Abstract
Behavior is controlled by both external instructions and internal motives, but the actions demanded by each may be different. A common consequence of such a conflict is a delay in decision making and subsequent motor responses. It is unknown, however, what neural mechanisms underlie motivational conflict and associated response delay. To answer this question, we recorded single-neuron activity in the superior colliculus (SC) as macaque monkeys performed a visually guided, asymmetrically rewarded saccade task. A peripheral spot of light at one of two opposing positions was illuminated to indicate a saccade target. In a given block of trials, one position was associated with a big reward and the other with a small reward. The big-reward position was alternated across blocks. Behavioral analyses revealed that small-reward trials created a conflict between the instructed saccade to one position and the internally motivated, yet invalid saccade to the opposite position. We found that movement neurons in the SC temporally exhibited bursting activity after the appearance of the small-reward target opposite their movement field. This transient activity predicted the amount of response delay for upcoming saccades. Our data suggest that motivational conflict activates movement neurons in both colliculi, thereby delaying saccade initiation through intercollicular inhibitory interactions.
INTRODUCTION
Conflict is a never-ending issue for both humans and animals. It often arises from a competition between social rules and individual internal motives, and often hinders decision making. How the conflict affects behavior and how it is resolved are of fundamental interest and importance in neuroscience. It is still unclear what neural mechanisms are responsible for the conflict resolution and the associated response delay.
Conflict research traditionally uses tasks characterized by interference between an invalid automatic response that is triggered by irrelevant stimulus information and a valid adaptive response that is determined by relevant stimulus information (Eriksen and Eriksen 1974; Hallett 1978; Isoda and Hikosaka 2007; Nakamura et al. 2005; Simon 1969; Stroop 1935). These studies have consistently shown that responses are particularly slow and error prone under conditions with interference compared with those without interference. It is suggested that delayed responses due to stimulus interference result from competition between concurrently processed, but mutually incompatible, motor programs (De Jong et al. 1994; DeSoto et al. 2001; Gratton et al. 1988; Ullsperger and von Cramon 2001). We shall refer to this type of conflict as sensory conflict.
However, behavior is not guided by external instructions alone. It can be controlled by both external instructions and internal motives (Mogenson et al. 1980). In real life, the actions demanded by each may be different, which we shall refer to as motivational conflict. A typical situation is that humans or animals are forced to choose a response option with small gains as opposed to another option that would lead to larger gains (Bowman et al. 1996; Minamimoto et al. 2005; Tremblay and Schultz 2000; Watanabe et al. 2003a). Although it is also known that motivational conflict produces response delay, the underlying neural mechanisms are not known.
To investigate how motivational conflict delays upcoming motor responses, we have devised a visually guided saccade task with unequal reward outcomes for macaque monkeys (“the visually guided biased saccade task”; Fig. 1A). In this task, the saccade target is randomly illuminated at one of two opposing positions, one position associated with a larger reward, the other with a smaller reward. The position-reward mapping remains constant during a given block of trials so that the animal knows the current association between target position and reward, but is frequently reversed across blocks (Fig. 1B). The animal has to make a correct response even if a small reward is expected, thereby creating a motivational conflict in small-reward trials. Using this model, we have recorded from movement-related neurons in the superior colliculus (SC). The SC is a paired structure in the midbrain and has a critical function in the control of saccades (Sparks and Hartwich-Young 1989; Wurtz and Albano 1980). Being a nodal point in ocular motor circuitry with convergent inputs from various cortical and subcortical structures, the SC appears to be an ideal place for studying the impact of motivational conflict on ocular motor behavior and underlying neural activity.
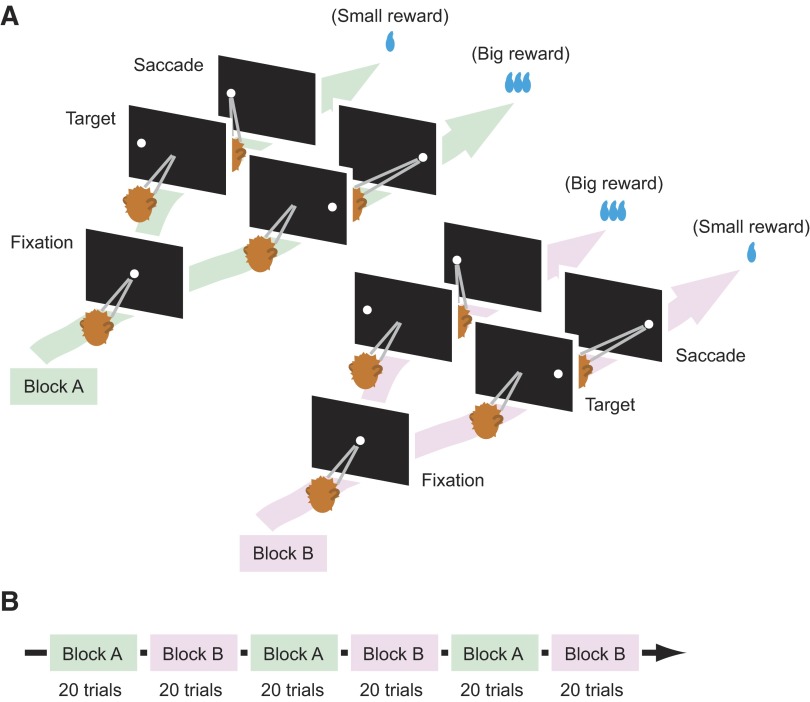
FIG. 1.
Visually guided biased saccade task. A: temporal sequences of events. The monkeys first fixated at the central spot of light (“Fixation”). After 1 s the fixation point went off and, simultaneously, the saccade target came on in the periphery (“Target”). The monkeys then made a saccade to the visible target (“Response”). The target was selected pseudorandomly from 2 possible locations, either in the response field of the neuron under study or in the diametrically opposite position. Throughout a block of 20 trials, the saccade to one position was associated with a large reward and the saccade to the other position was associated with a small reward or no reward. B: the 2 kinds of blocks with different position-reward contingencies were alternated while recording from individual neurons.
METHODS
Two rhesus monkeys (Macaca mulatta), T and S, were used as subjects. All animal care and experimental procedures were approved by the National Eye Institute Animal Care and Use Committee and complied with the Public Health Service Policy on the humane care and use of laboratory animals.
Surgical procedures
The monkey was anesthetized with intramuscular injections of ketamine HCl (10 mg/kg), diazepam (1 mg/kg), and glycopyrrolate (0.01 mg/kg), and then maintained at a general anesthetized state with isoflurane. After the skull was exposed, acrylic screws were installed to fasten the dental acrylic head implant to the skull. A plastic head holder and recording chambers were placed stereotaxically and secured with dental acrylic. Eye coils were implanted subconjunctivally into both eyes using the methods described previously (Judge et al. 1980). Craniotomy was performed after the monkey had been well trained for behavioral tasks described in the following text. Antibiotics and analgesics were administered after surgery.
Behavioral procedures
During experimental sessions, the monkey was placed in a sound-attenuated room and seated in a primate chair with its head immobilized. Behavioral tasks were under control of a QNX-based real-time experimentation data acquisition system [REX; Laboratory of Sensorimotor Research, National Eye Institute, National Institutes of Health (LSR/NEI/National Institutes of Health), Bethesda, MD]. Visual stimuli were rear-projected by an active matrix liquid crystal display projector (PJ550; ViewSonic, Walnut, CA) onto a frontoparallel screen 33 cm from the monkey's eyes. Water reward was delivered on a monkey's correct response through a spigot under the control of a solenoid valve, which was placed outside the sound-attenuated room.
The monkey was trained to perform the following two tasks using operant conditioning with positive reinforcement: the memory-guided saccade task (Hikosaka and Wurtz 1983) and the visually guided biased saccade task (Lauwereyns et al. 2002). In the memory-guided saccade task, a peripheral cue came on for 100 ms after the monkey had fixated on a central fixation point (FP) for 800 ms. The monkey was required to remember the cued location while maintaining central fixation for another 800–1,200 ms. The fixation point then disappeared and the monkey had to make a saccade to the cued location. This task was used to map the response field of a neuron under study.
In the visually guided biased saccade task (Fig. 1), after the monkey had fixated on the FP for 1 s, a peripheral target came on randomly either in the neuron's response field (50%) or in the diametrically opposite position (50%). Simultaneously, the FP went off. The monkey was then required to make a saccade to the visible target within 700 ms. In a block of 20 trials, the correct saccade to one direction was followed by a larger amount of reward (0.3 ml for monkey T and 0.4 ml for monkey S) and the correct saccade to the other direction was followed by a smaller amount of reward (0.075 ml for monkey T) or no reward (for monkey S). Even for the small or no reward direction, the monkey had to saccade correctly because otherwise the same trial was repeated. The position-reward contingency was reversed in the next block without any indication to the monkey. Note that the position-reward contingency was fixed in a given block of trials but the position of the target in each trial was unpredictable.
Recording procedures
Eye movements were recorded with the use of the magnetic search-coil technique with temporal resolution of 1 ms (Robinson 1963). Single-unit recordings were carried out using tungsten electrodes with impedances of 1.0–2.5 MΩ (FHC, Bowdoinham, ME). The electrode was driven by a hydraulic micromanipulator (MO-97A; Narishige, Tokyo, Japan) through a stainless steel guide tube, which was used to penetrate the dura and was held in place by a grid that was fixed inside the recording chamber (Crist et al. 1988). This grid system allowed the positioning of the electrodes at every 1 mm between penetrations. Neuronal activity was amplified, band-pass filtered (600 Hz to 8 kHz), and single units were isolated using custom voltage-time window discrimination software (MEX; LSR/NEI/National Institutes of Health, Bethesda, MD). The SC was identified, with the aid of magnetic resonance images, by its characteristic neuronal discharges in relation to visual stimuli or saccades made into a particular direction and amplitude.
Data analysis
We defined a neuron as movement related if the neuron showed a statistically reliable increase in the discharge rate 40 to 0 ms before a saccade made into the neuron's response field as compared with the discharge rate 100 to 0 ms before cue onset in the memory-guided saccade task (Wilcoxon signed-rank test, P < 0.05). We calculated a buildup index for individual movement-related neurons by averaging over time the values of the spike density function (see following text) 110 to 100 ms before the onset of saccades made into the response field in the memory-guided saccade task. Based on the buildup index, all of the movement-related neurons were classified into either burst neurons (buildup index ≤30 spikes/s) or buildup neurons (buildup index >30 spikes/s) (Munoz and Wurtz 1995).
We excluded misdirected saccades in the visually guided biased saccade task from the quantitative analyses subsequently described. Misdirected saccades were defined as those that were directed in error away from the saccade target. In addition, we treated the first trial in each block as a trial belonging to the previous block because the monkey was unable to recognize block changes until they had experienced at least one trial of reward outcomes in the new block.
As subsequently described, a subset of movement-related neurons exhibited a transient burst of activity when the animal had to make a saccade to the small-reward target that was opposite the response field. This transient activity is a focus of our study and is tentatively referred to as the “paradoxical posttarget activity” because these neurons became active, although the saccade target appeared outside their response field. To define the paradoxical posttarget activity, we compared neuronal activity 0 to 100 ms after target onset (“control period”) with activity 150 to 250 ms after target onset (“test period”) in small-reward trials in which the target was positioned away from the neuron's response field. A movement-related neuron that showed a statistically reliable increase in the discharge rate during the test period compared with the control period (Wilcoxon signed-rank test, P < 0.05) was defined as having the paradoxical posttarget activity. To illustrate the change of the paradoxical posttarget activity to a reversal of the position-reward contingency, we plotted the average firing frequency during the test period against the trial position within each block (Fig. 6).
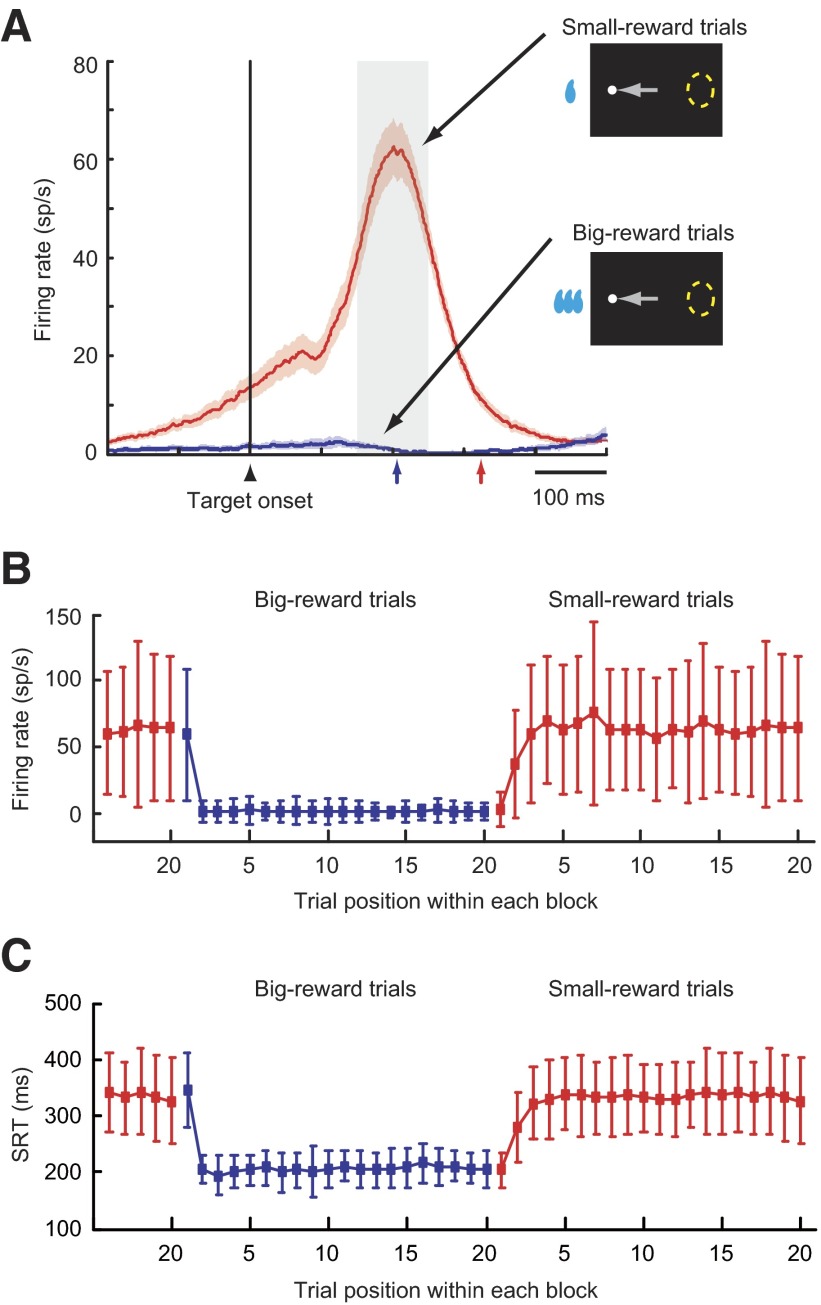
FIG. 6.
Selective occurrence of the paradoxical posttarget activity. A: the ensemble average activity for movement-related neurons having paradoxical posttarget activity (mean ± SE; n = 41). The activity is aligned on the onset of the target away from the response field and is shown separately for small-reward trials (red) and big-reward trials (blue). The median SRTs for small-reward trials (red arrow) and big-reward trials (blue arrow) are indicated on the horizontal axis. A gray rectangle denotes an epoch during which the neuronal firing rate was quantified in B. B: neuronal firing rate (mean ± 1SD) as a function of trial order from a reversal of the position-reward contingency. Shown are data from the same population of neurons as in A, in which the saccade was made away from the response field. Big-reward trials are indicated in blue and small-reward trials are in red. C: the SRT (mean ± 1SD) as a function of trial order from a reversal of the position-reward contingency. Shown are data from the same sessions as in B (n = 41). Both directions of saccades are combined. Big-reward trials are indicated in blue and small-reward trials in red.
To define the presence of anticipatory pretarget activity (Fig. 5), we calculated the mean firing rate in a 100-ms time window ending 50 ms after target onset (“peri-target period”). A movement-related neuron was judged to have the pretarget activity if activity in the peri-target period was significantly larger in blocks in which the contralateral target was associated with a big reward than in blocks in which the ipsilateral target was associated with a big reward (Mann–Whitney U test, P < 0.05).
FIG. 5.
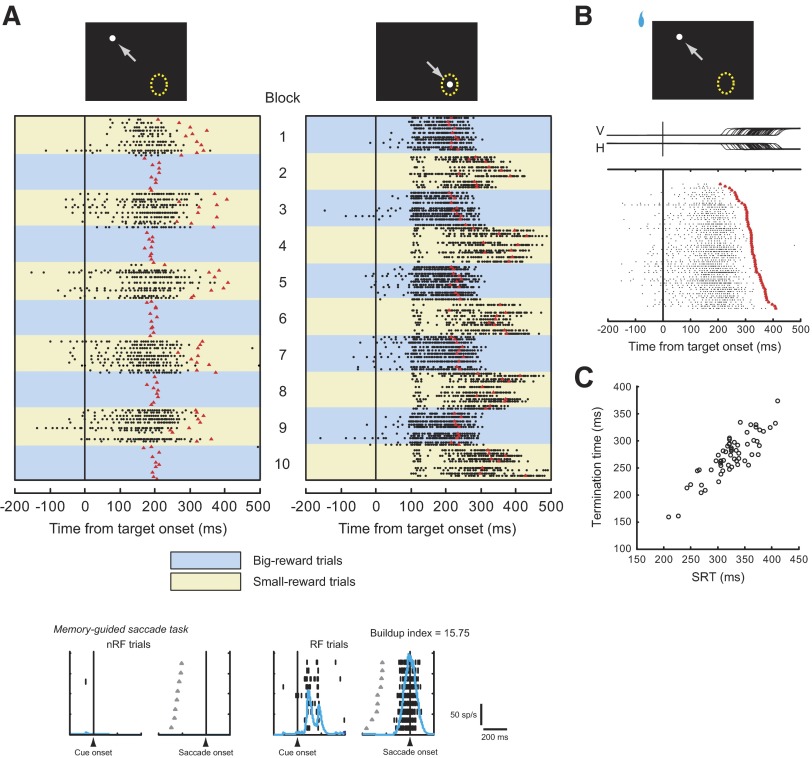
Another example of paradoxical posttarget activity for a movement-related neuron recorded in the left SC. A–C: same conventions as in Fig. 4. The buildup index was 15.75. Note that the paradoxical posttarget activity of this neuron was preceded by anticipatory pretarget activity.
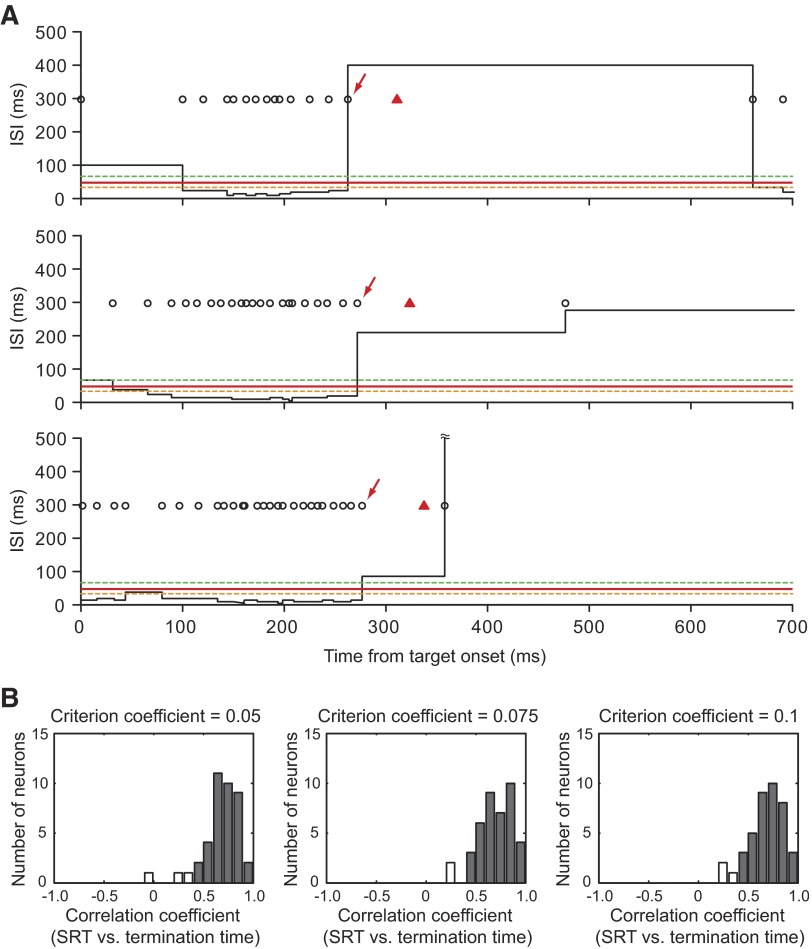
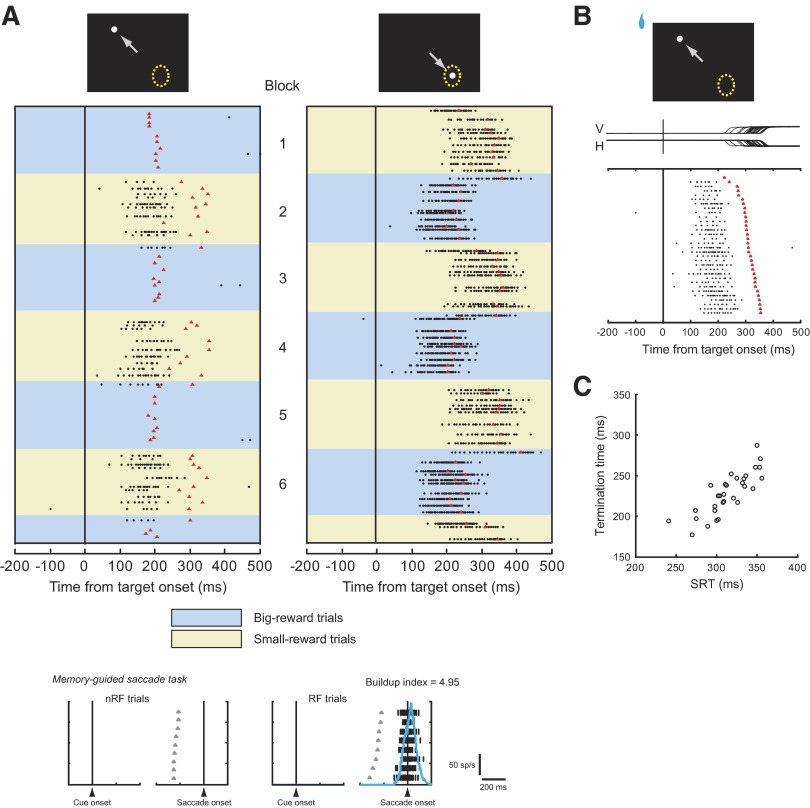
The termination time of the paradoxical posttarget activity for individual trials was defined as the time when the interspike interval (ISI) exceeded the criterion ISI for the first time after ISI reached the minimum value. To determine the criterion ISI for each neuron, we computed the average firing rate during a 40-ms presaccadic period when the big-reward target was presented within the response field of each neuron. We then multiplied the presaccadic firing rate by 0.075 (“criterion coefficient”) and took its reciprocal to yield the criterion ISI (Fig. 2). Qualitatively similar results were obtained by using the criterion coefficient = 0.10 or 0.05 (Fig. 2). If no action potential was observed between target onset and saccade onset on a particular trial, then we assigned 0 to the termination time for that trial. Correlations between the SRT and the termination time were assessed by the nonparametric Spearman rank correlation.
FIG. 2.
Determination of termination time. A: interspike interval (ISI) histograms for 3 examples of trials from the neuron shown in Fig. 4B. Black circles indicate neuronal spikes; red triangles denote the time of saccade initiation. The termination time (indicated by red arrows) was defined as the time when the ISI exceeded the criterion ISI for the first time after ISI reached the minimum value. The average firing rate during a 40-ms presaccadic period for this neuron was 311 spikes/s when the big-reward target was presented in the response field. Thus the criterion ISI (red solid line) was 42.9 ms with a criterion coefficient of 0.075. For reference, the criterion ISIs with the criterion coefficients of 0.05 and 0.1 are shown by green and orange broken lines, respectively. B: distribution of correlation coefficients between saccadic reaction time (SRT) and termination time. Filled bars indicate neurons with significant positive correlations between SRT and termination time. The means of correlation coefficients with 3 different criterion coefficients were not significantly different from one another (P = 0.79, one-way ANOVA after Fisher's z-transform).
Continuous neuronal activation functions (spike density functions [SDFs]) were constructed by convolving each action potential with an asymmetrical kernel resembling a postsynaptic potential (Thompson et al. 1996). In this kernel, the time constant for the growth phase was set to 1 ms and that for the decay phase was set to 20 ms. We used this kernel to avoid the influence of spikes backward in time.
RESULTS
Animal behavior
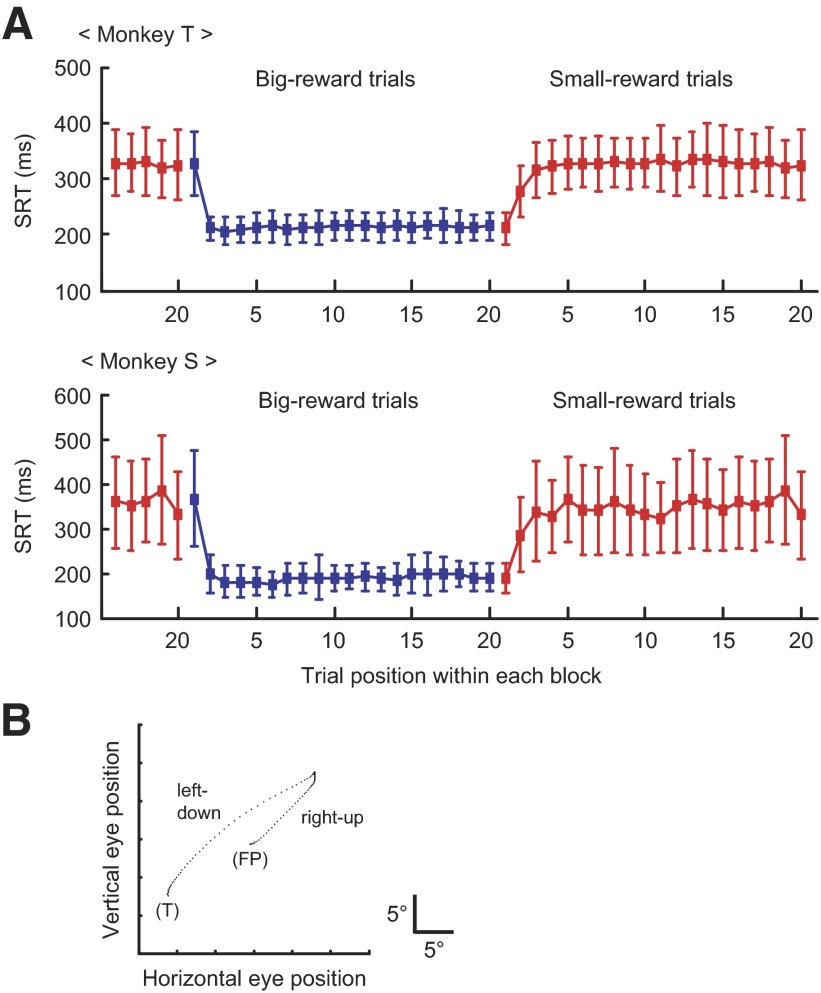
Consistent with previous reports (Lauwereyns et al. 2002; Watanabe et al. 2003a), saccadic reaction time (SRT) consistently changed depending on the expected outcome of reward: the SRT was reliably shorter when the saccade was followed by a big reward than when it was followed by a small reward (Fig. 3A). For monkey T, the difference in the average SRT between the two reward conditions was 118.0 ms and 103.6 ms for the leftward and rightward saccades, respectively (P < 0.001, t-test). For monkey S, the difference was 134.1 and 175.5 ms for the leftward and rightward saccades, respectively (P < 0.001, t-test). These behavioral data suggest that the animal was motivated to make a saccade to the big-reward position.
FIG. 3.
Animal performance. A: changes in SRT with changes in the position-reward mapping. The SRT (mean ± 1SD) is plotted as a function of the numerical position of trials in each block for monkey T (top) and monkey S (bottom). Big-reward trials are indicated in blue and small-reward trials in red. Both directions of saccades are combined. B: an example of occasional misdirected saccades observed in small-reward trials (x–y plot of eye position). The monkey first made a saccade in error to the position associated with a big reward (“right-up”) and then changed the saccade direction toward the correct visible target associated with a small reward (“left-down”). FP, fixation point; T, saccade target.
In addition to the difference in the average SRT, three behavioral findings are noteworthy. First, the variability in the SRT was substantially larger when a small reward was expected after the correct saccade than when a big reward was expected (Fig. 3A). Second, the SRT decreased quickly for a small-to-big reward transition but increased more slowly for a big-to-small reward transition (Fig. 3A). Third, the monkeys occasionally made misdirected saccades (Fig. 3B) almost exclusively on small-reward trials (125/2,313 for small-reward trials and 1/2,243 for big-reward trials in monkey T; 58/1,675 for small-reward trials and 1/1,570 for big-reward trials in monkey S; P < 0.001 for both monkeys, chi-square test). In those misdirected saccades, the saccade was directed in error to the position associated with a big reward (note that the target was not physically present at that location), which was usually followed by a second saccade toward the correct visible target (Fig. 3B). The occurrence of these misdirected saccades, along with longer SRT, strongly suggests that a motivational conflict was present in small-reward trials. More specifically, small-reward trials created a conflict between a saccade to the externally instructed position and a saccade to the internally desired position.
Activity of SC movement-related neurons
We found that about two thirds of movement-related neurons showed a transient burst of activity after target onset in small-reward trials in which the big-reward position was in their response field but the actual target was in the opposite position. Figure 4 illustrates an example of the responses of a single movement-related neuron recorded in the left SC. In Fig. 4A, each raster represents one trial and dots represent individual action potentials. The trials are displayed in actual temporal order beginning at the top, separately for contraversive saccades (the target was inside the neuron's response field; right column) and ipsiversive saccades (the target was opposite the neuron's response field; left column). In the first block, correct contraversive and ipsiversive saccades were, respectively, followed by a small reward (yellow background) and a big reward (blue background). In the next block, the position-reward contingency was reversed. In addition to a presaccadic burst of activity before contraversive saccades made into the response field, this neuron was also activated when the small-reward saccade had to be made away from the response field (Fig. 4A, left column with yellow background). This activation was surprising because the position of the target was opposite the neuron's response field, as shown in the neuron's activity in memory-guided saccades (Fig. 4A, bottom). We tentatively refer to this activity as the paradoxical posttarget activity, because the neuron responded despite the appearance of the target outside the response field. The paradoxical posttarget activity was transient: this neuron ceased firing before saccade initiation (Fig. 4, A and B).
FIG. 4.
An example of paradoxical posttarget activity. A: firing properties of a movement-related neuron recorded in the left superior colliculus (SC). The rastergram is aligned on the onset of the target (time = 0) and shown separately for the target within the response field (right) and away from it (left). Blue backgrounds depict big-reward trials and yellow backgrounds depict small-reward trials. In raster displays, black dots represent individual action potentials and red triangles denote the time of saccade onset. Misdirected saccades are not shown. Top: white dots represent the saccade target; yellow dotted circles represent the response field of this neuron; and gray arrows indicate the animal's saccade. Bottom: the activity during the memory-guided saccade task is shown separately for the saccade made into the response field (right, “RF trials”) and the saccade away from it (left, “nRF trials”); gray triangles denote the time of a GO signal for a saccade (offset of the fixation point); blue continuous lines represent the spike density function. The buildup index was 4.95 for this neuron. B: the raster display for trials in which the small-reward target was presented away from the response field. The trials are sorted according to SRT. The rastergram is aligned on the onset of the target. Red triangles indicate the time of saccade onset. V, vertical eye position; H, horizontal eye position. C: termination time (see methods) is plotted against SRT. One additional trial not shown has the termination time at 0 due to the lack of spikes; see the top trial in B.
Figure 5 illustrates the responses of another movement-related neuron recorded in the left SC. This neuron showed an anticipatory increase in activity, albeit weakly, before target onset in blocks where the contralateral target was associated with a big reward (Blocks 1, 3,…). This pretarget activity may reflect the animal's desire for the big-reward target to appear in the neuron's response field and an associated shift of attention to the big-reward target position (Hikosaka et al. 2006) because the modulation of activity started before the animal knew the amount of reward that would be given after the correct saccade. In addition to the pretarget activity, however, this neuron fired still further after target onset in small-reward trials, although the target was specified in the position away from the response field (Fig. 5A, left column with yellow background; see also Fig. 5B). Again, the saccade was initiated after the paradoxical posttarget activity terminated (Fig. 5, A and B).
Among 62 movement-related neurons studied, 41 neurons (66.1%) exhibited the paradoxical posttarget activity. A subset of these neurons (17/41, 42%) had both paradoxical posttarget activity and anticipatory pretarget activity (as the neuron in Fig. 5), whereas the remaining neurons had the paradoxical posttarget activity only (as the neuron in Fig. 4) (Table 1). The ensemble average activity for the 41 neurons showed a steep rise in activity starting about 100 ms after the onset of the small-reward target opposite the response field (Fig. 6A, red line). By contrast, the same population of neurons remained inactive when the big-reward target appeared at the same location (Fig. 6A, blue line). The change in the paradoxical posttarget activity on the reversal of the position-reward contingency was quicker for a small-to-big reward transition than for a big-to-small transition (Fig. 6B). This resembled the change in the SRT for the sessions in which movement-related neurons with the paradoxical posttarget activity were recorded (n = 41; Fig. 6C). These data raise the possibility that the paradoxical posttarget activity of SC neurons may cause long SRTs on small-reward trials.
TABLE 1.
Classification of saccade-related SC neurons
| Paradoxical Post-Target Activity | Total | ||
|---|---|---|---|
| (+) | (−) | ||
| Anticipatory pre-target activity | |||
| (+) | 17 | 11 | 28 |
| (−) | 24 | 10 | 34 |
| Total | 41 | 21 | 62 |
Values denote the number of neurons.
Paradoxical posttarget activity predicts SRT in small-reward trials
The above-described hypothesis predicts that the SRT is longer if the paradoxical posttarget activity lasts longer. This prediction appears to be supported by the paradoxical posttarget activity rank-ordered by SRTs (Figs. 4B and 5B). To examine this impression statistically, we computed the correlation between the SRT and the time at which paradoxical posttarget activity terminated (“termination time”; see methods). There was a strong positive correlation between the SRT and the termination time for the neuron shown in Fig. 4 (Spearman's r = 0.86, P < 0.001; Fig. 4C) and for the neuron shown in Fig. 5 (Spearman's r = 0.79, P < 0.001; Fig. 5C).
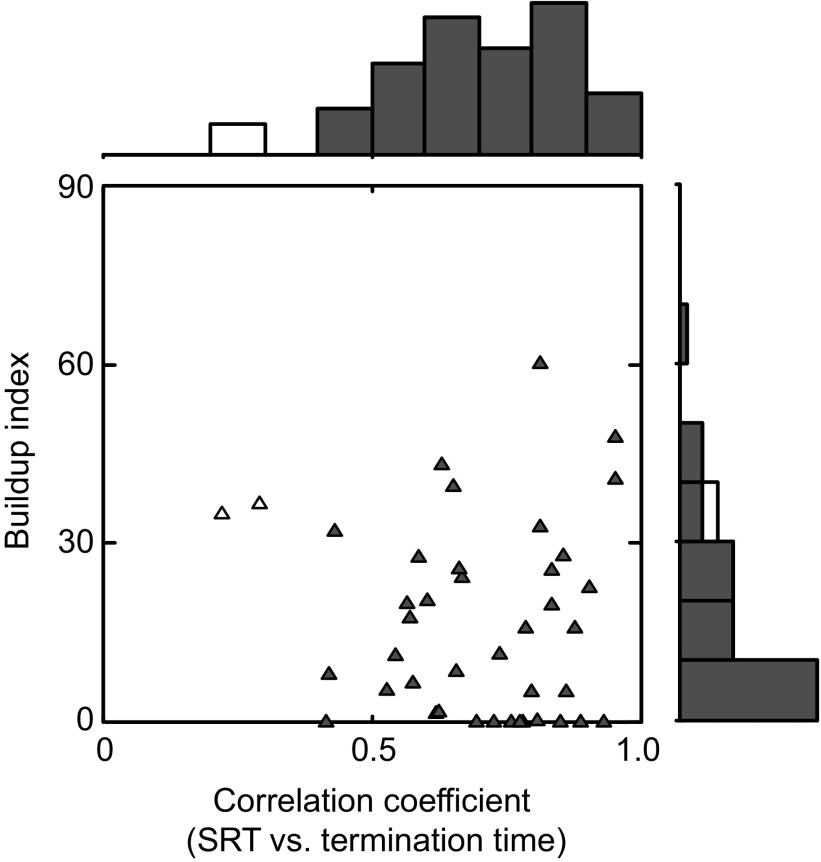
Out of 41 neurons that had paradoxical posttarget activity, 39 neurons showed a significant positive correlation between the SRT and the termination time (Fig. 7, abscissa). The average correlation coefficient for the population of neurons was significantly different from 0 (P < 0.001, t-test after Fisher's z-transform). These data suggest that paradoxical posttarget activity can account not only for a categorical difference in the SRT between big- and small-reward trials but also for a trial-by-trial difference in the SRT among small-reward trials. In particular, shorter termination times were associated with shorter SRTs and longer termination times were associated with longer SRTs.
FIG. 7.
Quantitative analyses of the paradoxical posttarget activity. The buildup index is plotted against the correlation coefficient between SRT and termination time. Filled triangles represent neurons with significant positive correlations between SRT and termination time (n = 39). The marginal histograms show the distribution of the correlation coefficient (top) and the buildup index (right). Filled bars indicate neurons with significant positive correlations between SRT and termination time.
Burst and buildup neurons show paradoxical posttarget activity
It is postulated that the primate SC contains two types of movement-related neurons: one population exhibits only a presaccadic burst of activity (burst neurons) and the other population exhibits a buildup of activity (buildup neurons) that is usually followed by a presaccadic burst (Munoz and Wurtz 1995). It has been shown that SC neurons tend to show buildup or prelude activity if their visual responses are modulated by reward expectation (Ikeda and Hikosaka 2003) and buildup neurons tend to show an enhancement of presaccadic activity when a big reward is expected (Ikeda and Hikosaka 2007). We therefore asked whether there was any relation between the paradoxical posttarget activity and the buildup activity.
For this purpose we calculated a buildup index for each neuron (see methods) and plotted in Fig. 7 the buildup index against the correlation coefficient between the SRT and the termination time, a measure that characterized the paradoxical activity (as shown in Figs. 4C and 5C). There was no significant correlation between these parameters (Spearman's r = −0.05, P = 0.77).
We then classified movement-related neurons into burst neurons (buildup index ≤30 spikes/s) or buildup neurons (buildup index >30 spikes/s) according to Munoz and Wurtz (1995). According to this criterion, 45 neurons were classified as burst neurons (including those shown in Figs. 4 and 5) and 17 neurons were classified into buildup neurons. We found that both burst and buildup neurons showed the paradoxical posttarget activity (burst neurons, 32/45; buildup neurons, 9/17; P = 0.18 by chi-square test). In addition, the correlation coefficient between the SRT and termination time was not significantly different between burst and buildup neurons (P = 0.98, t-test after Fisher's z-transform).
The distribution of movement-related neurons having the paradoxical posttarget activity considerably overlapped in depth with those without the paradoxical posttarget activity: the average (±SD) depth of the location of neurons with and without paradoxical posttarget activity was 2.1 (±0.5) mm and 2.2 (±0.4) mm from the SC surface, respectively (P = 0.50, t-test). This finding is consistent with the lack of apparent differences in the paradoxical posttarget activity between burst and buildup neurons in that burst neurons tend to be located more dorsally than buildup neurons in the intermediate layers of the SC (Munoz and Wurtz 1995).
Concurrent activation of bilateral movement neurons
The occurrence of misdirected saccades suggests that the monkeys faced a motivational conflict after the onset of the small-reward target: that is, the conflict between a saccade to the internally desired target (the position associated with a big reward) and a saccade to the externally instructed target (the position associated with a small reward). In contrast, these saccade goals were consistent with each other on the appearance of the big-reward target. How, then, is the motivational conflict reflected in collicular neuronal activity? To answer this question, it would be helpful to examine the activity of movement-related neurons in both colliculi, even though a saccade to a given location is controlled, in principle, by the contralateral SC (Sparks 1986).
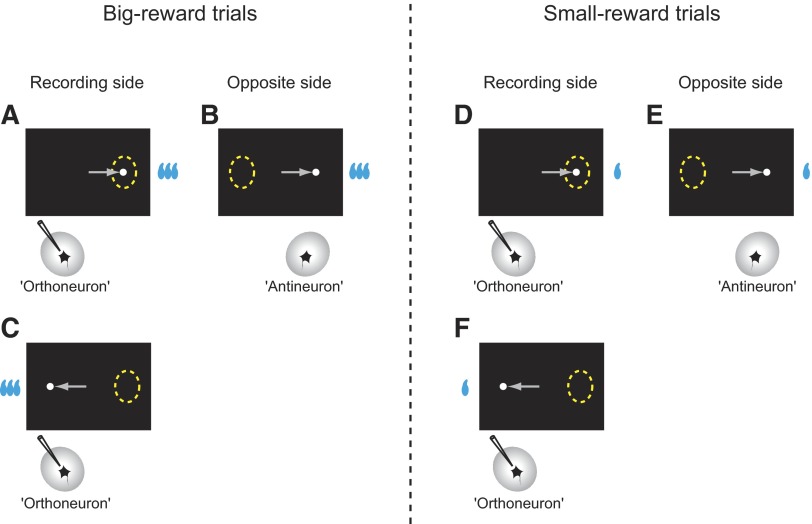
Although we recorded from only one neuron at a time, we could assume that for each neuron sampled (“orthoneuron”) its counterpart is present in the opposite SC with identical response properties to the orthoneuron but with the response field located in the opposite direction (“antineutron”) (Amador et al. 2004). Consider big-reward trials in which the saccade target appears in the response field of the orthoneuron (Fig. 8A) and assume that we have recorded its activity (e.g., Fig. 4A, right column, blue background). For the antineuron (Fig. 8B), the saccade target would appear opposite the response field. This is exactly the situation that occurs to the orthoneuron in the next block on big-reward trials (Fig. 8C) (e.g., Fig. 4A, left column, blue background). In other words, the antineuron, on big-reward trials, should behave identically as the orthoneuron behaves on big-reward trials in the next block. Following the same logic, when the saccade target appears in the response field of the orthoneuron on small-reward trials (Fig. 8D) (e.g., Fig. 4A, right column, yellow background), the antineuron (Fig. 8E) should behave identically as the orthoneuron behaves on small-reward trials in the next block (Fig. 8F) (e.g., Fig. 4A, left column, yellow background). We can now estimate the activity of movement-related neurons on the other (nonrecorded) side of the superior colliculus and compare it with the activity of movement-related neurons on the recorded side, as shown in the following text.
FIG. 8.
Comparison of neuronal activity between the sampled neuron (“orthoneuron”) and its theoretical counterpart (“antineutron”) for big-reward trials (A–C) and small-reward trials (D–F).
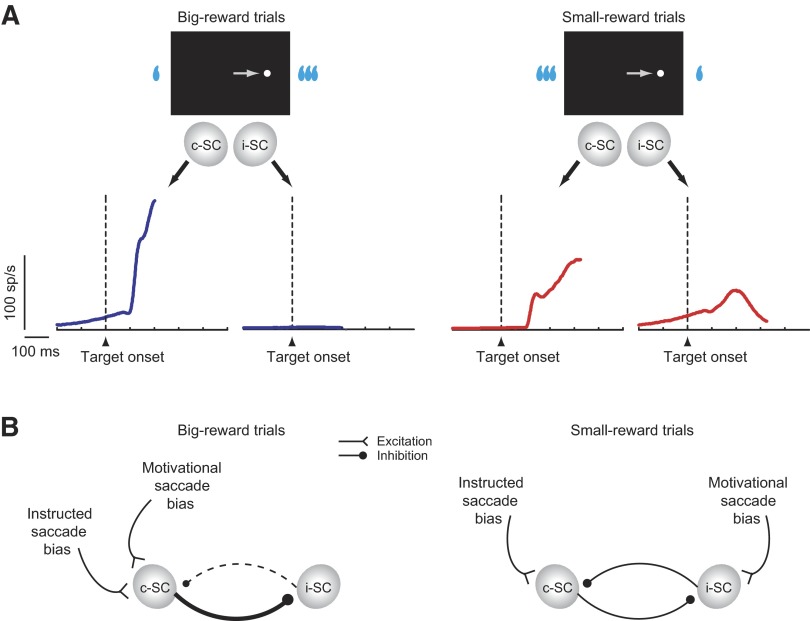
Figure 9A illustrates the ensemble average activity for all of the movement-related neurons sampled in this study (n = 62), separately for big-reward trials (left) and small-reward trials (right). On big-reward trials (Fig. 9A, left), movement-related neurons were active only in the SC contralateral to the target (c-SC), as exemplified in Figs. 4A and 5A, blue background. On small-reward trials (Fig. 9A, right), movement-related neurons were concurrently active on both sides approximately 100 ms after target onset, as exemplified in Figs. 4A and 5A, yellow background. Moreover, activity in c-SC was weaker and more protracted on small-reward trials (Fig. 9A, right) than that on big-reward trials (Fig. 9A, left), which may be discerned in Figs. 4A and 5A, right column. This protracted c-SC activity is likely to be a direct cause of the delayed saccades on small-reward trials because a saccade is thought to be controlled by the SC contralateral to the direction of the saccade (Sparks 1986). How then could the neurons in the ipsilateral SC (i-SC) determine the timing of the saccade initiation? One possibility, shown in Fig. 9B (right), is that the neurons in c-SC are inhibited by the neurons in i-SC through the mutual inhibitory mechanism between two sides of the SC (Munoz and Istvan 1998; Takahashi et al. 2005). Only after the inhibition caused by i-SC has been removed could the neurons in c-SC trigger a saccade, as demonstrated in Figs. 4B and 5B. The cause of the activation of neurons in i-SC (paradoxical posttarget activity) may be the motivational drive for a big reward, since the output of i-SC would cause a saccade to the position where a big reward should be available. By contrast, on big-reward trials (Fig. 9B, left) both the motivational drive and the task instruction would converge on the c-SC, which then inhibits i-SC completely and quickly causes a saccade to the target.
FIG. 9.
Effects of motivational conflict on neuronal activity. A: the ensemble average activity on big-reward trials (left) and small-reward trials (right) for all of the movement-related neurons studied (n = 62). The activity is aligned on the onset of the target and drawn until the median SRT. c-SC, SC contralateral to the target; i-SC, SC ipsilateral to the target. B: a schematic illustration of presumed behavioral processes influencing the intercollicular inhibitory interactions. The command for the saccade to the visual target is issued from c-SC. Presumed inhibitory interneurons are omitted.
DISCUSSION
In the present study, we investigated neural mechanisms of the conflict-induced response delay in the SC, a motor structure close to the oculomotor output. We observed that the appearance of the small-reward target in the visually guided biased saccade task consistently induced a substantial response delay. This response delay was likely to originate from motivational interference on task instructions, a discrepancy between what the animal wanted to do and what the animal had to do. Indeed, the animal occasionally made misdirected saccades to the position associated with a big reward, even though there was no visible target at that location; in fact, the saccade target had been illuminated away from it. Using this behavioral model of motivational conflict, we found that movement-related neurons in the SC exhibited a transient burst of activity after the onset of the small-reward target that was outside their response field (paradoxical posttarget activity). The time at which the paradoxical posttarget activity terminated was correlated with the time of saccade initiation on a trial-by-trial basis. Furthermore, the appearance of the small-reward target triggered simultaneous activation of movement-related neurons in the SC on both sides.
How might the paradoxical posttarget activity be related to cognitive or sensorimotor functions that have previously been characterized? First, the paradoxical posttarget activity might be related to a shift of attention. It is likely that the monkey paid attention to the position associated with a big reward when it was performing the visually guided biased saccade task. On the other hand, it is well known that, when a salient visual stimulus is presented abruptly, attention is quickly and involuntarily drawn to the position of the stimulus (Hikosaka et al. 1993; Remington et al. 1992). In our saccade task, attention should be drawn to the target immediately after its onset, irrespective of whether it is associated with a big reward or a small reward. According to the attention hypothesis, the paradoxical posttarget activity would then reflect a shift of attention to the ipsilateral, small-reward-associated target because the activity occurred just after its onset. However, this interpretation is inconceivable because there was no paradoxical posttarget activity when the same ipsilateral target appeared but associated with a big reward. Therefore the paradoxical posttarget activity does not seem to reflect a shift of attention.
Second, the paradoxical posttarget activity might be related to motor preparation. Saccade motor preparation appears to be represented in the SC by buildup neurons, rather than burst neurons (Munoz and Wurtz 1995). However, we did not find evidence that either buildup neurons or burst neurons preferentially exhibit the paradoxical posttarget activity. A problem in this analysis was that the buildup indices of SC movement-related neurons were distributed as a continuum and the classification into burst and buildup neurons is deemed arbitrary, as indicated previously (e.g., McPeek and Keller 2002b; Port and Wurtz 2003; Quaia et al. 1999). An alternative analysis we performed was to examine the correlation between the buildup index and a measure that characterized the paradoxical activity (Fig. 7) disregarding the cell classification. The result of this analysis showed no significant correlation and thus did not support the idea that the paradoxical posttarget activity is related to motor preparation.
After the preceding considerations, we feel that it is more fruitful to discuss the paradoxical posttarget activity as part of neural processes that are used to resolve motivational conflict. Our findings suggest that motivational conflict is represented in the SC, a structure close to the motor output, and therefore the correct motor response is inevitably delayed. This conclusion is in line with a presumed mechanism of response inhibition due to sensory conflict. Studies on human subjects, using lateralized readiness potentials in a stimulus–response incompatibility task or in versions of the Eriksen flanker task, have identified activation of the motor cortex that may correspond to the preparation of an invalid motor response before the execution of a correct motor response (De Jong et al. 1994; Gratton et al. 1998; Taylor et al. 2007; Ullsperger and von Cramon 2001). Furthermore, it has been shown that bilateral motor cortices are concurrently active under response conflict conditions such as a version of the Stroop task (DeSoto et al. 2001). These data, together with our findings, suggest that subthreshold activation of an incorrect response leads to a delay in the execution of a correct response through the reciprocal inhibitory mechanism between two sides of motor structures close to output, such as the primary motor cortex (Kujirai et al. 1993) and SC (Munoz and Istvan 1998; Takahashi et al. 2005). Yet, it is also possible that such reciprocal inhibition eventually contributes to the resolution of conflict once neural activity responsible for the correct response prevails over the other (Schlag et al. 1998).
It is generally thought, however, that response conflict is dealt with in the frontal cortical areas involved in higher-level planning. Specifically, the lateral prefrontal cortex (LPFC) and the medial frontal cortex including the anterior cingulate cortex (ACC), supplementary eye field (SEF), and presupplementary motor area (pre-SMA) are implicated in the monitoring or resolution of response conflict originating from stimulus interference (Isoda and Hikosaka 2007; MacDonald 3rd et al. 2000; Nachev et al. 2005; Stuphorn et al. 2000; Taylor et al. 2007) or emotional interference (Davis et al. 2005; Etkin et al. 2006). It is unclear whether conflict is resolved within these cortical areas so that only the final command is sent to downstream motor structures. It is proposed that the prefrontal-SC pathway has a mechanism that can prevent competing commands from arising simultaneously (Schlag-Rey et al. 1992). However, our data show that response conflict exists in the SC. It is also shown that parallel processing of different saccade programs can occur in the SC (McPeek and Keller 2002a). These observations suggest that, with respect to the ocular motor system, mutually competing motor commands can converge on the SC at the same time, without being fully resolved at the cortical level. Such a conflict may then be resolved in the SC, to allow a selected motor program to be issued. A role of cortical areas involved in conflict resolution may thus be to inhibit irrelevant motor programs on one hand while facilitating relevant motor programs on the other at the level close to motor output. Indeed, such a mechanism is shown in the limb-motor system under a sensory conflict (Taylor et al. 2007).
The present finding suggests that conflict-related cortical areas need to gain access to the SC to resolve motivational conflict of eye movement. How, then, can such top-down control signals reach the SC? There are two major pathways whereby the above-cited cortical areas can influence SC activity: direct cortico-SC routes and indirect cortico-basal ganglia-SC routes. It has been shown that the LPFC, ACC, and SEF have direct projections to the SC (Huerta and Kaas 1990; Leichnetz et al. 1981; Shook et al. 1990). A recent study shows that prefrontal neurons indeed send task-selective signals directly to the SC in an antisaccade task that invokes sensory conflict (Johnston and Everling 2006). The direct cortical routes are thought to mediate glutamatergic excitatory actions on target SC neurons (Creutzfeldt 1995). By contrast, the indirect routes through the basal ganglia are equipped with long-range GABAergic inhibitory mechanisms, which allow both facilitation of a relevant saccade program and inhibition of irrelevant saccade programs in the SC through the so-called direct and indirect pathways, respectively (Hikosaka et al. 2000; Mink 1996). It is known that the LPFC, ACC, SEF, and pre-SMA all project to an input station of the basal ganglia, the caudate nucleus (Huerta and Kaas 1990; Parthasarathy et al. 1992; Selemon and Goldman-Rakic 1985; Shook et al. 1991; Takada et al. 2001). Furthermore, a group of caudate neurons shows a higher level of saccade-related activity on small-reward trials than on big-reward trials, and this activity is inversely related to saccade latency (Watanabe et al. 2003b). This suggests that the caudate nucleus may be involved in the resolution of motivational conflict. In support of this, the blockade of the dopamine D2 receptor within the caudate nucleus results in a further response delay on small-reward trials (Nakamura and Hikosaka 2006). Further studies are necessary to clarify specific contributions of each of the cortical and basal ganglia structures to conflict resolution.
In summary, our data show that a conflict between a task demand and a motivational demand exists in the SC, a subcortical structure close to the motor output. Under this motivational conflict, overt saccade behavior continues to be suppressed by the competitive intercollicular interactions until a motor command for one action prevails against the other. A role of the conflict-related cortical areas may be to tip the balance between the two sides of the SC using their direct connections or indirect connections through the basal ganglia.
GRANTS
This work was supported by the intramural research program of the National Eye Institute.
Acknowledgments
We thank K. Nakamura and L. Ding for discussions; M. Matsumoto and E. Bromberg-Martin for comments; and M. K. Smith, J. W. McClurkin, T. W. Ruffner, A. M. Nichols, A. V. Hays, and L. P. Jensen for technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Amador et al. 2004.Amador N, Schlag-Rey M, Schlag J. Primate antisaccade. II. Supplementary eye field neuronal activity predicts correct performance. J Neurophysiol 91: 1672–1689, 2004. [DOI] [PubMed] [Google Scholar]
- Bowman et al. 1996.Bowman EM, Aigner TG, Richmond BJ. Neural signals in the monkey ventral striatum related to motivation for juice and cocaine rewards. J Neurophysiol 75: 1061–1073, 1996. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt 1995.Creutzfeldt OD Cortex Cerebri: Performance, Structural and Functional Organization of the Cortex. New York: Oxford Univ. Press, 1995.
- Crist et al. 1988.Crist CF, Yamasaki DS, Komatsu H, Wurtz RH. A grid system and a microsyringe for single cell recording. J Neurosci Methods 26: 117–122, 1988. [DOI] [PubMed] [Google Scholar]
- Davis et al. 2005.Davis KD, Taylor KS, Hutchison WD, Dostrovsky JO, McAndrews MP, Richter EO, Lozano AM. Human anterior cingulate cortex neurons encode cognitive and emotional demands. J Neurosci 25: 8402–8406, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong et al. 1994.De Jong R, Liang CC, Lauber E. Conditional and unconditional automaticity: a dual-process model of effects of spatial stimulus-response correspondence. J Exp Psychol Hum Percept Perform 20: 731–750, 1994. [DOI] [PubMed] [Google Scholar]
- DeSoto et al. 2001.DeSoto MC, Fabiani M, Geary DC, Gratton G. When in doubt, do it both ways: brain evidence of the simultaneous activation of conflicting motor responses in a spatial stroop task. J Cogn Neurosci 13: 523–536, 2001. [DOI] [PubMed] [Google Scholar]
- Eriksen and Eriksen 1974.Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys 16: 143–149, 1974. [Google Scholar]
- Etkin et al. 2006.Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron 51: 871–882, 2006. [DOI] [PubMed] [Google Scholar]
- Gratton et al. 1988.Gratton G, Coles MG, Sirevaag EJ, Eriksen CW, Donchin E. Pre- and poststimulus activation of response channels: a psychophysiological analysis. J Exp Psychol Hum Percept Perform 14: 331–344, 1988. [DOI] [PubMed] [Google Scholar]
- Hallett 1978.Hallett PE Primary and secondary saccades to goals defined by instructions. Vision Res 18: 1279–1296, 1978. [DOI] [PubMed] [Google Scholar]
- Hikosaka et al. 1993.Hikosaka O, Miyauchi S, Shimojo S. Focal visual attention produces illusory temporal order and motion sensation. Vision Res 33: 1219–1240, 1993. [DOI] [PubMed] [Google Scholar]
- Hikosaka et al. 2006.Hikosaka O, Nakamura K, Nakahara H. Basal ganglia orient eyes to reward. J Neurophysiol 95: 567–584, 2006. [DOI] [PubMed] [Google Scholar]
- Hikosaka et al. 2000.Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev 80: 953–978, 2000. [DOI] [PubMed] [Google Scholar]
- Hikosaka and Wurtz 1983.Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. III. Memory-contingent visual and saccade responses. J Neurophysiol 49: 1268–1284, 1983. [DOI] [PubMed] [Google Scholar]
- Huerta and Kaas 1990.Huerta MF, Kaas JH. Supplementary eye field as defined by intracortical microstimulation: connections in macaques. J Comp Neurol 293: 299–330, 1990. [DOI] [PubMed] [Google Scholar]
- Ikeda and Hikosaka 2003.Ikeda T, Hikosaka O. Reward-dependent gain and bias of visual responses in primate superior colliculus. Neuron 39: 693–700, 2003. [DOI] [PubMed] [Google Scholar]
- Ikeda and Hikosaka 2007.Ikeda T, Hikosaka O. Positive and negative modulation of motor response in primate superior colliculus by reward expectation. J Neurophysiol 98: 3163–3170, 2007. [DOI] [PubMed] [Google Scholar]
- Isoda and Hikosaka 2007.Isoda M, Hikosaka O. Switching from automatic to controlled action by monkey medial frontal cortex. Nat Neurosci 10: 240–248, 2007. [DOI] [PubMed] [Google Scholar]
- Johnston and Everling 2006.Johnston K, Everling S. Monkey dorsolateral prefrontal cortex sends task-selective signals directly to the superior colliculus. J Neurosci 26: 12471–12478, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge et al. 1980.Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res 20: 535–538, 1980. [DOI] [PubMed] [Google Scholar]
- Kujirai et al. 1993.Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol 471: 501–519, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauwereyns et al. 2002.Lauwereyns J, Watanabe K, Coe B, Hikosaka O. A neural correlate of response bias in monkey caudate nucleus. Nature 418: 413–417, 2002. [DOI] [PubMed] [Google Scholar]
- Leichnetz et al. 1981.Leichnetz GR, Spencer RF, Hardy SG, Astruc J. The prefrontal corticotectal projection in the monkey; an anterograde and retrograde horseradish peroxidase study. Neuroscience 6: 1023–1041, 1981. [DOI] [PubMed] [Google Scholar]
- MacDonald et al. 2000.MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288: 1835–1838, 2000. [DOI] [PubMed] [Google Scholar]
- Maunsell 2004.Maunsell JH Neuronal representations of cognitive state: reward or attention? Trends Cogn Sci 8: 261–265, 2004. [DOI] [PubMed] [Google Scholar]
- McPeek and Keller 2002a.McPeek RM, Keller EL. Superior colliculus activity related to concurrent processing of saccade goals in a visual search task. J Neurophysiol 87: 1805–1815, 2002a. [DOI] [PubMed] [Google Scholar]
- McPeek and Keller 2002b.McPeek RM, Keller EL. Saccade target selection in the superior colliculus during a visual search task. J Neurophysiol 88: 2019–2034, 2002b. [DOI] [PubMed] [Google Scholar]
- Minamimoto et al. 2005.Minamimoto T, Hori Y, Kimura M. Complementary process to response bias in the centromedian nucleus of the thalamus. Science 308: 1798–1801, 2005. [DOI] [PubMed] [Google Scholar]
- Mink 1996.Mink JW The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 50: 381–425, 1996. [DOI] [PubMed] [Google Scholar]
- Mogenson et al. 1980.Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol 14: 69–97, 1980. [DOI] [PubMed] [Google Scholar]
- Munoz and Istvan 1998.Munoz DP, Istvan PJ. Lateral inhibitory interactions in the intermediate layers of the monkey superior colliculus. J Neurophysiol 79: 1193–1209, 1998. [DOI] [PubMed] [Google Scholar]
- Munoz and Wurtz 1995.Munoz DP, Wurtz RH. Saccade-related activity in monkey superior colliculus. I. Characteristics of burst and buildup cells. J Neurophysiol 73: 2313–2333, 1995. [DOI] [PubMed] [Google Scholar]
- Nachev et al. 2005.Nachev P, Rees G, Parton A, Kennard C, Husain M. Volition and conflict in human medial frontal cortex. Curr Biol 15: 122–128, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura and Hikosaka 2006.Nakamura K, Hikosaka O. Role of dopamine in the primate caudate nucleus in reward modulation of saccades. J Neurosci 26: 5360–5369, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura et al. 2005.Nakamura K, Roesch MR, Olson CR. Neuronal activity in macaque SEF and ACC during performance of tasks involving conflict. J Neurophysiol 93: 884–908, 2005. [DOI] [PubMed] [Google Scholar]
- Parthasarathy et al. 1992.Parthasarathy HB, Schall JD, Graybiel AM. Distributed but convergent ordering of corticostriatal projections: analysis of the frontal eye field and the supplementary eye field in the macaque monkey. J Neurosci 12: 4468–4488, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port and Wurtz 2003.Port NL, Wurtz RH. Sequential activity of simultaneously recorded neurons in the superior colliculus during curved saccades. J Neurophysiol 90: 1887–1903, 2003. [DOI] [PubMed] [Google Scholar]
- Quaia et al. 1999.Quaia C, Lefevre P, Optican LM. Model of the control of saccades by superior colliculus and cerebellum. J Neurophysiol 82: 999–1018, 1999. [DOI] [PubMed] [Google Scholar]
- Remington et al. 1992.Remington RW, Johnston JC, Yantis S. Involuntary attentional capture by abrupt onsets. Percept Psychophys 51: 279–290, 1992. [DOI] [PubMed] [Google Scholar]
- Robinson 1963.Robinson DA A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng 10: 137–145, 1963. [DOI] [PubMed] [Google Scholar]
- Schlag et al. 1998.Schlag J, Dassonville P, Schlag-Rey M. Interaction of the two frontal eye fields before saccade onset. J Neurophysiol 79: 64–72, 1998. [DOI] [PubMed] [Google Scholar]
- Schlag-Rey et al. 1992.Schlag-Rey M, Schlag J, Dassonville P. How the frontal eye field can impose a saccade goal on superior colliculus neurons. J Neurophysiol 67: 1003–1005, 1992. [DOI] [PubMed] [Google Scholar]
- Selemon and Goldman-Rakic 1985.Selemon LD, Goldman-Rakic PS. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci 5: 776–794, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shook et al. 1990.Shook BL, Schlag-Rey M, Schlag J. Primate supplementary eye field. I. Comparative aspects of mesencephalic and pontine connections. J Comp Neurol 301: 618–642, 1990. [DOI] [PubMed] [Google Scholar]
- Shook et al. 1991.Shook BL, Schlag-Rey M, Schlag J. Primate supplementary eye field. II. Comparative aspects of connections with the thalamus, corpus striatum, and related forebrain nuclei. J Comp Neurol 307: 562–583, 1991. [DOI] [PubMed] [Google Scholar]
- Simon 1969.Simon JR Reactions toward the source of stimulation. J Exp Psychol 81: 174–176, 1969. [DOI] [PubMed] [Google Scholar]
- Sparks 1986.Sparks DL Translation of sensory signals into commands for control of saccadic eye movements: role of primate superior colliculus. Physiol Rev 66: 118–171, 1986. [DOI] [PubMed] [Google Scholar]
- Sparks and Hartwich-Young 1989.Sparks DL, Hartwich-Young R. The deep layers of the superior colliculus. In: The Neurobiology of Saccadic Eye Movements, edited by Wurtz RH, Goldberg ME. Amsterdam: Elsevier, 1989, p. 213–255. [PubMed]
- Stroop 1935.Stroop JR Studies of interference in serial verbal reactions. J Exp Psychol 18: 643–662, 1935. [Google Scholar]
- Stuphorn et al. 2000.Stuphorn V, Taylor TL, Schall JD. Performance monitoring by the supplementary eye field. Nature 408: 857–860, 2000. [DOI] [PubMed] [Google Scholar]
- Takada et al. 2001.Takada M, Tokuno H, Hamada I, Inase M, Ito Y, Imanishi M, Hasegawa N, Akazawa T, Hatanaka N, Nambu A. Organization of inputs from cingulate motor areas to basal ganglia in macaque monkey. Eur J Neurosci 14: 1633–1650, 2001. [DOI] [PubMed] [Google Scholar]
- Takahashi et al. 2005.Takahashi M, Sugiuchi Y, Izawa Y, Shinoda Y. Commissural excitation and inhibition by the superior colliculus in tectoreticular neurons projecting to omnipause neuron and inhibitory burst neuron regions. J Neurophysiol 94: 1707–1726, 2005. [DOI] [PubMed] [Google Scholar]
- Taylor et al. 2007.Taylor PC, Nobre AC, Rushworth MF. Subsecond changes in top down control exerted by human medial frontal cortex during conflict and action selection: a combined transcranial magnetic stimulation electroencephalography study. J Neurosci 27: 11343–11353, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson et al. 1996.Thompson KG, Hanes DP, Bichot NP, Schall JD. Perceptual and motor processing stages identified in the activity of macaque frontal eye field neurons during visual search. J Neurophysiol 76: 4040–4055, 1996. [DOI] [PubMed] [Google Scholar]
- Tremblay and Schultz 2000.Tremblay L, Schultz W. Reward-related neuronal activity during go-nogo task performance in primate orbitofrontal cortex. J Neurophysiol 83: 1864–1876, 2000. [DOI] [PubMed] [Google Scholar]
- Ullsperger and von Cramon 2001.Ullsperger M, von Cramon DY. Subprocesses of performance monitoring: a dissociation of error processing and response competition revealed by event-related fMRI and ERPs. Neuroimage 14: 1387–1401, 2001. [DOI] [PubMed] [Google Scholar]
- Watanabe et al. 2003a.Watanabe K, Lauwereyns J, Hikosaka O. Effects of motivational conflicts on visually elicited saccades in monkeys. Exp Brain Res 152: 361–367, 2003a. [DOI] [PubMed] [Google Scholar]
- Watanabe et al. 2003b.Watanabe K, Lauwereyns J, Hikosaka O. Neural correlates of rewarded and unrewarded eye movements in the primate caudate nucleus. J Neurosci 23: 10052–10057, 2003b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtz and Albano 1980.Wurtz RH, Albano JE. Visual-motor function of the primate superior colliculus. Annu Rev Neurosci 3: 189–226, 1980. [DOI] [PubMed] [Google Scholar]