Abstract
This study tested the role of the superior colliculus in generating movements of the mystacial vibrissae—whisking. First, we compared the kinematics of whisking generated by the superior colliculus with those generated by the motor cortex. We found that in anesthetized rats, microstimulation of the colliculus evoked a sustained vibrissa protraction, whereas stimulation of motor cortex produced rhythmic protractions. Movements generated by the superior colliculus are independent of motor cortex and can be evoked at lower thresholds and shorter latencies than those generated by the motor cortex. Next we tested the hypothesis that the colliculus is acting as a simple reflex loop with the neurons that drive vibrissa movement receiving sensory input evoked by vibrissa contacts. We found that most tecto-facial neurons do not receive sensory input. Not only did these neurons not spike in response to sensory stimulation, but field potential analysis revealed that subthreshold sensory inputs do not overlap spatially with tecto-facial neurons. Together these findings suggest that the superior colliculus plays a pivotal role in vibrissa movement—regulating vibrissa set point and whisk amplitude—but does not function as a simple reflex loop. With the motor cortex controlling the whisking frequency, the superior colliculus control of set point and amplitude would account for the main parameters of voluntary whisking.
INTRODUCTION
Palpatory vibrissa movements in rodents have emerged as an attractive model for motor control (Brecht et al. 1997; Kleinfeld et al. 2006; Vincent 1912). Voluntary vibrissa movements can be modulated and perhaps initiated by the motor cortex, which acts through a subcortical rhythm generator to control whisking kinematics (Cramer and Keller 2006; Cramer et al. 2007; Haiss and Schwarz 2005). In addition to the motor cortex, the superior colliculus is also likely to be involved in vibrissa motor control. The superior colliculus sends dense and direct projections to the facial nucleus, where the vibrissa motor neurons are located (Hattox et al. 2002; Miyashita and Mori 1995; Miyashita et al. 1994; Vidal et al. 1988), and stimulation of the superior colliculus has been shown to produce movements of the vibrissae (McHaffie and Stein 1982). These findings suggest that the superior colliculus may also have a role in controlling whisking kinematics. Indeed the superior colliculus may have a unique role in whisking behavior by virtue of its function as a sensorimotor loop. We have recently shown that colliculus neurons respond to vibrissae contacts with short-latency and reliable spikes by virtue of its direct and potent inputs from trigeminal nuclei (Hemelt and Keller 2007; and see Drager and Hubel 1976; Huerta et al. 1983). This, coupled with its direct projections to the facial nucleus, implies that the superior colliculus functions as a closed loop (Kleinfeld et al. 1999) through which vibrissae contacts reliably evoke vibrissae movements.
We had two aims in this study. First, we aimed to test the hypothesis that collicular outputs regulate specific parameters of whisking kinematics. To test this aim, we used microstimulation in the superior colliculus to generate naturalistic movements. This approach was pioneered by Schwarz and collaborators (Haiss and Schwarz 2005) and adapted by us for studying the role of motor cortex in regulating whisking (Cramer and Keller 2006; Cramer et al. 2007). Here we compared whisking kinematics generated by microstimulation of the superior colliculus and the motor cortex.
Our second aim was to test the hypothesis that the trigemino-collicular-facial loop acts as a simple reflex arc with the collicular neurons that drive vibrissa movement receiving sensory input from the trigeminal nuclei. To test this, we identified isolated tecto-facial neurons (i.e., neurons in the superior colliculus that project directly to the facial nucleus) and tested their suprathreshold response to sensory stimulation. We also tested subthreshold inputs to tecto-facial neurons using field potentials.
METHODS
Surgical procedures
We performed experiments using 19 female Sprague-Dawley rats weighing 200–300 g. All procedures strictly adhered to institutional and federal guidelines. Rats were anesthetized with isoflurane (0.6–1.5%), administered through a tracheal tube (16 rats), or with urethan injection (1.2 g/kg, 3 rats). Body temperature was maintained at 37°C with a servo-controlled heating blanket. Following infusion of local anesthetics at surgical sites, we performed a craniotomy (1.5–2.5 mm diam) over motor cortex and cortex covering the superior colliculus. Exposed cortex was kept moist with saline. Motor cortex lesions were produced by suctioning away motor cortex and surrounding tissue.
Electrical stimulation
To antidromically identify tecto-facial neurons, concentric platinum/iridium bipolar electrodes (Frederick Haer and Co., Bowdoinham, ME) were inserted in the brain stem at an angle of 64°, 1.9 mm from the midline, and lowered 5 mm to the facial nucleus. Low-threshold vibrissa movements confirmed electrode placement in the facial nucleus. Current injections for identifying tecto-facial neurons were 10–150 μA for 100–200 μs. We varied current intensities to ensure activation of the entire facial nucleus to maximize the probability of identifying tecto-facial neurons. Antidromic thresholds were typically <80 μA.
To evoke vibrissa movements, custom-made platinum/quartz electrodes (tip 20 μm) were inserted over superior colliculus (0.3–2.5 mm lateral, −5.5 to −7.8 mm from Bregma) and lowered to a depth of 3 mm before stimulating. Stimulation in the superior colliculus consisted of trains of stimuli, 200-μs pulse width, at 50–1,000 Hz delivered for 1 or 2 s. Alternately the electrode was lowered into the rhythmic whisking region of the motor cortex as previously described (Cramer and Keller 2006; Haiss and Schwarz 2005).
Sensory stimulation
To test for somatosensory responses, we manually deflected the vibrissae with a probe, and, once a responsive unit was identified, directed an air-puff at the vibrissae using a Picospritzer (General Valve, Fairfield, NJ), as previously described (Hemelt and Keller 2007; Masri et al. 2006). We also manually stroked the face and body with a wooden probe to identify somatosensory-responsive units with low spontaneous activity. To test for auditory and visual responses, we used loud noises, such as clapping, and a light-emitting diode (LED) passed close to the eye.
Recording
We obtained extracellular single unit recordings with either quartz-insulated platinum electrodes (0.5–4 MΩ) or a 16-channel Michigan Probe multi-array (0.5–4 MΩ, Anne Arbor). Electrodes were advanced perpendicular to the cortical surface. We continuously stimulated vibrissae on the contralateral face during electrode penetrations to detect collicular units with low or no spontaneous activity. Waveforms recorded from well-isolated units were digitized at 40 kHz through an AlphaLab data-acquisition system (Alpha Omega) or through a Plexon data-acquisition system (Plexon, Dallas, TX). We isolated units off-line with a combination of threshold and waveform component analysis using Off-Line Sorter (Plexon). Field potentials were recorded simultaneously with the single units through the same electrodes. These were digitized at 10 kHz.
To record the vibrissal EMGs, a pair of bipolar EMG electrodes (76-μm Teflon-coated stainless steel wire) was tunneled subcutaneously into the deep intrinsic muscles through a small incision in the face. EMG recordings were sampled at 25 kHz and filtered (0.1 Hz to 20 kHz) using an A-M Systems differential amplifier (Sequim, WA) and Alpha-Omega data-acquisition system.
Recording sites were marked with electrolytic lesions (5–10 μA for 20 s). The animals were deeply anesthetized and perfused transcardially with buffered saline followed by 4% buffered paraformaldehyde. Recording sites were identified in Nissl-stained coronal sections.
Data analysis
Electromyographic (EMG) analysis was performed as in our previous studies (see Cramer and Keller 2006) using a custom-written program (Igor, Lake Oswego, OR). In brief, data were filtered (1 Hz to 1 kHz differential filter), and EMG envelopes were generated by smoothing the data, sub-sampled to 500 Hz, using a sliding box algorithm with a width of 6 points. For data sampled at 25 kHz, the smoothing algorithm had a corner frequency of 166 Hz. (In Fig. 1, A and B, a width of 131 points was used for better visualization of Fig. 1A). We defined whisking onset as an increase in activity that significantly (99.5% confidence interval) exceeded the amplitude of baseline activity (Fig. 2A). Threshold was defined as the minimal stimulus intensity at and above which significant EMG activity was evoked. Protraction magnitude was defined as the area under the filtered, smoothed EMG curve from the whisking onset to the offset of activity, defined as the point when activity fell below the 99.5% confidence interval. In cases where activity did not fall back to baseline, the area was measured from the onset of activity until the next stimulation.
FIG. 1.
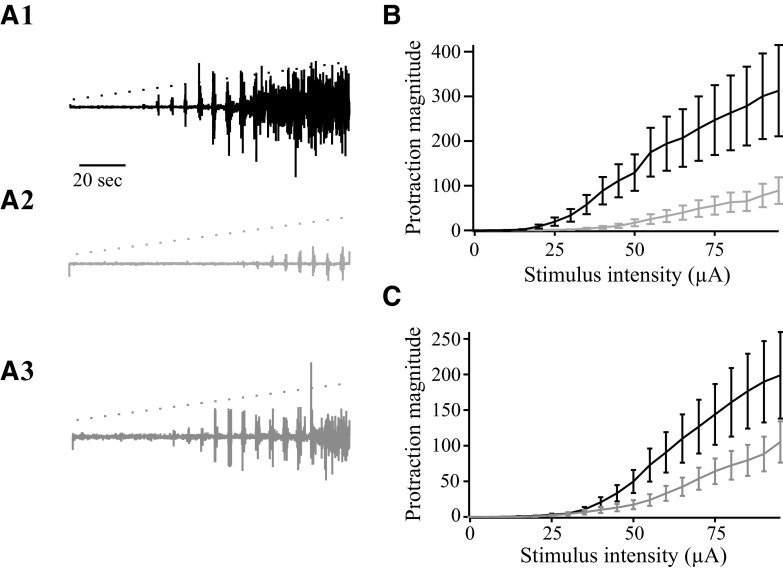
Movements evoked by stimulation in the superior colliculus are distinct from those evoked by motor cortex. A and B: collicular stimulation evokes sustained protractions (A), whereas cortical stimulation evokes rhythmic whisking (B). The rectified electromyographic (EMG) envelope (top) and raw EMG (bottom) are shown. Stimulus (250–350 μA at 50 Hz) timing and duration is indicated below the EMG. C: EMG activity was recorded during stimulation (60 μA) of superior colliculus with motor cortex intact. D: after motor cortex lesion, identical stimulation (as in C) evokes similar EMG activity. E: EMG activity evoked by various intensity stimulations in the superior colliculus shows the stimulus-response relationship with motor cortex intact. F: identical stimulation in the same experiment (as in E) after motor cortex lesion shows a similar stimulus-response relationship.
FIG. 2.
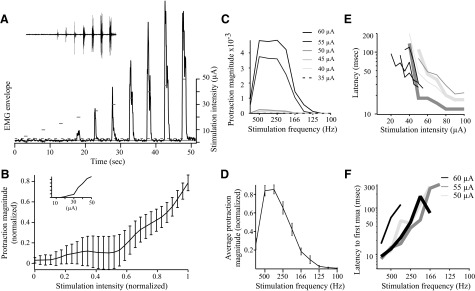
Kinematics of vibrissa movements evoked by stimulation in the superior colliculus. A: vibrissa pad EMGs were recorded during superior colliculus stimulation (333 Hz for 1 s). EMG envelope (smoothed, rectified EMG trace) represents whisker protraction. Sustained protractions beginning shortly after stimulation can be seen with both duration and angle of protraction increasing with stimulus intensity. The solid line is the baseline mean, and the dotted line is the 99% confidence interval. Lines above the trace indicate stimulation timing and intensity. Inset: filtered EMG with stimulus artifact blanked. B: protraction magnitude increases with increasing stimulus intensity. Magnitude of vibrissa protraction is measured as the area under the EMG envelope. Protraction magnitude and stimulus intensity are both normalized to the maximum. Error bars are SE. n = 21. Inset: Individual example of increasing protraction. C: effect of stimulation frequency on protraction magnitude at a single location, testing several stimulation intensities. Stimulation with 30 μA gave no response. D: stimulation frequency has a large effect on the magnitude of protraction at all sites tested. The stimulation frequency was varied from 100 Hz to 1 kHz, using a 1-s train. Error bars represent SE; n = 23. E: latencies decrease with increasing stimulation intensity. Bold lines are contralateral movements, thin lines are ipsilateral. Matching light gray and dark gray lines were recorded simultaneously. F: latencies decrease with increasing stimulation frequency. Bold lines are contralateral movements, thin lines are ipsilateral. Matching black lines were recorded simultaneously. Maximum frequency is 1,000 Hz.
Statistical analysis was performed in Microsoft Excel or Stata with the tests specified in the text. Data are presented as means ± SE.
RESULTS
The regulation of movement by brain structures can be effectively studied by applying microstimulation to a structure and monitoring the resultant EMG activity (Cheney and Fetz 1985; Graziano et al. 2002; Stoney et al. 1968). When applied to the appropriate locus in the rat motor cortex—the rhythmic whisking region—microstimulation evokes large, rhythmic protractions of the vibrissae, mimicking voluntary whisking (Cramer and Keller 2006; Cramer et al. 2007; Haiss and Schwarz 2005). These vibrissae movements can be reliably monitored by recording EMG activity from the vibrissal pad (Cramer and Keller 2006).
The superior colliculus sends a dense and direct projection to the facial motor neurons controlling vibrissae movements (Hattox et al. 2002). To determine how output from the superior colliculus might regulate vibrissae movements, we applied microstimulation (50–1,000 Hz, 10–100 μA) in the superior colliculus of anesthetized rats while recording EMGs bilaterally from the vibrissa pad. In all cases, we confirmed the location of the stimulation site post hoc (see methods). We found that stimulation throughout the intermediate and deep layers of the superior colliculus evokes vigorous unilateral or bilateral vibrissa protraction. The topography of effective stimulation sites is discussed further in the following text. Figure 1 depicts typical examples of EMGs recorded during stimulation-evoked vibrissa movement. Here, and at all effective sites, stimulation in the superior colliculus produced a sustained protraction of the vibrissae, which frequently outlasted the stimulation period (Fig. 1, A and C, and see Supplemental Movie 11). As described in the following text, the magnitude of these sustained protractions increased with increasing stimulation frequency or intensity; at the highest stimulation settings used, the protractions typically ranged between 20 and 30°.
To compare these stimulus-evoked movements with previously characterized vibrissae movements evoked by motor cortex stimulation, we applied, in the same animal, identical stimulus parameters to both the superior colliculus and the rhythmic whisking region of motor cortex (Cramer and Keller 2006; Haiss and Schwarz 2005). As previously reported, motor cortex stimulation evoked rhythmic protractions, in contrast to the sustained protractions evoked by collicular stimulation (cf. Fig. 1, A and B, and Supplemental Movies 1 and 2). The maximum protraction of motor cortex evoked movements was smaller than those evoked from superior colliculus, typically ranging between 2 and 12° (Fig. 1, A and B, and Supplemental Movies 1 and 2).
Independence from motor cortex
We considered the possibility that the movements evoked by collicular stimulation might result from antidromic activation of neurons projecting from motor cortex to superior colliculus. To test this possibility, we ablated the motor cortex ipsilaterally (3 rats) or bilaterally (3 rats; see methods). Figure 1D shows an example of EMG activity evoked by colliculus stimulation 30 min following motor cortex ablation. Note that the movements were similar to those evoked—from the same site—prior to the lesion using identical stimulus parameters (Fig. 1C). Cortical ablation had no effect on movement amplitude or stimulus threshold, as evidenced by comparing traces in Fig. 1, E and F. In every experiment, stimulation in the colliculus evoked sustained vibrissae movements following motor cortex lesion. These findings suggest that the sustained protractions evoked by collicular stimulation are independent of the motor cortex.
Vibrissa kinematics
Movements evoked by microstimulation often require trains of stimuli at relatively high frequencies (Stoney et al. 1968). We tested the effects of stimulus frequency on the amplitude of vibrissae protraction at 15 collicular stimulation sites. As depicted in Fig. 2, C and D, stimulus trains at 333 Hz evoked the largest protractions. Stimulus trains at lower or higher frequencies produced progressively smaller vibrissae movements. This dependency of movement amplitude on stimulus frequency distinguishes the superior colliculus from the motor cortex; in the latter, stimulus frequency has relatively little effect on the amplitude of vibrissae movements (Cramer and Keller 2006). This finding further supports the notion that the superior colliculus evokes vibrissae movements that are independent of the motor cortex.
The kinematics of vibrissae protraction were also affected by changing the stimulus intensity. Increasing stimulus intensity increased the protraction magnitude by increasing both the maximal angle of protraction and the duration of protraction (Fig. 2, A and B; unless otherwise indicated, all stimuli were applied at 333 Hz). In Fig. 2B, larger error bars at intermediate intensities reflect variable thresholds. The average threshold for evoked movement was 27.2 ± 2.5 μA (n = 23, see methods for threshold criteria). The finding that protractions increased with increasing stimulus intensity indicates that, unlike saccades evoked by collicular stimulation in cat and monkey (McIlwain 1986; Schiller and Stryker 1972), evoked vibrissa protractions are not all-or-none. Vibrissa movements were typically quite small at threshold stimulus intensities but increased with increasing stimulus intensity and frequently did not saturate until the physical limits of vibrissa movement were reached (Fig. 2B).
In addition to positively affecting protraction magnitude, increased stimulus intensity or frequency decreased the onset latency of the evoked movements (Fig. 2, E and F). To accurately determine onset latency, we extracted motor unit action potentials from the EMG as in our previous study (Cramer and Keller 2006). We defined onset latency as the latency to the first motor unit action potential evoked by stimulation. Because it was not always possible to reliably extract individual motor units from the EMG records, we performed this analysis on only 11 samples. At the highest range of stimulus intensity tested (≤100 μA), stimuli delivered at 333 Hz produced movements with onset latency ranging from 12 and 31 ms (median = 21, n = 7). At the highest stimulus frequency tested (1 kHz), stimuli delivered at 50–60 μA resulted in movement onsets between 8 and 17 ms (median = 9, n = 4) after the onset of the stimulus train.
Bilateral movements
Stimulation in the superior colliculus could evoke vibrissae movements ipsilaterally, contralaterally, or bilaterally. There was no difference in thresholds to evoke ipsi- or contralateral protractions (ipsilateral = 27.3 ± 2.6 μA, contralateral = 27.1 ± 4.4 μA, n = 11 and 12 respectively, P = 0.5 unpaired t-test).
The laterality of movement was dependent on stimulus location within the intermediate or deep layers of the superior colliculus. A typical example is depicted in Fig. 3 where contralateral movements were evoked by stimulating more dorsal regions, and ipsilateral movements were evoked from deeper layers. This relationship between stimulation site and movement laterality varied predictably throughout the superior colliculus. We obtained similar results in every animal, suggesting a modular organization in the colliculus where different regions project to ipsilateral or contralateral facial nuclei.
FIG. 3.
EMG recordings were evoked by stimulating in the superior colliculus (1-s trains at 333 Hz with 5–100 μA). Timing and intensity is indicated above EMG traces. While descending through the superior colliculus, contralateral movement (left) begins at 4 mm below the brain surface, whereas ipsilateral movement (right) begins at 4.5 mm below the surface. Ipsilaterally evoked movement also persisted in deeper levels (down to 7.0 mm below the surface), whereas no contralateral movement could be evoked <6 mm. The schematic shows the stimulation sites.
State dependence of movements
Movements evoked by microstimulation in the motor cortex are affected by the animal's arousal state or depth of anesthesia (Berg and Kleinfeld 2003; Stoney et al. 1968). To determine whether anesthetic level also affects movements evoked by collicular stimulation, we varied the concentration of isoflurane between 0.6 and 1.3% (per 500 ml/min of 95% oxygen), and monitored vibrissal EMG activity. Figure 4A shows movements evoked by a sequence of stimuli (5–100 μA) while the animal received 0.7% isoflurane (Fig. 4A1) or 1.1% isoflurane (Fig. 4A2). Deeper anesthesia resulted in decreased movement amplitude and duration evoked by the same stimulation intensity. Group data (n = 16) are shown in Fig. 4B. Each stimulation site was tested twice, at a “low” (0.6–1.1%) and “high” (0.9–1.3%) isoflurane concentration. The absolute concentrations were determined empirically for each rat to maintain adequate anesthetic plane, such that there were no withdrawal reflexes to noxious stimuli. Lower levels of anesthesia resulted in significantly increased magnitude of evoked movement (ANOVA, P < 0.0001, 640 measurements).
FIG. 4.
Responses to stimulation are state-dependent. Deeper anesthesia levels result in lower amplitude protractions, while tail pinch slightly decreases response. A: an example of changes in protraction resulting from different states of arousal. EMG activity is evoked by 1-s stimulation trains from 5 to 100 μA (indicated above EMG traces). A1: EMG evoked with 0.7% isoflurane, without tail pinch. A2: 1.1% anesthesia, no tail pinch. A3: 0.7% isoflurane, with tail pinch. B: for each stimulation location, stimulations were made at 2 anesthesia levels: a lighter and a deeper level. Recordings were made 5 min after changing the anesthesia. Gray line, the light anesthesia trials; black line, the deeper anesthesia trials. All animals were adequately anesthetized at both levels. n = 16 (8 on each side). C: comparison is of the same stimulation trains in the same location, with and without tail pinch. Tail pinch appears to decrease evoked whisker movements; n = 22 trials (11 on each side).
An alert state of arousal can be mimicked in anesthetized animals by stimulating the cholinergic reticular activating system (Kayama et al. 1991). We took advantage of the fact that this system can be activated simply by pinching the rat's tail (Kayama et al. 1991; Masri et al. 2006). Figure 4A compares stimulation-evoked movement during tail-pinch evoked “alert state” (Fig. 4A3) or under control conditions (Fig. 4A1), both recorded under 0.7% isoflurane. Unexpectedly, tail pinch resulted in a small decrease in protraction magnitude. Group data (Fig. 4C, n = 22) demonstrates that tail pinch consistently and significantly suppressed the magnitude of stimulation-evoked protraction (ANOVA, P < 0.0001, 880 measurements).
Trigeminal input to tecto-facial neurons
The tecto-facial pathway has been proposed to participate in a closed loop in which trigeminal (sensory) inputs directly activate tecto-facial neurons to create a simple reflex loop (Kleinfeld et al. 1999). It is not known, however, whether the trigemino-tecto-facial loop forms a simple reflex arc, where tecto-facial neurons receive direct inputs from trigeminal nuclei to form the proposed reflex arc. Two predictions arise from this proposal: that collicular neurons projecting to the facial nucleus respond to vibrissae stimuli and that trigeminal inputs target tecto-facial neurons.
We tested the first prediction by recording single units from 127 collicular neurons and assessed each of these for antidromic activation from the facial nucleus. Facial stimulation evoked spikes in 27 of these neurons. These neurons had no or very low spontaneous firing rates, and in most, the evoked spikes had short (<2 ms) onset latencies (Fig. 5). As a result, we were able to conclusively apply the collision test for only one neuron (Fig. 5A). As an alternative, we defined those spikes that could entrain to high-frequency stimuli as antidromically evoked (Swadlow 1998). Of 16 neurons tested in this manner, including the 1 depicted in Fig. 5A, 15 could follow stimulus trains ≥50 Hz. We considered seven additional neurons to be putative tecto-facial neurons based only on the short latency (≤5 ms) of their evoked spikes. Four other neurons of the 27 that responded to facial nucleus stimulation did not meet our criteria for putative tecto-facial neurons, responding inconsistently and with long and variable latencies (10–20 ms). In total, 22 of the 127 (17%) neurons recorded were identified as putative tecto-facial neurons.
FIG. 5.
Tecto-facial neurons respond to facial nucleus stimulation with short-latency action potentials. A: spontaneous spikes in the superior colliculus (open arrows) can be collided with spikes evoked from stimulation in facial nucleus. Stimulus artifact (*) is truncated. Top trace: the filled arrow marks the antidromically-evoked spike. Bottom trace: the antidromically evoked spike has been collided with the spontaneous spike. B: facial nucleus stimulation evokes short-latency (2 ms) responses. Shown is the peri-stimulus time histogram of a single unit and a simultaneously recorded field potential. The stimulus artifact can be seen in the field potential at the time of stimulation (arrow). C: histogram of antidromic latencies of putative tecto-facial neurons.
Two of these 22 (9%) identified tecto-facial neurons responded to vibrissa deflections. One of these could only be driven by manually deflecting the vibrissae; the other responded with one spike/stimulus at an 11-ms latency. We tested all identified tecto-facial neurons for responses to other sensory stimuli. These neurons did not respond to visual, auditory, or nonvibrissa somatosensory stimulation (see methods for details). We tested 12 nonresponsive neurons with tail-pinch to simulate an “alert state” (see preceding text), but this did not affect the responses.
We tested the response properties of the other 105 neurons that were not identified as antidromic. Twenty-five of them responded to contralateral vibrissae stimuli, and these responses were similar to those we recently described (Hemelt and Keller 2007). Fourteen neurons responded only to other tactile (nonvibrissae) stimuli, and twelve responded only to auditory stimuli. Fifty-four spontaneously active neurons did not respond to somatosensory, auditory, or visual stimuli.
Thus 9% of the presumptive tecto-facial neurons in our sample responded to vibrissae stimuli. Further, the majority (96%) of the neurons in the superior colliculus that responded to sensory stimuli were not antidromically activated by stimulation of the facial nucleus and most likely do not project to the facial nucleus.
Our second prediction was that trigemino-tectal neurons target tecto-facial neurons in the superior colliculus. Inputs from the trigeminal nucleus terminate in the superior colliculus in honeycomb-like patches (Mana and Chevalier 2001), but the distribution of tecto-facial neurons is unknown. While our previous experiment shows that trigeminal inputs do not reliably drive tecto-facial neurons to firing threshold (at least in the anesthetized animal), trigeminal neurons may target tecto-facial neurons to provide sub-threshold inputs. To test this, we used an array of 16 electrodes (with 70–150 μM spacing) in the superior colliculus to record field potentials. Potentials were evoked either antidromically, using facial nucleus stimulation; or orthodromically, by stimulating the vibrissae (see methods). To activate a large percentage of tecto-facial neurons, we used stimulation intensities similar to those that evoked vibrissa movements during electrode placement in the facial nucleus. We reasoned that if trigeminal inputs target tecto-facial neurons, antidromically evoked and stimulus-evoked field potentials might overlap in the same collicular regions.
Facial nucleus stimulation evokes vibrissae movements that result in neuronal firing in trigeminal nuclei (Nguyen and Kleinfeld 2005; Szwed et al. 2003). This trigeminal activity is likely to evoke orthodromic activation of the superior colliculus. To disambiguate this orthodromic response from the antidromic responses, we transected the facial nerve to eliminate vibrissae movements and thus orthodromic activity. This resulted in a suppression of the later—presumably orthodromic—component of the responses, whereas the short-latency, presumably antidromic, responses were unaffected.
Figure 6 depicts two examples of pairs of field potentials evoked by antidromic and vibrissae stimulation. Antidromic stimulation evoked focal activation in the superior colliculus with large-amplitude potentials recorded from one or two adjacent electrodes in the array. For example, in Fig. 6A, antidromic activity (gray trace) peaks in electrode 3A. Figure 6B shows another example where the largest antidromic field potentials are recorded through electrode 3B. Vibrissae-evoked field potentials were more broadly distributed throughout the colliculus. However, the largest magnitude potentials always occurred at loci different from the foci of antidromically evoked potentials (compare Fig. 6, A and B). We obtained similar results from seven recording trials in three animals using either ipsi- and contralateral facial nucleus stimulation (six and one trials, respectively). In five of the trials, we tested responses to vibrissae stimulation both ipsi- and contralateral to the recording electrodes. Ipsilaterally evoked responses were smaller that contralaterally evoked ones, but in both conditions, there was no overlap between the foci of anti- and orthodromically evoked responses. These findings suggest that trigeminal inputs do not preferentially target collicular regions containing tecto-facial neurons.
FIG. 6.
Trigeminal inputs to the superior colliculus do not appear to target tecto-facial neurons. A: spatial distribution of field potentials in response to ipsilateral antidromic stimulation (gray, 80 μA) compared with contralateral sensory stimulation (air puff to vibrissae, black). Electrode configuration shown in sagittal schematic. The arrow points to the stimulation artifact. The time of air puff application was aligned to time of electrical stimulation although the time scales are different. Antidromic peak is largest in 3A, while orthodromic peaks are largest in columns 1 and 2. B: as in A, but electrode array is oriented in the coronal plane. Both antidromic stimulation and vibrissa stimulation are contralateral. The antidromic peak is largest in 3B, while orthodromic peaks are largest in columns 1 and 2.
DISCUSSION
Microstimulation-evoked movements
Microstimulation in the superior colliculus produced sustained vibrissa protractions, frequently outlasting the stimulus duration. These movements were distinct from the rhythmic movements evoked by identical stimulation in the motor cortex and were unaffected by motor cortex lesion (Fig. 1).
The effective stimulation area in these experiments is difficult to delineate due to the potential for current spread and possible activation of axons of passage. However, the superior colliculus contains no known axons of passage projecting to the facial nucleus (May 2005). The low thresholds for evoked movements (27.2 ± 2.5 μA), the large size of the superior colliculus, and the fact that specific patterns of movement (e.g., ipsi- or contralateral) could be evoked from adjacent stimulation sites, all suggest that our stimuli affected a relatively restricted region. Although unlikely, we cannot exclude the possibility that collicular stimulation antidromically activated axon collaterals of an unknown pathway that projects to the facial nucleus.
Bilateral movements
Microstimulation at different sites in the colliculus produced ipsi-, contra-, or bilateral movements (Fig. 3). While contralateral movements were evoked by stimulating dorsal sites, ipsilateral movements were evoked from ventral collicular regions. This suggests that the colliculus contains discrete modules the outputs of which control different vibrissae pads.
Bilateral movements were evoked from sites intermediate to the ipsi- and contralateral sites. It was not possible to determine whether the latter were produced by individual neurons that project bilaterally or are the result of concurrent stimulation of ipsi- and contralaterally projecting neurons.
This topographical organization of collicular outputs was described first by Stein and collaborators in their studies of tecto-spinal pathways (McHaffie et al. 1989). In the rat, the superior colliculus mediates two types of behavior: orienting, assumed to be generated through the crossed tecto-spinal pathway, and aversion, thought to be mediated through the ipsilateral tecto-spinal pathway (Ellard and Goodale 1988; Sahibzada et al. 1986; but see Dean et al. 1986). Stein and collaborators show that deeper layers of the colliculus are more likely to contain nociceptive neurons and hypothesize that deep layers are those projecting ipsilaterally to initiate aversion movements. Conversely, they show that more superficial layers of the superior colliculus process innocuous information, and suggest these layers project contralaterally to initiate orienting movements (McHaffie et al. 1989). Our findings are consistent with this idea: We find that the deeper layers preferentially drive the ipsilateral pathway, while more superficial layers drive contralateral movement.
State dependence of movements
We tested the effect of the animal's state of arousal on stimulus-evoked movements in two ways: by changing the level of anesthetic and by pinching the tail to stimulate the cholinergic reticular activating system. At lower anesthetic levels, evoked protraction magnitudes were larger with increases in both the angle and duration of protraction (Fig. 4). This may be the result of decreased inhibition or increased excitation of neurons in the facial nucleus activated by electrical stimulation in the superior colliculus (Grelot and Bianchi 1987).
Tail pinch increases both spontaneous and evoked activity in several brain regions, presumably through stimulation of the cholinergic reticular activating system (Castro-Alamancos 2004; Kayama et al. 1991; Masri et al. 2006). Cholinergic neurons project to the superior colliculus (Kobayashi and Isa 2002), and acetylcholine has been shown to activate neurons in the superior colliculus in vitro (Li et al. 2004; Sooksawate and Isa 2006) and in vivo (Aizawa et al. 1999; Watanabe et al. 2005). In addition, acetylcholine excites facial motor neurons in vitro (Zaninetti et al. 1999). However, in contrast to the effects of anesthesia, tail pinch decreased the magnitude of evoked movements (Fig. 4). It is possible that in addition to releasing acetylcholine, tail pinch resulted in release of norepinephrine (Kayama et al. 1991), a substance known to suppress activity in the superior colliculus (Faingold and Casebeer 1999; Takemoto et al. 1978).
Trigemino-tectal-facial loop
The short latency of stimulation-evoked movements (as low as 8 ms), and the low thresholds (27.2 ± 2.5 μA) required for movement, imply that the superior colliculus can act directly on vibrissa motor neurons and drive them to fire. Further, tecto-facial neurons respond to stimulation in the facial nucleus with short-latency antidromic spikes (median = 2 ms). Together, these data imply that the tecto-facial pathway contains fast-conducting and excitable axons. By contrast, cortical stimulation under similar conditions evokes vibrissae movements at latencies between 30 and 300 ms (200 ± 100 ms) (Cramer and Keller 2006; compare with Fig. 2, G and H).
The inputs that drive these rapidly conducting tecto-facial neurons are unknown. We and others have previously shown that superior colliculus neurons respond to vibrissa input (Drager and Hubel 1976; Hemelt and Keller 2007; McHaffie et al. 1989). However, we report here that these trigeminal inputs activate a small proportion (9%) of tecto-facial neurons. Field potential analysis also suggests that trigeminal inputs do not preferentially target the tecto-facial pathway. It is possible that under anesthesia, somatosensory inputs that normally drive these neurons are suppressed (Populin 2005). We find this possibility unlikely because collicular neurons recorded under identical conditions respond robustly and reliably to vibrissae stimuli (Hemelt and Keller 2007). Further, tail-pinch, applied to simulate an “alert state” while testing tecto-facial neurons for sensory inputs failed to unmask sensory responses.
These findings suggest that the superior colliculus does not act as a simple, closed sensorimotor loop. Rather, trigeminal inputs originating from the vibrissae preferentially target neurons that do not project directly to the facial nucleus. The transformation between sensory inputs and motor outputs that drive the vibrissae presumably involves the rich intrinsic connections characterizing the circuitry of the superior colliculus (Lee and Hall 2006; Saito and Isa 2003).
Superior colliculus in vibrissa movements
The superior colliculus has long been studied as a center for eye and gaze orientation (Isa and Sasaki 2002; King 2004; McIlwain 1991). Collicular stimulation evokes orienting movements of the eyes, head, and pinnae in monkey, hamster, rat, and bat (McHaffie and Stein 1982; Moschovakis 1996; Valentine et al. 2002). We find that colliculus-evoked vibrissa movements increase in magnitude with increasing stimulus intensity; this is similar to the effects of stimulation on saccadic amplitudes in the rat (McHaffie and Stein 1982). This effect of intensity is similar to that seen with circling behavior elicited by stimulation in the superior colliculus of the awake rat (Tehovnik 1989). Thus collicular outputs orient different mobile sensors toward salient stimuli. These orienting vibrissa movements might be positioning this important sensory organ relative to an object of interest (Barth and Schallert 1987), or they might be acting to coordinate vibrissa movements with head movements (Towal and Hartmann 2006). These results fit well with the recent study suggesting that the superior colliculus is involved in behaviors important for attending to salient stimuli (Bittencourt et al. 2005).
The vibrissae movements evoked by stimulating the motor cortex or the superior colliculus are substantially different. Whereas stimulating the rhythmic whisking region of cortex mainly produces cycles of protraction and retraction (Cramer and Keller 2006; Haiss and Schwarz 2005), stimulation in the superior colliculus produced a sustained protraction (Fig. 1). Minimum latency to motor-unit activity evoked by cortical stimulation is close to 100 ms (Cramer and Keller 2006), whereas we report latencies as short as 8 ms. Thus collicular and cortical outputs are likely to have substantially different roles in regulating whisking. While motor cortex appears to regulate whisking frequency, acting through a brain stem central patter generator (Cramer et al. 2007), the superior colliculus may be controlling the amplitude and set point of whisking. Thus outputs from these structures could account for the main kinematic variables in whisking.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants NS-051799 and NS-31078 to A. Keller and Fellowship F31-NS-060506 to M. E. Hemelt.
Supplementary Material
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Aizawa 1999.Aizawa H, Kobayashi Y, Yamamoto M, Isa T. Injection of nicotine into the superior colliculus facilitates occurrence of express saccades in monkeys. J Neurophysiol 82: 1642–1646, 1999. [DOI] [PubMed] [Google Scholar]
- Barth 1987.Barth TM, Schallert T. Somatosensorimotor function of the superior colliculus, somatosensory cortex, and lateral hypothalamus in the rat. Exp Neurol 95: 661–678, 1987. [DOI] [PubMed] [Google Scholar]
- Berg 2003.Berg RW, Kleinfeld D. Vibrissa movement elicited by rhythmic electrical microstimulation to motor cortex in the aroused rat mimics exploratory whisking. J Neurophysiol 90: 2950–2963, 2003. [DOI] [PubMed] [Google Scholar]
- Bittencourt 2005.Bittencourt AS, Nakamura-Palacios EM, Mauad H, Tufik S, Schenberg LC. Organization of electrically and chemically evoked defensive behaviors within the deeper collicular layers as compared to the periaqueductal gray matter of the rat. Neuroscience 133: 873–892, 2005. [DOI] [PubMed] [Google Scholar]
- Brecht 1997.Brecht M, Preilowski B, Merzenich MM. Functional architecture of the mystacial vibrissae. Behav Brain Res 84: 81–97, 1997. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos 2004.Castro-Alamancos MA Absence of rapid sensory adaptation in neocortex during information processing states. Neuron 41: 455–464, 2004. [DOI] [PubMed] [Google Scholar]
- Cheney 1985.Cheney PD, Fetz EE. Comparable patterns of muscle facilitation evoked by individual corticomotoneuronal (CM) cells and by single intracortical microstimuli in primates: evidence for functional groups of CM cells. J Neurophysiol 53: 786–804, 1985. [DOI] [PubMed] [Google Scholar]
- Cramer 2006.Cramer NP, Keller A. Cortical control of a whisking central pattern generator. J Neurophysiol 96: 209–217, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer 2007.Cramer NP, Li Y, Keller A. The whisking rhythm generator: a novel mammalian network for the generation of movement. J Neurophysiol 97: 2148–2158, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean 1986.Dean P, Redgrave P, Sahibzada N, Tsuji K. Head and body movements produced by electrical stimulation of superior colliculus in rats: effects of interruption of crossed tectoreticulospinal pathway. Neuroscience 19: 367–380, 1986. [DOI] [PubMed] [Google Scholar]
- Drager 1976.Drager UC, Hubel DH. Topography of visual and somatosensory projections to mouse superior colliculus. J Neurophysiol 39: 91–101, 1976. [DOI] [PubMed] [Google Scholar]
- Ellard 1988.Ellard CG, Goodale MA. A functional analysis of the collicular output pathways: a dissociation of deficits following lesions of the dorsal tegmental decussation and the ipsilateral collicular efferent bundle in the Mongolian gerbil. Exp Brain Res 71: 307–319, 1988. [DOI] [PubMed] [Google Scholar]
- Faingold 1999.Faingold C, Casebeer D. Modulation of the audiogenic seizure network by noradrenergic and glutamatergic receptors of the deep layers of superior colliculus. Brain Res 821: 392–399, 1999. [DOI] [PubMed] [Google Scholar]
- Graziano 2002.Graziano MS, Taylor CS, Moore T. Complex movements evoked by microstimulation of precentral cortex. Neuron 34: 841–851, 2002. [DOI] [PubMed] [Google Scholar]
- Grelot 1987.Grelot L, Bianchi AL. Differential effects of halothane anesthesia on the pattern of discharge of inspiratory and expiratory neurons in the region of the retrofacial nucleus. Brain Res 404: 335–338, 1987. [DOI] [PubMed] [Google Scholar]
- Haiss 2005.Haiss F, Schwarz C. Spatial segregation of different modes of movement control in the whisker representation of rat primary motor cortex. J Neurosci 25: 1579–1587, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattox 2002.Hattox AM, Priest CA, Keller A. Functional circuitry involved in the regulation of whisker movements. J Comp Neurol 442: 266–276, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemelt 2007.Hemelt ME, Keller A. Superior sensation: superior colliculus participation in rat vibrissa system. BMC Neurosci 8: 12, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta 1983.Huerta MF, Frankfurter A, Harting JK. Studies of the principal sensory and spinal trigeminal nuclei of the rat: projections to the superior colliculus, inferior olive, and cerebellum. J Comp Neurol 220: 147–167, 1983. [DOI] [PubMed] [Google Scholar]
- Isa 2002.Isa T, Sasaki S. Brain stem control of head movements during orienting; organization of the premotor circuits. Prog Neurobiol 66: 205–241, 2002. [DOI] [PubMed] [Google Scholar]
- Kayama 1991.Kayama Y, Ito S, Koyama Y. Properties of “possibly” cholinergic neurons ascending from the rat ponto-mesencephalic area: comparison with noradrenergic and serotonergic neurons. Fukushima J Med Sci 37: 75–93, 1991. [PubMed] [Google Scholar]
- King 2004.King AJ The superior colliculus. Curr Biol 14: R335–338, 2004. [DOI] [PubMed] [Google Scholar]
- Kleinfeld 2006.Kleinfeld D, Ahissar E, Diamond ME. Active sensation: insights from the rodent vibrissa sensorimotor system. Curr Opin Neurobiol 16: 435–444, 2006. [DOI] [PubMed] [Google Scholar]
- Kleinfeld 1999.Kleinfeld D, Berg RW, O'Conner SM. Anatomical loops and their electrical dynamics in relation to whisking by rat. Somatosens Motor Res 16: 69–88, 1999. [DOI] [PubMed] [Google Scholar]
- Kobayashi 2002.Kobayashi Y, Isa T. Sensory-motor gating and cognitive control by the brainstem cholinergic system. Neural Netw 15: 731–741, 2002. [DOI] [PubMed] [Google Scholar]
- Lee 2006.Lee P, Hall WC. An in vitro study of horizontal connections in the intermediate layer of the superior colliculus. J Neurosci 26: 4763–4768, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li 2004.Li F, Endo T, Isa T. Presynaptic muscarinic acetylcholine receptors suppress GABAergic synaptic transmission in the intermediate grey layer of mouse superior colliculus. Eur J Neurosci 20: 2079–2088, 2004. [DOI] [PubMed] [Google Scholar]
- Mana 2001.Mana S, Chevalier G. Honeycomb-like structure of the intermediate layers of the rat superior colliclulus: afferent and efferent connections. Neuroscience 103: 673–693, 2001. [DOI] [PubMed] [Google Scholar]
- Masri 2006.Masri RM, Trageser JC, Bezdudnaya T, Li Y, Keller A. Cholinergic regulation of the posterior medial thalamic nucleus. J Neurophysiol 96: 2265–2273, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May 2005.May P The mammalian superior colliculus: laminar structure and connections. Prog Brain Res 151: 321–378, 2005. [DOI] [PubMed] [Google Scholar]
- McHaffie 1989.McHaffie JG, Kao CQ, Stein BE. Nociceptive neurons in rat superior colliculus: response properties, topography, and functional implications. J Neurophysiol 62: 510–525, 1989. [DOI] [PubMed] [Google Scholar]
- McHaffie 1982.McHaffie JG, Stein BE. Eye movements evoked by electrical stimulation in the superior colliculus of rats and hamsters. Brain Res 247: 243–253, 1982. [DOI] [PubMed] [Google Scholar]
- McIlwain 1986.McIlwain JT Effects of eye position on saccades evoked electrically from superior colliculus of alert cats. J Neurophysiol 55: 97–112, 1986. [DOI] [PubMed] [Google Scholar]
- McIlwain 1991.McIlwain JT Distributed spatial coding in the superior colliculus: a review. Vis Neurosci 6: 3–13, 1991. [DOI] [PubMed] [Google Scholar]
- Miyashita 1994.Miyashita E, Keller A, Asanuma H. Input-output organization of the rat vibrissae motor cortex. Exp Brain Res 99: 223–232, 1994. [DOI] [PubMed] [Google Scholar]
- Miyashita 1995.Miyashita E, Mori S. The superior colliculus relays signals descending from the vibrissal motor cortex to the facial nerve nucleus in the rat. Neurosci Lett 195: 69–71, 1995. [DOI] [PubMed] [Google Scholar]
- Moschovakis 1996.Moschovakis AK The superior colliculus and eye movement control. Curr Opin Neurobiol 6: 811–816, 1996. [DOI] [PubMed] [Google Scholar]
- Nguyen 2005.Nguyen QT, Kleinfeld D. Positive feedback in a brainstem tactile sensorimotor loop. Neuron 45: 447–457, 2005. [DOI] [PubMed] [Google Scholar]
- Populin 2005.Populin LC Anesthetics change the excitation/inhibition balance that governs sensory processing in the cat superior colliculus. J Neurosci 25: 5903–5914, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahibzada 1986.Sahibzada N, Dean P, Redgrave P. Movements resembling orientation or avoidance elicited by electrical stimulation of the superior colliculus in rats. J Neurosci 6: 723–733, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito 2003.Saito Y, Isa T. Local excitatory network and NMDA receptor activation generate a synchronous and bursting command from the superior colliculus. J Neurosci 23: 5854–5864, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller 1972.Schiller PH, Stryker M. Single-unit recording and stimulation in superior colliculus of the alert rhesus monkey. J Neurophysiol 35: 915–924, 1972. [DOI] [PubMed] [Google Scholar]
- Sooksawate 2006.Sooksawate T, Isa T. Properties of cholinergic responses in neurons in the intermediate grey layer of rat superior colliculus. Eur J Neurosci 24: 3096–3108, 2006. [DOI] [PubMed] [Google Scholar]
- Stoney 1968.Stoney SD, Thompson WD, Asanuma H. Excitation of pyramidal tract cells by intracortical microstimulation: effective extent of stimulating current. J Neurophysiol 31: 659–669, 1968. [DOI] [PubMed] [Google Scholar]
- Swadlow 1998.Swadlow HA Neocortical efferent neurons with very slowly conducting axons: strategies for reliable antidromic identification. J Neurosci Methods 79: 131–141, 1998. [DOI] [PubMed] [Google Scholar]
- Szwed 2003.Szwed M, Bagdasarian K, Ahissar E. Encoding of vibrissal active touch. Neuron 40: 621–630, 2003. [DOI] [PubMed] [Google Scholar]
- Takemoto 1978.Takemoto I, Sasa M, Takaori S. Role of the locus coeruleus in transmission onto anterior colliculus neurons. Brain Res 158: 269–278, 1978. [DOI] [PubMed] [Google Scholar]
- Tehovnik 1989.Tehovnik EJ Head and body movements evoked electrically from the caudal superior colliculus of rats: pulse frequency effects. Behav Brain Res 34: 71–78, 1989. [DOI] [PubMed] [Google Scholar]
- Towal 2006.Towal RB, Hartmann MJ. Right-left asymmetries in the whisking behavior of rats anticipate head movements. J Neurosci 26: 8838–8846, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine 2002.Valentine DE, Sinha SR, Moss CF. Orienting responses and vocalizations produced by microstimulation in the superior colliculus of the echolocating bat,Eptesicus fuscus. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 188: 89–108, 2002. [DOI] [PubMed] [Google Scholar]
- Vidal 1988.Vidal PP, May PJ, Baker R. Synaptic organization of the tectal-facial pathways in the cat. I. Synaptic potentials following collicular stimulation. J Neurophysiol 60: 769–797, 1988. [DOI] [PubMed] [Google Scholar]
- Vincent 1912.Vincent SB The function of the vibrissae in the behavior of the white rat. Behav Monogr 1: 7–86, 1912. [Google Scholar]
- Watanabe 2005.Watanabe M, Kobayashi Y, Inoue Y, Isa T. Effects of local nicotinic activation of the superior colliculus on saccades in monkeys. J Neurophysiol 93: 519–534, 2005. [DOI] [PubMed] [Google Scholar]
- Zaninetti 1999.Zaninetti M, Tribollet E, Bertrand D, Raggenbass M. Presence of functional neuronal nicotinic acetylcholine receptors in brain stem motoneurons of the rat. Eur J Neurosci 11: 2737–2748, 1999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.