Abstract
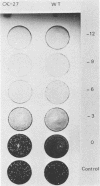
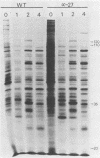
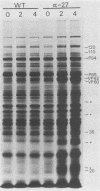
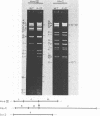
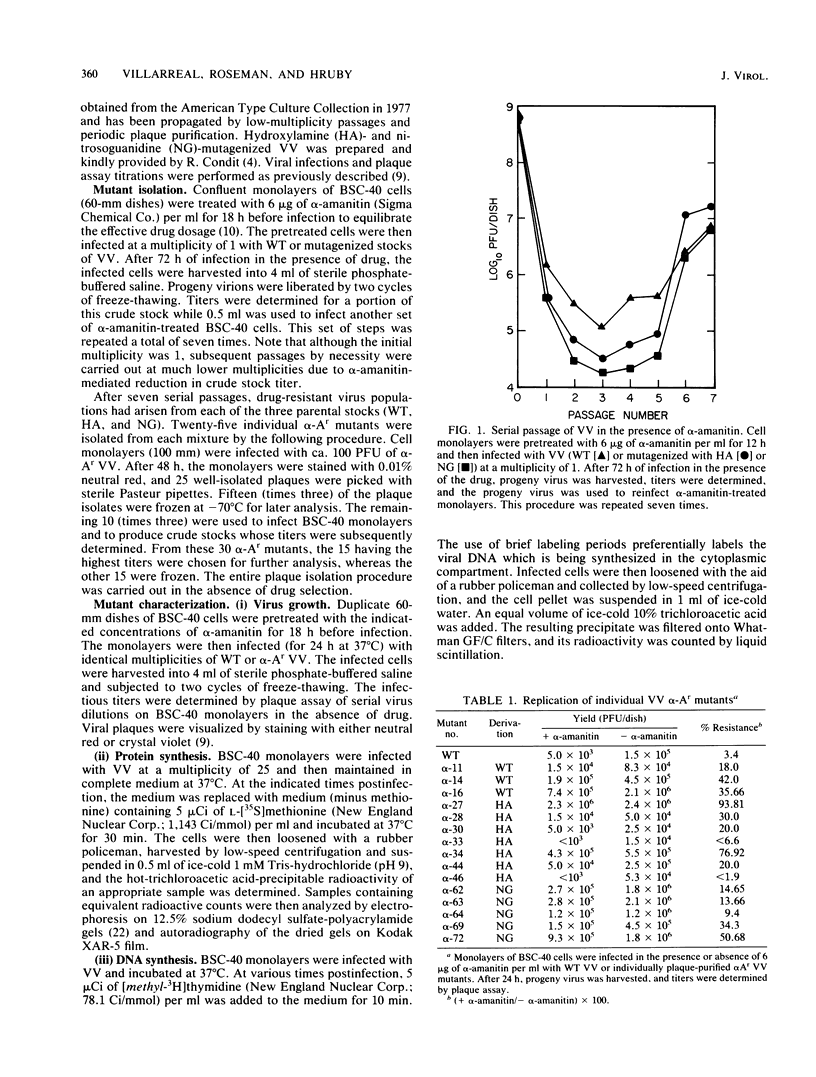
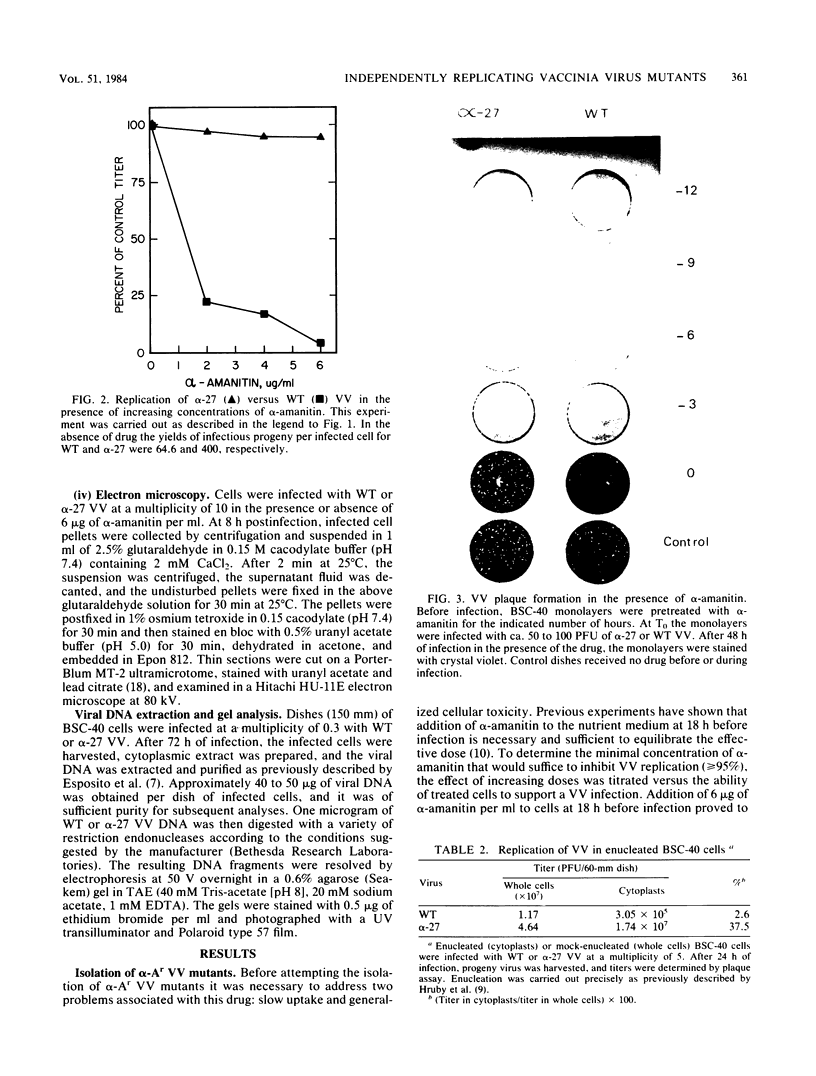
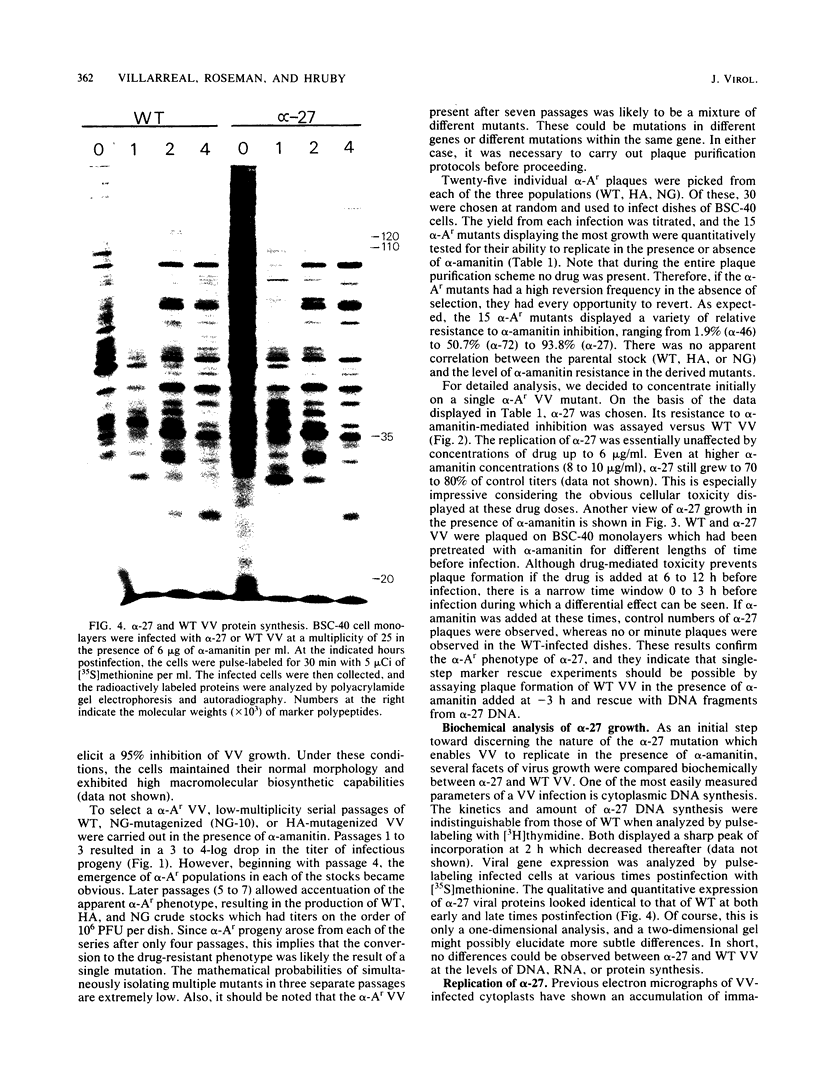
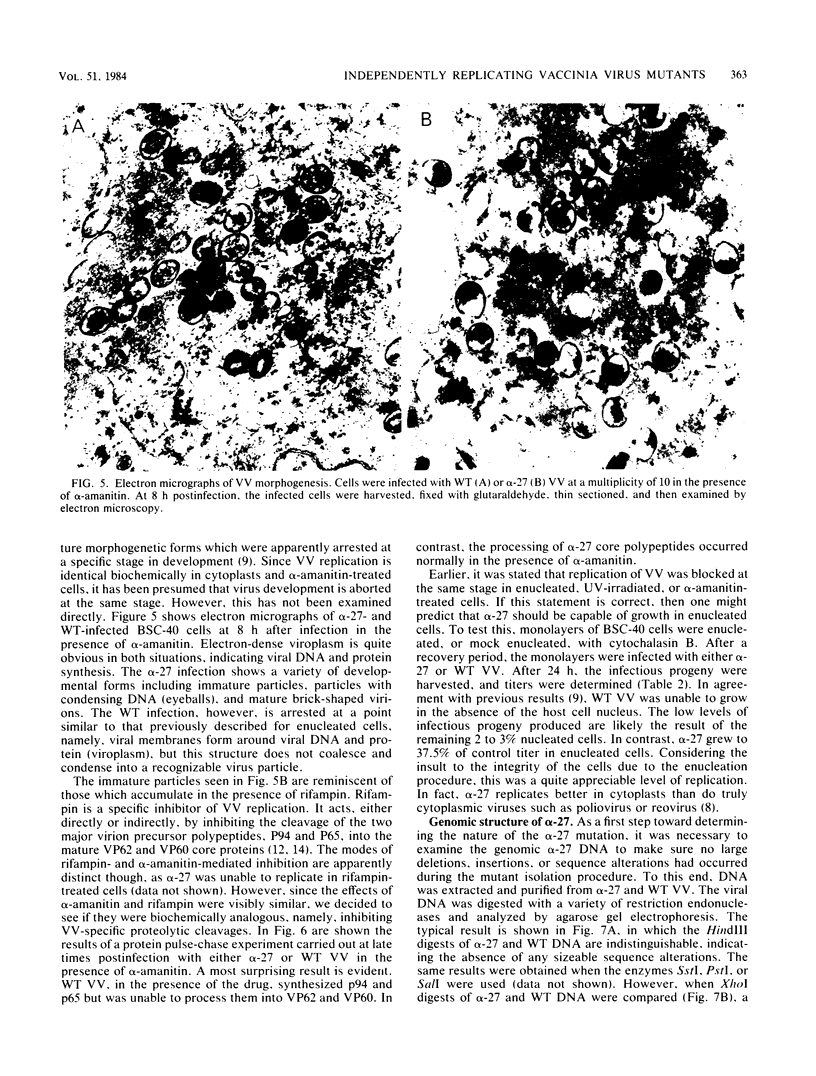
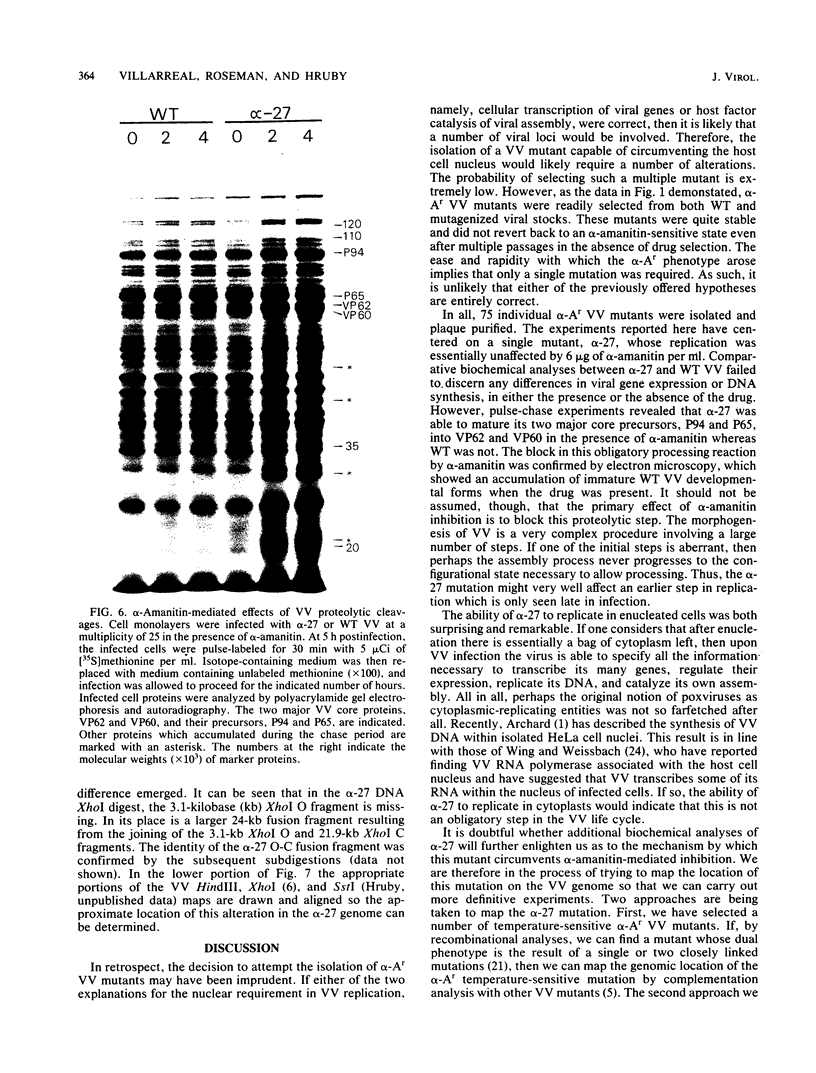
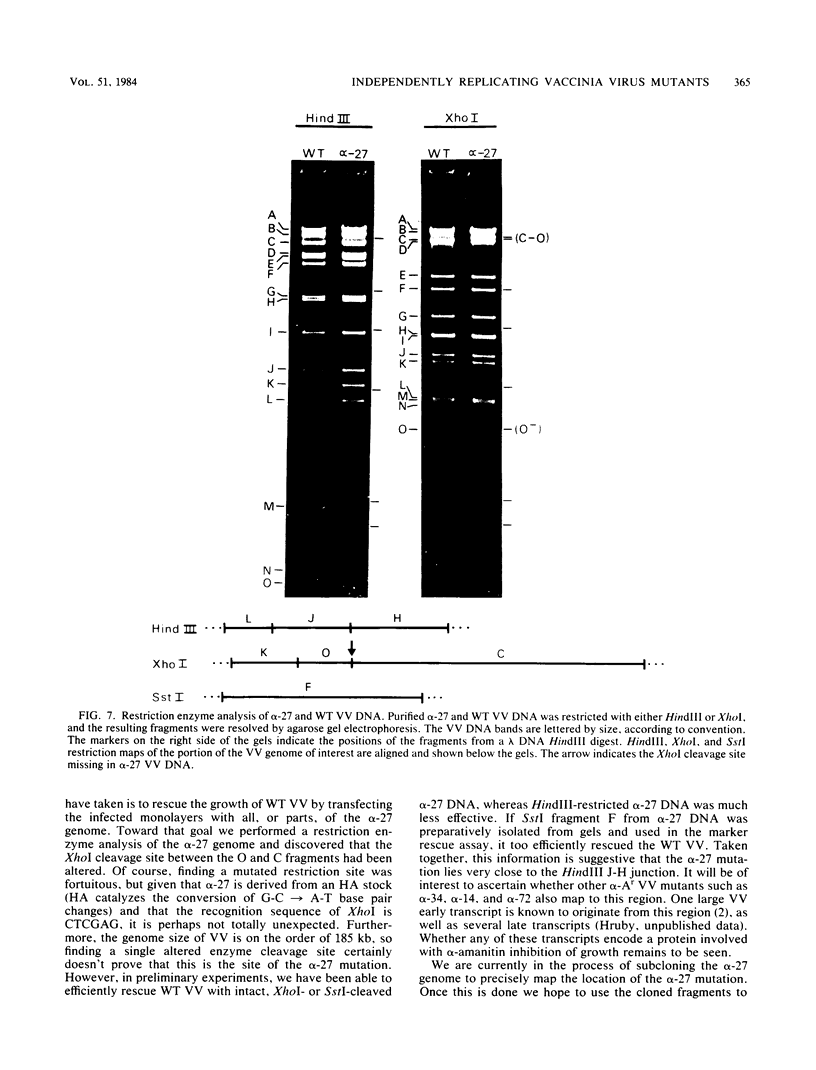
alpha-Amanitin-resistant vaccinia virus mutants were isolated after serial viral passages in BSC-40 cells that were carried out in the presence of inhibitory levels (6 micrograms/ml) of alpha-amanitin. One such mutant, alpha-27, was highly refractory (greater than 95%) to alpha-amanitin-mediated inhibition and was selected for further study. In the absence of drug, the phenotypes of alpha-27 and wild-type vaccinia virus were indistinguishable with respect to growth kinetics. DNA synthesis, protein synthesis, and morphogenesis. Infections in the presence of alpha-amanitin revealed two striking differences, however. First, wild-type virus was unable to catalyze proteolytic processing of the two major capsid proteins VP62 and VP60, whereas alpha-27 was most efficient at this process. Second, wild-type viral morphogenesis within the infected cells was arrested by alpha-amanitin at an apparently analogous step to that previously described for enucleated cells. This observation was supported by the ability of alpha-27 virus to replicate in enucleated BSC-40 cells. Restriction enzyme analyses of alpha-27 versus wild-type genomes revealed that a XhoI cleavage site was altered in the alpha-27 DNA molecule, suggesting a possible location for the alpha-amanitin resistance locus.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archard L. C. Synthesis of full-length, virus genomic DNA by nuclei of vaccinia-infected HeLa cells. J Gen Virol. 1983 Dec;64(Pt 12):2561–2575. doi: 10.1099/0022-1317-64-12-2561. [DOI] [PubMed] [Google Scholar]
- Bajszár G., Wittek R., Weir J. P., Moss B. Vaccinia virus thymidine kinase and neighboring genes: mRNAs and polypeptides of wild-type virus and putative nonsense mutants. J Virol. 1983 Jan;45(1):62–72. doi: 10.1128/jvi.45.1.62-72.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolden A., Noy G. P., Weissbach A. Vaccinia virus infection of HeLa cells. II. Disparity between cytoplasmic and nuclear viral-specific RNA. Virology. 1979 Apr 15;94(1):138–145. doi: 10.1016/0042-6822(79)90444-6. [DOI] [PubMed] [Google Scholar]
- Condit R. C., Motyczka A. Isolation and preliminary characterization of temperature-sensitive mutants of vaccinia virus. Virology. 1981 Aug;113(1):224–241. doi: 10.1016/0042-6822(81)90150-1. [DOI] [PubMed] [Google Scholar]
- Condit R. C., Motyczka A., Spizz G. Isolation, characterization, and physical mapping of temperature-sensitive mutants of vaccinia virus. Virology. 1983 Jul 30;128(2):429–443. doi: 10.1016/0042-6822(83)90268-4. [DOI] [PubMed] [Google Scholar]
- DeFilippes F. M. Restriction enzyme mapping of vaccinia virus DNA. J Virol. 1982 Jul;43(1):136–149. doi: 10.1128/jvi.43.1.136-149.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito J., Condit R., Obijeski J. The preparation of orthopoxvirus DNA. J Virol Methods. 1981 Feb;2(3):175–179. doi: 10.1016/0166-0934(81)90036-7. [DOI] [PubMed] [Google Scholar]
- Follett E. A., Pringle C. R., Pennington T. H. Virus development in enucleate cells: echovirus, poliovirus, pseudorabies virus, reovirus, respiratory syncytial virus and Semliki Forest virus. J Gen Virol. 1975 Feb;26(2):183–196. doi: 10.1099/0022-1317-26-2-183. [DOI] [PubMed] [Google Scholar]
- Hruby D. E., Guarino L. A., Kates J. R. Vaccinia virus replication. I. Requirement for the host-cell nucleus. J Virol. 1979 Feb;29(2):705–715. doi: 10.1128/jvi.29.2.705-715.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby D. E., Lynn D. L., Kates J. R. Vaccinia virus replication requires active participation of the host cell transcriptional apparatus. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1887–1890. doi: 10.1073/pnas.76.4.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. V., Moss B. Mapping of the vaccinia virus DNA polymerase gene by marker rescue and cell-free translation of selected RNA. J Virol. 1984 Jan;49(1):72–77. doi: 10.1128/jvi.49.1.72-77.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E., Moss B. Formation of a vaccinia virus structural polypeptide from a higher molecular weight precursor: inhibition by rifampicin. Proc Natl Acad Sci U S A. 1970 Jul;66(3):677–684. doi: 10.1073/pnas.66.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N., Katz E., Grimley P. M. Rifampicin: a specific inhibitor of vaccinia virus assembly. Nature. 1969 Dec 27;224(5226):1280–1284. doi: 10.1038/2241280a0. [DOI] [PubMed] [Google Scholar]
- Pennington T. H., Follett E. A. Vaccinia virus replication in enucleate BSC-1 cells: particle production and synthesis of viral DNA and proteins. J Virol. 1974 Feb;13(2):488–493. doi: 10.1128/jvi.13.2.488-493.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott D. M., Kates J., Kirkpatrick J. B. Replication of vaccinia virus DNA in enucleated L-cells. J Mol Biol. 1971 Aug 14;59(3):505–508. doi: 10.1016/0022-2836(71)90313-5. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raczynski P., Condit R. C. Specific inhibition of vaccinia virus growth by 2'-O-methyladenosine: isolation of a drug-resistant virus mutant. Virology. 1983 Jul 30;128(2):458–462. doi: 10.1016/0042-6822(83)90270-2. [DOI] [PubMed] [Google Scholar]
- Silver M., Dales S. Evidence against involvement of host transcription in the replication of vaccinia and herpes simplex viruses. Virology. 1982 Apr 15;118(1):214–218. doi: 10.1016/0042-6822(82)90334-8. [DOI] [PubMed] [Google Scholar]
- Silver M., McFadden G., Wilton S., Dales S. Biogenesis of poxviruses: role for the DNA-dependent RNA polymerase II of the host during expression of late functions. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4122–4125. doi: 10.1073/pnas.76.8.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar P., Condit R. C. Selection for temperature-sensitive mutations in specific vaccinia virus genes: isolation and characterization of a virus mutant which encodes a phosphonoacetic acid-resistant, temperature-sensitive DNA polymerase. Virology. 1983 Jul 30;128(2):444–457. doi: 10.1016/0042-6822(83)90269-6. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Traktman P., Sridhar P., Condit R. C., Roberts B. E. Transcriptional mapping of the DNA polymerase gene of vaccinia virus. J Virol. 1984 Jan;49(1):125–131. doi: 10.1128/jvi.49.1.125-131.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing D., Weissbach A. Vaccinia virus RNA polymerase associated with nuclei of infected HeLa cells. J Virol. 1984 Jan;49(1):26–34. doi: 10.1128/jvi.49.1.26-34.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]