Abstract
The opioid receptor-like 1 (NOP or ORL1) receptor is a G-protein-coupled receptor the endogenous ligand of which is the heptadecapeptide, nociceptin (Noc). NOP receptors are known to modulate pain processing at spinal, supraspinal, and peripheral levels. Previous work has demonstrated that NOP receptors inhibit N-type Ca2+ channel currents in rat sympathetic stellate ganglion (SG) neurons via pertussis toxin (PTX)-sensitive Gαi/o subunits. However, the identification of the specific Gα subunit that mediates the Ca2+ current modulation is unknown. The purpose of the present study was to examine coupling specificity of Noc-activated NOP receptors to N-type Ca2+ channels in SG neurons. Small interference RNA (siRNA) transfection was employed to block the expression of PTX-sensitive Gα subunits. RT-PCR results showed that siRNA specifically decreased the expression of the intended Gα subunit. Evaluation of cell surface protein expression and Ca2+ channel modulation were assessed by immunofluorescence staining and electrophysiological recordings, respectively. Furthermore, the presence of mRNA of the intended siRNA target Gα protein was examined by RT-PCR experiments. Fluorescence imaging showed that Gαi1, Gαi3, and Gαo were expressed in SG neurons. The transfection of Gαi1-specific siRNA resulted in a significant decrease in Noc-mediated Ca2+ current inhibition, while silencing of either Gαi3 or Gαo was without effect. Taken together, these results suggest that in SG neurons Gαi1 subunits selectively couple NOP receptors to N-type Ca2+ channels.
INTRODUCTION
The opioid receptor-like 1 (NOP, or ORL1) receptor belongs to the opioid receptor subfamily of the G-protein-coupled receptor (GPCR) superfamily. The heptadecapeptide nociceptin (Noc) is the endogenous NOP receptor ligand that mediates its effects by coupling NOP receptors to effectors via members of the Gαi/o family of heterotrimeric G proteins. The Gαi1-3 and GαoA/B protein subunits are pertussis toxin (PTX)-sensitive; GαoA and GαoB are splice variants, whereas Gαi1-3 is not. Stimulation of NOP receptors by Noc results in inhibition of voltage-gated Ca2+ channels, activation of G protein inwardly rectifying K+ (GIRK) channels and negative coupling to adenylyl cyclase (for review, see Connor and Christie 1999; Mogil and Pasternak 2001; New and Wong 2002). NOP receptors have been shown to regulate pain processing as well as cardiovascular functions (Kapusta 2000; Mogil and Pasternak 2001). The Noc-mediated inhibition of N-type Ca2+ channel currents occurs in a voltage-dependent and membrane-delimited manner (Larsson et al. 2000; Vaughan et al. 2001). More recently, it has been reported that NOP receptors, when expressed at a high density, are capable of forming a complex with N-type Ca2+ channels that results in tonic inhibition of the channels in the absence of agonist (Beedle et al. 2004). Previously, we reported that adult rat sympathetic stellate ganglion (SG) neurons express NOP receptors that modulate N-type Ca2+ channels following exposure to Noc (Ruiz-Velasco et al. 2005). Pretreatment with PTX abolished the nociceptin-mediated Ca2+ current inhibition.
Coupling specificity of GPCR and Gα subunits has been studied in a number of expression systems, including primary neurons and established cell lines. For instance, coupling of various GPCR with PTX-sensitive Gαi/o subunits has been examined in rat superior cervical ganglion (SCG) and hippocampal neurons by pretreating the cells with PTX and heterologously expressing mutationally modified PTX-resistant Gα mutants (Chen and Lambert 2000; Jeong and Ikeda 2000; Kammermeier et al. 2003; Straiker et al. 2002; Tian and Kammermeier 2006). The rescue of coupling between GPCR and effector (i.e., ion channels) indicates that the receptor is capable of coupling to the heterologously expressed PTX-resistant Gα subunit. Another technique used to study receptor-G protein coupling involves heterologous expression of PTX-sensitive Gα subunits fused to the C-termini of GPCR (Bertin et al. 1994). Under these conditions, a stoichiometric ratio of 1:1 between GPCR and Gα subunit is achieved (Moon et al. 2001). This approach has been applied successfully in various expression systems (for review, see Seifert et al. 1999). Finally, the use of antisense oligonucleotides has also been shown to be an effective tool used to probe G protein coupling specificity between Ca2+ channels with either Gα or Gβγ subunits (Gollasch et al. 1993; Hescheler et al. 1987; Kleuss et al. 1991, 1992).
The purpose of the present study was to determine the specific PTX-sensitive Gα protein subunits that couple NOP receptors with N-type Ca2+ channels in SG neurons by employing small interference RNA (siRNA). This is a new and powerful technique that provides an efficient means for blocking expression of a specific gene in a variety of cell types and organisms (Milhavet et al. 2003). In this report, we describe the transfection of acutely dissociated rat SG neurons with siRNA targeting each of the natively expressed PTX-sensitive Gα subunits. Thereafter the Noc-mediated modulation of N-type Ca2+ channel currents was examined in freshly replated SG neurons.
METHODS
Stellate ganglion neuron isolation
The experiments performed were approved by the Penn State College of Medicine Animal Care and Use Committee (IACUC). Adult rat SG neurons were prepared employing methods previously described (Ruiz-Velasco et al. 2005). Briefly, male Wistar rats (150–225 g) were killed by CO2 anesthesia and decapitated using a laboratory guillotine. The SG was removed and cleared of connective tissue in ice-cold Hanks' balanced salt solution. Thereafter the SG neurons were enzymatically dissociated as described (Ruiz-Velasco et al. 2005). The isolated SG neurons were resuspended in minimal essential medium (MEM), supplemented with 10% fetal calf serum, 1% Pen-Strep, and 1% glutamine (all from Invitrogen, Carlsbad, CA). The neurons were plated onto 35-mm polystyrene tissue culture plates coated with poly-l-lysine and stored in a humidified incubator (95% air-5% CO2) at 37°C. The media in the cells was not replaced while the neurons were kept in culture.
Gα subunit immunofluorescence and cell imaging
For all imaging assays, acutely isolated SG neurons were cultured in 35-mm glass-bottom dishes (MatTek, Ashland, MA) coated with poly-l-lysine. SG neurons that were stained for expression of the PTX-sensitive Gα subunits (i.e., Gαi1-3 and Gαo) were kept in culture for 24 h (shown in Fig. 1), while the siRNA-transfected neurons were kept in culture for 110–120 h (shown in Figs. 3, 5, and 7). Prior to imaging, the cells were rinsed five times with 1× phosphate-buffered saline (PBS), then fixed with 2% formaldehyde and 2% sucrose for 20 min. The neurons were then permeabilized in a solution containing 0.05% Tween 20 (EMD Biosciences, San Diego, CA) and 5% goat serum (Vector Laboratories, Burlingame, CA) for 10 min at 37°C. The cells were preincubated in 5% goat serum for 15 min at room temperature prior to addition of the primary antibody. Primary antibodies were diluted in PBS containing 5% goat serum. The primary antibodies employed were mouse anti-Gαi1 (1:200; Biomol, Plymouth Meeting, PA), mouse anti-Gαi2 (1:200; Biomol; Lab Vision, Fremont, CA; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti Gαi2 (1:200; Abcam Cambridge, MA), chicken anti-Gαi3 (1:200; Chemicon International, Temecula, CA) and mouse anti-Gαo (1:200; Biomol). The mouse anti-Gαo monoclonal antibody was designed to recognize both Gαo splice variants. Neurons were incubated with the primary antibodies for 60 min at room temperature and placed in a rocker platform. Following the 60-min incubation period, the cells were preincubated in 5% goat serum for 60 min at room temperature prior to addition of the secondary antibodies. The secondary antibodies employed were Alexa Fluor 488 and Alexa Fluor 568 goat anti-mouse or goat anti-chicken (Invitrogen) at a final concentration of 3–5 μg/ml. Secondary antibodies were added to the cells for 45 min and placed on a rocker kept at ∼4°C. Finally, the cells were rinsed three times. In some experiments, micronuclear injection of acutely dissociated SG neurons with Gαi2 (0.2 μg/μl), and enhanced green fluorescent protein (pEGFP) (0.005 μg/μl) cDNA constructs was performed to obtain staining with the rabbit anti-Gαi2 antibody (Abcam).
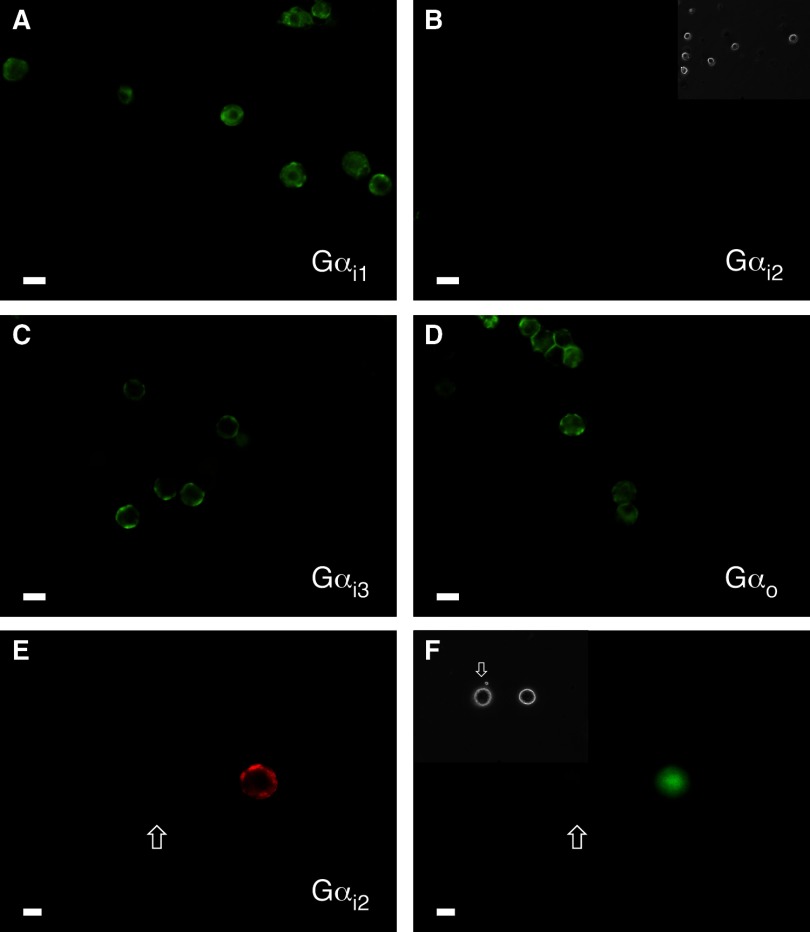
FIG. 1.
Immunofluorescence imaging reveals stellate ganglion (SG) neurons express pertussis toxin (PTX)-sensitive Gαi1, Gαi3, and Gαo but not Gαi2 subunits. Shown are fluorescence images of SG neurons fixed, permeabilized, and stained with primary antibodies against Gαi1 (A), Gαi2 (B), Gαi3 (C), and Gαo (D) followed by Alexa Fluor 488-conjugated IgG secondary antibody. The neurons were imaged at 20 × with a filter set containing an excitation filter at 480 ± 15 nm, a dichroic beam splitter of 505 nm (LP) and an emission filter at 535 ± 20 nm. The images were pseudocolored; scale bars represents 20 μm. B, inset: the phase image for the corresponding fluorescence field shown. As positive controls for Gαi2 staining, fluorescence images of SG neurons transfected with Gαi2 and enhanced green fluorescent protein (pEGFP) cDNA constructs are shown in E and F. The secondary antibody employed in E was Alexa Fluor 568 and imaged with a filter set containing an excitation filter at 540 ± 15 nm, a dichroic beam splitter of 585 nm (LP) and an emission filter at 620 ± 30 nm. F, inset: the phase image of the corresponding fluorescence field. Scale bars represent 20 μm. Arrow in all 3 images points to non-Gαi2- and GFP-expressing neuron.
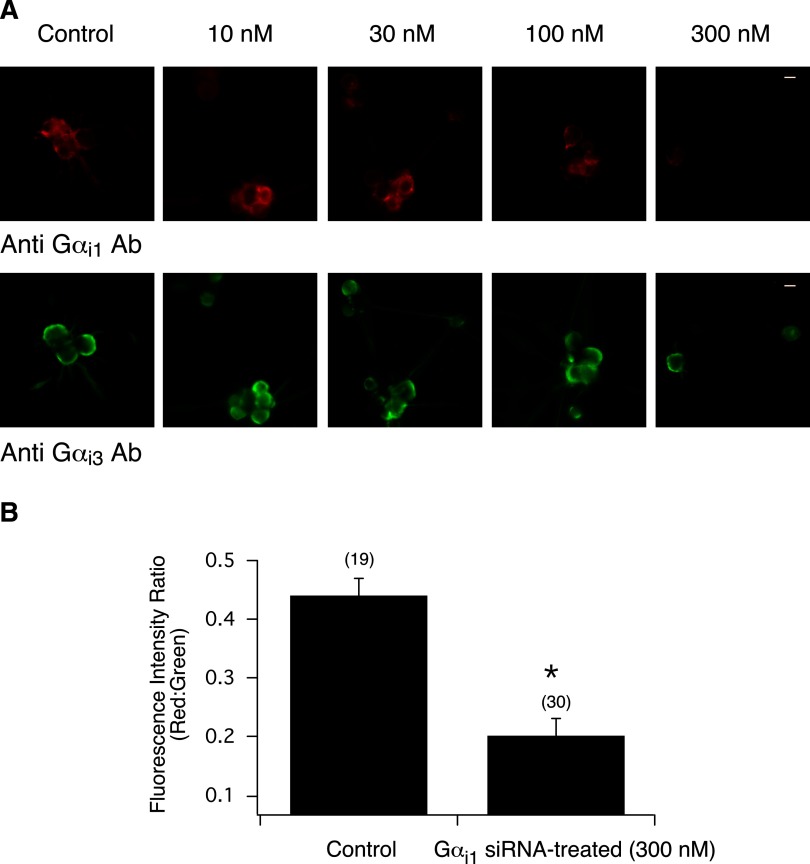
FIG. 3.
Inhibition of Gαi1 expression in rat SG neurons after siRNA transfection. A: siRNA concentration-response relationship 110–120 h post transfection in control and Gαi1 siRNA-treated (10–300 nM) SG neurons. Neurons were double-stained for Gαi1 (red) and Gαi3 (green) subunits. Green and red fluorescence images were acquired as described in Fig. 1. The images were pseudocolored; scale bar represents 20 μm. B: summary graph showing the mean ± SE fluorescence intensity ratio of red:green in control and Gαi1 siRNA-transfected (300 nM) neurons. Numbers in parenthesis indicate the number of cells in all acquired images followed by ratio determination. *P < 0.01 vs. control, Student's t-test.
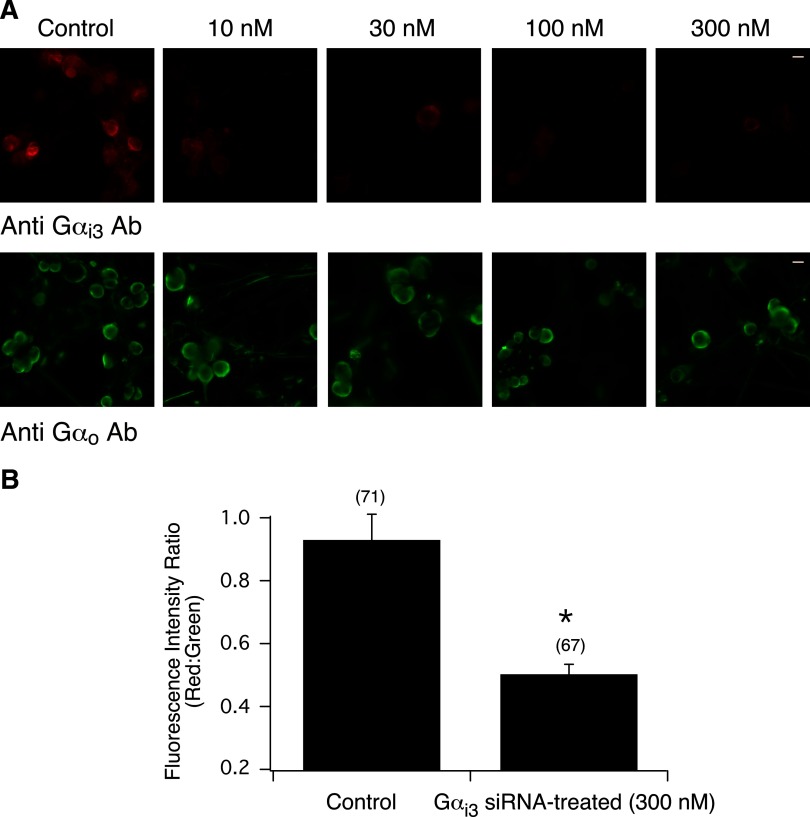
FIG. 5.
Inhibition of Gαi3 expression in rat SG neurons following siRNA transfection. A: siRNA concentration-response relationship 110–120 h post transfection in control and Gαi3 siRNA-treated (10–300 nM) SG neurons. Neurons were double-stained for Gαi3 (red) and Gαo (green) subunits. Green and red fluorescence images were acquired as described in Fig. 1. The images were pseudocolored; scale bar represents 20 μm. B: summary graph showing the mean ± SE fluorescence intensity ratio of red:green in control and Gαi3 siRNA-transfected (300 nM) neurons. Numbers in parenthesis indicate the number of cells in all acquired images followed by ratio determination. *P < 0.01 vs. control, Student's t-test.
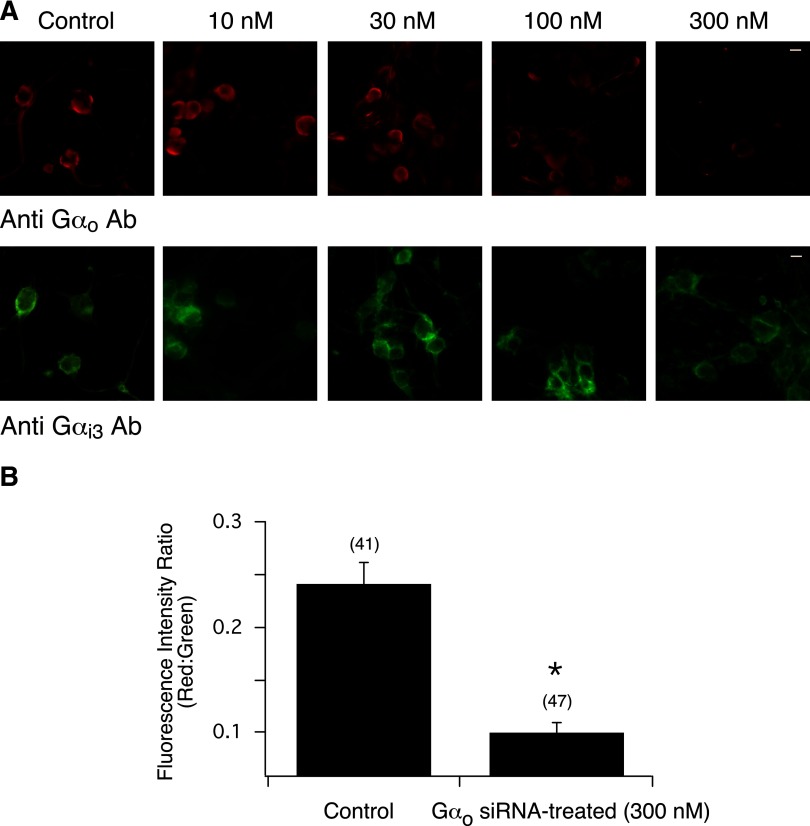
FIG. 7.
Inhibition of Gαo expression in rat SG neurons following siRNA transfection. A: siRNA concentration-response relationship 110–120 h post transfection in control and Gαo siRNA-treated (10–300 nM) SG neurons. Neurons were double-stained for Gαo (red) and Gαi3 (green) subunits. Green and red fluorescence images were acquired as described in Fig. 1. The images were pseudocolored; scale bar represents 20 μm. B: summary graph showing the mean ± SE fluorescence intensity ratio of red:green in control and Gαo siRNA-transfected (300 nM) neurons. Numbers in parenthesis indicate the number of cells in all acquired images followed by ratio determination. *P < 0.01 vs. control, Student's t-test.
Images were obtained with a Nikon TE2000 microscope using a ×20 objective, the X-Cite 120 (EXFO Life Sciences Group, Ontario, Ontario, Canada) for illumination and acquired with an Orca-ER 1394 digital CCD camera (Hamamatsu Photonics) and iVision software (Biovision Tech, Exton, PA). Fluorescence images of Alexa Fluor 488-labeled neurons were obtained with a filter set containing an excitation filter at 480 ± 15 nm, a dichroic beam splitter of 505 nm (LP), and an emission filter at 535 ± 20 nm (B-2E/C, Nikon). The filter set (G-2E/C; Nikon) containing an excitation filter at 540 ± 15 nm, a dichroic beam splitter of 585 nm (long pass, LP) and an emission filter at 620 ± 30 nm was employed for Alexa Fluor 568-labeled neurons. Although the exposure time employed for cells stained with Alexa Fluor 488 and Alexa Fluor 568 was different, it remained constant for each group. The acquired images were pseudocolored with iVision software and were postprocessed under the same conditions. Analysis of the red:green intensity ratios were performed with the ImageJ software package (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov/ij/, 1997–2007). Optical sections of Silencer Cy™3-labeled-neurons were obtained with a ×60 oil objective and the G-2E/C filter set and collected in 1.0-μm steps covering the z-axis field employing the Pro Scan II (Prior Scientific, Cambridge, UK) motorized stage. The acquired images were processed with the Huygens Essential software package (Scientific Volume Imaging b.v., Hilbersum, The Netherlands) and pseudocolored with iVision software.
siRNA sequence design and transfection
The siRNA sequences were chosen by employing a macro written by Stephen R. Ikeda (National Institutes of Health/NIAAA) on Igor Pro software (Lake Oswego, OR) and based on criteria with a scoring system (10 being best and 1 worse) recently described (Reynolds et al. 2004). The target sequences for rat Gα subunits and scores were as follows.
Gαi1, 5′-TAA CGG ACG TCA TCA TAA A-3′ (10) and 5′-GAA TAG CAC AAC CAA ATT A-3′ (9) corresponding to nucleotide positions 1016-1034 and 482-500, respectively; Gαi3, 5′-TCA AGG AGC TCT ACT TCA A-3′ (9) corresponding to nucleotide positions 572-590; and GαoA, 5′-TCA ACG ACT CTG CCA AAT A-3′ (9) and 5′-CCA TCT GCT TTC CTG AAT A-3′ (9) corresponding to nucleotide positions 446-464 and 854-872, respectively. This last siRNA sequence was also designed to target the GαoB splice variant as well. All siRNA oligonucleotides were purchased from Ambion/Applied Biosystems, Foster City, CA. In another separate set of experiments, labeled control siRNA, Silencer Cy™3-labeled Negative Control 1 (Ambion/Applied Biosystems), was used to assess the uptake efficiency and localization of siRNA oligonucleotides by SG neurons.
Five hours after plating the acutely dissociated SG neurons, the supplemented MEM media was replaced with premixed Opti-MEM (Invitrogen), Lipofectamine 2000 (15–20 μl/ml, Invitrogen), 2 mM 2,3-butanedione monoxime, and the appropriate amount of siRNA (10–300 nM). The total volume of transfection media was 1 ml. After the fourth h of transfection, 1 ml of MEM (supplemented with 2% FBS and 1% glutamine) was added to each dish. Thus the neurons were transfected with siRNA for a total of 5 h. Afterward, the transfection media was removed, and the dishes were rinsed three times with MEM (2% FBS, 1% Pen-Strep, 1% glutamine). The neurons were incubated in MEM (2% FBS, 1% Pen-Strep, 1% glutamine) and returned to the incubator. The amount of serum was decreased from 10 to 2% to keep the growth of processes at a minimum. As the transfection protocol was developed, we found that it was necessary to transfect the neurons twice to obtain the loss of protein expression as measured with our immunofluorescence staining protocol. This approach has also been reported by others to be necessary for silencing G proteins (i.e., α, β, and γ) in cultured cells (Krummins and Gilman 2006) and other signaling proteins in sympathetic neurons (Davidson et al. 2004). Thus in the present study, SG neurons were retransfected 48 h after the initial transfection. However, for the second transfection, the incubation period was reduced. That is, the MEM was again replaced with premixed Opti-MEM, Lipofectamine 2000; 2 mM 2,3-butanedione monoxime, and the appropriate amount of siRNA (10–300 nM). Following the second h of transfection, 1 ml of MEM (supplemented with 2% FBS and 1% glutamine) was added to each dish. The total transfection period was 3 h. Thereafter the cells were rinsed three times as described in the preceding text, and MEM (2% FBS, 1% Pen-Strep, 1% glutamine) was added to each dish and returned to the incubator. SG neurons were kept in culture for a total of 110–120 h at which time they were prepared for imaging (described in the preceding text) or electrophysiological recording (described in the following text).
Electrophysiology and data analysis
Whole cell Ca2+ channel currents of siRNA-transfected neurons kept in culture a total period of 110–120 h were recorded at room temperature (21–24°C) employing the whole cell patch-clamp technique. Recording pipettes were pulled from borosilicate glass capillaries (Corning 7052; Garner Glass, Claremont, CA) on a Flaming-Brown (P-97) micropipette puller (Sutter Instrument, Novato, CA), coated with silicone elastomer (Sylgard; Dow Corning, Midland, MI) and fire polished with a microforge. Ca2+ channel currents were acquired with a patch-clamp amplifier (Axopatch 200B, Axon Instruments), analog filtered at 2–5 kHz (-3 dB; 4-pole low-pass Bessel filter). and digitized employing custom-designed software (S5) on a PowerMacG4 computer (Apple Computer, Cupertino, CA) equipped with an 18-bit A/D converter board (ITC18, Instrutech, Port Washington, NY). The cells' series resistance and membrane capacitance were electronically compensated (80–85%). Data and statistical analyses were performed with the Igor Pro and Prism 4 (GraphPad Software, San Diego, CA) software packages, respectively, with P < 0.05 considered statistically significant. Graphs and current traces were produced with the Igor Pro, Canvas 8.0 (Deneba Software, Miami, FL), and GraphPad Prism (GraphPad) software packages.
The internal pipette solution contained (in mM): 120 N-methyl-d-glucamine, 20 tetraethylammonium hydroxide (TEA-OH), 11 EGTA, 10 HEPES, 1 CaCl2, 4 Mg-ATP, 0.3 Na2GTP, 20 HCl, and 14 tris creatine phosphate. The pH was adjusted to 7.2 with methanesulfonic acid and the osmolality was 293–302 mOsmol/kg. The external solution consisted of (in mM): 145 TEA-OH, 140 methanesulfonic acid, 10 HEPES, 15 glucose, 10 CaCl2, and 0.0003 tetrodotoxin. The pH was adjusted to 7.4 with TEA-OH and the osmolality was 320–325 mOsmol/kg. Stock solutions of nociceptin (Noc; Tocris Cookson, Ellisville, MO), ω-conotoxin GVIA (Bachem, Torrance, CA), and norepinephrine (NE; Sigma) were prepared in water and diluted in the external solution to the final concentration employed.
SG neuron replating
For electrophysiological recording studies, it was necessary to replate the SG neurons to eliminate the processes formed during extended culture times (i.e., 110–120 h). As a result, space-clamp conditions were improved. The neurons were incubated for 5 min in Earle's balanced salt solution (EBSS) containing 0.2 mg/ml collagenase type D (Boehringer Mannheim, Indianapolis, IN). During the 5-min exposure to collagenase, the dishes were placed in an incubator shaker set at 38°C and rotating at 50 rpm. Thereafter the dishes were tapped several times to facilitate neuron detachment and 1 ml of MEM (10% FBS, 1% glutamine and 1% Pen-Strep) was added to each dish. The cells were then transferred to sterile 5 ml microtubes and an additional 3 ml of MEM (10% FBS, 1% glutamine, and 1% Pen-Strep) was added to each microtube. After a 6-min centrifugation to pellet the neurons, the supernatant was removed leaving ∼150 μl of media remaining per microtube. The pellet of cells was gently broken up after the addition of 250 μl of MEM (10% FBS, 1% glutamine, and 1% Pen-Strep). The cell suspension from each microtube was plated onto 35-mm dishes. Electrophysiological recordings of the neurons were begun ≥5 h after plating to allow for recovery from enzyme digestion.
Single-cell RT-PCR analysis
RT-PCR experiments were carried out employing the OneStep RT-PCR Kit (Qiagen, Valencia, CA) and the following primer sequences (Integrated DNA Technologies, Coralville, IA), employed previously (Kammermeier et al. 2003), with expected product sizes in parenthesis were used.
Glyceraldehydes-3-phosphate dehydrogenase (GAPDH) forward: CCA AAA GGG TCA TCA TCT CCG, GAPDH backward: AGA CAA CCT GGT CCT CAG TGT AGC (501 bp). Gαi1 forward: TGA CTA TGA CCT GGT TCT TGC TGA G, Gαi1 backward: ACA CTA CAT TCT CTG TTG CTG GGA G (482 bp). Gαi3 forward: TGA GTA AAG AGC CCA GGA TTG C, Gαi3 backward: CAA AGC AGT TCT GAC CAC CAA CC (453 bp). Gαo forward: CGT GGA GTA TGG TGA CAA GGA GAG, Gαo backward: AAG GTG AAG TGG GTT TCT ACG ATG (300 bp).
In this set of experiments, the siRNA-transfected neurons required replating because of their strong adherence to the poly-l-lysine-coated dishes. Thus the transfected cells were replated (described in the preceding text) in new poly-l-lysine dishes and allowed to adhere to the bottom no more than 60 min prior to neuron collection. First, the cells were rinsed twice in a solution containing (in mM) 130 NaCl, 5.4 KCl , 10 HEPES, 0.8 MgCl2, 10 CaCl2, 15 glucose, and 15 sucrose. Thereafter neurons were collected into SigmaCote-coated (Sigma) glass capillaries (Corning 7052) with a final volume of 10–15 μl. The number of neurons collected per capillary ranged from 10 to 15. The cells were placed in PCR tubes containing reagents supplied by the manufacturer (Qiagen). The tubes were placed in a PCR thermocycler (Eppendorf) and subjected to the following protocol: 94°C for 1 min, 58°C for 1 min, and 74°C for 1.5 min, for a total of 44 cycles. The PCR products were analyzed by agarose gel electrophoresis, and visualized by ethidium bromide staining and UV illumination. Gel images were captured using a digital gel imaging system (Eastman Kodak, Rochester, NY) and processed with iVision software.
RESULTS
PTX-sensitive Gα subunits expressed in SG neurons couple N-type Ca2+ channels to NOP receptors
In the present study, we examined the coupling specificity between NOP receptors and PTX-sensitive Gαi/o subunits in rat SG neurons. N-type Ca2+ channel currents are inhibited following Noc-mediated NOP receptor activation. Previous reports have shown that this channel subtype is the main carrier of Ca2+ ions in SG neurons (Fuller et al. 2004; Kukwa et al. 1998). We have previously demonstrated that overnight pretreatment of SG neurons with PTX significantly decreases the Noc-mediated Ca2+ current inhibition (Ruiz-Velasco et al. 2005). The first set of experiments was performed to determine which PTX-sensitive Gα subunit was expressed in SG neurons. Figure 1 shows immunofluorescence images of acutely dissociated SG neurons exposed to Gαi1 (A), Gαi2 (B), Gαi3 (C), and Gαo (D) antibodies. Of the four subunits, Gαi2 immunofluorescence was not detected. Four different antibodies to Gαi2 were tested, and no staining was observed with any of the antibodies. The inset in Fig. 1B shows a phase image of the cells that did not stain for the Gαi2 antibody. Consequently, SG neurons were transfected with a Gαi2 cDNA construct and served as a positive control. The cells were co-transfected with the GFP cDNA construct to identify the cells that had been successfully transfected. Figure 1E shows an immunofluorescence image of an SG neuron stained with the Gαi2 antibody, and the image in Fig. 1F shows expression of GFP in the same neuron. The inset shown in Fig. 1F is a phase image of the corresponding SG neuron expressing both constructs (indicated by arrow) and a separate cell that did not stain with the antibody or express GFP. These results indicate that SG neurons do not express Gαi2 and that NOP receptors employ one or more of the other PTX-sensitive Gα subunits to modulate Ca2+ channel currents.
siRNA of PTX-sensitive Gα subunits decreases mRNA expression
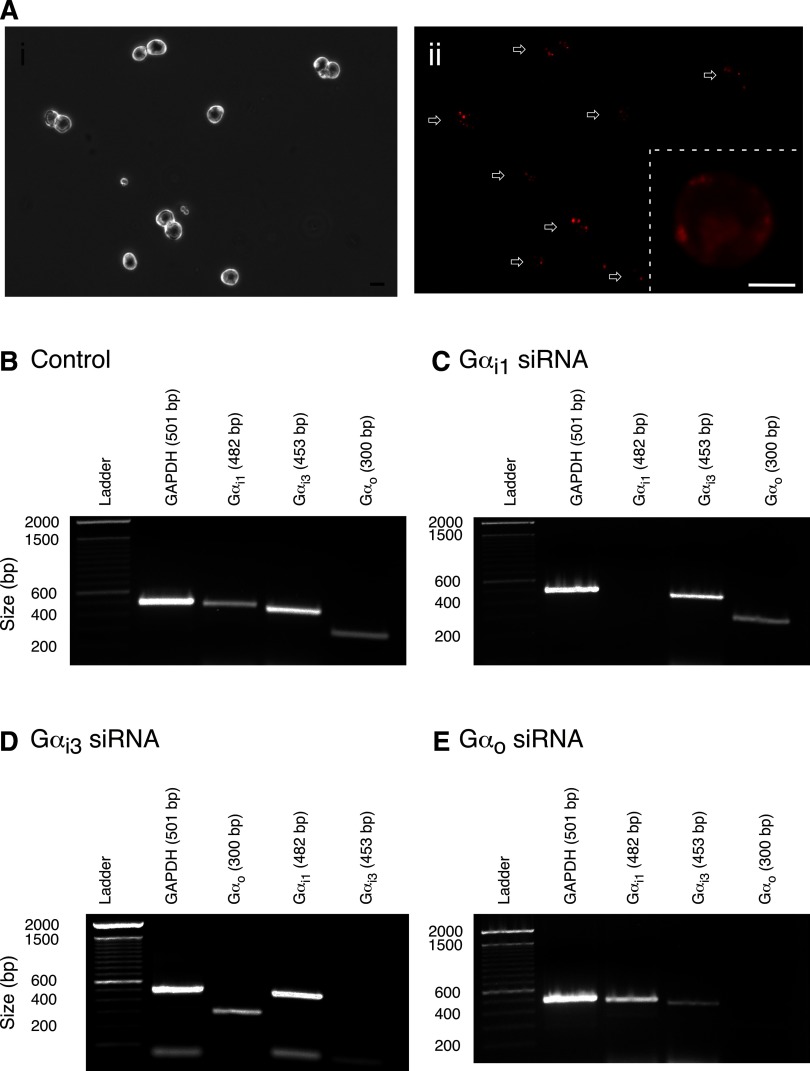
We first optimized conditions to effectively transfect SG neurons with siRNA. To determine the uptake, cellular distribution and uptake efficiency of siRNA, SG neurons were transfected with a negative control Cy™3-labeled siRNA for 5 h. Figure 2 A, i and ii, shows phase contrast and fluorescence images of SG neurons, respectively, following transfection with the Cy™3-labeled oligonucleotide (300 nM). The fluorescence image demonstrates that all SG neurons internalized the labeled siRNA. The inset in Fig. 2Aii is a deconvolved fluorescence image of a neuron transfected with Cy™3-labeled siRNA showing the oligonucleotide is dispersed in the cytoplasm.
FIG. 2.
Monitoring transfection efficiency and detection of Gα subunit mRNA expression in SG neurons by RT-PCR. Phase (Ai) and fluorescence (Aii) images of SG neurons transfected with Cy™3-labeled negative control small interference RNA (siRNA; 300 nM) for 5 h. Neurons were imaged at ×10 and acquired with the filter set described for Fig. 1. The images were pseudocolored; scale bar in Ai represents 20 μm. Arrows in Aii point to all SG neurons shown in phase image in Ai. Aii, inset: a deconvolution fluorescence image of a single neuron imaged at ×60 (1.5 Mag); scale bar = 10 μm. Single-cell reverse transcription-PCR analyses of RNA isolated from control (B), Gαi1 siRNA (C)-, Gαi3 siRNA (D)-, and Gαo siRNA-treated (E) SG neurons employing primer pairs for glyceraldehydes-3-phosphate dehydrogenase (GAPDH), Gαi1, Gαi3, and Gαo subunits. PCR products were separated by 1.5% agarose gel electrophoresis. The expected molecular size for each PCR product is indicated on the top of each gel. The number of SG neurons tested for each Gα protein ranged from 10 to 15 cells. The gels in each panel were obtained from the same animal. This protocol was performed with ≥4 separate rat dissections, and the results observed were similar.
With knockdown experiments, it was necessary to transfect the neurons a second time to significantly reduce Gα subunit expression, an approach reported to be successful (Davidson et al. 2004; Krummins and Gilman 2006). To test for the siRNA-mediated gene silencing of the Gα subunits, RT-PCR experiments were performed in control (Fig. 2B) and SG neurons following 110–120 h transfection with 300 nM of either Gαi1 (C) or Gαi3 (D) or Gαo siRNA (E). The cells were replated in 35 mm dishes (as described in methods). Soon thereafter, 10–15 cells were collected in glass pipettes and RT-PCR was performed. The primer pairs employed for each Gα subunit are described in methods and have been successfully employed in other neuron types (Kammermeier et al. 2003; Tian and Kammermeier 2006). Figure 2B shows a control gel for the three Gα subunits and the housekeeping gene, GAPDH. The SG neurons transfected with Gαi1 siRNA showed that mRNA for this subunit was absent, while the message of the other three proteins was present (Fig. 2C). Similarly, Gαi3- and Gαo-treated SG neurons showed downregulation of their respective mRNA and not of the other subunits tested (Fig. 2, D and E). The gel images also show that in some cases the intensity of the Gα subunit mRNA was different (i.e., Gαi1, A and D). Because the number of cells collected in each tube varied from 10 to 15, it is possible that the difference in band intensity is a result of greater mRNA loading in one lane than another. Nevertheless, these semi-quantitative results indicate that siRNA for each Gα subunit did not affect mRNA expression of the nontargeted PTX-sensitive Gα subunits and suggests that targeting was specific at the nucleotide level. These findings were observed in four separate rat preparations.
siRNA directed to Gαi1, Gαi3, and Gαo reveals that NOP receptors couple to Gαi1 subunits to Ca2+ channels
In this set of experiments, we optimized conditions (i.e., siRNA concentration response and time to silence) to transfect SG neurons with siRNA targeting Gαi1, Gαi3, and Gαo. Immunofluorescence imaging was initially employed to determine whether the targeted protein was in fact knocked down. Figure 3A shows immunofluorescence images of SG neurons stained for Gαi1 and Gαi3 in cells that were transfected with 10, 30, 100, and 300 nM siRNA directed to rat Gαi1. Following a 110–120 h posttransfection period, Gαi1 expression diminished (as shown by decreasing fluorescence intensity) as siRNA concentration was increased. A final concentration of 300 nM was necessary to observe a significant decrease in Gαi1 expression. On the other hand, Fig. 3A shows that in the same cells Gαi3 expression was unaffected by the Gαi1 siRNA treatment at all concentrations employed. The summary plot in Fig. 3B shows the mean fluorescence intensity ratio (red:green) of control and Gαi1 siRNA-transfected neurons. The ratio was determined from all acquired images.
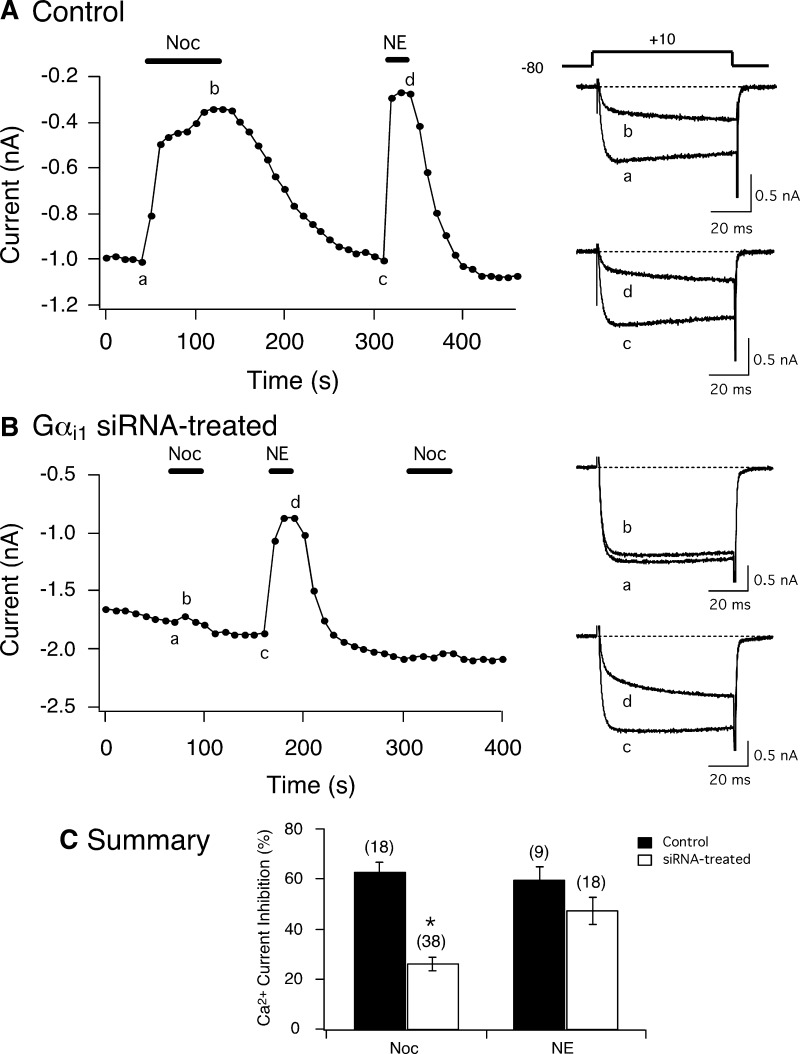
Next, Ca2+ currents were recorded in siRNA-transfected neurons. Figure 4, A and B, shows time courses of Ca2+ currents of a control and a Gαi1 siRNA-treated (300 nM) SG neuron, respectively, obtained before and during application of Noc (3 μM). The currents were evoked every 10 s with a depolarizing step pulse to +10 mV for 70 ms from a holding potential of −80 mV (Fig. 4B, top right). In the control neuron, the current amplitude was blocked by ∼65% after Noc exposure. The superimposed Ca2+ current traces before (a) and during (b) Noc exposure are shown to the right. Following recovery to the peak Ca2+ current, the cell was exposed to 10 μM NE (current traces c and d). Application of NE resulted in inhibition of Ca2+ currents as well. Figure 4B shows that Noc application to the Gαi1 siRNA-treated neuron did not result in significant coupling between Noc-stimulated NOP receptors and Ca2+ channels. The Ca2+ current amplitude was blocked by ∼12% following Noc application (see current traces a and b on right). As a positive control, NE was next applied to the same neuron and Ca2+ currents in a similar fashion as control neurons. Figure 4C is a summary graph of the Noc- and NE-mediated Ca2+ current inhibition in control and Gαi1 siRNA-transfected SG neurons. During Noc exposure, the inhibition of Ca2+ currents was significantly (P < 0.01) lower than control SG neurons, while the effect of NE was not different in both group of neurons. As noted in methods, at least two siRNA sequences were employed separately for each Gα subunit. Similar results were obtained for both siRNA sequences and the results shown in Fig. 4C were pooled. Specifically, the Noc-mediated Ca2+ current inhibition was 25 ± 3.3, n = 26, and 28.7 ± 5.3%, n = 12 (P = 0.51) for the first and second siRNA sequences, respectively. It should also be noted that in 9 of the 38 siRNA-treated neurons with either nucleotide sequence, we did not observe a decrease in Ca2+ current modulation following Noc application. That is, the Ca2+ current inhibition was >50%. We assume that the siRNA transfection was not as efficacious in silencing Gαi1 in this number of neurons (discussed in the following text). In a separate set of experiments, control and Gαi1 siRNA-transfected neurons were exposed to ω-conotoxin GVIA (10 μM) to determine if N-type Ca2+ channels were the main carriers of Ca2+ ions in SG neurons, as has been previously reported (Fuller et al. 2004; Kukwa et al. 1998). In control neurons, exposure to the N-type Ca2+ channel blocker resulted in Ca2+ current inhibition of 62.4 ± 3.7% (n = 9). The Noc- and NE-mediated current inhibition measured in the presence of the toxin was significantly (P < 0.01) decreased to 14.8 ± 2.8 (n = 9) and 16.4 ± 4.7% (n = 6), respectively. Application of ω-conotoxin GVIA to transfected cells caused a 59.6 ± 4.5% (n = 11) inhibition of Ca2+ currents. Subsequent exposure of these neurons to Noc and NE also resulted in a significant (P < 0.01) decrease of Ca2+ current inhibition to 22.3 ± 4.8 (n = 11) and 20.9 ± 2.8% (n = 7), respectively. These results suggest that the Ca2+ current inhibition mediated by activated NOP and α2-adrenergic receptors (α2-AR) occurs primarily through N-type Ca2+ channels.
FIG. 4.
Effect of siRNA targeting Gαi1 subunits on nociceptin (Noc)-mediated Ca2+ current inhibition in SG neurons. Time courses of Ca2+ current amplitude inhibition acquired from Noc (3 μM) and norepinephrine (NE, 10 μM) application in a control (A) and a Gαi1 siRNA-treated (300 nM) neuron (B). The currents were evoked every 10 s by a single 70-ms test pulse to +10 mV from a holding potential of −80 mV (shown A, top right). ▪, application of Noc or NE. Right: the superimposed Ca2+ currents before (bottom, a and c) and during (top, b and d) application of Noc or NE. C: summary graph showing the mean ± SE Ca2+ current inhibition produced by Noc and NE in control and siRNA-treated neurons. Numbers in parenthesis indicate the number of experiments, *P < 0.01 vs. control, Student's t-test.
This experimental paradigm was employed systematically to determine the coupling specificity of Gαi3 and Gαo and Ca2+ channel modulation. Next SG neurons were transfected with Gαi3 siRNA to determine whether silencing this subunit would also disrupt coupling of NOP receptors and Ca2+ channels. Figure 5A shows the immunofluorescence images of control and neurons treated with increasing concentration of Gαi3 siRNA. As seen with SG neurons transfected with Gαi1 siRNA, 300 nM was the RNA concentration required to silence Gαi3 expression 110–120 h posttransfection. The images shown in Fig. 5A also demonstrate that Gαo expression was unaffected by siRNA transfection. The summary graph in Fig. 5B shows that the fluorescence intensity ratio was significantly (P < 0.01) lower in siRNA-tranfected neurons than control cells.
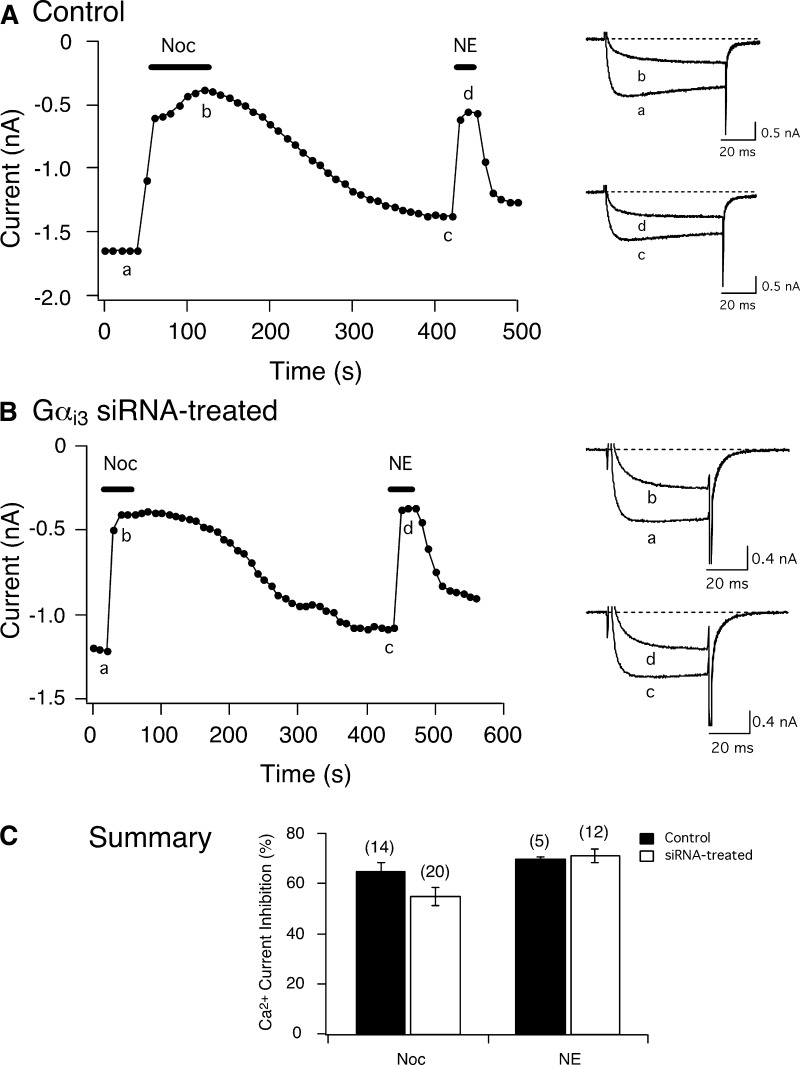
Figure 6, A and B, depicts peak Ca2+ currents as a function of time in a control and a Gαi3 siRNA-treated neuron, respectively. The Ca2+ currents were evoked as described in the preceding text for Fig. 4. In the control neuron, application of Noc decreased Ca2+ currents by ∼64% (see current traces a and b on right). Exposure of the cell to NE following washout also resulted in inhibition of the Ca2+ currents (traces c and d). The time course in Fig. 6B shows that both Noc and NE exerted similar effects on Ca2+ currents as the control group. Figure 6C summarizes the Noc- and NE-mediated Ca2+ current inhibition in control and siRNA-treated neurons. Unlike the Gαi1 siRNA-transfected neurons, Gαi3 protein knock-down produced no significant alteration on the Noc-mediated Ca2+ current inhibition when compared with control neurons. Also NE had similar effects in both group of cells. Again, the Ca2+ current inhibition following Noc exposure was similar (P = 0.2) in both siRNA-treated neurons and the results were pooled (58.7 ± 4.5, n = 13, vs. 48.7 ± 5.4%, n = 7).
FIG. 6.
Effect of siRNA targeting Gαi3 subunits on Noc-mediated Ca2+ current inhibition in SG neurons. Time courses of Ca2+ current amplitude inhibition acquired from Noc (3 μM) and NE (10 μM) application in a control (A) and a Gαi3 siRNA-treated neuron (B). The currents were evoked every 10 s by a single 50-ms test pulse to +10 mV from a holding potential of −80 mV (shown Fig. 3A, top). ▪, application of either Noc or NE. Right: superimposed Ca2+ currents indicate the effect of Noc or NE prior to (bottom, a and c) and during (top, b and d) agonist application. C: summary graph showing the mean ± SE Ca2+ current inhibition produced by noc and NE in control and siRNA-treated neurons. Numbers in parenthesis indicate the number of experiments.
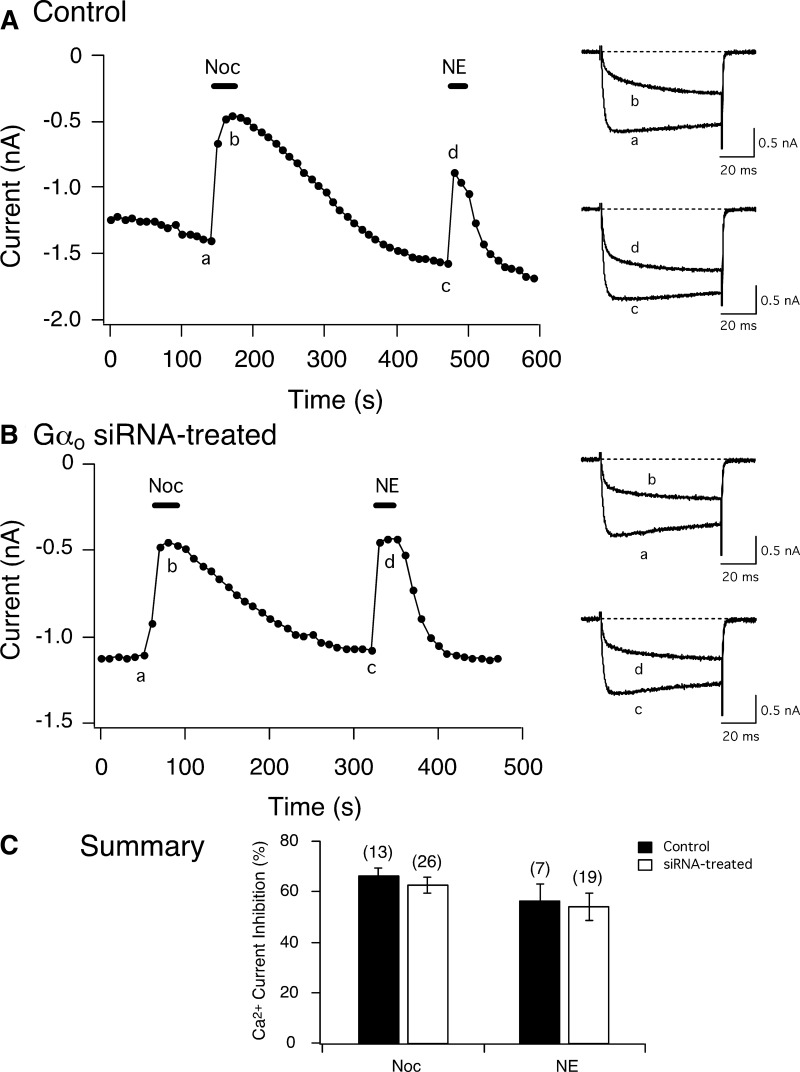
The next set of experiments were designed to examine whether silencing of native Gαo subunits would also disrupt coupling of NOP receptors and N-type Ca2+ channels. The Gαo siRNA concentration-response relationship in Fig. 7A shows that 300 nM was also the most efficient concentration required to remove expression of this subunit. On the other hand, the fluorescence images demonstrate that expression of Gαi3 was unaffected. The fluorescence intensity ratio plotted in Fig. 7B shows that transfection of SG neurons with Gαo siRNA resulted in decreased levels of Gαo subunits. The time courses of peak Ca2+ currents shown in Fig. 8, A and B, correspond to a control and an siRNA-treated SG neuron, respectively, before and after Noc and NE application. Exposure of Noc to the control and siRNA-treated neurons resulted in >60% inhibition of the Ca2+ currents (traces a and b, right side). The superimposed Ca2+ current traces (a–d) for each neuron are shown to the right. When NE was applied externally to either cell, Ca2+ current inhibition was also observed. The mean Noc- and NE-mediated Ca2+ current inhibition in control and siRNA-transfected cells is illustrated in Fig. 8C. There were no significant differences between both groups of neurons. The NE-mediated Ca2+ current inhibition values obtained in control and in all three groups of siRNA-transfected neurons were similar to those described earlier (Fuller et al. 2004). It should also be noted that there are two known Gαo splice variants (Hepler and Gilman 1992). One of the siRNA sequences employed targeted GαoA while the second sequence was designed to silence both GαoA and GαoB. As with the findings described in the preceding text, the Noc-mediated Ca2+ current inhibition magnitude was similar for both siRNA sequences employed (65.7 ± 4.8, n = 15, vs. 57.7 ± 2.8%, n = 11, P = 0.2). Finally, both Gαi1 and Gαo subunits were silenced to determine if α2-AR coupling to Ca2+ channels would be altered. The NE-mediated Ca2+ current inhibition was not significantly (P = 0.52) different between control and Gαi1/Gαo siRNA-transfected cells (53.8 ± 10.8, n = 4, vs. 46.0 ± 6.3, n = 7). These results suggest that α2-AR are capable of compensating for the silenced Gα subunits or SG neurons express splice variants that couple to all PTX-sensitive Gα subunits. In this study, coupling specificity of α2-AR was not further examined. Taken together, these findings demonstrate that Noc-activated NOP receptors couple to PTX-sensitive Gαi1 subunits to modulate N-type Ca2+ currents, whereas α2-AR appear to employ more than one Gα subunit.
FIG. 8.
Effect of Gαo siRNA treatment on Noc-mediated Ca2+ current inhibition in SG neurons. Time courses of Ca2+ current amplitude inhibition acquired from Noc (3 μM) and NE (10 μM) application in a control (A) and a Gαo siRNA-treated neuron (B). The currents were evoked every 10 s by a single 70-ms test pulse to +10 mV from a holding potential of −80 mV (shown Fig. 3A, top). ▪, application of Noc. Right: superimposed Ca2+ currents indicate the effect of Noc or NE prior to (bottom, a and c) and during (top, b and d) agonist application. C: summary graph showing the mean ± SE Ca2+ current inhibition produced by Noc and NE in control and siRNA-treated neurons. Numbers in parenthesis indicate the number of experiments.
DISCUSSION
The goal of the present study was to determine whether signaling specificity occurred between Noc-activated NOP receptors and N-type Ca2+ channels by employing RNA interference. Our efforts were focused at the NOP receptor–Gα subunit junction. The initial immunofluorescence results showed that of the five PTX Gα subunits identified (Gαi1-3 and the splice variants GαoA/B), the Gαi2 subunit was not expressed in the soma of SG neurons. This suggests that Gαi2 does not influence NOP signaling in rat SG neurons. However, the role of Gαi2 in coupling NOP receptors with Ca2+ channels should not be discounted altogether as this G protein subunit is expressed in rat superior cervical ganglion (SCG) neurons (Kammermeier et al. 2003), and NOP receptor activation results in Ca2+ current inhibition in SCG neurons (Larsson et al. 2000). The commercial Gαo antibody employed in these same experiments was generated to recognize the expression of both Gαo splice variants. The unavailability of antibodies that recognize each variant prevented us from identifying the specific Gαo subunit present in SG neurons. Therefore Gα signaling specificity was examined for Gαi1, Gαi3, and GαoA/B subunits.
Immunofluorescence assays demonstrated that 110–120 h post siRNA transfection were sufficient to observe a significant decrease in expression of the targeted Gα without affecting the other PTX-sensitive Gα subunits. The results showed that siRNA directed toward the PTX-sensitive Gαi1 subunit led to a significant decrease in Ca2+ channel modulation following Noc application when compared with control neurons. The magnitude of Noc-mediated Ca2+ current inhibition was similar to that we previously reported to occur with PTX-treated SG neurons (Ruiz-Velasco et al. 2005). In addition, silencing of Gαi1 subunits did not affect the NE-mediated Ca2+ current inhibition. On the other hand, when either Gαi3 or Gαo was silenced, coupling between NOP receptors and Ca2+ channels was unaffected. Furthermore, the results from RT-PCR experiments showed that siRNA treatment affected expression of the intended siRNA target and not the other PTX-sensitive Gα subunits. Therefore these findings suggest that coupling of NOP receptors and N-type Ca2+ channels occurs specifically via the PTX-sensitive Gαi1 subunits. Similar observations have been reported to occur with the human δ opioid receptor (Moon et al. 2001). In that study, the opioid receptor cDNA was fused in-frame with PTX-resistant Gαi1 or Gαo subunits and GTPase activity was measured in HEK293 cells. It was found that cells expressing the Gαi1-receptor fusion construct exhibited a three-fold greater efficiency of agonist-stimulated GTPase activity than the Gαo-receptor construct. Conversely, in hippocampal neurons, GABAB, cannabinoid (CB1) and somatostatin receptors—all PTX-coupled GPCR—have been shown to be unable to couple with heterologously expressed PTX-resistant Gαi1 subunits (Straiker et al. 2002).
The results presented in this study differ from those previously reported in another sympathetic neuron type by Brown and colleagues (Caulfield et al. 1994; Delmas et al. 1998, 1999). The studies were performed with rat SCG neurons to examine coupling specificity between N-type Ca2+ channels and α2-AR or M4 muscarinic acetylcholine receptors (mAChR) employing site-directed antibody injection or antisense RNA expression. It was shown that Gαo subunits are primarily responsible for mediating the inhibition of Ca2+ channels. Similar findings also identified Gαo as the subunit coupling L-type Ca2+ channels and α-AR in rat pituitary GH3 cells (Hescheler et al. 1987; Kleuss et al. 1991). In the present study, silencing Gαo subunits did not appear to affect the coupling of NOP receptors and N-type Ca2+ channels. The disparity between coupling specificity observed between α2-AR and NOP receptors is possibly relevant regarding the critical function α2-AR play in regulating the overall sympathetic tone centrally and neurotransmitter release peripherally. The lack of Gα subunit specificity may be to preserve this crucial function of α2-AR. On the other hand, SG neurons provide the main sympathetic innervation to cardiac muscle. The discriminating coupling of NOP receptors in SG neuron may be associated with controlling blood pressure and/or heart rate (Kapusta 2000).
As previously mentioned, other techniques have been employed to study selective coupling between Gα subunits and GPCR in several cell systems that require heterologous expression of PTX-resistant Gα subunits alone or fused in-frame with the receptor. Thereafter, the rescue of coupling is tested. Coupling specificity has been reported to occur with δ opioid receptors (Moon et al. 2001), metabotropic glutamate receptor 2 (mGluR2) (Kammermeier et al. 2003), mGluR6 (Tian and Kammermeier 2006), and GABAB, CB1, somatostatin receptors (Straiker et al. 2002). Although these studies have demonstrated coupling specificity, one drawback is that it can only be known which subunit is capable of coupling and not which subunit actually couples under physiological conditions. In the present study, with RNA interference, the heterologous expression of signaling proteins was circumvented and the role of natively expressed Gα subunits in Ca2+ channel modulation could be determined. Thus expression levels and nonspecific effects of heterologously expressed proteins were not a concern. For instance, one report has shown that coupling can occur among heterologously expressed Gα14, Gα16, and NOP receptors, yet both natively expressed Gα subunits are unable to do so with NOP receptors (Yung et al. 1999). Therefore under certain conditions NOP receptors are capable of coupling to other non-PTX-sensitive Gα subunits. Whether this occurs under physiological or pathophysiological conditions remains to be determined.
Here we have also presented evidence suggesting that α2-AR expressed in SG neurons couple to more than one PTX-sensitive Gα subunit to modulate Ca2+ channels. That is, when any of the three Gα subunits were silenced, the NE-mediated Ca2+ current inhibition was similar to nontransfected neurons. This is not surprising given that two published studies have demonstrated that the NE-mediated Ca2+ current inhibition in rat SCG neurons is mediated via Gαo (Caulfield et al. 1994) or Gαo and Gαi2 (Jeong and Ikeda 2000). Our data showed, however, that silencing Gαi1 alone or Gαi1 and Gαo in SG neurons did not disrupt coupling of α2-AR and Ca2+ channels. Moreover, it has been reported that both Gαo and Gαi contribute to the α2-AR-mediated inhibition of Ca2+ currents with a greater contribution of the former than the latter (Delmas et al. 1999). Interestingly, another report found that α2-AR couple to all three Gαi protein subunits in COS-7 cells (Wise et al. 1997). Overall the results imply that the nonsilenced PTX-sensitive Gα subunits (i.e., Gαi3) are capable of compensating for the knocked down Gα protein such that α2-AR signaling remains unaltered in SG neurons. Alternatively, SG neurons express α2-AR splice variants that can couple to all PTX-sensitive Gα subunits.
As pointed out in the preceding text, although silencing of Gαi1 resulted in a significant decrease in Noc-mediated Ca2+ current inhibition (n = 38), coupling (i.e., >60% inhibition) was observed in nine siRNA-treated neurons. There may be several reasons for this. First, it could be that siRNA transfection in these neurons was not as effective as with most cells. Second, it may be that silencing Gαi1 in this group of neurons resulted in compensatory expression of other Gα subunits. In a recent report, silencing of specific Gαβγ subunits was performed in HeLa cells (Krummins and Gilman 2006). It was found that when some of the Gαi1 subunits were silenced, modest increases in expression of Gαi2 and Gαi3 occurred. Thus it may be that an increased expression of either of these subunits or a separate one was sufficient to overcome the loss of Gαi1 and allow coupling of NOP receptors and N-type Ca2+ channels. If this is the case, then it would be expected that this compensatory effect be observed in all neurons transfected with Gαi1 siRNA. An explanation for this may be that although SG neurons are sympathetic, those that innervate sweat glands are cholinergic (Habecker and Landis 1994) while those that innervate the heart and lungs are adrenergic (Fuller et al. 2004; Pardini et al. 1990) and signaling of NOP receptors may differ. In the present study, however, we did not attempt to delineate the origin (i.e., retrograde label) of the SG neurons. Interestingly, the report by Krummins and Gilman (2006) showed that silencing each of the Gαi1-3 subunits alone or in combination did not affect the α2-adrenergic-mediated adenylyl cyclase inhibition or forskolin-stimulated cAMP synthesis. On the other hand, knockdown of Gαi1 alone did significantly decrease the prostaglandin E1 (PGE1) receptor-mediated stimulated cAMP synthesis, suggesting that in HeLa cells signaling specificity occurs with PGE1 receptors and not with α-AR. A limitation of our experimental approach is that there is presently no available method to measure protein expression levels in single isolated neurons.
In conclusion, this is the first study to demonstrate coupling specificity of NOP receptors with N-type Ca2+ channels in SG neurons by successfully employing siRNA to knock-down specific PTX-sensitive Gαi/o subunits. Selective silencing showed that the Noc-activated NOP receptors couple to Ca2+ channels via Gαi1 subunits and not through Gαi3 or Gαo proteins. Gαi2 protein subunits do not appear to be expressed in the soma of acutely dissociated SG neurons. In addition, silencing of any PTX-sensitive Gα subunit did not result in loss of coupling between α2-AR and Ca2+ channels. Thus specificity of Gα subunit coupling between NOP receptors and Ca2+ channels neurons seems to be less promiscuous when compared with α2-AR in SG neurons.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-074311 to V. Ruiz-Velasco.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Arias et al. 2006.Arias A, Feinn R, Kranzler HR. Association of an Asn40Asp (A118G) polymorphism in the mu-opioid receptor gene with substance dependence: a meta analysis. Drug Alcohol Depend 83: 262–268, 2006. [DOI] [PubMed] [Google Scholar]
- Beedle et al. 2004.Beedle AM, McRory JE, Poirot O, Doering CJ, Altier C, Barrere C, Hamid J, Nargeot J, Bourinet E, Zamponi GW. Agonist-independent modulation of N-type calcium channels by ORL1 receptors. Nat Neurosci 7: 118–125, 2004. [DOI] [PubMed] [Google Scholar]
- Bertin et al. 1994.Bertin B, Freissmuth M, Jockers R, Strosberg AD, Marullo S. Cellular signaling by an agonist-activated receptor/Gs alpha fusion protein. Proc Natl Acad Sci USA 91: 8827–8831, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield et al. 1994.Caulfield MP, Jones S, Vallis Y, Buckley NJ, Kim GD, Milligan G, Brown DA. Muscarinic M-current inhibition via G alpha q/11 and alpha-adrenoceptor inhibition of Ca2+ current via G alphao in rat sympathetic neurons. J Physiol 477: 415–422, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen and Lambert 2000.Chen H, Lambert NA. Endogenous regulators of G protein signaling proteins regulate presynaptic inhibition at rat hippocampal synapses. Proc Natl Acad Sci USA 9: 12810–12815, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor and Christie 1999.Connor M, Christie MD. Opioid receptor signalling mechanisms. Clin Exp Pharmacol Physiol 26: 493–499, 1999. [DOI] [PubMed] [Google Scholar]
- Davidson and Harel et al. 2004.Davidson TJ, Harel S, Arboleda VA, Prunnell GF, Shelanski ML, Greene LA, Troy CM. Highly efficient small interfering RNA delivery to primary mammalian neurons induces microRNA-like effects before mRNA degradation. J Neurosci 24: 10040–10046, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas et al. 1998.Delmas P, Abogadie FC, Dayrell M, Haley JE, Milligan G, Caulfield MP, Brown DA, Buckley NJ. G-proteins and G-protein subunits mediating cholinergic inhibition of N-type calcium currents in sympathetic neurons. Eur J Neurosci 10: 1654–1666, 1998. [DOI] [PubMed] [Google Scholar]
- Delmas et al. 1999.Delmas P, Abogadie FC, Milligan G, Buckley NJ, Brown DA. βγ dimers derived from Go and Gi proteins contribute different components of adrenergic inhibition of Ca2+ channels in rat sympathetic neurons. J Physiol 518: 23–36, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller et al. 2004.Fuller BC, Sumner AD, Kutzler MA, Ruiz-Velasco V. A novel approach employing ultrasound guidance for percutaneous cardiac muscle injection to retrograde label rat stellate ganglion neurons. Neurosci Lett 363: 252–256, 2004. [DOI] [PubMed] [Google Scholar]
- Gollasch et al. 1993.Gollasch M, Kleuss C, Hescheler J, Wittig B, Schultz G. Gi2 and protein kinase C are required for thyrotropin-releasing hormone-induced stimulation of voltage-dependent Ca2+ channels in rat pituitary GH3 cells. Proc Natl Acad Sci USA 90: 6265–6269, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habecker and Landis 1994.Habecker BA, Landis SC. Noradrenergic regulation of cholinergic differentiation. Science 264: 1602–1604, 1994. [DOI] [PubMed] [Google Scholar]
- Hepler and Gilman 1992.Hepler JR, Gilman AG. G proteins. Trends Biochem Sci 17: 383–387, 1992. [DOI] [PubMed] [Google Scholar]
- Hescheler et al. 1987.Hescheler J, Rosenthal W, Trautwein W, Schultz G. The GTP-binding protein, Go, regulates neuronal calcium channels. Nature 325: 445–447, 1987. [DOI] [PubMed] [Google Scholar]
- Ikeda 1991.Ikeda SR Double-pulse calcium channel current facilitation in adult rat sympathetic neurons. J Physiol 439: 181–214, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong and Ikeda 2000.Jeong SW, Ikeda SR. Effect of G protein heterotrimer composition on coupling of neurotransmitter receptors to N-type Ca(2+) channel modulation in sympathetic neurons. Proc Natl Acad Sci USA 97: 907–912, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermeier et al. 2003.Kammermeier PJ, Davis MI, Ikeda SR. Specificity of metabotropic glutamate receptor 2 coupling to G proteins. Mol Pharmacol 63: 183–191, 2003. [DOI] [PubMed] [Google Scholar]
- Kapusta 2000.Kapusta DR Neurohumoral effects of orphanin FQ/nociceptin: relevance to cardiovascular and renal function. Peptides 21: 1081–1099, 2000. [DOI] [PubMed] [Google Scholar]
- Kleuss et al. 1991.Kleuss C, Hescheler J, Ewel C, Rosenthal W, Schultz G, Wittig B. Assignment of G-protein subtypes to specific receptors inducing inhibition of calcium currents. Nature 353: 43–48, 1991. [DOI] [PubMed] [Google Scholar]
- Kleuss et al. 1992.Kleuss C, Scherübl H, Hescheler J, Schultz G, Wittig B. Different beta-subunits determine G-protein interaction with transmembrane receptors. Nature 358: 424–426, 1992. [DOI] [PubMed] [Google Scholar]
- Krummins and Gilman 2006.Krummins AM, Gilman AG. Targeted knockdown of G protein subunits selectively prevents receptor-mediated modulation of effectors and reveals complex changes in non-targeted signaling proteins. J Biol Chem 281: 10250–10262, 2006. [DOI] [PubMed] [Google Scholar]
- Kukwa et al. 1998.Kukwa W, Macioch T, Szulczyk PJ. Stellate neurons innervating the rat heart express N, L and P/Q calcium channels. J Auton Ner Sys 74: 143–151, 1998. [DOI] [PubMed] [Google Scholar]
- Larsson et al. 2000.Larsson KP, Olsen UB, Hansen AJ. Nociceptin is a potent inhibitor of N-type Ca(2+) channels in rat sympathetic ganglion neurons. Neurosci Lett 296: 121–124, 2000. [DOI] [PubMed] [Google Scholar]
- Milhavet et al. 2003.Milhavet O, Gary DS, Mattson MP. RNA interference in biology and medicine. Pharmacol Rev 55: 629–648, 2003. [DOI] [PubMed] [Google Scholar]
- Mogil and Pasternak 2001.Mogil JS, Pasternak GW. The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol Rev 53: 381–415, 2001. [PubMed] [Google Scholar]
- Moon et al. 2001.Moon HE, Bahia DS, Cavalli A, Hoffmann M, Milligan. Control of the efficiency of agonist-induced information transfer and stability of the ternary complex containing the delta opioid receptor and the alpha subunit of G(i1) by mutation of a receptor/G protein contact interface. Neuropharmacology 41: 321–330, 2001. [DOI] [PubMed] [Google Scholar]
- New and Wong 2002.New DC, Wong YH. The ORL1 receptor: molecular pharmacology and signalling mechanisms. Neurosignals 11: 197–212, 2002. [DOI] [PubMed] [Google Scholar]
- Pardini et al. 1990.Pardini BJ, Lund DD, Schmid PG. Innervation patterns of the middle cervical-stellate ganglion complex in the rat. Neurosci Lett 117: 300–306, 1990. [DOI] [PubMed] [Google Scholar]
- Reynolds et al. 2004.Reynolds A, Leake D, Boese Q, Scaringe S, Marshall WS, Khvorova A. Rational siRNA design for RNA interference. Nat Biotechnol 22: 326–330, 2004. [DOI] [PubMed] [Google Scholar]
- Ruiz-Velasco and Ikeda 2000.Ruiz-Velasco V, Ikeda SR. Multiple G-protein betagamma combinations produce voltage-dependent inhibition of N-type calcium channels in rat superior cervical ganglion neurons. J Neurosci 20: 2183–2191, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Velasco et al. 2005.Ruiz-Velasco V, Puhl HL, Fuller BC, Sumner AD. Modulation of Ca2+ channels by opioid receptor-like 1 receptors natively expressed in rat stellate ganglion neurons innervating cardiac muscle. J Pharmacol Exp Ther 314: 987–994, 2005. [DOI] [PubMed] [Google Scholar]
- Seifert et al. 1999.Seifert R, Wenzel-Seifert K, Kobilka BK. GPCR-Galpha fusion proteins: molecular analysis of receptor-G-protein coupling. Trends Pharmacol Sci 20: 383–389, 1999. [DOI] [PubMed] [Google Scholar]
- Straiker et al. 2002.Straiker AJ, Borden CR, Sullivan JM. G-protein alpha subunit isoforms couple differentially to receptors that mediate presynaptic inhibition at rat hippocampal synapses. J Neurosci 22: 2460–2468, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian and Kammermeier 2006.Tian L, Kammermeier PJ. G protein coupling profile of mGluR6 and expression of G alpha proteins in retinal ON bipolar cells. Vis Neurosci 23: 909–916, 2006. [DOI] [PubMed] [Google Scholar]
- Vaughan et al. 2001.Vaughan CW, Connor M, Jennings EA, Marinelli S, Allen RG, Christie MJ. Actions of nociceptin/orphanin FQ and other prepronociceptin products on rat rostral ventromedial medulla neurons in vitro. J Physiol 534: 849–859, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise and Milligan 1997.Wise A, Watson-Koken MA, Rees S, Lee M, Milligan G. Interactions of the alpha2A-adrenoceptor with multiple Gi-family G-proteins: studies with pertussis toxin-resistant G-protein mutants. Biochem J 321: 721–728, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung and Wong 1999.Yung LY, Joshi SA, Chan RY, Chan JS, Pei G, Wong YH. GalphaL1 (Galpha14) couples the opioid receptor-like1 receptor to stimulation of phospholipase C. J Pharmacol Exp Ther 288: 232–238, 1999. [PubMed] [Google Scholar]