisms likely to regulate inhibition within the IC, although these other mechanisms have not been explored in terms of their regulation of frequency responsiveness.
Potential additional mechanisms of 5-HT1B action
We focused on the GABAA receptor because of its well-studied relationship to frequency tuning in the IC, and our results support the GABAA receptor as one pathway for the effects of the 5-HT1B receptor. Our results do not preclude additional effector mechanisms of 5-HT1B activation not tested in this study, however. In fact, a logical additional consequence of 5-HT1B activation decreasing GABA release would be to decrease the activation of GABAB as well as GABAA receptors. In the IC, activation of the GABAB receptor has an inhibitory effect and block of the GABAB receptor has an excitatory effect on auditory responses, although these effects are relatively modest (Burger and Pollak 1998; Faingold et al. 1989; Vaugn et al. 1996). Thus a decrease of GABAB receptor activation could potentially contribute to the overall increase in excitability we observed during application of the 5-HT1B agonist. The 5-HT1B receptor itself, in addition to being a heteroreceptor on nonserotonergic neurons, acts as an autoreceptor on serotonergic fibers in several brain regions, leading to a diminished release of serotonin (Roberts et al. 2001; Sari 2004). It is therefore possible that a decrease in the activation of a serotonin receptor with inhibitory effects could have accounted for some of the effects of 5-HT1B activation. One such receptor is the 5-HT1A receptor, which often hyperpolarizes neurons (Barnes and Sharp 1999; Hoyer et al. 2002). Activation of this receptor within the IC does in fact induce widespread decreases in auditory responses (Hurley 2006, 2007). A final possibility that has not been excluded is that the 5-HT1B receptor reduces glycinergic inhibition, which also shapes frequency tuning (LeBeau et al. 2001; Lu and Jen 2001). The presence of any of these additional mechanisms of 5-HT1B activation in the IC would raise the interesting possibility that this receptor regulates the balance of multiple signaling pathways through targeted presynaptic effects.
Functional implications of 5-HT1B modulation
SEROTONERGIC CONTEXT INFLUENCES THE EFFECT OF THE 5-HT1B RECEPTOR.
The functional consequences of 5-HT1B activation will depend to a large extent on the conditions during which serotonin is released and on the other infrastructure of the serotonergic system within the IC. Because the release of serotonin is not triggered by auditory stimuli in the same way as inhibitory inputs, the function of the 5-HT1B receptor is not likely to be the simple opposite of the function of the inhibition it reduces. Serotonergic neurons that innervate the IC, located outside the auditory system in the dorsal and median raphe nuclei (Klepper and Herbert 1991), have higher rates of firing during waking than during sleeping states (Trulson and Jacobs 1979, 1981), presumably correlated with an increase in serotonin level in the IC across this behavioral transition. Serotonin levels also fluctuate in some brain regions in response to social stimuli or stressful situations (Boutelle et al. 1990; Clement et al. 1998; Mas et al. 1995). Measurements of serotonin levels within the IC show activation of the serotonergic system in response to systemic injection of salicylate (Liu et al. 2003), but not following a 45-min exposure to white noise (Cransac et al. 1998), although small increases in serotonin levels have been measured during the presentation of broadband noise as well as during waking from anesthesia (Hall and Hurley 2007). In general, the effects of 5-HT1B activation are likely to be greatest during internal states or situations evoking a high level of behavioral arousal.
Another important contextual consideration is the presence of additional types of serotonin receptors in the IC. Members of at least four families of serotonin receptor have been localized within the IC (Cornea-Hebert 1999; Harlan et al. 2000; Heidmann et al. 1998; Morales et al. 1998; Peruzzi and Dut 2004; Thompson et al. 1994; To et al. 1995; Vilaró et al. 2005), but the effects of only a few of these on auditory responses have been measured. Other than the 5-HT1B receptor, the best-characterized serotonin receptor is the 5-HT1A receptor, which usually has a somatodendritic localization (Barnes and Sharp 1999; Hoyer et al. 2002). In keeping with its hyperpolarizing effect in other brain regions, activation of this receptor usually decreases the overall auditory responsiveness of IC neurons (Barnes and Sharp 1999; Hoyer et al. 2002; Hurley 2006, 2007). Because both the 5-HT1A and the 5-HT1B receptors have a relatively high affinity for serotonin (Hoyer et al. 1994), they should both be activated with increasing levels of serotonin. Because both 5-HT1A and 5-HT1B agonists affect relatively large proportions of the neuron population (Hurley 2006, 2007), it seems reasonable to predict that they affect the same neural circuits in at least some cases. This has never been directly assessed, however, nor has whether these receptors act in opposition to each other or interact in complementary ways.
Is variability in the effects of the 5-HT1B receptor functional?
Variability among neurons in the effects of 5-HT1B activation was observed at multiple levels in the current study, including variation in the specific pattern of effects across the frequency response curves (Figs. 2–4), and even whether neurons responded to 5-HT1B activation at all (Figs. 1, 2, and 5). Activation of the 5-HT1B receptor caused complex patterns of reduction in inhibition that should influence responses to spectrally rich signals such as vocalizations in neuron-specific ways. Perhaps the simplest type of response transformation observed was for neurons that did show changes in response level without accompanying changes in frequency response curve bandwidth (Fig. 2A, top neuron) or in relative two-tone spike reduction (Fig. 3, neuron 1). Because changes in the responses of IC neurons to vocalizations are correlated with changes in frequency tuning (Klug et al. 2002; Hurley and Pollak 2005), these neurons would be expected to maintain their relative selectivity for spectrally complex signals despite a higher overall response level. In contrast, other types of changes in frequency tuning or spectral integration would be expected to alter the selectivity for complex signals in ways that would match their spectral content and temporal structure (Nataraj and Wenstrup 2006; Portfors 2004); Portfors and Wenstrup 1999; Taniguchi et al. 1986). For example, the neurons that exhibited expansions of their frequency response curves (Fig. 2A, lower neuron) would be expected to show an increase in response to vocalizations containing these unmasked frequencies. Similarly, for neurons in which discrete notches of spike reduction were unchanged despite a general increase in spikes (Fig. 3, neuron 2), a greater contrast might emerge between the reduced responses to stimuli containing these “notch” frequencies relative to the facilitation of those that do not.
Another type of variation was simply that 5-HT1B activation decreased two-tone effects for some neurons but not others. Because differences among the patterns of response to 5-HT1B activation may be influenced by differences in inputs or intrinsic properties among the neurons themselves, it is tempting to speculate that these different classes of neurons, and their responses to 5-HT1B activation, also fulfill distinct functional roles. An interesting comparison exists in visual cortex, in which different classes of inhibitory neurons can be distinguished not only on the basis of their spike waveforms but also by their differential sensitivity to serotonin receptor agonists (Xiang and Prince 2003). A higher proportion of fast-spiking interneurons than low-threshold spike interneurons are sensitive to the 5-HT3 receptor, whereas a higher percentage of low-threshold spike interneurons are sensitive to the 5-HT1A receptor. Because these receptors have opposite effects on neuron excitability, the effects of serotonin application on the sIPSCs received by pyramidal neurons are complex. In many systems, neurons targeted by the 5-HT1B receptor can also be projection neurons arising from particular sources. For example, in the nearby superior colliculus, retinotectal projections, as opposed to corticotectal projections, express the 5-HT1B receptor (Mooney et al. 1994, 1996), potentially regulating the balance between ascending and descending inputs in vivo. Likewise during development of the somatosensory and visual systems, thalamocortical neurons express the 5-HT1B receptor, potentially contributing to the patterning of barrel cortex (Bennett-Clarke et al. 1993; Leslie et al. 1992). Indeed, in frog optic tectum, manipulation of the 5-HT1B receptors of projections from retinal ganglion cells disrupts the topographic map of the tectum (Butt et al. 2002). Whether classes of inhibitory inputs with differential sensitivity to 5-HT1B activation are further distinguished by their physiological characteristics or nuclei of origin in the IC, however, is not yet known.
Implications for plasticity
A final interesting possibility is a role for the 5-HT1B receptor in plasticity in frequency tuning, which occurs in the IC in response to associative learning, or acoustic damage related to trauma or aging (Barsz et al. 2007; Bartels et al. 2007; Felix and Portfors 2007; Gao and Suga 1998; Turner et al. 2005). This possibility arises because changes in inhibition have been implicated in such plasticity, whether it expressed in a widespread way across the tonotopic map of the IC or is associated with targeted changes in tonotopy, as in associative learning or tinnitus (Bartels et al. 2007; Gerken 1996; Wang et al. 1996, 2002; Willott 1988; Xiao and Suga 2002). Suggestively, several serotonin receptors have already been implicated in learning-related frequency tuning plasticity (5-HT2A) (Ji and Suga 2007) or are regulated in level in response to deafening (5-HT5B) (Holt et al. 2005) or aging (5-HT2B) (Tadros et al. 2007).
In summary, we have identified a receptor mechanism that can link a diffuse signal of state, serotonin, with the encoding of frequency in the auditory midbrain through its specific effects on excitatory-inhibitory circuitry. Although the functional implications of this link are not entirely defined, it could contribute to the affective regulation of auditory processing in the IC.
GRANTS
These experiments were funded in part by National Institute of Deafness and Other Communication Disorders Grant DC-006608 plus supplement 02S1.
Acknowledgments
The authors thank Dr. Youssef Sari for helpful comments and M. Eversman, K. Harris, and M. Smith for assistance in data analysis.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Arthur et al. 1971.Arthur RM, Pfeiffer RR, Suga N. Properties of “two-tone inhibition” in primary auditory neurone. J Physiol 212: 593–609, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astl et al. 1996.Astl J, Popelar J, Kvasnak E, Syka J. Comparison of response properties of neurons in the inferior colliculus of guinea pigs under different anesthetics. Audiology 35: 335–345, 1996. [DOI] [PubMed] [Google Scholar]
- Barnes and Sharp 1999.Barnes N, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology 38: 1083–1152, 1999. [DOI] [PubMed] [Google Scholar]
- Barsz et al. 2007.Barsz K, Wilson WW, Walton JP. Reorganization of receptive fields following hearing loss in inferior colliculus neurons. Neuroscience 147: 532–545, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels et al. 2007.Bartels H, Staal MJ, Albers FW. Tinnitus and neural plasticity of the brain. Otol Neurotol 28: 178–184, 2007. [DOI] [PubMed] [Google Scholar]
- Bennett-Clarke et al. 1993.Bennett-Clarke CA, Leslie MJ, Chiaia NL, Rhoades RW. Serotonin 1B receptors in the developing somatosensory and visual cortices are located on thalamocortical axons. Proc Natl Acad Sci USA 90: 153–157, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutelle et al. 1990.Boutelle MG, Zetterstrom T, Pei Q, Svensson L, Fillenz M. In vivo neurochemical effects of tail pinch. J Neurosci Methods 34: 151–157, 1990. [DOI] [PubMed] [Google Scholar]
- Burger and Pollak 1998.Burger RM, Pollak GD. Analysis of the role of inhibition in shaping responses to sinusoidally amplitude-modulated signals in the inferior colliculus. J Neurophysiol 80: 1686–1701, 1998. [DOI] [PubMed] [Google Scholar]
- Butt et al. 2002.Butt CM, Zhao B, Duncan MJ, Debski EA. Sculpting the visual map: the distribution and function of serotonin-1A and serotonin-1B receptors in the optic tectum of the frog. Brain Res 931: 21–31, 2002. [DOI] [PubMed] [Google Scholar]
- Chadha et al. 2000.Chadha A, Sur C, Atack J, Duty S. The 5HT(1B) receptor agonist, CP-93129, inhibits [(3)H]-GABA release from rat globus pallidus slices and reverses akinesia following intrapallidal injection in the reserpine-treated rat. Br J Pharmacol 130: 1927–1932, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement et al. 1998.Clement HW, Kirsch M, Hasse C, Opper C, Gemsa D, Wesemann W. Effect of repeated immobilization on serotonin metabolism in different rat brain areas and on serum corticosterone. J Neural Transm 105: 1155–1170, 1998. [DOI] [PubMed] [Google Scholar]
- Cornea-Hebert et al. 1999.Cornea-Hebert V, Riad M, Wu C, Singh S, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol 409: 187–209, 1999. [DOI] [PubMed] [Google Scholar]
- Cransac et al. 1998.Cransac H, Cottet-Emard J, Hellstrom S, Peyrin L. Specific sound-induced noradrenergic and serotonergic activation in central auditory structures. Hear Res 118: 151–156, 1998. [DOI] [PubMed] [Google Scholar]
- Faingold et al. 1989.Faingold CL, Gehlbach G, Caspary DM. On the role of GABA as an inhibitory neurotransmitter in inferior colliculus neurons: iontophoretic studies. Brain Res 500: 302–312, 1989. [DOI] [PubMed] [Google Scholar]
- Felix and Portfors 2007.Felix RA 2nd, Portfors CV. Excitatory, inhibitory and facilitatory frequency response areas in the inferior colliculus of hearing impaired mice. Hear Res 228: 212–229, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuzessery and Hall 1996.Fuzessery ZM, Hall JC. Role of GABA in shaping frequency tuning and creating FM sweep selectivity in the inferior colliculus. J Neurophysiol 76: 1059–1073, 1996. [DOI] [PubMed] [Google Scholar]
- Gerken 1996.Gerken GM Central tinnitus and lateral inhibition: an auditory brainstem model. Hear Res 97: 75–83, 1996. [PubMed] [Google Scholar]
- Gao and Suga 1998.Gao E, Suga N. Experience-dependent corticofugal adjustment of midbrain frequency map in bat auditory system. Proc Natl Acad Sci USA 95: 12663–12670, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall and Hurley 2007.Hall IC, Hurley LM. The serotonin releaser fenfluramine alters the auditory responses of inferior colliculus neurons. Hear Res 228: 82–94, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall 1999.Hall JC GABAergic inhibition shapes frequency tuning and modifies response properties in the auditory midbrain of the leopard frog. J Comp Physiol [A] 185: 479–491, 1999. [DOI] [PubMed] [Google Scholar]
- Harlan et al. 2000.Harlan R, Yuan Y, Garcia M. Serotonin 5-HT2C receptors in central auditory pathways. ARO Abstr 23: 113, 2000. [Google Scholar]
- Havey and Caspary 1980.Havey DC, Caspary DM. A simple technique for constructing “piggy-back” multibarrel microelectrodes. Electroencephalogr Clin Neurophysiol 48: 249–251, 1980. [DOI] [PubMed] [Google Scholar]
- Heidmann et al. 1998.Heidmann D, Szot P, Kohen R, Hamblin M. Function and distribution of three rat 5-hydroxytryptamine7 (5-HT7) receptor isoforms produced by alternative splicing. Neuropharmacology 37: 1621–1632, 1998. [DOI] [PubMed] [Google Scholar]
- Holt et al. 2005.Holt AG, Asako M, Lomax CA, MacDonald JW, Tong L, Lomax MI, Altschuler RA. Deafness-related plasticity in the inferior colliculus: gene expression profiling following removal of peripheral activity. J Neurochem 93: 1069–1086, 2005. [DOI] [PubMed] [Google Scholar]
- Hoyer et al. 1994.Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin). Pharmacol Rev 46: 157–203, 1994. [PubMed] [Google Scholar]
- Hoyer et al. 2002.Hoyer D, Hannon J, and Martin G. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav 71: 533–534, 2002. [DOI] [PubMed] [Google Scholar]
- Hurley 2006.Hurley LM Different serotonin receptor agonists have distinct effects on sound-evoked responses in inferior colliculus. J Neurophysiol 96: 2177–2188, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley 2007.Hurley LM Activation of the serotonin 1A receptor alters the temporal characteristics of auditory responses in the inferior colliculus. Brain Res 1181: 21–29, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley et al. 2004.Hurley LM, Devilbiss D, Waterhouse B. A matter of focus: monoaminergic modulation of stimulus coding in mammalian sensory networks. Curr Opin Neurobiol 14: 488–495, 2004. [DOI] [PubMed] [Google Scholar]
- Hurley and Pollak 1999.Hurley LM, Pollak GD. Serotonin differentially modulates responses to tones and frequency-modulated sweeps in the inferior colliculus. J Neurosci 19: 8071–8082, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley and Pollak 2001.Hurley LM, Pollak GD. Serotonin effects on frequency tuning of inferior colliculus neurons. J Neurophysiol 85: 828–842, 2001. [DOI] [PubMed] [Google Scholar]
- Hurley and Pollak 2005.Hurley LM, Pollak GD. Serotonin shifts first-spike latencies of inferior colliculus neurons. J Neurosci 25: 7876–86, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley et al. 2002.Hurley LM, Thompson AM, Pollak GD. Serotonin in the inferior colliculus. Hear Res 168: 1–11, 2002. [DOI] [PubMed] [Google Scholar]
- Hwang and Dun 1999.Hwang LL, Dun NJ. Serotonin modulates synaptic transmission in immature rat ventrolateral medulla neurons in vitro. Neurosci 91: 959–970, 1999. [DOI] [PubMed] [Google Scholar]
- Ji and Suga 2007.Ji W, Suga N. Serotonergic modulation of plasticity of the auditory cortex elicited by fear conditioning. J Neurosci 27: 4910–4918, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson et al. 1992.Johnson SW, Mercuri NB, North RA. 5-hydroxytryptamine1B receptors block the GABAB synaptic potential in rat dopamine neurons. J Neurosci 12: 2000–2006, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyuzhny et al. 2000.Kalyuzhny AE, Dooyema J, Wessendorf MW. Opioid- and GABA(A)-receptors are co-expressed by neurons in rat brain. Neuroreport 11: 2625–2628, 2000. [DOI] [PubMed] [Google Scholar]
- Klepper and Herbert 1991.Klepper A, Herbert H. Distribution and origin of noradrenergic and serotonergic fibers in the cochlear nucleus and inferior colliculus of the rat. Brain Res 557: 190–201, 1991. [DOI] [PubMed] [Google Scholar]
- Klug et al. 2002.Klug A, Bauer EE, Hanson JT, Hurley LM, Meitzen J, Pollak GD. Response selectivity for species-specific calls in the inferior colliculus of Mexican free-tailed bats is generated by inhibition. J Neurophysiol 88: 1941–1954, 2002. [DOI] [PubMed] [Google Scholar]
- LeBeau et al. 2001.LeBeau FE, Malmierca MS, Rees A. Iontophoresis in vivo demonstrates a key role for GABA(A) and glycinergic inhibition in shaping frequency response areas in the inferior colliculus of guinea pig. J Neurosci 21: 7303–7312, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy and Wenstrup 2000.Leroy S, Wenstrup J. Spectral integration in the inferior colliculus of the mustached bat. J Neurosci 20: 8533–8541, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie et al. 1992.Leslie MJ, Bennett-Clarke CA, Rhoades RW. Serotonin 1B receptors form a transient vibrissa-related pattern in the primary somatosensory cortex of the developing rat. Brain Res Dev Brain Res 69: 143–148, 1992. [DOI] [PubMed] [Google Scholar]
- Liu et al. 2003.Liu J, Li X, Wang L, Dong Y, Han H, and Liu G. Effects of salicylate on serotoninergic activities in rat inferior colliculus and auditory cortex. Hear Res 175: 45–53, 2003. [DOI] [PubMed] [Google Scholar]
- Lu and Jen 2001.Lu Y, Jen PH. GABAergic and glycinergic neural inhibition in excitatory frequency tuning of bat inferior collicular neurons. Exp Brain Res 141: 331–339, 2001. [DOI] [PubMed] [Google Scholar]
- Ma et al. 2002.Ma CL, Kelly JB, Wu SH. Presynaptic modulation of GABAergic inhibition by GABA(B) receptors in the rat's inferior colliculus. Neuroscience 114: 207–215, 2002. [DOI] [PubMed] [Google Scholar]
- Mas et al. 1995.Mas M, Fumero B, Gonzalez-Mora JL. Voltammetric and microdialysis monitoring of brain monoamine neurotransmitter release during sociosexual interactions. Behav Brain Res 71: 69–79, 1995. [DOI] [PubMed] [Google Scholar]
- Matsuoka et al. 2004.Matsuoka T, Hasuo H, Akasu T. 5-Hydroxytryptamine 1B receptors mediate presynaptic inhibition of monosynaptic IPSC in the rat dorsolateral septal nucleus. Neurosci Res 48: 229–238, 2004. [DOI] [PubMed] [Google Scholar]
- Mittmann and Wenstrup 1995.Mittmann DH, Wenstrup JJ. Combination-sensitive neurons in the inferior colliculus. Hear Res 90: 185–191, 1995. [DOI] [PubMed] [Google Scholar]
- Mooney et al. 1996.Mooney R, Huang X, Shi M, Bennett-Clarke C, Rhoades R. Serotonin modulates retinotectal and corticotectal convergence in the superior colliculus. Prog Brain Res 112: 57–69, 1996. [DOI] [PubMed] [Google Scholar]
- Mooney et al. 1994.Mooney R, Shi M, Rhoades R. Modulation of retinotectal transmission by presynaptic 5-HT1B receptors in the superior colliculus of the adult hamster. J Neurophysiol 72: 3–13, 1994. [DOI] [PubMed] [Google Scholar]
- Morales et al. 1998.Morales M, Battenberg E, Bloom F. Distribution of neurons expressing immunoreactivity for the 5HT3 receptor subtype in the rat brain and spinal cord. J Comp Neurol 402: 385–401, 1998. [PubMed] [Google Scholar]
- Nataraj and Wenstrup 2005.Nataraj K, Wenstrup JJ. Roles of inhibition in creating complex auditory responses in the inferior colliculus: facilitated combination-sensitive neurons. J Neurophysiol 93: 3294–312, 2005. [DOI] [PubMed] [Google Scholar]
- Nataraj and Wenstrup 2006.Nataraj K, Wenstrup JJ. Roles of inhibition in complex auditory responses in the inferior colliculus: inhibited combination-sensitive neurons. J Neurophysiol 95: 2179–2192, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombi and Caspary 1996.Palombi PS, Caspary DM. GABA inputs control discharge rate primarily within frequency receptive fields of inferior colliculus neurons. J Neurophysiol 75: 2211–2219, 1996. [DOI] [PubMed] [Google Scholar]
- Peruzzi and Dut 2004.Peruzzi D, Dut A. GABA, serotonin and serotonin receptors in the rat inferior colliculus. Brain Res 998: 247–250, 2004. [DOI] [PubMed] [Google Scholar]
- Pickles 1982.Pickles JO An Introduction to the Physiology of Hearing. New York: Academic, 1982, p. 92–98.
- Portfors 2004.Portfors CV Combination sensitivity and processing of communication calls in the inferior colliculus of the Moustached Bat Pteronotus parnellii. An Acad Bras Cienc 76: 253–7, 2004. [DOI] [PubMed] [Google Scholar]
- Portfors and Wenstrup 1999.Portfors C, Wenstrup JJ. Delay-tuned neurons in the inferior colliculus of the mustached bat: implications for analyses of target distance. J Neurophysiol 82: 1326–1338, 1999. [DOI] [PubMed] [Google Scholar]
- Portfors and Wenstrup 2001.Portfors CV, Wenstrup JJ. Responses to combinations of tones in the nuclei of the lateral lemniscus. J Assoc Res Otolaryngol 2: 104–117, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portfors and Wenstrup 2002.Portfors CV, Wenstrup JJ. Excitatory and facilitatory frequency response areas in the inferior colliculus of the mustached bat. Hear Res 168: 131–138, 2002. [DOI] [PubMed] [Google Scholar]
- Roberts et al. 2001.Roberts C, Allen L, Langmead CJ, Hagan JJ, Middlemiss DN, Price GW. The effect of SB-269970, a 5-HT(7) receptor antagonist, on 5-HT release from serotonergic terminals and cell bodies. Br J Pharmacol 132: 1574–1580, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero et al. 1992.Ruggero MA, Robles L, Rich NC. Two-tone suppression in the basilar membrane of the cochlea: mechanical basis of auditory-nerve rate suppression. J Neurophysiol 68: 1087–99, 1992. [DOI] [PubMed] [Google Scholar]
- Sanchez et al. 2008.Sanchez JT, Gans D, Wenstrup JJ. Glycinergic “inhibition” mediates selective excitatory responses to combinations of sounds. J Neurosci 28(1): 80–90, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari 2004.Sari Y Serotonin1B receptors: from protein to physiological function and behavior. Neurosci Biobehav Rev 28: 565–582, 2004. [DOI] [PubMed] [Google Scholar]
- Schuller 1997.Schuller G A cheap earphone for small animals with good frequency response in the ultrasonic frequency range. J Neurosci Methods 71: 187–190, 1997. [DOI] [PubMed] [Google Scholar]
- Schuller et al. 1986.Schuller G, Radtke-Schuller S, Betz M. A stereotaxic method for small animals using experimentally determined reference profiles. J Neurosci Methods 18: 339–350, 1986. [DOI] [PubMed] [Google Scholar]
- Stanford and Lacey 1996.Stanford IM, Lacey MG. Differential actions of serotonin, mediated by 5-HT1B and 5-HT2C receptors, on GABA-mediated synaptic input to rat substantia nigra pars reticulata neurons in vitro. J Neurosci 16: 7566–7573, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga 1965.Suga N Analysis of frequency-modulated sounds by auditory neurones of echo-locating bats. J Physiol 179: 26–53, 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga et al. 1975.Suga N, Simmons JA, Jen PHS. Peripheral specialization for fine analysis of doppler-shifted echoes in the auditory system of the “CF-FM” bat Pteronotus parnellii. J Exp Biol 63: 161–92, 1975. [DOI] [PubMed] [Google Scholar]
- Tadros et al. 2007.Tadros SF, D'Souza M, Zettel ML, Zhu X, Lynch-Erhardt M, Frisina RD. Serotonin 2B receptor: upregulated with age and hearing loss in mouse auditory system. Neurobiol Aging 28: 1112–1123, 2007. [DOI] [PubMed] [Google Scholar]
- Taniguchi et al. 1986.Taniguchi I, Niwa H, Wong D, Suga N. Response properties of FM-FM combination-sensitive neurons in the auditory cortex of the mustached bat. J Comp Physiol [A] 159: 331–7, 1986. [DOI] [PubMed] [Google Scholar]
- Thompson et al. 1994.Thompson GC, Thompson AM, Garrett KM, Britton BH. Serotonin and serotonin receptors in the central auditory system. Otolaryngology Head Neck Surg 110: 93–102, 1994. [DOI] [PubMed] [Google Scholar]
- To et al. 1995.To Z, Bonhaus D, Eglen R, Jakeman L. Characterization and distribution of putative 5-ht7 receptors in guinea-pig brain. Br J Pharmacol 115: 107–116, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tongjaroenbungam et al. 2004.Tongjaroenbungam W, Jongkamonwiwat N, Cunningham J, Phansuwan-Pujito P, Dodson HC, Forge A, Govitrapong P, Casalotti SO. Opioid modulation of GABA release in the rat inferior colliculus. BMC Neurosci 5: 31, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tongjaroenbuangam et al. 2006.Tongjaroenbuangam W, Jongkamonwiwat N, Phansuwan-Pujito P, Casalotti SO, Forge A, Dodson H, Govitrapong P. Relationship of opioid receptors with GABAergic neurons in the rat inferior colliculus. Eur J Neurosci 24: 1987–1994, 2006. [DOI] [PubMed] [Google Scholar]
- Trulson and Jacobs 1979.Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain Res 163: 135–150, 1979. [DOI] [PubMed] [Google Scholar]
- Trulson and Jacobs 1981.Trulson ME, Jacobs BL. Activity of serotonin-containing neurons in freely moving cats. In: Serotonin Neurotransmission and Behavior, edited by Jacobs BL, Gelperin A. Cambridge, MA: The MIT Press, 1981, p. 360–363.
- Turner et al. 2005.Turner JG, Hughes LF, Caspary DM. Affects of aging on receptive fields in rat primary auditory cortex layer V neurons. J Neurophysiol 94: 2738–2747, 2005. [DOI] [PubMed] [Google Scholar]
- Vaughn et al. 1996.Vaughn MD, Pozza MF, Lingenhohl K. Excitatory acoustic responses in the inferior colliculus of the rat are increased by GABAB receptor blockade. Neuropharmacology 35: 1761–1767, 1996. [DOI] [PubMed] [Google Scholar]
- Vilaró et al. 2005.Vilaró M, Cortes R, Mengod G. Serotonin 5-HT4 receptors and their mRNAs in rat and guinea pig brain: distribution and effects of neurotoxic lesions. J Comp Neurol 484: 418–439, 2005. [DOI] [PubMed] [Google Scholar]
- Wang et al. 2002.Wang J, Ding D, Salvi RJ. Functional reorganization in chinchilla inferior colliculus associated with chronic and acute cochlear damage. Hear Res 168: 238–249, 2002. [DOI] [PubMed] [Google Scholar]
- Wang et al. 1996.Wang J, Salvi RJ, Powers N. Plasticity of response properties of inferior colliculus neurons following acute cochlear damage. J Neurophysiol 75: 171–183, 1996. [DOI] [PubMed] [Google Scholar]
- Willott et al. 1988.Willott JF, Parham K, Hunter KP. Response properties of inferior colliculus neurons in middle-aged C57BL/6J mice with presbycusis. Hear Res 37: 15–27, 1988. [DOI] [PubMed] [Google Scholar]
- Xiang and Prince 2003.Xiang Z, Prince DA. Heterogeneous actions of serotonin on interneurons in rat visual cortex. J Neurophysiol 89: 1278–1287, 2003. [DOI] [PubMed] [Google Scholar]
- Xiao and Suga 2002.Xiao Z, Suga N. Reorganization of the cochleotopic map in the bat's auditory system by inhibition. Proc Natl Acad Sci USA 99: 15743–15748, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al. 1992.Yang L, Pollak GD, Resler C. GABAergic circuits sharpen tuning curves and modify response properties in the mustache bat inferior colliculus. J Neurophysiol 68: 1760–1774, 1992. [DOI] [PubMed] [Google Scholar]
- Yigit et al. 2003.Yigit M, Keipert C, Backus KH. Muscarinic acetylcholine receptors potentiate the GABAergic transmission in the developing rat inferior colliculus. Neuropharmacol 45: 504–513, 2003. [DOI] [PubMed] [Google Scholar]