Abstract
Vision of newborn infants is limited by immaturities in their visual brain. In adult primates, the transient onset discharges of visual cortical neurons are thought to be intimately involved with capturing the rapid succession of brief images in visual scenes. Here we sought to determine the responsiveness and quality of transient responses in individual neurons of the primary visual cortex (V1) and visual area 2 (V2) of infant monkeys. We show that the transient component of neuronal firing to 640-ms stationary gratings was as robust and as reliable as in adults only 2 wk after birth, whereas the sustained component was more sluggish in infants than in adults. Thus the cortical circuitry supporting onset transient responses is functionally mature near birth, and our findings predict that neonates, known for their “impoverished vision,” are capable of initiating relatively mature fixating eye movements and of performing in detection of simple objects far better than traditionally thought.
INTRODUCTION
Human infants can “see” the world as early as their eyes open. However, infant's spatial vision is crude and sensitivity to low contrast images is relatively poor for several months after birth (Blakemore 1990; Boothe et al. 1985; Chino et al. 2004; Kiorpes and Movshon 2004). The retina and visual brain of infant monkeys exhibit immaturities that are thought to limit their perceptual development. The photoreceptors and their distribution in the retina are not fully developed near birth (Hendrickson 1993; Hendrickson and Yuodelis 1984). Neuronal responses in the visual thalamus (LGN) (Hawken et al. 1997; Movshon et al. 2005) visual cortex (V1) (Blakemore and Vital-Durand 1981; Chino et al. 1997; Endo et al. 2000; Hatta et al. 1998; Rust et al. 2002; Zhang et al. 2005) are “sluggish,” and the spatial resolving power and contrast sensitivity of neurons in the infant monkey's visual brain are substantially lower than in adults (Chino et al. 1997; Zhang et al. 2005; Zheng et al. 2007).
There are lines of evidence, however, suggesting that the visual capacities of human infants may be better than previously thought. Specifically in simple detection tasks, visual performance appears much better if the spatial grain of high-contrast targets are optimized for an infant's age and fixating saccades are used for the response measure (Dobkins et al. 1999; Matsuzawa and Shimojo 1997; Regal 1981; Taga et al. 2002). The neural basis for this relatively “mature” visual performance in human infants is not known. If the responses of cortical neurons are exceedingly sluggish as previous studies indicated, it is unclear how neonates and infants can competently detect even simple objects.
In adult monkeys, the responses of typical cortical neurons to brief stationary stimuli consist of a robust transient onset discharge followed by steady but reduced sustained firing (Geisler 1983; Hegde and Van Essen 2003; Mechler et al. 1998; Muller et al. 2001; Palanca and DeAngelis 2003). Mature primates make frequent saccades and typically fixate on a single object <200 ms, emphasizing the importance of the transient onset discharges of visual cortical neurons in capturing a rapid succession of brief images and in saccade initiation (Dragoi et al. 2002; Epelboim et al. 1994; Keesey 1960; Muller et al. 2001; Palanca and DeAngelis 2003; Tovee et al. 1993; Zohary et al. 1990). These observations in infants and adults together predict that the transient onset discharges of neurons in infant visual cortex may be relatively well developed soon after birth. However, to this date, no published data are available to examine this possibility.
In this study we examined the temporal patterns of firing in V1 and V2 neurons of 2- and 4-wk-old infant monkeys (roughly equivalent to 2- and 4-mo-old humans) and compared the relative maturation of the transient versus the sustained components of their spike trains. We found that as early as 2 wk of age, the transient onset responses of V1 and V2 neurons to brief presentation of stationary gratings were as robust and as reliable as in adults while their sustained responses were relatively poor. These observations provide evidence that the cortical circuits in “low-level” processing (i.e., V1/V2) that are required for detection of simple objects and for fixating eye movements are functionally mature only weeks after birth if not at birth.
METHODS
Subjects and physiological preparation
Seven 2-wk-old and seven 4-wk-old infant monkeys and five adult monkeys (Macaca mulatta) served as subjects. The weight of infant monkeys was between 480 and 600 g in 2-wk-old and 500 and 525 g in 4-wk-old infants. The adult monkeys varied in age between 2 and 4 yr and in weight between 3.9 and 5.75 kg. All experimental procedures conformed to the National Institute of Health guidelines for the use of animals in research and were approved by the University of Houston's Institutional Animal Care and Use Committee.
The surgical preparation and recording procedures have been described in detail elsewhere (Zhang et al. 2005; Zheng et al. 2007). The monkeys were initially anesthetized with an intramuscular injection of ketamine hydrochchloride (15–20 mg/kg) and acepromazine malerate (0.15–0.2 mg/kg). A superficial vein was cannulated, and all subsequent surgical procedures were carried out with additional anesthesia as needed (Propofol, 4–6 mg·kg−1·h−1). A tracheotomy was performed to facilitate artificial respiration, and after securing the subjects in a stereotaxic instrument, a small craniotomy and durotomy were made over the lunate sulcus. After all surgical procedures were completed, the animals were paralyzed by an intravenous injection of vercuronium bromide (Norcuron; 0.1 mg·kg−1·h−1) and artificially ventilated with a mixture of 59% N2O-39% O2-2% CO2. Anesthesia was maintained by the continuous infusion of a mixture of Propofol (4 mg·kg−1·h−1) and sufentanyl citrate (0.05 μg·kg−1·h−1). Core body temperature was kept at 37.6°C. Cycloplegia was produced by topical instillation of 1% atropine, and the animals' corneas were protected with rigid gas-permeable, extended-wear contact lenses. Retinoscopy was used to determine the contact lens parameters required to focus the eyes on the stimulus screens. Additional spectacle lenses were also used if necessary.
Extracellular recording and visual stimulation
Tungsten-in-glass microelectrodes were used to isolate the activity from individual cortical neurons. Action potentials were extracellularly recorded and amplified using conventional technology. A typical penetration for V1 recording began several millimeters posterior to the lunate sulcus and ∼1.5 cm from the midline and ended when the electrode tip entered the white matter. The tangential penetrations for V2 recording (the angle of deviations from the perpendicular penetration was ∼20°) were typically started right behind the blood vessel running along the lunate sulcus and also ∼1.5 cm from the midline. The penetration ended when the electrode tip exited V2. In rare cases where V2 was hidden below the cortical surface (typically detected by obvious imbalance of ocular dominance in consecutive units), we proceeded with experiments in V1 and continued with the experiments in V2. All receptive fields were located within 5.0° of the center of the projected fovea.
For each isolated neuron, the receptive fields for both eyes were mapped, and its ocular dominance was initially determined using hand-held stimuli. Initially responses to drifting sine wave gratings were measured to determine the preferred orientation and spatial frequency for each unit. The visual stimuli were generated on a monochrome monitor (VRG) with ultra-short persistence (frame rate = 140 Hz; 800 × 600 pixels, screen size = 20 × 15° at 114 cm and mean luminance = 50 cd/m2). Cells were classified as simple or complex on the basis of the temporal characteristics of their responses to a drifting sine wave grating of the optimal spatial frequency and orientation (Skottun et al. 1991). Following the determination of the preferred orientation, direction of drift, and optimal spatial frequency of each neuron, we determined its receptive-field center size. Specifically, the center of the receptive field was found by locating the position where the largest response was evoked by a 0.5° grating patch, responses were measured as a function of the diameter of the optimized circular grating patch that was positioned at the RF center. For simple cells only, an optimal spatial phase was found with contrast reversing stationary gratings.
Transient and sustained responses
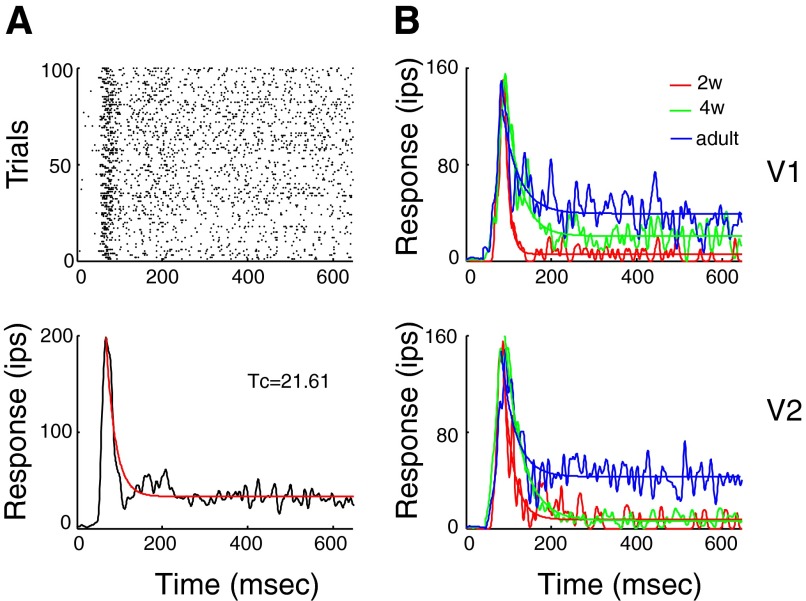
To study the temporal pattern of neuronal firing, the stationary sine wave gratings that were optimized in each unit for spatial frequency, orientation, and spatial phase (simple cells only) were presented for 640 ms, and responses were recorded. The stimulation was confined to the receptive-center (RFC), which was determined by selecting the stimulus size that initiated the largest discharge during the measurement of the area-response functions. PSTHs were constructed from 100 spike trains (duration = 640 ms) with a bin width of 1.0 ms and smoothed by Gaussian filter with SD of 2.0 ms (Bair et al. 2003) (Fig. 1A).
FIG. 1.
Temporal dynamics of responses and developmental changes in firing rates of transient and sustained components of cell's discharge in V1 and V2. A, top: raster plot showing responses of an adult V1 neuron to 640 ms presentation of a high contrast (80%) stationary gratings optimized for orientation/direction, spatial frequency, spatial phase, and size for 100 trials. Bottom: poststimulus time histogram (PSTH) constructed with resolution of 1.0 ms and smoothed by a Gaussian filter with SD of 2.0 ms. Firing rate R was fitted into an exponential decay function (red line). R = (Rmax − Rmin)*exp(−t/tc) + Rmin, where Rmax is the peak firing rate, Rmin is the asymptotic firing rate, tc is the time constant, and t is the time since peak. B: temporal patterns of responses in V1 (top) and V2 (bottom) neurons of 2 (red)- and 4-wk-old-infant (green) and adult (blue) monkeys.
Onset latency was determined from PSTHs by measuring the time between stimulus onset and the time at which unit's response significantly exceeded the background noise distribution (P = 0.01) over three consecutive bins. More specifically, “noise” preceding responses to stimulus onset was calculated by counting spontaneous activity for a period of 250 ms and was described by a Poisson distribution. Visual latency was determined by measuring the time between stimulus onset and the time at which unit's response significantly exceeded the background noise distribution over three consecutive bins (i.e., exceeded a level corresponding to a probability of P = 0.01). Onset-to-peak was determined by measuring the time required for unit's responses to reach 95% of its peak firing rate after the response level rose above noise to minimize potentially high variability (jitter) in locating ‘peak’ responses (Bair et al. 2003) (Fig. 4A).
FIG. 4.

Detectability (d′) of V1 and V2 neurons in infants and adults. A: raster plot of responses of an adult V1 complex cell to stimulus presentation. B: raster plot showing spontaneous activity of the same unit during blank trials. C: PSTH for the unit in A during 640-ms stimulus presentation. D: growth of cumulative d′ during 640 stimulus presentation. Time for d′ to reach 1.0 (Td′=1) and 2.0 (Td′=2) are also illustrated. E: growth of cumulative d′ in representative V1 neurons in 2 (red)- and 4-wk-old (green) infants and adult (blue) monkeys. F: growth of cumulative d′ in representative V2 neurons.
Histology
At the end of each penetration, small electrolytic lesions (5 μA, 5 s, electrode negative) were made at three points along the track for later reconstruction. Experiments were terminated by administering an overdose of sodium pentobarbital (100 mg/kg), and the animals were killed by perfusion through the heart with an aldehyde fixtative. Frozen sections were stained for Nissl substance and cytochrome oxidase. The sites of recording and laminar distribution of individual units were estimated from recording depths and electrode tracks (Zheng et al. 2007). Our sampling was in general uniform and similar in all subject groups.
RESULTS
PSTHs were constructed from 100 spike trains (duration = 640 ms) with a bin width of 1.0 ms and smoothed by Gaussian filter with SD of 2.0 ms (Bair et al. 2003) (Fig. 1A). We specifically examined the strength (discharge rate) and reliability (variance-to-mean ratios and d′) and contrast sensitivity of the transient and sustained components of the neuronal responses. Stimuli, unless specified otherwise, were 640-ms stationary, high-contrast (80%) sine wave gratings that were optimized for each neuron with respect to orientation, spatial frequency, size, and, for simple cells, spatial phase.
In a V1 complex cell from an adult monkey, cell's discharge rose rapidly from its relatively low spontaneous firing level until it reached its peak firing (Fig. 1A). The peak transient discharge was followed by a rapid decline of firing and response depression lasting ∼40–50 ms. Weaker sustained firing of spikes continued afterward until the stimulus offset. Firing rate (R) after the peak discharge rate was fitted into an exponential decay function. R = (Rmax − Rmin)*exp(−t/tc) + Rmin, where Rmax is the peak firing rate, Rmin is the asymptotic firing rate, tc is the time constant, and t is the time since peak.
Adult-like transient onset discharges near birth
As early as 2 wk of age, the amplitude of the transient onset discharge was adult-like in representative V1 and V2 neurons (Fig. 1B, red). However, their firing rate dropped more rapidly and deeply from the peak than in adults. The sustained firing during the subsequent 400 ms barely exceeded the noise level. The sustained firing component of the V1 unit from a 4-wk-old infant improved modestly, but the overall sustained discharge remained substantially lower than in adults (Fig. 1B, green).
To determine the generality of the observed differences between the transient and sustained responses in the infant visual cortex, we characterized these response components for a population of V1 (n = 108 in 2-wk-old, n = 91 in 4-wk-old, and n = 193 in adult monkeys; Fig. 2A) and V2 neurons (n = 124 in 2-wk-old, n = 162 in 4-wk-old, and 204 in adult monkeys; Fig. 2B). For both V1 and V2, the median amplitudes (colored triangles) of the transient onset discharge (Rmax) in 2- and 4-wk-old infants were very similar to those in adults (Kruskal-Wallis test, P = 0.14 for V1 and P = 0.08 for V2).
FIG. 2.

A: frequency histograms showing the distribution of the firing rate of individual V1 neurons during the peak transient firing (Rmax, □) and during the sustained firing (Rmin,  ). B: comparable frequency histograms for V2 neurons. C: frequency histograms showing the spontaneous activity of individual V1 neurons (
). B: comparable frequency histograms for V2 neurons. C: frequency histograms showing the spontaneous activity of individual V1 neurons ( ) and their overall responses to 640-ms step stimuli (□). D: comparable frequency histograms for V2 neurons. The median values (numbers) are indicated with ▾ and | for each age group. 2W: 2-wk-old-infants, 4W: 4-wk-old-infants.
) and their overall responses to 640-ms step stimuli (□). D: comparable frequency histograms for V2 neurons. The median values (numbers) are indicated with ▾ and | for each age group. 2W: 2-wk-old-infants, 4W: 4-wk-old-infants.
The median amplitudes (dotted lines) of the sustained firing component (Rmin) for both 2- and 4-wk-old infants were significantly lower in both V1 and V2 compared with adults (Kruskal-Wallis test, P < 0.01 for V1 and P < 0.01 for V2), although the sustained responses were much lower than transient responses for all ages ( , Fig. 2, A and B). At 4 wk of age, there was substantial improvement for the amplitude of the sustained firing especially in V1 due to an increase in the proportion of cells exhibiting higher response rates. However, the overall sustained responses at 4 wk of age were still significantly lower in both areas than those in adults (Kruskal-Wallis test, P < 0.01 for V1 and P < 0.01 for V2). The high transient and low sustained firing rate of neurons in infants resulted in an overall decrease in the sustained/transient response ratios, i.e., sustained responses were disproportionately weaker than the transient responses in both V1 and V2 of 2- and 4-wk-old infants compared with adults.
, Fig. 2, A and B). At 4 wk of age, there was substantial improvement for the amplitude of the sustained firing especially in V1 due to an increase in the proportion of cells exhibiting higher response rates. However, the overall sustained responses at 4 wk of age were still significantly lower in both areas than those in adults (Kruskal-Wallis test, P < 0.01 for V1 and P < 0.01 for V2). The high transient and low sustained firing rate of neurons in infants resulted in an overall decrease in the sustained/transient response ratios, i.e., sustained responses were disproportionately weaker than the transient responses in both V1 and V2 of 2- and 4-wk-old infants compared with adults.
The developmental changes in spontaneous activity and the overall response amplitude of individual neurons to our 640-ms step stimuli (i.e., onset transient firing, sustained discharge and small off responses) are shown for V1 (Fig. 2C) and for V2 (D). The spontaneous activity in 2- and 4- wk-old infants ( ) was significantly lower than in adults (P < 0.01), and this lower spontaneous firing is likely to influence the detectability (d′) of these neurons (see following text). Also our result is in line with similar observations in the LGN of infant macaque monkeys (Movshon et al. 2005).
) was significantly lower than in adults (P < 0.01), and this lower spontaneous firing is likely to influence the detectability (d′) of these neurons (see following text). Also our result is in line with similar observations in the LGN of infant macaque monkeys (Movshon et al. 2005).
Because of the sluggish sustained responses (Fig. 2, A and B), the overall responsiveness of V1 and V2 neurons to step stimuli was also significantly lower in 2- and 4-wk-old infants than in adults (P < 0.01).
Low variability during transient firing in infants
In adult cortical neurons, the trial-to-trial variability of the responses to drifting gratings is known to increase proportionally with response amplitude (Rust et al. 2002; Shadlen and Newsome 1998; Tolhurst et al. 1983). However, the variability of discharge during the transient onset responses of V1 (Muller et al. 2001) and V2 neurons (Hegde and Van Essen 2003) to stationary gratings is lower than that for sustained responses. To measure the trial-to-trial variability of the discharge in infants, we calculated the variance-to-mean ratios (σ2/μ) for the transient and sustained components of the spike trains. For the measurement window, transient firing was sampled for a period of 50 ms around the peak and the sustained discharge was sampled for the same duration (50 ms) at 100 ms prior to the end of the 640 ms stimulation period (Fig. 3A).
FIG. 3.

Developmental changes in variance-to-mean ratios during the transient and sustained firing of V1 and V2 neurons. A: PSTH for an adult V1 complex cell from which its variance-to-mean ratio was calculated for transient and sustained components. For the measurement window, the transient firing was sampled for a period of 50 ms around the peak (left  ) and the sustained discharge was sampled for the same duration (50 ms) at 100 ms prior to the end of 640-ms stimulation (right
) and the sustained discharge was sampled for the same duration (50 ms) at 100 ms prior to the end of 640-ms stimulation (right  ). B: scatter plots of the variance-to-mean ratios of individual V1 (top) and V2 (bottom) neurons during transient discharge against their variance-to-mean ratios during sustained firing. Frequency histograms and median values (▾) are also illustrated for the transient and sustained components.
). B: scatter plots of the variance-to-mean ratios of individual V1 (top) and V2 (bottom) neurons during transient discharge against their variance-to-mean ratios during sustained firing. Frequency histograms and median values (▾) are also illustrated for the transient and sustained components.
Figure 3B plots the variance-to-mean ratio in each neuron for its sustained component as a function of its transient component. Both in infants and adults, the transient responses of individual V1 and V2 neurons (V1: 0.65 for 2-wk-old, 0.64 for 4-wk-old, and 0.76 for adults, V2: 0.67 for 2-wk-old, 0.70 for 4-wk-old, and 0.80 for adults) were substantially less variable (i.e., exhibiting less variance-to-mean ratios) than their sustained responses (V1: 0.96 for 2-wk-old, 0.88 for 4-wk-old, and 0.90 for adults V2: 0.90 for 2-wk-old, 0.93 for 4-wk-old, and 0.93 for adults; sign-rank test, P < 0.01). The age-matched comparisons of variance-to-mean ratios between V1 and V2 yielded no significant differences (Kruskal-Wallis test, P > 0.3). The ratios in our adults are similar to the previously published values (e.g., Hedge and Van Essen 2003; Muller et al. 2001; Rust et al. 2002).
Interestingly, the median variance-to-mean ratios for the transient responses of V1 and V2 neurons were clearly lower in both 2- and 4-wk-old infants than in adults (Kruskal-Wallis test, P < 0.01). However, the median ratios for sustained responses of either V1 or V2 neurons were virtually the same for infants and adults (Kruskal-Wallis test, P > 0.1). These results for the transient and sustained responses together suggest that the “better” variance-to-mean ratios in V1 of neonatal monkeys previously found by averaging responses over the entire stimulation period of 640 ms (Rust et al. 2002) reflect less noisy spike trains during the transient firing.
High detectability (d′) of transient responses in infants
In psychophysical detection tasks, subjects must know when or if stimuli appear against a background. Similarly cortical neurons must be able to initiate impulses that reliably rise above spontaneous impulses for successful detection of stimuli. To determine whether or not the observed robust transient responses of cortical neurons in infants can detect stimuli as precisely as in adults, we compared the firing rates during stimulus presentation (signal) with the firing rate in the absence of stimuli (noise) and calculated the detectability (d′) for individual neurons using the following formula: d′ = (μ1 − μ2)/√(σ12 + σ22)/2 (Muller et al. 2001) (Fig. 4). For this we assumed that the distribution of spikes in responses to stimulus presentation was largely Gaussian and thus the formula represents the difference between the mean firing rates, divided by SDs (Green and Swets 1966). The d′was calculated as a function of integration time from the response onset, defined as the time at which a unit's response significantly exceeded the noise level for three consecutive bins. The raster plots of responses during stimulation with a stationary grating (Fig. 4A) and during maintained discharge (B) and a resulting PSTH (C) are illustrated for a complex cell in V1 from an adult monkey to demonstrate how the detectability (d′) grows during stimulus presentation (Fig. 4D). The growth of d′ from response onset is rapid, reaching the value of 1.0 (i.e., 76% correct detection) in 21.5 ms (Td′1) and 2.0 (92% correct) in 39.5 ms (Td′2). The maximum d′ value of ∼4.0 was attained around the time when the peak firing was reached (compare Fig. 4, C and D) after which d′ remained relatively high. Consistent with previous observations (Muller et al. 2001), the maximum detectability was obtained during the transient discharge, and d′ declined slightly with longer stimulation.
In 2- and 4-wk-old infants, the transient onset discharges of V1 and V2 neurons were capable of detecting stimuli as well as in adults. The d′ values in representative V1 and V2 neurons increased at about a same rate and were as high as in adults during onset transients (Fig. 4, E and F). However, the d′ scores decreased substantially during sustained firing (by nearly 40% in 2-wk-old infants and by 20% in 4-wk-old infants), whereas in adults, the decrease in d′ with longer stimulation was negligible.
To characterize the time course of changes in detectability for our populations of V1 and V2 neurons, we compared the maximum d′ values achieved during the first 150 ms following response onset and the d′ value during the period of 150 ms at the end of 640-ms stimulus presentation (Fig. 5, A and B). The frequency distribution of the population of V1 and V2 neurons and the median maximum d′ values (colored lines) achieved during the first 150 ms following response onset in 2- and 4-wk-old infants were nearly identical to those in adults (Kruskal-Wallis test, P > 0.2 for V1 and P > 0.5 for V2; Fig. 5A). The time required to reach d′ = 1.0 (Td′1) and d′ = 2.0 (Td′2) in V1 and V2 was virtually the same for infants and adults (Kruskal-Wallis test, P > 0.9 and P > 0.4 for V2; Fig. 5C).
FIG. 5.

Population data for detectability (d′) of V1 and V2 neurons in infants and adults A: frequency distributions of maximum d′ achieved during the 1st 150 ms following response onset (transient) in V1 (left) and V2 (right) neurons in 2 (top)- and 4-wk-old (middle) infants and adults (bottom). Median values are indicated with colored lines. B: frequency histograms of d′ achieved during a period of 150 ms at the end of 640 ms of stimulus presentation (sustained) in V1 (left) and V2 (right) neurons in 2 (top)- and 4-wk-old (middle) infants and adults (bottom). |, median values. C: quartile values of Td′=1 (top) and Td′=2 (bottom) for V1 (left) and V2 (right) neurons in infants and adults. Numbers on top of the figure indicate sample size for each group.
During the period of 150 ms at the end of stimulus presentation, the median d′ values for V1 and V2 neurons in 2- and 4-wk-old infants (colored lines) were significantly lower than in adults (Kruskal-Wallis test, P < 0.01; Fig. 5B). Equally important was that the d′ values for V1 and V2 neurons significantly decreased as stimulation continued in all animal groups, i.e., the detectability during the sustained discharge was significantly poorer than that during the transient responses in infants and adults (Kruskal-Wallis test, P < 0.01 for both V1 and V2). Taken together, the maximum d′ achieved by V1 or V2 neurons largely occurred during the first 150 ms following response onset in both infants and adults, and, more importantly for this study, the d′ during the transient discharge in 2- and 4-wk-old infants were as high as in adults.
Complex timing of onset transient responses in infants
DELAYED ONSET LATENCIES.
Although the onset discharge was robust and reliable, infant's neurons exhibited small but significant delays in onset latencies. Onset latency was defined as the time between stimulus onset and the time at which a unit's response significantly exceeded the noise level for three consecutive bins (Fig. 6A) (Maunsell and Gibson 1992). The median onset latencies of V1 and V2 neurons (colored lines) were substantially longer (by ∼15 ms) in 2- and 4-wk-old infants than in adults (Kruskal-Wallis test, P < 0.01; Fig. 6B).
FIG. 6.
Complex timing of transient responses in infant V1 and V2. A: methods to measure onset latency and time-to-peak (time required for response to reach 95% of the peak from onset), and time constant. Top: raster plot of responses in an adult complex V2 cell during the first 200 ms of 640-ms stationary stimulus presentation. Bottom: PSTH for the same unit. See the text for details. B: frequency histograms of onset latencies in individual V1 (left) and V2 (right) units in 2 (top)- and 4-wk-old (middle) infants and adults (bottom). |, median onset latency. C: frequency histograms of time-to-peak latencies and median values for infants and adults. D: frequency histograms of time constants and median values for infants and adults.
MATURE ONSET-TO-PEAK LATENCIES.
The time required for a neuron's firing rate to reach its peak from response onset, onset-to-peak latency, in both V1 and V2 of 2- and 4-wk-old infants was not different from that in adults (Kruskal-Wallis test, P > 0.5 in V1 and V2; Fig. 6C). Thus once responses were initiated, the timing of the rising portion of transient responses in infants was adult-like. Interestingly, the onset-to-peak latencies of V2 neurons were significantly longer than in V1 for both infants and adults (Kruskal-Wallis test, P < 0.01).
FASTER DECAY OF DISCHARGES.
The response decay following the peak firing was more rapid in infants (shorter time constant, tc) than in adult units. Our population analysis supports this observation (Fig. 6D). The median decay time constants for both V1 and V2 of 2- and 4-wk-old infants were significantly shorter than in adults (Kruskal-Wallis test, P < 0.01). The delayed onset latency, adult-like peak firing rate, and faster decay of responses together resulted in shorter durations of transient discharges in infant V1 and V2 neurons compared with adults.
Contrast sensitivity of transient versus sustained discharges
Shorter decay constants revealed with high contrast (80%) stimuli suggest that the mechanisms that rapidly turn off the rising neuronal firing at stimulus onset could be more active in infants than in adults. Such robust mechanisms soon after birth may be closely associated with the lower response rates during the sustained firing and predict lower contrast sensitivity of the sustained discharges in infants. To determine whether lowering stimulus contrasts from 80% differentially affects the transient and sustained components of spike trains in infants and adults, we examined contrast versus response relations of individual V1 and V2 neurons (Fig. 7).
FIG. 7.

Developmental changes in contrast sensitivity during the onset transient and sustained firing of V1 and V2 neurons. A: analysis methods. PSTHs for responses of an adult V1 complex cell as a function of stimulus contrast. The discharge during a period of 150 ms following the response onset was analyzed for the transient component and the responses during 150 ms at the end of stimulation were analyzed for the sustained component. B: receiver operating characteristic (ROC) curves for the transient (left) and sustained (right) responses of the same cell as a function of contrast. C: probability functions for the transient (left) and sustained (right) component of responses from which threshold (α) and slope (β) values were determined. ···, the probability of 82% correct for determining the threshold value. See the detail in the text. D: representative probability functions of V1 (left) and V2 (right) neurons for 2 (top)- and 4-wk-old (middle) infants and adults (bottom). •, the transient responses; ○, the sustained responses for each unit.
Figure 7A illustrate our analysis methods using responses of a typical adult V1 complex cell. For each unit, the discharge during a period of 150 ms following the response onset was analyzed for the transient component and the responses during 150 ms at the end of stimulation were analyzed for the sustained component (Fig. 7A). To take noise in neuronal firing into consideration and to simulate neuronal “performance” as a psychophysical observer, we determined the “threshold” and the slope of each function by receiver operating characteristic (ROC) methods (Tolhurst et al. 1983). At each stimulus contrast and for each choice of a criterion for the unit, the percentage of trials with responses greater than the criterion (true positive) was plotted against the percentage of blank trials with response greater than the criterion (false positive; Fig. 7B). The probability of correctly discriminating a stimulus trial from blank trials was computed by integrating the ROCs and plotted against stimulus contrast and fitted into an accumulative form of Weibull function: P = 1 –0.5*exp [−(x/α)β], where P is the value of probability that the number of spikes in an interval will equal or exceed the certain criterion value, x is contrast, α represents contrast threshold, and β is the slope of the function. For units with no spontaneous activity, P is computed as P = 0.5 + 0.5*Pyn. Pyn is the probability that responses to stimulus trials are greater than one spike per trial. For this V1 neuron, the threshold (α) and slope (β) during the transient firing were much better (α = 0.08 and β = 2.86) than those during the sustained responses (α = 0.21 and β = 1.21; Fig. 7C). The estimate of threshold values (α) was made by selecting the lowest stimulus contrast at which the neuron's responses during stimulus presentation significantly exceeded its responses during blank presentation on 82% of trials (···).
For our representative V1 and V2 neuron from 2- and 4-wk-old infants, the unit's ability to detect “weak” stimuli (threshold) during their transient discharge (○) was as good as in adults (Fig. 7D). However, the threshold values for the sustained firing (•) were elevated compared with the thresholds for transient responses and were substantially higher in infants than in adults. For our population of units, scatter plots in Fig. 8 compare the values of α for the transient discharge with those for the sustained discharge for each unit. In nearly all units, the value of α for transient responses was significantly lower than that for sustained responses in infants and adults (sign rank test, P < 0.001 for both V1 and V2). More importantly, the median α values of V1 (12.57% for 2 wk and 13.66% for 4 wk) and V2 (12.72% for 2 wk and 11.53% for 4 wk) for transient responses of these infants were not significantly different from those in adults (11.16% for V1 and 12.62% for V2; Kruskal-Wallis test, P > 0.05).
FIG. 8.
Scatter plots of α values for the transient responses of V1 (left) and V2 (right) units against α values for their sustained responses in infants and adults. Frequency histograms and median values (▾) are also illustrated.
For the sustained responses, however, the median α values for 2-wk (52.66% for V1 and 63.70% for V2) and 4-wk-old infants (50.85% for V1 and 55.32% for V2) were significantly higher than in adults (34.11% for V1 and 31.02% for V2). Also a relatively large proportion of V1 and V2 neurons in infants had the highest α values during their sustained responses while thresholds for their transient discharge were adult like, i.e., between 8 and 30%. A small proportion of adult V2 units (7%) had similarly elevated α values for their sustained discharges, but these units also showed exceptionally high α values for their transient responses.
For nearly all units in infants and adults, the values of β for the transient responses of V1 and V2 units in infants were significantly better than those for the sustained responses (sign rank tests, P < 0.01; Fig. 9). As a result, the median β values of the transient discharges were significantly greater than those for the sustained responses for all age groups (Kruskal-Wallis test, P < 0.01). However, the median β values for both the transient and sustained responses of infant V1 or V2 neurons were similar to those in adults (Kruskal-Wallis test, P > 0.1).
FIG. 9.
Scatter plots of β values for the transient responses of V1 (left) and V2 (right) units against β values for their sustained responses in 2 (top)- and 4-wk-old (middle) infants and adults (bottom). Frequency histograms and median values (▾) are also illustrated.
The value of β for a given unit signifies the slope of the cell's contrast probability function around its contrast threshold value of α. However, the ability of this unit (as an “observer”) to detect small increments in stimulus contrast (contrast discriminability) must take its α value into consideration in addition to its β value. Here we define the contrast discriminability as the increase in detection probability caused by 1% increment in stimulus contrast at the steepest portion of the function (Fig. 10A). For example, two units with black and blue probability functions had very different β values (2.39 and 5.36) but exhibited the identical threshold α values (0.12). As a result, their contrast discriminability values were same as their β values 2.39 and 5.36, respectively. In contrast, two units with black and orange probability functions had the same β values (1.56) but their α values were very different (0.12 and 0.42). Consequently, their contrast discriminability values were substantially different from each other (2.39 and 0.68).
FIG. 10.

Contrast discriminability of transient and sustained discharge. A: discriminability vs. slope constant (β) values. Two units (black and blue) had the same α value (0.12) but different β values (2.39 and 6.39). Their discriminability values, defined as the increase in detection probability caused by 1.0% increment in stimulus contrast at the steepest portion of each function (right small panels) were same as their β values (2.39 and 6.39, respectively). Two units (black and orange) had the same β value (2.39) but their α values were considerably different (0.12 and 0.42). Consequently, their contrast discriminability values were also very different (2.39 and 0.68). B: scatter plots of discriminability values for the transient responses of V1 (left) and V2 (right) units against discriminability values for their sustained responses in 2 (top)- and 4-wk-old (middle) infants and adults (bottom). Frequency histograms and median values (▾) are also illustrated.
For all age groups, the contrast discriminability of nearly all units during the transient firing was far greater than that during the sustained responses (Kruskal-Wallis test, P < 0.01; Fig. 10B). More importantly the median contrast discriminability during the transient firing was as high in infants as in adults (Kruskal-Wallis test, P > 0.1 in V1 and P > 0.7 in V2). However, the median contrast discriminability during the sustained firing of V1 and V2 units was significantly lower in infants than in adults (Kruskal-Wallis test, P < 0.01).
Considered together, the contrast sensitivity of V1 and V2 neurons measured with transient discharges was largely adult-like near birth whereas the contrast sensitivity for sustained responses was remarkably low during the first 4 wk of monkey's life.
DISCUSSION
Development of transient versus sustained responses
At only 14 days after birth, the onset transient responses of V1 neurons are as responsive and as reliable as those in adults. The present results substantially differ from the sluggish responses commonly attributed to V1 neurons during the first several postnatal weeks in macaque monkeys (Blakemore and Vital-Durand 1981; Chino et al. 1997; Rust et al. 2002; Zhang et al. 2005). In most of these earlier studies, the visual stimuli consisted of high-contrast sine-wave gratings that were drifted for several seconds, and the responses were typically averaged over the entire stimulation period. Here we also found that the responsiveness and contrast sensitivity of V1 neurons during the sustained firing were relatively more sluggish in infants than that in adults although stimulus duration was only for a period of 640 ms. Thus the impoverished responses of infant's V1 neurons in the previous studies are likely to have reflected the reduced responsiveness and contrast sensitivity of their sustained responses.
The observed differential maturation of transient versus sustained responses in V1 is maintained and somewhat amplified in V2. Our previous work has found that the average response rates of V2 neurons to stimulation of their RF centers with drifting gratings are ∼40% lower for 640-ms stimulation (Zheng et al. 2007) and 70% lower for 3-s stimulation compared with V1 in 2-wk-old infant monkeys (Zhang et al. 2005). However, here we found that the transient onset discharges of V2 neurons in 2-wk-old infants to brief stationary gratings were as responsive as the transient responses in V1 of the same or age-matched infants and, more importantly, were as responsive as in V2 of adult monkeys. This result is remarkable because at this age, several other receptive-field properties of V2 neurons, when studied by averaging responses over the entire stimulation periods, are far more immature than V1 neurons (Zhang et al. 2005; Zheng et al. 2007).
Our results give strong evidence that the functional circuitry in V1 and V2 supporting the responsiveness and the reliability of transient onset discharges is surprisingly mature near birth. Apparently the known immaturities of retina (Hendrickson 1993; Hendrickson and Yuodelis 1984; Packer et al. 1990) and/or the LGN (Hawken et al. 1997; Movshon et al. 2005) have small impact on the transient onset responses of V1 or V2 neurons except for delays in onset latency for 2- and 4-wk-old infants (Fig. 4B). This latency delay may have resulted from longer integration time of neural signals within the retina and/or the LGN, delayed conduction between the retina and cortex, and from immaturities within cortical areas. Pinning down a source(s) of the delay is not possible because there are no comparable data in the literature for the precortical structures of age-matched monkeys. However, considering that once responses were initiated in V1 or V2 neurons, the time course of responses (onset-to-peak latency) was adult-like (Fig. 6C) and that the average integration time in the LGN measured with drifting gratings are slower by ∼10–15 ms in infants (similar delay to that in this study) than in adults (Movshon et al. 2005), the observed longer onset latencies may have largely reflected retinal and/or LGN immaturities.
Why are the sustained responses in infants sluggish? For adult V1 neurons, there are several mechanisms previously proposed to explain the rapid decline of firing rate following the peak discharge in response to sudden onset of stationary gratings. These physiological mechanisms include synaptic depression (Abbott et al. 1997; Boudreau and Ferster 2005; Carandini et al. 2002; ; Chance et al. 1998; Markram and Tsodyks 1996; Muller et al. 2001; Stratford et al. 1996; Varela et al. 1997), intrinsic membrane properties associated with conductance changes and membrane hyperpolarization (Carandini and Ferster 1997, 2000; Sanchez-Vives et al. 2000a,b) that are presumably responsible for contrast adaptation (Albrecht et al. 1984; Allison et al. 1993; Maffei et al. 1973; Marlin et al. 1988; Movshon and Lennie 1979; Ohzawa et al. 1985; Sclar et al. 1989), and a cortical network of inhibitory neurons (Ahmed et al. 1997; Boudreau and Ferster 2005; Dealy and Tolhurst 1974; Ohzawa et al. 1985; Vidyasagar 1990; Wilson and Humanski 1993). Obviously these mechanisms are not mutually exclusive. Also the degree to which some of these mechanisms are involved in rapid decline of firing for in vivo preparations, e.g., thalamocortical synaptic depression, is a matter of considerable debate (Boudreau and Ferster 2005; Muller et al. 2001).
Regardless, one or more of these suppressive mechanisms are likely to be more active in visual cortex soon after birth. For short-term (e.g., <1–2 s) suppressive effects, synaptic depression (Boudreau and Ferster 2005; Chance et al. 1998; Muller et al. 1999, 2001) appears to be a more plausible mechanism for the depressed responsiveness of the sustained responses in infants of this study. This type of synaptic depression is thought to be associated with rapid depletion of neurotrasmitters at thalamocoritcal and/or corticocortical synapses (Boudreau and Ferster 2005; Chance et al. 1998; Varela et al. 1997). The shorter decay constants in 2- and 4-wk-old infants compared with that in adults (Fig. 6D) could be a sign of more robust synaptic depression in infant visual cortex, reflected in the observed weaker sustained responses. Elevated contrast threshold and lower contrast discriminability of the sustained responses of infant units (Figs. 8 and 10B, respectively) or their enhanced transient responses in this study and surprisingly high temporal resolutions V1 and V2 neurons in our previous study (Zheng et al. 2007) are all consistent with the presence of high levels of synaptic depression and nonlinear temporal dynamics in V1 and V2 of our infants (Geisler 1983; Chance et al. 1998; Muller et al. 2001).
If short-term synaptic depression is largely responsible for the impoverished sustained responses in infants of this study, synaptic depression at corticocortical synapses rather than thalamocortical synapses could be involved (Boudreau and Ferster 2005). The great majority of V1 units in this study were located either in supra- or infragranular layers, and most V2 neurons typically do not receive direct LGN inputs (Bullier 2004). Depression at corticocortical synapses, however, may also involve “polysynaptic” excitatory circuits and intrinsic local inhibitory interneurons (Boudreau and Ferster 2005; Chung and Ferster 1998; Kara et al. 2002), and some or all of these elements in infant visual cortex could be immature. More specifically, synaptic depression at corticocortical synapses could be more robust due to larger depletion of excitatory transmitters. Alternatively, inhibitory network that presumably “shunts” excitatory polysynaptic neurons or inhibits presynaptic excitatory neurons (Boudreau and Ferster 2005) are more functionally active in visual cortex soon after birth. There is some evidence that inhibitory circuitry in V1 matures relatively earlier than excitatory neuronal circuitry during the first several weeks of monkey's life (Endo et al. 2000; Shaw et al. 1991).
The sluggish sustained responses in V1 units of neonatal monkeys previously reported during prolonged exposure to high-contrast drifting gratings (Blakemore and Vital-Durand 1981; Chino et al. 1997; Rust et al. 2002; Zhang et al. 2005) could also reflect “immature” intrinsic membrane properties of cortical neurons that initiate more robust membrane hyperpolarization and hence, stronger and longer-lasting contrast adaptation (Carandini and Ferster 1997, 2000; Carandini et al. 2002; Sanchez-Vives et al. 2000a,b). Some investigators, however, suggested that a slow form of synaptic depression could also contribute to contrast adaptation in adult V1 (Abbott et al. 1997; Chance et al. 1998; Nelson 1991; Todorov et al. 1997). Considered together, all of these mechanisms, synaptic depression, intrinsic membrane properties, and a network of inhibitory neurons, could potentially contribute to a different degree in lowering the responsivity and contrast sensitivity of sustained responses in V1 and V2 during early development.
Perceptual significance
The present findings provide a considerably different perspective on early neural and perceptual development in primates from traditional views on this issue (Blakemore 1990; Boothe et al. 1985; Chino et al. 2004; Kiorpes and Movshon 2004). Our results suggest that depending on the nature of visual stimuli, visual cortical neurons in young infants are capable of functioning at near adult-like levels. Consequently, infants may be able to perform in certain visual detection or discrimination tasks far better than previously thought. Temporal resolving power or critical flicker-fusion frequency (CFF) measured with stationary high-contrast, low-frequency gratings is surprisingly mature in 2–4 mo-old human infants (2–4 wk-old monkeys) (Regal 1981; Dobkins et al. 1999). In monkeys, the qualitatively adult like CFFs were recently found in 1- to 4-wk-old infants (Stavros and Kiorpes 2008). Consistent with this behavioral observation, we previously found that a considerable proportion of V1 and V2 neurons exhibit the temporal resolution >40 Hz as early as 2 wk of age, although the average resolution in these infants is substantially lower than in adults (Zheng et al. 2007).
Fixating eye movements (saccades) of human infants are relatively mature near birth. Although saccade latency is substantially slower in infants than in adults (Aslin and Salapatek 1975; Reznick et al. 2000; Richards and Holley 1999), qualitatively adult-like saccades are present in newborn babies (Aslin and Salapatek 1975) and as early as 2–3 mo of age, adult-like amplitudes and velocities are attained in “reactive saccades” (Garbutt et al. 2006; Hainline et al. 1984; Matsuzawa and Shimojo 1997; Richards and Holley 1999; Taga et al. 2002). The present results represent the first and only concrete evidence that the visual signals in the early stages of cortical processing (i.e., V1 and V2) that are required for fixating saccade initiation are robust and very reliable by ≥14 days after birth, if not at birth, in monkeys.
Visual fixation and saccades are most frequently used as effective tools to measure visual sensitivity and cognitive development in neonates and infants. As mentioned earlier, newborn infants can competently discriminate between visual objects made up of simple shapes of high-contrast and/or colors (Banks and Salapatek 1978; Frantz 1963; Taga et al. 2002). However, saccades that require cognitive discrimination and/or attention may not develop as early as fixating saccades (Aslin 2007; Garbutt et al. 2006; Kourtzi and DiCarlo 2006). This is because cognitive skills and visual attention presumably depend on information processing in cortical mechanisms of higher-order visual areas (Blake and Logothetis 2002; Britten et al. 1996; Celebrini and Newsome 1994; Cook and Maunsell 2002; Nienborg and Cumming 2006; Tong et al. 1998), and its functional maturation is known to be considerably delayed during early development (Barone et al. 1995; Distler et al. 1996; Kiorpes and Bassin 2003; Kiorpes and Movshon 2004; Kovacs et al. 1999; Rodman et al. 1991; Zhang et al. 2005).
Considered together, it is safe to conclude that the low-level mechanisms in the visual brain of infant primates are sufficiently well developed to detect many “simple” objects in their visual scenes and that infant's visual world could be much more robust than traditionally thought.
GRANTS
This work was supported by National Institutes of Health Grants EY-08128, EY-03611, and RR-07146.
Acknowledgments
We thank S. Stevenson and H. Bedell for comments on an early draft.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Abbott et al. 1997.Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science 275: 220–224, 1997. [DOI] [PubMed] [Google Scholar]
- Ahmed et al. 1997.Ahmed B, Allison JD, Douglas RJ, Martin KA. An intracellular study of the contrast-dependence of neuronal activity in cat visual cortex. Cereb Cortex 7: 559–570, 1997. [DOI] [PubMed] [Google Scholar]
- Albrecht et al. 1984.Albrecht DG, Farrar SB, Hamilton DB. Spatial contrast adaption characteristics of neurons recorded in cat's visual cortex. J Physiol 347: 713–739, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison et al. 1993.Allison JD, Casagrande VA, Debruyn EJ, Bonds AB. Contrast adaptation in striate cortical neurons of the nocturnal primate bush baby (Galago crassicaudatus). Vis Neurosci 10: 1129–1139, 1993. [DOI] [PubMed] [Google Scholar]
- Aslin 2007.Aslin RN What's in a look? Dev Sci 10: 48–53, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslin and Salapatek 1975.Aslin RN, Salapatek P. Saccadic localization of visual targets by the very young human infants. Percept Psychophys 17: 293–302, 1975. [Google Scholar]
- Bair et al. 2003.Bair W, Cavanaugh JR, et al. Time course and time-distance relationships for surround suppression in macaque V1 neurons. J Neurosci 23: 7690–7701, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks and Salapatek 1978.Banks MS, Salapatek P. Acuity and contrast sensitivity in 1-, 2-, and 3-month-old human infants. Invest Ophthalmol Vis Sci 17: 361–365, 1978. [PubMed] [Google Scholar]
- Barone et al. 1995.Barone P, Dehay C, Berland M, Bullier J, Kennedy H. Developmental remodeling of primate visual cortical pathways. Cereb Cortex 5: 22–38, 1995. [DOI] [PubMed] [Google Scholar]
- Blake and Logothetis 2002.Blake R, Logothetis NK. Visual competition. Nat Rev Neurosci 3: 13–21, 2002. [DOI] [PubMed] [Google Scholar]
- Blakemore 1990.Blakemore C Maturation of mechanisms for efficient spatial vision. In: Vision: Coding and Efficiency, edited by Blakemore C. Cambridge, UK: Cambridge Univ. Press, 1990, p. 254–256.
- Blakemore and Vital-Durand 1981.Blakemore C, Vital-Durand F. Postnatal development of the monkey's visual system. Ciba Found Symp 86: 151–171, 1981. [DOI] [PubMed] [Google Scholar]
- Boothe et al. 1985.Boothe RG, Dobson V, Teller DY. Postnatal development of vision in human and non-human primates. Annu Rev Neurosci 8: 495–545, 1985. [DOI] [PubMed] [Google Scholar]
- Boudreau and Ferster 2005.Boudreau CE, Ferster D. Short-term depression in thalamocortical synapses of cat primary visual cortex. J Neurosci 25: 7179–7190, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten et al. 1996.Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis Neurosci 13: 87–100, 1996. [DOI] [PubMed] [Google Scholar]
- Bullier et al. 2004.Bullier J, Chalupa LM, Werner JS. Communication between cortical areas of the visual system. In: The Visual Neuroscience. Cambridge, MA: 2004, p. 522–540.
- Carandini and Ferster 1997.Carandini M, Ferster D. A tonic hyperpolarization underlying contrast adaptation in cat visual cortex. Science 276: 949–952, 1997. [DOI] [PubMed] [Google Scholar]
- Carandini and Ferster 2000.Carandini M, Ferster D. Membrane potential and firing rate in cat primary visual cortex. J Neurosci 20: 470–484, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandini et al. 2002.Carandini RG, Heeger DJ, Senn W. A synaptic explanation of suppression in visual cortex. J Neurosci 22: 10053–10065, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celebrini and Newsome 1994.Celebrini S, Newsome WT. Neuronal and psychophysical sensitivity to motion signals in extrastriate area MST of the macaque monkey. J Neurosci 14: 4109–4124, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance et al. 1998.Chance FS, Nelson SB, Abbott LF. Synaptic depression and the temporal response characteristics of V1 cells. J Neurosci 18: 4785–4799, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chino, et al. 2004.Chino, Y, Bi H, Zhang B. Normal and abnormal development of the neuronal response properties in primate visual cortex. In: The Primate Visual System, edited by Kaas JH, Collins CE. Boca Raton, FL: CRC, 2004, p. 81–108.
- Chino et al. 1997.Chino YM, Smith WL 3rd, Hatta S, Cheng H. Postnatal development of binocular disparity sensitivity in neurons of the primate visual cortex. J Neurosci 17: 296–307, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung and Ferster 1998.Chung S, Ferster D. Strength and orientation tuning of the thalamic input to simple cells revealed by electrically evoked cortical suppression. Neuron 20: 1177–1189, 1998. [DOI] [PubMed] [Google Scholar]
- Cook and Maunsell 2002.Cook EP, Maunsell JH. Attentional modulation of behavioral performance and neuronal responses in middle temporal and ventral intraparietal areas of macaque monkey. J Neurosci 22: 1994–2004, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dealy and Tolhurst 1974.Dealy RS, Tolhurst DJ. Is spatial adaptation an aftereffect of prolonged inhibition? J Physiol 241: 261–270, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler et al. 1996.Distler C, Bachevalier J, Kennedy C, Mishkin M, Ungerleider LG. Functional development of the corticocortical pathway for motion analysis in the macaque monkey: a 14C-2-deoxyglucose study. Cereb Cortex 6: 184–195, 1996. [DOI] [PubMed] [Google Scholar]
- Dobkins et al. 1999.Dobkins KR, Anderson CM, Lia B. Infant temporal contrast sensitivity functions (tCSFs) mature earlier for luminance than for chromatic stimuli: evidence for precocious magnocellular development? Vision Res 39: 3223–3239, 1999. [DOI] [PubMed] [Google Scholar]
- Dragoi et al. 2002.Dragoi V, Sharma J, Miller EK, Sur M. Dynamics of neuronal sensitivity in visual cortex and local feature discrimination. Nat Neurosci 5: 883–891, 2002. [DOI] [PubMed] [Google Scholar]
- Endo et al. 2000.Endo M, Kaas JH, Jain N, Smith EL 3rd, Chino Y. Binocular cross-orientation suppression in the primary visual cortex (V1) of infant rhesus monkeys. Invest Ophthalmol Vis Sci 41: 4022–4031, 2000. [PubMed] [Google Scholar]
- Epelboim et al. 1994.Epelboim J, Booth JR, Steinman M. Reading unspaced text: implications for theories of reading eye movements. Vision Res 34: 1735–1766, 1994. [DOI] [PubMed] [Google Scholar]
- Frantz 1963.Frantz RL Pattern vision in newborn infants. Science 140: 296–297, 1963. [DOI] [PubMed] [Google Scholar]
- Garbutt et al. 2006.Garbutt S, Harwood MR, Harris CM. Infant saccades are not slow. Dev Med Child Neurol 48: 662–667, 2006. [DOI] [PubMed] [Google Scholar]
- Geisler 1983.Geisler WS Mechanisms of visual sensitivity: backgrounds and early dark adaptation. Vision Res 23: 1423–1432, 1983. [DOI] [PubMed] [Google Scholar]
- Green 1966.Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York: Wiley, 1966.
- Hainline et al. 1984.Hainline L, Turkel J, Abramov I, Lemerise E, Harris CM. Characteristics of saccades in human infants. Vision Res 24: 1771–1780, 1984. [DOI] [PubMed] [Google Scholar]
- Harwerth and Levi 1978.Harwerth RS, Levi DM. Reaction time as a measure of suprathreshold grating detection. Vision Res 18: 1579–1586, 1978. [DOI] [PubMed] [Google Scholar]
- Hatta et al. 1998.Hatta S, Kumagami T, Qian J, Thornton M, Smith EL, 3rd, Chino YM. Nasotemporal directional bias of V1 neurons in young infant monkeys. Invest Ophthalmol Vis Sci 39: 2259–2267, 1998. [PubMed] [Google Scholar]
- Hawken et al. 1997.Hawken MJ, Blakemore C, Morley JW. Development of contrast sensitivity and temporal-frequency selectivity in primate lateral geniculate nucleus. Exp Brain Res 114: 86–98, 1997. [DOI] [PubMed] [Google Scholar]
- Hegde and Van Essen 2003.Hegde J, Van Essen DC. Strategies of shape representation in macaque visual area V2. Vis Neurosci 20: 313–328, 2003. [DOI] [PubMed] [Google Scholar]
- Hendrickson 1993.Hendrickson AE Morphological development of the primate retina. In: Early Visual Development, Normal and Abnormal, edited by Simons K. New York: Oxford, 1993, p. 287–295.
- Hendrickson and Yuodelis 1984.Hendrickson AE, Yuodelis C. The morphological development of the human fovea. Ophthalmology 91: 603–612, 1984. [DOI] [PubMed] [Google Scholar]
- Kara et al. 2002.Kara P, Pezaris JS, Yurgenson S, Reid RC. The spatial receptive field of thalamic inputs to single cortical simple cells revealed by the interaction of visual and electrical stimulation. Proc Natl Acad Sci USA 99: 16261–16266, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesey 1960.Keesey UT Effects of involuntary eye movements on visual acuity. J Opt Soc Am 50: 769–774, 1960. [DOI] [PubMed] [Google Scholar]
- Kiorpes and Bassin 2003.Kiorpes L, Bassin SA. Development of contour integration in macaque monkeys. Vis Neurosci 20: 567–575, 2003. [DOI] [PubMed] [Google Scholar]
- Kiorpes et al. 2004.Kiorpes L, Movshon JA, Chalupa LM, Werner JS. The Visual Neuroscience. Cambridge, MA, 2004, p. 159–73.
- Kiorpes and Movshon 2004.Kiorpes L, Movshon JA. Development of sensitivity to visual motion in macaque monkeys. Vis Neurosci 21: 851–859, 2004. [DOI] [PubMed] [Google Scholar]
- Kourtzi and DiCarlo 2006.Kourtzi Z, DiCarlo JJ. Learning and neural plasticity in visual object recognition. Curr Opin Neurobiol 16: 152–158, 2006. [DOI] [PubMed] [Google Scholar]
- Kovacs et al. 1999.Kovacs I, Kozma P, Feher A, Benedek G. Late maturation of visual spatial integration in humans. Proc Natl Acad Sci USA 96: 12204–12209, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei et al. 1973.Maffei L, Fiorentini A, Bisti S. Neural correlate of perceptual adaptation to gratings. Science 182: 1036–1038, 1973. [DOI] [PubMed] [Google Scholar]
- Markram and Tsodyks 1996.Markram H, Tsodyks M. Redistribution of synaptic efficacy between neocortical pyramidal neurons. Nature 382: 807–810, 1996. [DOI] [PubMed] [Google Scholar]
- Marlin et al. 1988.Marlin SG, Hasan SJ, Cynader MS. Direction-selective adaptation in simple and complex cells in cat striate cortex. J Neurophysiol 59: 1314–1330, 1988. [DOI] [PubMed] [Google Scholar]
- Matsuzawa and Shimojo 1997.Matsuzawa M, Shimojo S. Infant's fast saccades in the gap paradigm and development of visual attention. Infant Behav Dev 20: 449–455, 1997. [Google Scholar]
- Maunsell and Gibson 1992.Maunsell JH, Gibson JR. Visual response latencies in striate cortex of the macaque monkey. J Neurophysiol 68: 1332–1344, 1992. [DOI] [PubMed] [Google Scholar]
- Mechler et al. 1998.Mechler F, Victor JD, Purpura KP, Shapley R. Robust temporal coding of contrast by V1 neurons for transient but not for steady-state stimuli. J Neurosci 18: 6583–6598, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon et al. 2005.Movshon JA, Kiorpes L, Hawken MJ, Cavanaugh JR. Functional maturation of the macaque's lateral geniculate nucleus. J Neurosci 25: 2712–2722, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon and Lennie 1979.Movshon JA, Lennie P. Pattern-selective adaptation in visual cortical neurons. Nature 278: 850–852, 1979. [DOI] [PubMed] [Google Scholar]
- Muller et al. 1999.Muller JR, Metha AB, Krauskopf J, Lennie P. Rapid adaptation in visual cortex to the structure of images. Science 285: 1405–1408, 1999. [DOI] [PubMed] [Google Scholar]
- Muller et al. 2001.Muller JR, Metha AB, Krauskopf J, Lennie P. Information conveyed by onset transients in responses of striate cortical neurons. J Neurosci 21: 6978–6990, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson 1991.Nelson SB Temporal interactions in the cat visual system. II. Suppressive and facilitatory effects in the lateral geniculate nucleus. J Neurosci 11: 357–368, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienborg and Cumming 2006.Nienborg H, Cumming BG. Macaque V2 neurons, but not V1 neurons, show choice-related activity. J Neurosci 26: 9567–9578, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohzawa et al. 1985.Ohzawa I, Sclar G, Freeman RD. Contrast gain control in the cat's visual system. J Neurophysiol 54: 651–667, 1985. [DOI] [PubMed] [Google Scholar]
- Packer et al. 1990.Packer O, Hendrickson AE, Curcio CA. Developmental distribution of photoreceptors across the Macaca nemestrina (pigtail macaque) retina. J Comp Neurol 298: 472–493, 1990. [DOI] [PubMed] [Google Scholar]
- Palanca and DeAngelis 2003.Palanca BJ, DeAngelis GC. Macaque middle temporal neurons signal depth in the absence of motion. J Neurosci 23: 7647–7658, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regal 1981.Regal DM Development of critical flicker frequency in human infants. Vision Res 21: 549–555, 1981. [DOI] [PubMed] [Google Scholar]
- Reznick et al. 2000.Reznick JS, Chawarska K, Betts S. The development of visual expectations in the first year. Child Dev 71: 1191–1204, 2000. [DOI] [PubMed] [Google Scholar]
- Richards and Holley 1999.Richards JE, Holley FB. Infant attention and the development of smooth pursuit tracking. Dev Psychol 35: 856–867, 1999. [DOI] [PubMed] [Google Scholar]
- Rodman et al. 1991.Rodman HR, Skelly JP, Gross CG. Stimulus selectivity and state dependence of activity in inferior temporal cortex of infant monkeys. Proc Natl Acad Sci USA 88: 7572–7575, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roufs 1972.Roufs JA Dynamic properties of vision. I. Experimental relationships between flicker and flash thresholds. Vision Res 12: 261–278, 1972. [DOI] [PubMed] [Google Scholar]
- Rust et al. 2002.Rust NC, Schultz SR, Movshon JA. A reciprocal relationship between reliability and responsiveness in developing visual cortical neurons. J Neurosci 22: 10519–10523, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vives et al. 2000.Sanchez-Vives MV, Nowak LG, McCormick DA. Cellular mechanisms of long-lasting adaptation in visual cortical neurons in vitro. J Neurosci 20: 4286–4299, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vives et al. 2000.Sanchez-Vives MV, Nowak LG, McCormick DA. Membrane mechanisms underlying contrast adaptation in cat area 17 in vivo. J Neurosci 20(11):4267–85, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclar et al. 1989.Sclar G, Lennie P, DePriest DD. Contrast adaptation in striate cortex of macaque. Vision Res 29: 747–755, 1989. [DOI] [PubMed] [Google Scholar]
- Shadlen and Newsome 1998.Shadlen MN, Newsome WT. The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J Neurosci 18: 3870–3896, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw et al. 1991.Shaw C, Cameron L, March D, Cynader M, Zielinski B, Hendrickson A. Pre- and postnatal development of GABA receptors in Macaca monkey visual cortex. J Neurosci 11: 3943–3959, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortess and Krauskopf 1961.Shortess GK, Krauskopf J. Role of involuntary eye-movement in stereoscopic acuity. J Opt Soc Am 51: 555–559, 1961. [Google Scholar]
- Skottun et al. 1991.Skottun BC, De Valois RL, Grosof DH, Movshon JA, Albrecht DG, Bonds AB. Classifying simple and complex cells on the basis of response modulation. Vision Res 31: 1079–1086, 1991. [DOI] [PubMed] [Google Scholar]
- Stratford et al. 1996.Stratford KJ, Tarczy-Hornoch K, Martin KA, Bannister NJ, Jack JJ. Excitatory synaptic inputs to spiny stellate cells in cat visual cortex. Nature 382: 258–261, 1996. [DOI] [PubMed] [Google Scholar]
- Taga et al. 2002.Taga G, Ikejiri T, Tachibana T, Shimojo S, Soeda A, Takeuchi K, Konishi Y. Visual feature binding in early infancy. Perception 31: 273–286, 2002. [DOI] [PubMed] [Google Scholar]
- Todorov et al. 1997.Todorov EV, Siapas AG, Somers DC, Nelson SB. Modeling visual cortical contrast adaptation effects. In: Computational Neuroscience, Trends in Research, edited by Bower JM. New York: Plenum, 1997, p. 525–531.
- Tolhurst et al. 1983.Tolhurst DJ, Movshon JA, Dean AF. The statistical reliability of signals in single neurons in cat and monkey visual cortex. Vision Res 23: 775–785, 1983. [DOI] [PubMed] [Google Scholar]
- Tong et al. 1998.Tong F, Nakayama K, Vaughan JT, Kanwisher N. Binocular rivalry and visual awareness in human extrastriate cortex. Neuron 21: 753–759, 1998. [DOI] [PubMed] [Google Scholar]
- Tovee et al. 1993.Tovee MJ, Rolls ET, Treves A, Bellis RP. Information encoding and the responses of single neurons in the primate temporal visual cortex. J Neurophysiol 70: 640–654, 1993. [DOI] [PubMed] [Google Scholar]
- Varela et al. 1997.Varela JA, Sen K, Gibson J, Fost J, Abbott LF, Nelson SB. A quantitative description of short-term plasticity at excitatory synapses in layer 2/3 of rat primary visual cortex. J Neurosci 17: 7926–7940, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidyasagar 1990.Vidyasagar TR Pattern adaptation in cat visual cortex is a co-operative phenomenon. Neuroscience 36: 175–179, 1990. [DOI] [PubMed] [Google Scholar]
- Wilson and Humanski 1993.Wilson HR, Humanski R. Spatial frequency adaptation and contrast gain control. Vision Res 33: 1133–1149, 1993. [DOI] [PubMed] [Google Scholar]
- Zhang et al. 2005.Zhang B, Zheng J, Watanabe I, Maruko I, Bi H, Smith EL 3rd, Chino YM. Delayed maturation of receptive field center/surround mechanisms in V2. Proc Natl Acad Sci USA 102: 5862–5867, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng et al. 2007.Zheng J, Zhang B, Bi H, Maruko I, Watanabe I, Nakatsuka C, Smith EL 3rd, Chino YM. Development of temporal response properties and contrast sensitivity of V1 and V2 neurons in macaque monkeys. J Neurophysiol 97: 3905–3916, 2007. [DOI] [PubMed]
- Zohary et al. 1990.Zohary E, Hillman P, Hochstein S. Time course of perceptual discrimination and single neuron reliability. Biol Cybern 62: 475–486, 1990. [DOI] [PubMed] [Google Scholar]