Abstract
Dopamine-glutamate interactions in the striatum are critical for normal basal ganglia-mediated control of movement. Although regulation of glutamatergic transmission by dopamine is increasingly well understood, regulation of dopaminergic transmission by glutamate remains uncertain given the apparent absence of ionotropic glutamate receptors on dopaminergic axons in dorsal striatum. Indirect evidence suggests glutamatergic regulation of striatal dopamine release is mediated by a diffusible messenger, hydrogen peroxide (H2O2), generated downstream from glutamatergic AMPA receptors (AMPARs). The mechanism of H2O2-dependent inhibition of dopamine release involves activation of ATP-sensitive K+ (KATP) channels. However, the source of modulatory H2O2 is unknown. Here, we used whole cell recording, fluorescence imaging of H2O2, and voltammetric detection of evoked dopamine release in guinea pig striatal slices to examine contributions from medium spiny neurons (MSNs), the principal neurons of striatum, and dopamine axons to AMPAR-dependent H2O2 generation. Imaging studies of H2O2 generation in MSNs provide the first demonstration of AMPAR-dependent H2O2 generation in neurons in the complex brain-cell microenvironment of brain slices. Stimulation-induced increases in H2O2 in MSNs were prevented by GYKI-52466, an AMPAR antagonist, or catalase, an H2O2 metabolizing enzyme, but amplified by mercaptosuccinate (MCS), a glutathione peroxidase inhibitor. By contrast, dopamine release evoked by selective stimulation of dopamine axons was unaffected by GYKI-52466 or MCS, arguing against dopamine axons as a significant source of modulatory H2O2. Together, these findings suggest that glutamatergic regulation of dopamine release via AMPARs is mediated through retrograde signaling by diffusible H2O2 generated in striatal cells, including medium spiny neurons, rather than in dopamine axons.
INTRODUCTION
As a central component of the basal ganglia (Albin et al. 1989; Kemp and Powell 1971a), the striatum receives glutamatergic input from most of the cerebral cortex as well as from the thalamus (Berendse and Groenewegen 1990; Gerfen and Wilson 1996; Graybiel et al. 1994; Kemp and Powell 1971b; McGeorge and Faull 1989). This excitatory input forms asymmetric synapses on the heads of dendritic spines of medium spiny neurons (MSNs), which are the principal neurons of the striatum that integrate incoming cortical activity and encode striatal output (Graybiel et al. 1994; Wilson 1993). Importantly, MSNs also receive synaptic dopamine input from midbrain dopamine neurons (Freund et al. 1984; Smith and Bolam 1990). Converging glutamatergic and dopaminergic afferents control striatal network output at the level of individual spines to regulate motor and cognitive function (e.g., Cagniard et al. 2006; Costa et al. 2006). Despite the lack of direct synaptic associations between glutamatergic and dopaminergic axons (Freund et al. 1984; Smith and Bolam 1990), their apposition on MSN spines and their complementary functions suggest a reciprocal modulatory relationship.
Evidence for dopamine-dependent regulation of glutamate transmission is well established, including inhibition of glutamate release via D2 dopamine receptors on corticostriatal afferents (Bamford et al. 2004a,b; Cepeda et al. 2001). This regulation by dopamine occurs through volume transmission (Cragg and Rice 2004; Fuxe and Agnati 1991; Garris et al. 1994; Rice 2000; Rice and Cragg 2008) with synaptic spillover of dopamine facilitated by the location of dopamine transporters only on dopaminergic axons and its actions mediated by predominantly extrasynaptic dopamine receptors (Cragg and Rice 2004; Nirenberg et al. 1996; Rice and Cragg 2008; Sesack et al. 1994; Yung et al. 1995). How synaptically released glutamate regulates striatal dopamine release is much less clear. Glutamatergic transmission is primarily “hard-wired” with synapses surrounded by neuronal and glial transporters that limit glutamate spillover and ensure primary activation of subsynaptic ionotropic receptors (Barbour 2001; Bergles et al. 1999; Danbolt 2001; Galvan et al. 2006; Rusakov et al. 1999). When glutamate spillover does occur, e.g., after uptake inhibition, dopamine release can be inhibited via metabotropic glutamate receptors on dopaminergic axons (Zhang and Sulzer 2003). However, any effect of ionotropic glutamate-receptor activation on dopamine release must be indirect given that dopaminergic axons lack these receptors (Bernard and Bolam 1998; Bernard et al. 1997; Chen et al. 1998).
Indirect evidence suggests that glutamate-dependent regulation of striatal dopamine release involves a diffusible messenger, hydrogen peroxide (H2O2), which is generated downstream from glutamatergic AMPA receptors (AMPARs) and which activates ATP-sensitive K+ channels (KATP) to inhibit dopamine release (Avshalumov et al. 2003; Avshalumov and Rice 2003). The source of modulatory H2O2 is unknown, although the AMPAR dependence suggests that generation is unlikely to occur in dopaminergic axons. Here we tested the hypothesis that MSNs are an important source of modulatory H2O2 in striatum given that these cells constitute 95% of striatal neurons (Kemp and Powell 1971a) and express functional AMPARs (Bernard and Bolam 1998; Bernard et al. 1997; Carter and Sabatini 2004; Chen et al. 1998; Kita 1996). Using real-time fluorescence imaging of intracellular H2O2 in MSNs with simultaneous whole cell recording in striatal slices, we show that glutamate input to dorsolateral striatum acts via AMPARs to generate H2O2 in MSNs, which could provide a retrograde signal to inhibit axonal dopamine release. Companion voltammetric and imaging data indicate that dopaminergic axons do not contribute to the generation of modulatory H2O2.
METHODS
All animal handling procedures were in accordance with the National Institutes of Health guidelines and were approved by the New York University School of Medicine Animal Care and Use Committee.
Slice preparation
Procedures for slice preparation were similar to previously published methods (Avshalumov et al. 2005; Bao et al. 2005; Koós and Tepper 1999). Young adult male guinea pigs (Hartley, 150–250 g) were deeply anesthetized with sodium pentobarbital (50 mg/kg ip) and perfused transcardially with ∼30 mL of ice-cold modified artificial cerebrospinal fluid (ACSF) containing (in mM) 225 sucrose, 2.5 KCl, 0.5 CaCl2, 7 MgSO2, 28 NaHCO3, 1.25 NaH2PO4, 7 glucose, 1 ascorbate, and 3 pyruvate. After perfusion and decapitation, the brain was rapidly removed and immersed in this ice-cold modified ACSF for 1–2 min, then bisected and blocked before slicing on a Vibratome (Ted Pella, St. Louis, MO). Most whole cell recording and imaging studies were done in coronal slices. However, some slices were also cut in an angled parasagittal plane, at a 20–25° rostrolateral angle to the midline to preserve nigrostriatal dopaminergic fibers entering the striatum from the median forebrain bundle (see Fig. 4A). It should be noted that this angle is roughly perpendicular to incoming thalamostriatal fibers (Smeal et al. 2007), so that this slice orientation permits selective stimulation of the nigrostriatal dopamine pathway. Angled parasagittal slices were used for all dopamine recording experiments.
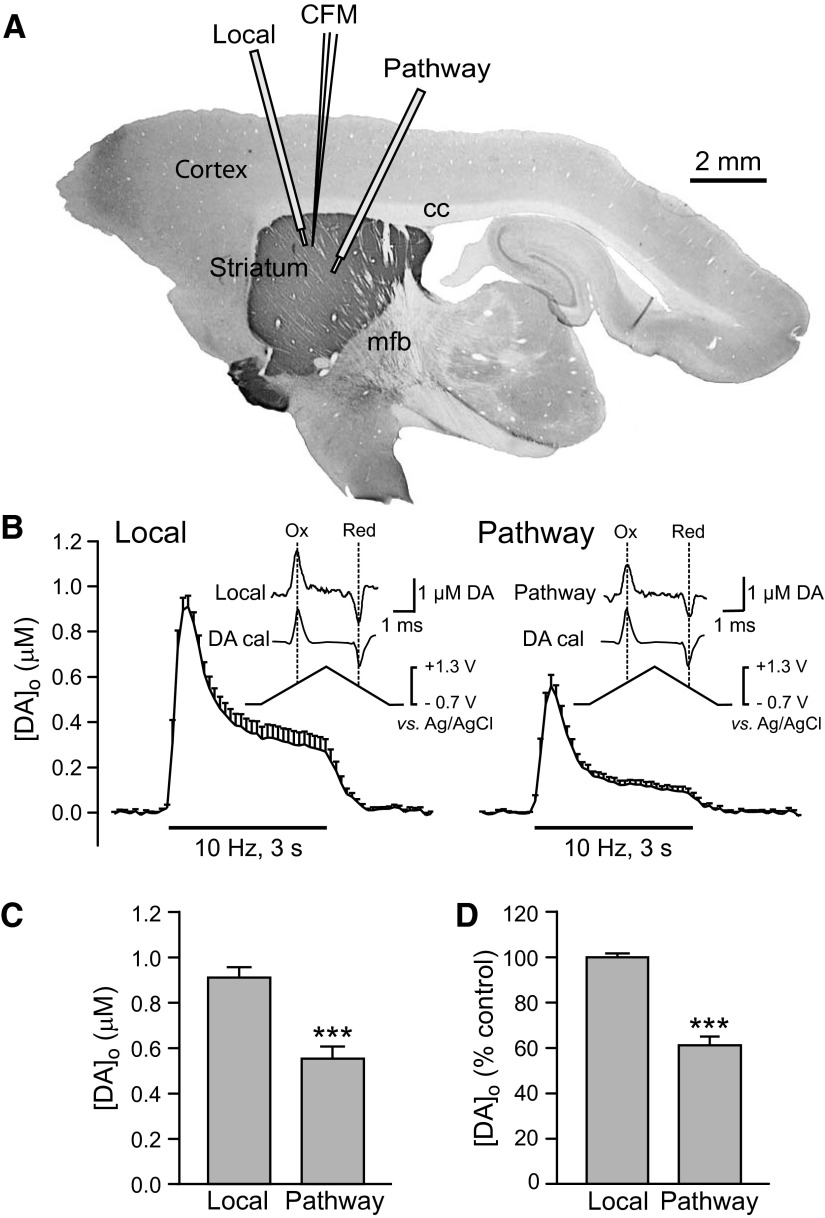
FIG. 4.
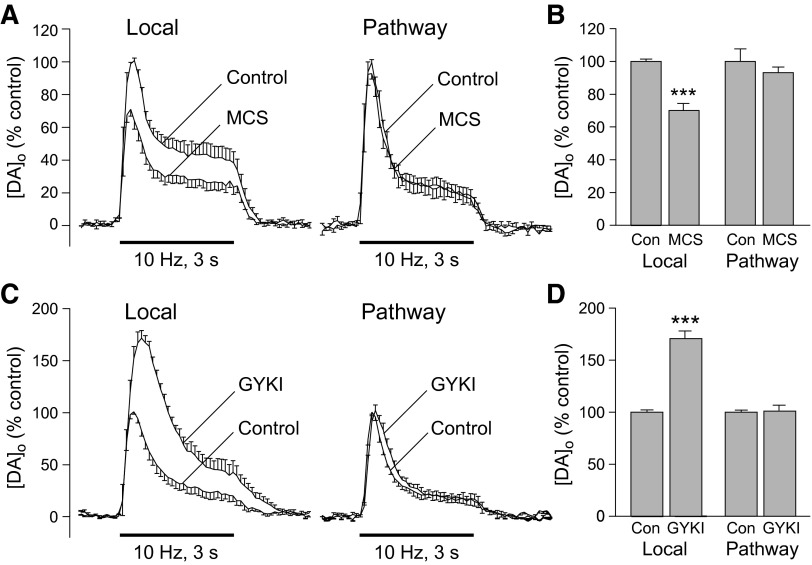
Comparison of striatal dopamine release evoked by local stimulation and by pathway stimulation of dopaminergic axons. A: orientation of stimulation electrodes and recording electrode for local stimulation and pathway stimulation of dopaminergic axons in an angled parasagittal section of guinea pig brain stained for tyrosine-hydroxylase immunoreactivity using methods published previously (Rice et al. 1997). B: average [DA]o vs. time profiles evoked at a single site by alternating local and pathway stimulation (30 pulses, 10 Hz). Data are means ± SE. Insets: the applied triangular waveform for fast-scan cyclic voltammetry at a carbon-fiber microelectrode with representative voltammograms for evoked [DA]o and dopamine calibration (DA cal) showing characteristic oxidation (Ox) and reduction (Red) peak potentials that confirm dopamine identity. C: average peak evoked [DA]o recorded at a given site with local or pathway stimulation; pathway evoked [DA]o was significantly lower than that evoked by local stimulation. (n = 12; ***P < 0.001 vs. local). D: average peak [DA]o evoked by local or pathway stimulation normalized to locally evoked release with locally evoked peak [DA]o taken as 100% (n = 12; ***P < 0.001 vs. local).
Visualized whole cell recording and H2O2 imaging
Striatal slices (300 μm thickness) were kept for 30 min at 34°C in a holding solution containing (in mM) 125 NaCl, 2.5 KCl, 1.0 MgCl2, 25 NaHCO3, 1.25 NaH2PO4, 25 glucose, 1 ascorbate, 3 pyruvate, and 2 CaCl2 at pH 7.3–7.4, saturated with 95% O2-5% CO2. The holding solution was then allowed to cool slowly to room temperature over ≥30 min before experimentation. For recording, slices were transferred to a submersion chamber (Warner Instruments LLC, Holliston, MA) maintained at 32°C and superfused at 1.2 mL/min with bicarbonate-buffered ACSF containing (in mM) 124 NaCl, 3.7 KCl, 26 NaHCO3, 1.3 MgSO2, 1.3 KH2PO4, 10 glucose, and 2.4 CaCl2 and saturated with 95% O2-5% CO2.
MSNs in dorsolateral striatum were identified visually under infrared differential interference contrast (IR-DIC) microscopy using an Olympus BX51WI fixed-stage microscope (New York/New Jersey Scientific, Middlebush, NJ) with a ×40 water-immersion objective. Patch pipettes for whole cell recording and dye loading were pulled from borosilicate glass capillaries (1.5 mm OD, 0.86 mm ID) on a Flaming/Brown model P-97 micropipette puller (Sutter Instruments, Novato, CA). Pipettes had open tip diameters of <2 μm and resistances of 3–8 MΩ. The intracellular filling solution contained (in mM) 120 K-gluconate, 20 KCl, 2 MgCl, 10 Na-HEPES, 10 EGTA, 2 Na2-ATP, and 0.2 GTP; pH adjusted to 7.2–7.3 with KOH, 280–290 mosM (Avshalumov et al. 2005; Bao et al. 2005; Koós and Tepper 1999). The intracellular solution also contained Alexa Red (0.1%) for cell visualization plus the fluorescent dye 2′7′ dichlorodihydrofluorescein (H2DCF) diacetate (7 μM) for H2O2 imaging (Avshalumov et al. 2005, 2007).
Imaging of intracellular H2O2 was as described previously (Avshalumov et al. 2005; Bao et al. 2005). Briefly, H2DCF diacetate was loaded into a given cell via the patch pipette used for simultaneous whole cell recording. Once a whole cell configuration was obtained, cell physiology was monitored for 15–20 min in current-clamp mode before images were taken to allow sufficient time for the dye to infiltrate the cell and cleavage by intracellular esterases to form H2DCF; H2DCF becomes fluorescent DCF after oxidation by H2O2 or other ROS (Avshalumov et al. 2005, 2007; Reynolds and Hastings 1995; Sah and Schwartz-Bloom 1999). Excitation wavelength for DCF was 488 nm with emission at 535 nm. To minimize DCF photooxidation, images were obtained at 500-ms intervals with 30-ms exposure using eight-frame averaging. Background fluorescence from an area adjacent to the recorded cell was subtracted from each averaged image for that cell. Fluorescence data are presented as [(stimulated intensity − basal)/(basal)] × 100%. Basal DCF fluorescence was taken as the average intensity recorded for 2 s immediately before stimulation. Stimulated intensity was determined from a 2-s average taken after the stimulus ended for comparison with the average basal fluorescence for each cell.
Activity-dependent H2O2 generation in MSNs was elicited using surface bipolar stimulating electrodes. In most experiments, local stimulation (<200 μm from a recorded cell) was used in coronal slices. However, angled parasagittal slices were used to compare the effects of local stimulation with stimulation of the nigrostriatal dopamine pathway. For these experiments, one bipolar electrode was positioned locally, then a second electrode was positioned >1.5 mm from a recorded cell with an orientation that was shown in voltammetric studies to elicit reliable dopamine release. Stimulation parameters were 30 pulses at 10 Hz with pulse duration of 100 μs and amplitude of 0.6–0.8 mA for local and 2–4 mA for pathway stimulation as optimized in studies of evoked dopamine release as described in the following text.
Voltammetric monitoring of dopamine release
All voltammetric recording of evoked dopamine release in dorsolateral striatum was done in angled parasagittal slices. Slices (400 μm) were maintained at room temperature for ≥1 h before experimentation in HEPES-buffered ACSF containing (in mM) 120 NaCl, 5 KCl, 20 NaHCO3, 6.7 HEPES acid, 3.3 HEPES salt, 2 MgSO2, 10 glucose, and 2 CaCl2, saturated with 95% O2-5% CO2, which minimizes slice edema (MacGregor et al. 2001; Rice et al. 1997). Dopamine release was examined in submerged slices maintained at 32°C and superfused with the same bicarbonate-buffered ACSF used for whole cell recording and H2O2 imaging.
Evoked extracellular dopamine concentration ([DA]o) was monitored with carbon-fiber microelectrodes (8 μm tip diameter, 30–50 μm length; made in-house using methods modified from Millar and Pelling 2001) and fast-scan cyclic voltammetry using a Millar voltammeter (available from Dr. Julian Millar at Queen Mary University of London, UK). Data acquisition and analysis were as described previously (Chen et al. 2001). Carbon-fiber microelectrodes were inserted in the dorsolateral striatum with the tip 50–100 μm below the slice surface. Surface bipolar stimulating electrodes were positioned to elicit optimal [DA]o at a single recording site for both local and dopamine axon pathway stimulation (see Fig. 4). After 30-min equilibration in the slice chamber, dopamine release was evoked at 10-min intervals by alternating local and dopamine pathway stimulation (30 pulses, 10 Hz; 100-μs pulse duration). The stimulation intensity that evoked perimaximal [DA]o was determined for each stimulation site; stimulus amplitude was 0.6–0.8 mA for local stimulation and 2–4 mA for dopamine pathway stimulation. Evoked [DA]o was quantified by postexperimental calibration with known concentrations of dopamine at 32°C in all media used in a given experiment.
HPLC analysis of striatal dopamine tissue content
In separate experiments, the striatal dopamine tissue content of coronal slices (400 μm) was determined using HPLC with electrochemical detection as described previously (Chen et al. 2001). Slice pairs were allowed to equilibrate for 30 min at 32°C in bicarbonate-buffered ACSF before experimentation as in dopamine release studies. One slice of each pair was then incubated for a further 30 min at 32°C in bicarbonate-buffered ACSF alone while the other was incubated in ACSF containing a glutathione (GSH) peroxidase inhibitor mercaptosuccinate (MCS, 1 mM) (Avshalumov et al. 2003) for 30 min. After incubation, a sample of striatal tissue from each slice was weighed, frozen on dry ice, and stored at −80°C for subsequent HPLC analysis. On the day of analysis, samples were diluted 100-fold with ice-cold, deoxygenated mobile-phase, sonicated, spun at 13,000 rpm for 2 min, then the supernatant (10 μL) injected directly into the HPLC system for determination of dopamine content.
Drugs and chemicals
All experimental solutions were prepared immediately before use. MCS (as mercaptosuccinic acid) and components of all ACSF solutions used were from Sigma Chemical (St. Louis, MO). Catalase was from Calbiochem (San Diego, CA). For some experiments, catalase was heat-inactivated as described previously (Avshalumov et al. 2003). Other drugs, including AP5, 1-(4-aminophenyl)-4-methyl-7,8-methylenedioxy-5H-2,3-benzodiazepine hydrochloride (GYKI-52466), and tetrodotoxin (TTX) were from Tocris Cookson (Ellisville, MO). Alexa Red and 2′7′-H2DCF diacetate were from Invitrogen (Carlsbad, CA). Stock solutions of H2DCF diacetate were prepared in dimethylsulfoxide (DMSO) (Avshalumov et al. 2003, 2005); final DMSO levels were <0.1%, which alone had no effect on control responses.
Statistics
Data are expressed as means ± SE where n equals the number of cells for whole cell recording and imaging data or slices for dopamine release and dopamine content data. Significance of differences was assessed using paired or unpaired Student's t-test for comparison of two groups as appropriate, one-way ANOVA for comparison of three groups, or two-way ANOVA for comparison of time versus drug-dependent effects.
RESULTS
MSN identification
Striatal MSNs were identified by their somatodendritic morphology and their electrophysiological characteristics in coronal or angled parasagittal brain slices; these characteristics were independent of plane of section. Recorded cells (n = 61) were not spontaneously active in vitro but did exhibit spike activity with depolarizing current injection as described previously (Bao et al. 2005); mean resting membrane potential was −77.0 ± 0.9 mV and input resistance was 78.1 ± 2.0 MΩ. Additionally, all neurons exhibited slow depolarization ramps in response to depolarizing currents and inward rectification with hyperpolarizing currents consistent with previously described properties of striatal MSNs (Bao et al. 2005; Kitai et al. 1979; Koós and Tepper 1999; Nisenbaum and Wilson 1995).
Stimulus-induced H2O2 generation in striatal MSNs
To monitor activity-dependent ROS generation, MSNs were loaded with H2DCF diacetate via the recording pipette; H2DCF becomes fluorescent DCF after oxidation by H2O2 or other ROS (Avshalumov et al. 2005, 2007; Reynolds and Hastings 1995; Sah and Schwartz-Bloom 1999). Although MSNs did not exhibit spontaneous activity, basal DCF fluorescence, reflecting tonic ROS production, was seen in all recorded MSNs (Fig. 1A). During local electrical stimulation, each pulse of the stimulus train (30 pulses, 10 Hz) elicited a single action potential in MSNs (Fig. 1A). This stimulus paradigm also produced a significant 25–35% increase in simultaneously recorded DCF fluorescence intensity (FI) in a majority of MSNs monitored under these conditions (P < 0.01; paired t-test vs. basal DCF FI; n = 7 of 11 recorded cells). In contrast, 4 of 11 cells showed no change in DCF FI (P > 0.05 paired t-test vs. basal) although physiological responses to stimulation were indistinguishable between these two groups as discussed further at the end of results. Interestingly, there was no intermediate response between these two extremes. When seen, increases in DCF FI were rapid, beginning after 5–10 stimulus pulses and reaching an average maximal level of 29 ± 1% (n = 7; Fig. 1, A and D). It should be noted that H2DCF is irreversibly activated by ROS, such that a plateau in DCF FI persists after stimulation, precluding evaluation of ROS clearance (e.g., Avshalumov et al. 2005).
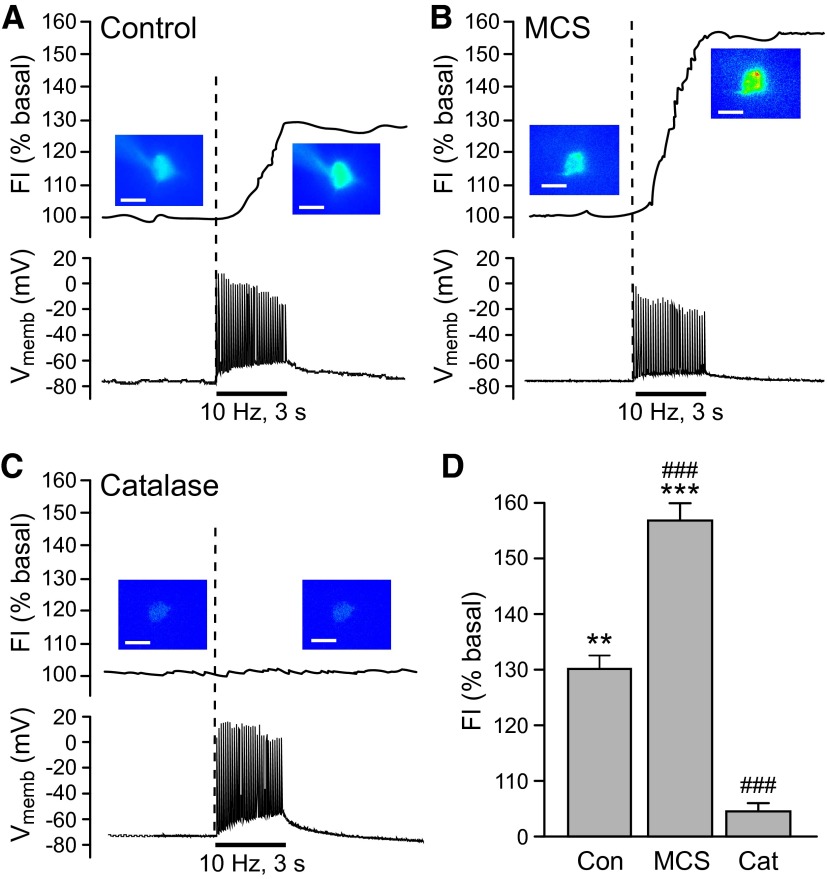
FIG. 1.
Activity-dependent generation of endogenous H2O2 in striatal medium spiny neurons (MSNs). A–C: representative examples of intracellular H2O2 (top) and membrane voltage (Vmemb; bottom) monitored simultaneously in identified MSNs during local pulse-train stimulation (30 pulses, 10 Hz). The time course of stimulus-induced changes in dichlorofluorescein (DCF) fluorescence intensity (FI) is shown along with pseudocolor images of DCF FI recorded under basal conditions and at the end of stimulation (scale bar = 20 μm). A: stimulus-induced increase in DCF FI in an MSN during local stimulation; under control conditions, 7 of 11 MSNs showed a significant increase in DCF FI (P < 0.01 vs. basal). Simultaneous current-clamp recording indicated that a single action potential was generated with each stimulus pulse in all recorded MSNs. B: inhibition of glutathione (GSH) peroxidase by mercaptosuccinate (MCS, 1 mM) amplified the stimulus-evoked increases in DCF FI, consistent with selective detection of H2O2, with no effect on action potential generation in recorded MSNs. In the presence of MCS, 7 of 7 MSNs showed a significant increase in DCF FI (P < 0.001). C: the presence of the H2O2 metabolizing enzyme catalase (Cat; 500 U/mL) did not alter spike generation during stimulation but prevented the stimulus-induced increase in DCF FI in 5 of 5 MSNs. D: average stimulus-induced changes in DCF FI in H2O2 source MSNs under control conditions (Con; n = 7), in the presence of MCS (n = 7), or in the presence of catalase (n = 5; **P < 0.01 vs. basal; ***P < 0.001 vs. basal). The increase in DCF FI in MCS was nearly twofold greater than under control conditions, whereas the presence of catalase markedly attenuated the usual control response (###P < 0.001 vs. control), confirming the H2O2-dependence of the evoked increases in DCF FI.
Given that H2DCF can react with other ROS as well as H2O2, it was critical to ascertain whether or not the monitored increase in DCF FI was H2O2 dependent. Endogenous H2O2 levels are regulated primarily by the cellular enzymes GSH peroxidase and catalase (Cohen 1994; Stults et al. 1977). Therefore we examined whether stimulus-induced H2O2 generation in striatal MSNs could be modulated by manipulation of these peroxidase enzymes. When GSH peroxidase activity was inhibited by MCS (1 mM) (Avshalumov et al. 2003, 2005), the stimulus-induced increase in DCF FI was amplified to nearly twice that seen under control conditions (Fig. 1, B and D; P < 0.001 vs. control, n = 7 per group). In striking contrast to the control population, all MSNs recorded in the presence of MCS showed an increase in DCF FI during local stimulation, suggesting that the antioxidant status of individual MSNs dictates the magnitude of activity-dependent H2O2 levels. Confirming that H2O2 is the primary ROS generated in MSNs during local stimulation, no increase in DCF FI was seen in any recorded MSN in the presence of catalase (n = 5; 500 U/mL) (Avshalumov et al. 2003), although action potential generation was unaltered (Fig. 1, C and D). By contrast, a typical proportion of MSNs (3 of 5) showed an increase in DCF FI with stimulation in the presence of heat-inactivated catalase (26 ± 1%; P < 0.01 vs. basal; n = 3). These data not only confirmed that H2O2 was the monitored ROS in MSNs but also showed that activity-dependent H2O2 levels are governed by cellular peroxidase activity.
Stimulus-induced H2O2 generation in striatal MSNs requires AMPAR activation
In the presence of the Na+-channel blocker TTX (1 μM) (Avshalumov et al. 2005), local stimulation in dorsolateral striatum failed to elicit either action potentials or an associated increase in intracellular H2O2 in any recorded MSN (n = 5; Fig. 2, A and C), demonstrating that H2O2 generation requires action potentials. To determine whether the effect of TTX was primarily the result of inhibition of spike generation per se or inhibition of synaptic transmitter release, we compared the effect of depolarizing current injection in individual MSNs on DCF FI with that evoked by local stimulation. Using a current injection paradigm designed to mimic the effect of local stimulation (30 pulses, 10 Hz), we found no detectable increase in endogenous intracellular H2O2 levels despite the induction of spike firing of each recorded MSN (n = 5; Fig. 2B). In the same cells, however, subsequent local stimulation caused the usual ∼30% increase in DCF FI (Fig. 2, B and C). Taken together, these data suggest that activity-dependent generation of modulatory H2O2 in striatum requires synaptic input.
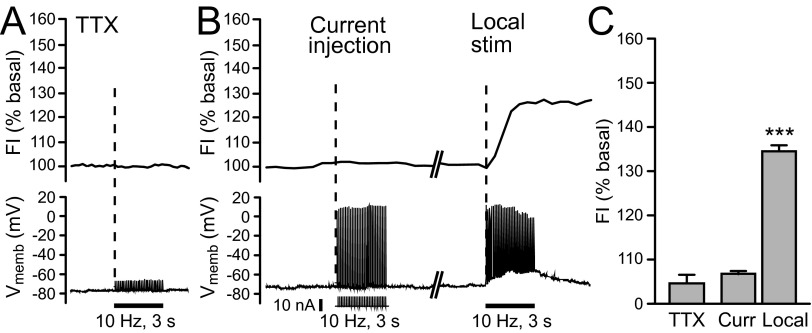
FIG. 2.
Endogenous H2O2 generation in striatal MSNs requires coherent synaptic activation. A: intracellular H2O2 (top) and membrane voltage (Vmemb; bottom) monitored simultaneously in MSNs during local pulse-train stimulation (30 pulses, 10 Hz) in tetrodotoxin (TTX, 1 μM). In the presence of TTX, stimulus-induced action potential generation was prevented and no increase in DCF FI was seen in any recorded MSN (5 of 5). B: current injection (30 pulses, 10 Hz; pulse amplitude: 5–10 nA; pulse duration: 100 μs) alone elicited action potentials in individual MSNs, but no increase in DCF FI was detected in any MSN examined (n = 5), whereas subsequent local stimulation (30 pulses, 10 Hz) caused the usual 30% increase in DCF FI in the same cells (n = 5; P < 0.001 vs. basal). This suggests that synaptic activation of MSNs, rather than action potential generation alone, is required to generate modulatory H2O2. C: average DCF FI in the presence of TTX (n = 5) or with current injection with subsequent local stimulation, as described for panel B (n = 5; ***P < 0.001 vs. basal).
Previous studies in cultured neurons indicate that intracellular ROS production increases in response to exogenous glutamate agonists (Bindokas et al. 1996; Carriedo et al. 2001; Dugan et al. 1995; Lafon-Cazal et al. 1993; Reynolds and Hastings 1995). We therefore examined whether endogenous glutamate released during local pulse-train stimulation was required for H2O2 generation in striatal MSNs. Striatal MSNs express both NMDA receptors (NMDARs) and AMPARs (Bernard and Bolam 1998; Chen et al. 1998). However, excitation is largely mediated by AMPARs rather than NMDARs with mild stimulation (Jiang and North 1991; Kita 1996; Pennartz et al. 1991). Consistent with these data, blockade of AMPARs with GYKI-52466 (50–100 μM) (Avshalumov et al. 2003) prevented both the generation of action potentials and stimulus-induced increases in DCF FI in all recorded MSNs during local stimulation (n = 7; Fig. 3, A and C). By contrast, stimulus-induced action potentials were unaltered by blockade of NMDARs by AP5 (100 μM) (Avshalumov et al. 2003) and an increase in DCF FI persisted in recorded MSNs (n = 5, Fig. 3, B and C). These data are the first to show that endogenous glutamate acting via AMPARs leads to H2O2 generation in CNS neurons.
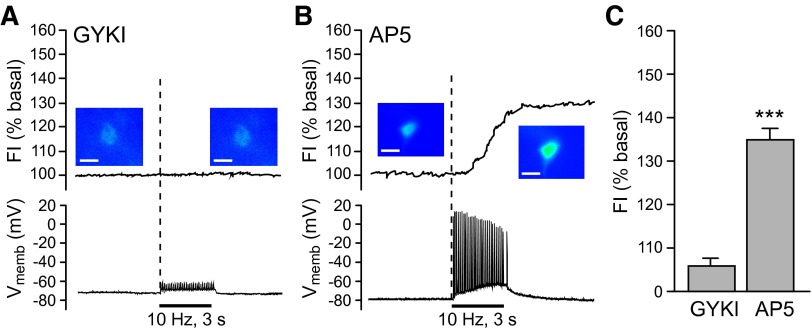
FIG. 3.
Glutamate-dependent H2O2 generation in striatal MSNs requires AMPAR activation. A and B: intracellular H2O2 (top) and membrane voltage (Vmemb; bottom) monitored simultaneously in MSNs during local pulse-train stimulation (30 pulses, 10 Hz). Time course of stimulus-induced changes in DCF FI are shown with pseudocolor images of DCF FI recorded under basal conditions and at the end of stimulation (scale bar = 20 μm). A: the usual stimulus-induced increase in DCF FI in MSNs was prevented by GYKI-52466 (50–100 μM), an AMPAR antagonist as were stimulus-evoked action potentials monitored during simultaneous current-clamp recording (n = 7; P > 0.05 vs. basal). B: blockade of NMDARs by AP5 (100 μM) had no effect on either DCF FI or action potential generation in recorded MSNs with 5 of 5 MSNs showing a significant increase in DCF FI (P < 0.001 vs. basal). C: summary of average changes in DCF-FI in MSNs in the presence of GYKI (n = 7) or AP5 (n = 5) (***P < 0.001 vs. basal).
Does activation of dopaminergic axons produce autoinhibitory H2O2?
Dopaminergic axons are another potential source of modulatory H2O2, either directly as a consequence of increased mitochondrial oxygen consumption during action potential generation (Boveris and Chance 1973; Kennedy et al. 1992) and/or indirectly via dopamine metabolism or autoxidation (Berman and Hastings 1999; Cohen 1994; Kulagina and Michael 2003) or via AMPAR activation of MSNs by glutamate co-released from dopaminergic axon terminals (Chuhma et al. 2004; Kaneko et al. 1990; Sulzer et al. 1998; Trudeau 2004). We examined the role of these possible contributing factors to H2O2-dependent suppression of striatal dopamine release using angled parasagittal slices in which dopaminergic axon tracts within the striatum are sufficiently preserved to permit dopamine pathway stimulation 1.5–2 mm distal to a recording site. Dopamine release evoked by alternating local and pathway pulse-train stimulation (30 pulses, 10 Hz) was monitored voltammetrically at a single recording site with a carbon-fiber microelectrode (Fig. 4A) and identified by the single oxidation and single reduction peak potentials that define the signature voltammogram for dopamine (Fig. 4B, insets). Dopamine release evoked by distal stimulation was dopamine pathway specific; an increase in [DA]o was only seen when the pathway-stimulating electrode was positioned ventrocaudally to the recording electrode but not from sites that were perpendicular to this location. The average [DA]o evoked by local stimulation was ∼40% higher than that evoked by dopamine pathway stimulation (Fig. 4, C and D; P < 0.001; n = 12), reflecting the efficacy of local stimulation to activate most, if not all, dopaminergic axons surrounding a recording site, contrasted with pathway stimulation, which activates dopaminergic axons that diverge from the stimulus site.
To elucidate whether selective activation of dopaminergic axons generates H2O2 that might serve as an autoinhibitory signal to regulate dopamine release, we amplified endogenous H2O2 levels by inhibiting GSH peroxidase using MCS, then compared the effect on [DA]o evoked locally and by pathway stimulation. Consistent with the elevation of H2O2 levels in MSNs during local stimulation in the presence of MCS (Fig. 1), locally evoked [DA]o was suppressed throughout the stimulus after 30 min exposure to MCS (2-way ANOVA, P < 0.001 vs. same-site control, n = 6; Fig. 5, A and B) as described previously (Avshalumov et al. 2003). Release suppression was reversible on MCS washout (not shown). In contrast to the effect of MCS on locally evoked [DA]o, however, MCS did not alter either the peak amplitude or the time course of [DA]o evoked at the same recording site by dopamine pathway stimulation (2-way ANOVA, P > 0.05, n = 6; Fig. 5, A and B). This implies the absence of modulatory H2O2 generation during selective stimulation of dopaminergic axons, including that from dopamine metabolism or autoxidation.
FIG. 5.
Modulatory H2O2 is not generated in dopaminergic axons. A: average [DA]o vs. time profiles evoked at a single site by alternating local and pathway stimulation (30 pulses, 10 Hz) in the absence and presence of the GSH peroxidase inhibitor mercaptosuccinate (MCS; 1 mM; n = 6). B: summary of the effect of MCS on peak [DA]o at a given site evoked by local vs. dopamine pathway stimulation; control peak evoked [DA]o for either local or pathway stimulation was taken as 100%. Increasing endogenous H2O2 availability by inhibiting GSH peroxidase caused a significant decrease in [DA]o evoked by local stimulation but had no effect on pathway evoked [DA]o (n = 6; ***P < 0.001 vs. local control). C: average [DA]o vs. time profiles evoked at a single site by alternating local and pathway stimulation (30 pulses, 10 Hz) in the absence and presence of an AMPAR blocker, GYKI-52466 (GYKI; 50–100 μM; n = 6). D: summary of the effect of GYKI on peak [DA]o at a given site evoked by local and pathway stimulation; control peak evoked [DA]o for either local or pathway stimulation is taken as 100%. Blockade of AMPARs caused a significant increase in [DA]o evoked by local stimulation but had no effect on that evoked by selective stimulation of dopaminergic axons (n = 6; ***P < 0.001 vs. local control).
The lack of effect of GSH peroxidase inhibition on dopamine pathway-evoked [DA]o also argues against the possibility that acutely elevated H2O2 levels might deplete the releasable pool of dopamine, e.g., by direct oxidation. Separate companion studies of striatal dopamine content confirmed that dopamine levels were unaltered by exposure to MCS (1 mM, 30 min) with dopamine content of 55 ± 3 nmol/g tissue wet weight (n = 16) in slices exposed to MCS versus 58 ± 1 nmol/g (n = 16) in control slices (P > 0.05 MCS vs. control, unpaired t-test).
Given evidence for co-release of glutamate and dopamine from dopaminergic neurons in culture and from mesolimbic dopaminergic input to ventral striatum (nucleus accumbens) in brain slices (Chuhma et al. 2004; Kaneko et al. 1990; Sulzer et al. 1998; Trudeau 2004), we next examined whether co-released glutamate during stimulation of nigrostriatal dopaminergic input to dorsolateral striatum might contribute to glutamate-dependent H2O2 generation and consequent dopamine release modulation. Previous studies show that striatal dopamine release evoked by local pulse-train stimulation is suppressed by concurrent glutamate release acting at AMPARs (Avshalumov et al. 2003). We therefore compared the effect of AMPAR blockade by GYKI-52466 on [DA]o evoked by local versus dopamine pathway stimulation (30 pulses, 10 Hz). As previously, GYKI-52466, caused an increase of ∼70% in peak amplitude of [DA]o evoked by local stimulation (Fig. 5, C and D) with amplification of evoked [DA]o throughout the pulse train (2-way ANOVA, P < 0.001 vs. same-site control, n = 6), confirming AMPAR-dependent modulation of dopamine release. At the same recording site, however, GYKI-52466 had no effect on the amplitude of [DA]o evoked by dopamine pathway stimulation (Fig. 5, C and D; 2-way ANOVA, P > 0.05 vs. control, n = 6). These data suggest that if co-release of dopamine and glutamate from dopaminergic axons does occur in dorsolateral striatum, this source of glutamate input does not contribute to AMPAR-mediated suppression of dopamine release. These data also support the absence of concurrent activation of thalamostriatal input during dopamine pathway stimulation.
Lack of H2O2 generation in MSNs during dopamine pathway stimulation
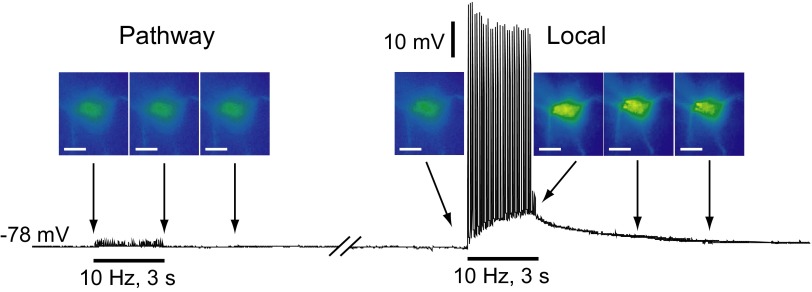
To examine possible contributions from concurrent thalamostriatal activation in angled parasagittal slices directly and to test whether H2O2 generation consequent to stimulation of dopaminergic axons might contribute to elevated H2O2 levels in striatal MSNs, we compared DCF FI in MSNs during local versus dopamine pathway stimulation. Using the same orientation of stimulating and recording electrodes found to elicit reliable dopamine release (Fig. 4), dopamine pathway stimulation (30 pulses, 10 Hz) failed to evoke action potentials in any recorded MSN (n = 13; Fig. 6). This not only confirms the absence of thalamostriatal pathway activation but also argues further against glutamate co-release from dopamine axons. Moreover simultaneously recorded DCF FI did not differ from basal DCF FI in these cells (n = 13, P > 0.05 vs. basal). The lack of detectable H2O2 elevation in MSNs, here used as “reporter cells,” suggests minimal production of diffusible H2O2 during selective dopamine axon stimulation. By contrast, subsequent local pulse-train stimulation elicited a single action potential for each stimulus pulse delivered in the same MSNs (Fig. 6), accompanied by an increase of 31 ± 1% in DCF FI in 10 of 13 recorded cells (P < 0.001 vs. basal; n = 10).
FIG. 6.
Absence of detectable H2O2 generation in MSNs during dopaminergic axon pathway stimulation. Representative example of H2O2 imaging (top) and simultaneous current-clamp (bottom) recording in an MSN during pathway and local pulse-train (30 pulses, 10 Hz) stimulation; the break in the current-clamp record indicates an interval of 2 min. Corresponding pseudocolor DCF FI images were taken at the time points indicated by the arrows; scale bars = 20 μm. Pathway stimulation of dopaminergic axons did not induce MSN firing or a change in DCF FI, whereas subsequent local stimulation induced firing of action potentials and ∼30% increase in DCF FI in these same cells (n = 7).
H2O2 source versus nonsource MSNs
Throughout these studies, a population of MSNs showed no detectable increase in DCF FI during local stimulation under control conditions or conditions in which action potential generation was unaltered (e.g., in heat-inactivated catalase). Of 39 cells recorded under these conditions, 30 produced a detectable increase in H2O2 (77%), whereas 9 did not (23%). This implies that a majority of MSNs are H2O2 “source” cells whereas a smaller population are “nonsource” (or possibly “sink”) cells. The occurrence of nonsource cells was not simply a technical artifact from insufficient dye loading because exogenously applied H2O2 caused an increase in DCF FI that was typically >70% of basal in these cells and was similar to that seen in source MSNs (not illustrated). A comparison of basic electrophysiological properties in subpopulations of each group indicated no difference in resting membrane potential (−77.2 ± 1.1 mV source vs. −76.2 ± 1.5 mV nonsource) or input resistance (79.9 ± 2.3 MΩ source vs. 72.9 ± 3.6 MΩ nonsource; unpaired t-test, P > 0.05 for both comparisons, n = 15 source and 5 nonsource MSNs). Excitability also appeared similar in both source and nonsource cells, given that local stimulation evoked action potentials that faithfully followed stimulation frequency in both populations. Last the viability of both populations was similar with similar recording times possible for both groups. Importantly, however, when GSH peroxidase was inhibited by MCS (e.g., Fig. 1B), all MSNs tested (7/7) showed a significant increase in DCF FI, as noted in the preceding text. This implies that differences in peroxidase enzyme activity in MSN subpopulations determine intracellular H2O2 levels in a given cell and, therefore whether the cell will be a source of glutamate-dependent, modulatory H2O2.
DISCUSSION
How synaptically released transmitters affect adjacent release sites that lack discrete point-to-point synaptic connections, like dopaminergic and glutamatergic inputs to striatal MSNs, is a long-standing question. In the case of dopamine, the answer is that it acts by volume transmission (Cragg and Rice 2004; Fuxe and Agnati 1991; Garris et al. 1994; Rice 2000; Rice and Cragg 2008). However, glutamate acts primarily within a synapse (Barbour 2001; Rusakov et al. 1999) with spillover limited by avid uptake. Thus whether synaptically released glutamate acting at ionotropic glutamate receptors regulates dopamine release is unresolved. We show here that glutamatergic excitation of AMPARs in striatal MSNs increases generation of the diffusible signaling molecule, H2O2, in a large population of these cells. This is the first direct demonstration of H2O2 generation in neurons by endogenous glutamate. By contrast, we found no evidence for generation of modulatory H2O2 by dopamine axons or released dopamine. Together, these data support a model of glutamatergic regulation of striatal dopamine release that is mediated in large part by AMPAR-dependent H2O2 generated as a retrograde messenger in striatal neurons, including MSNs, rather than dopamine axons.
H2O2 as a signaling molecule
In contrast to other ROS, H2O2 is neither a free radical nor an ion, which limits its reactivity (Cohen 1994) and increases its membrane permeability (Bienart et al. 2006, 2007; Makino et al. 2004; Ramasarma 1982), so that it is well-suited as a diffusible messenger. Recent evidence suggests that cell-specific membrane permeability to H2O2 governs its efflux and entry (Bienart et al. 2007; Makino et al. 2004). Cellular levels, therefore, reflect the balance among generation, primarily by mitochondrial respiration (Boveris and Chance 1973; Dugan et al. 1995; Kennedy et al. 1992; Liu et al. 2002), metabolism, primarily by GSH peroxidase and catalase (Cohen 1994; Stults et al. 1977) but also peroxiredoxins (Hofmann et al. 2002; Rhee et al. 2001), and H2O2 diffusion into and out of cells (Makino et al. 2004). The role of peroxide metabolism in managing cellular H2O2 levels in MSNs was shown by the marked amplification of activity-dependent H2O2 levels in all recorded MSNs after GSH peroxidase inhibition (Fig. 1, B and D). The finding that all recorded MSNs showed a stimulation-induced increase in H2O2 in the presence of MCS also suggests that GSH peroxidase helps determine whether individual MSNs are H2O2-source or nonsource cells. Importantly, demonstration that AMPAR-dependent generation of modulatory H2O2 occurs in MSNs (Fig. 3A), but not dopaminergic axons (Fig. 5), further indicates that H2O2 must diffuse from an external cellular source to inhibit axonal dopamine release.
No generation of modulatory H2O2 by dopamine axons
In our initial report showing that endogenously produced H2O2 inhibited dopamine release, we suggested that dopamine axons might be the primary source of activity-dependent H2O2 (Chen et al. 2001), given the abundance of mitochondria within a few hundred nanometers of dopamine axon terminals (Nirenberg et al. 1997). In that case, H2O2 would be an autoinhibitor of dopamine release as it is in dopamine cell bodies (Avshalumov et al. 2005). However, the hypothesis of direct generation of modulatory H2O2 by dopamine axons is not supported by our subsequent work, including the present studies. Most obviously, we found that dynamic regulation of dopamine release by endogenous H2O2 requires AMPAR activation (Avshalumov et al. 2003). As noted in the introduction, this implies that dopamine axons cannot be the primary source of modulatory H2O2 because they lack AMPARs (Bernard and Bolam 1998; Bernard et al. 1997; Chen et al. 1998). Indeed GSH peroxidase inhibition by MCS has no effect on locally evoked [DA]o when AMPARs are blocked (Avshalumov et al. 2003), indicating that there is no remaining H2O2 signal to amplify. The present finding that MCS has no effect on pathway-evoked [DA]o (Fig. 5A) confirms that there is little, if any, direct AMPAR-independent contribution from dopamine axons to the generation of modulatory H2O2.
These and other data also imply that there are no indirect contributions to dynamically generated H2O2 from metabolism or autoxidation of released dopamine (Berman and Hastings 1999; Cohen 1994; Kulagina and Michael 2003) or from glutamate co-released from dopamine axons (Chuhma et al. 2004; Kaneko et al. 1990; Sulzer et al. 1998; Trudeau 2004). The findings that MCS has no effect on pathway-evoked dopamine release (Fig. 5A) or on locally evoked release when AMPARs are blocked and evoked [DA]o is nearly twofold higher than control (Avshalumov et al. 2003) argue against a modulatory role for H2O2 formed from released dopamine. Indirect support for this argument comes from the absence of detectable elevation in DCF FI in MSNs during selective dopamine pathway stimulation, which could have reflected H2O2 diffusion from dopamine axons (Fig. 6). The inability of dopamine pathway stimulation to increase DCF FI or to alter MSN physiology (Fig. 6) coupled with the absence of an effect of AMPAR blockade on dopamine pathway-evoked [DA]o (Fig. 5C) further shows that glutamate co-released from dopamine axons does not contribute to AMPAR-dependent generation of modulatory H2O2.
Glutamate-dependent generation of H2O2 in MSNs
In contrast to the lack of evidence for self-regulation of dopamine release by H2O2 generated in dopamine axons, the present studies clearly demonstrate glutamate-dependent H2O2 generation in MSNs. The findings and approaches used differ in three main ways from earlier work on glutamate-receptor agonist-induced increases in H2O2 or other ROS levels in cultured neurons (e.g., Bindokas et al. 1996; Carriedo et al. 2001; Dugan et al. 1995; Lafon-Cazal et al. 1993; Reynolds and Hastings 1995). First, in previous studies, exogenous glutamate agonists were used to elicit increases in ROS, whereas here endogenously released glutamate was the trigger. Second, the earlier focus was on glutamate neurotoxicity, whereas the present studies demonstrate that AMPAR-dependent H2O2 generation is a component of normal glutamate signaling in striatum. Third, because CNS neurons in culture lack glia to provide glutamate uptake and limit excitotoxicity, as well as the glial antioxidant network that limits oxidative damage (e.g., Avshalumov et al. 2004), it is surprising and significant that increases in intracellular H2O2 in MSNs also occur in the complex microenvironment of brain slices during AMPAR activation by endogenous glutamate.
It is also notable that action potential generation alone in MSNs was not sufficient to generate H2O2. Although the factors underlying this difference are not yet known, a requirement for glutamatergic input has been reported previously for another class of diffusible messengers, the endocannabinoids. Kreitzer and Malenka (2005) found that detectable endocannabinoid release from MSNs when postsynaptic depolarization was paired with presynaptic stimulation but not with postsynaptic depolarization alone.
Are MSNs the only source of dynamic, modulatory H2O2 generation? The role of other striatal cells has not yet been examined; however, it is likely that MSNs are an important source of modulatory H2O2 given that MSNs constitute 90–95% of the neuron population of the striatum (Kemp and Powell 1971a). Moreover, glutamate synapses are closely apposed to dopaminergic synapses on the dendrites of MSNs (Freund et al. 1984; Smith and Bolam 1990) and are therefore ideally positioned to modulate dopamine release via postsynaptically generated H2O2. Consistent with this anatomical evidence, stimulated H2O2 generation in MSNs is dependent on AMPAR activation, amplified by inhibition of GSH peroxidase, and eliminated by catalase (Figs. 1 and 3) in a pattern consistent with the previously reported consequences of these manipulations on dopamine release (Avshalumov et al. 2003). Thus H2O2 produced in MSNs alone could be sufficient to mediate glutamate-dependent regulation of dopamine release. Of course, contributions from the remaining 5–10% of striatal neurons could also occur as most of these express AMPAR subunits (Deng et al. 2007). Striatal glia might contribute, as well, with the caveat that although cultured striatal astrocytes express AMPAR protein (Fan et al. 1999), there are no reports of functional consequences of AMPAR activation in these cells in striatum.
How does H2O2 inhibit dopamine release? We have reported previously that H2O2 signaling in the striatum requires the activation of KATP channels (Avshalumov and Rice 2003; Avshalumov et al. 2003), which are found throughout the nigrostriatal dopamine pathway (Avshalumov et al. 2003, 2005; Liss et al. 1999; Mourre et al. 1989; Xia and Haddad 1991; Zini et al. 1993). Although other H2O2 sensing systems from phosphatases and kinases to transcription factors can also regulate cell function (Finkel et al. 2003; Kamsler and Segal 2004; Kishida and Klann 2007; Rhee 2006; Rhee et al. 2005; Veal et al. 2007), these processes are very slow (minutes to hours) compared with the rapid, dynamic H2O2 regulation of striatal dopamine release which occurs on a subsecond time scale.
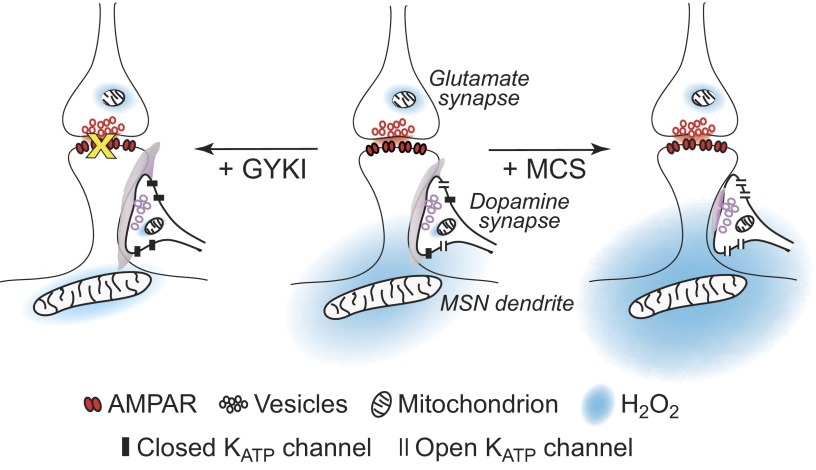
Based on these findings, we propose a model of glutamate-dependent modulation of axonal dopamine by AMPAR-dependent H2O2 generation in MSNs in which AMPAR activation of dendrites and somata of these cells leads to generation of H2O2 that diffuses to adjacent dopamine axons and inhibits dopamine release via opening of KATP channels (Fig. 7, center). When AMPARs are blocked (+ GYKI), H2O2-dependent regulation of dopamine release via KATP channels is lost (Avshalumov and Rice 2003), and dopamine release is enhanced (Fig. 7, left). Conversely, when activity-dependent levels of H2O2 are amplified by inhibiting GSH peroxidase with MCS, this leads to enhanced H2O2-dependent KATP-channel activation (Avshalumov and Rice 2003), and further suppression of dopamine release (Fig. 7, right).
FIG. 7.
Model of axonal dopamine release regulation by glutamate acting via AMPARs and generation of diffusible H2O2 in striatal MSNs. Center: activation of AMPARs on MSN dendrites generates H2O2 that diffuses to adjacent dopamine axons and inhibits dopamine release via opening of KATP channels. Left: when AMPARs are blocked (+ GYKI), H2O2-dependent regulation of dopamine release via KATP channels is lost and dopamine release is enhanced. Right: when activity-dependent levels of H2O2 are amplified by inhibiting GSH peroxidase with MCS, this leads to enhanced H2O2-dependent KATP-channel activation and further suppression of dopamine release.
Conclusions and implications
What are the implications of diffusible H2O2 as the mediator of striatal glutamate-dopamine interactions? As an inhibitory intermediate, endogenously generated H2O2 reverses conventional glutamatergic excitation and leads to inhibition of dopamine release via H2O2-sensitive KATP channels (Avshalumov and Rice 2003). Generation of detectable levels of H2O2 in a majority of MSNs following AMPAR activation would necessarily contribute to dopamine release inhibition given that this diffusible messenger will readily leave the cell in which it is produced. It would be expected that H2O2 is produced in all MSNs, and potentially all AMPAR-expressing cells, during glutamatergic activation. Whether a particular MSN or other cell is an H2O2 source, however, apparently depends largely on the peroxidase activity in that cell, inasmuch that all recorded MSNs showed an increase in DCF FI when GSH peroxidase was inhibited, whereas stimulus-evoked H2O2 levels were kept below detection limits in ∼25% of recorded MSNs under control conditions. Does detection indicate functionally relevant levels of H2O2? Our previous studies of the effect of endogenous H2O2 on the physiology of dopaminergic neurons in the substantia nigra indicate that the answer is yes. In those studies, we compared increases in DCF FI when endogenous H2O2 was amplified by increasing concentrations of the GSH peroxidase inhibitor, MCS. Low concentration of MCS, even those sufficient to cause a significant increase in DCF FI, had no effect on KATP channels in dopamine neurons, implying that there is a threshold for KATP channel modulation (i.e., a functional H2O2 concentration) that is above DCF detection limits (Avshalumov et al. 2005). Thus DCF sensitivity to H2O2 appears to be even greater than the sensitivity of KATP channels to endogenous H2O2 levels. These data therefore imply that glutamatergic activation of source MSNs could produce levels of H2O2 sufficient to inhibit nigrostriatal dopamine input to those cells (Fig. 7), whereas nonsource MSNs lack this capacity. This would also hold true for other striatal cells that might serve as sources of H2O2.
Differential regulation of H2O2 generation in MSNs could be important physiologically, given differential dopaminergic regulation of striatonigral versus striatopallidal MSNs (Surmeier et al. 2007). For example, in striatonigral MSNs in which D1 dopamine-receptor activation enhances responsiveness to glutamatergic input (Albin et al. 1989; Surmeier et al. 2007), a consequent increase in H2O2 generation would provide an inhibitory signal to decrease dopamine input, which would then reverse D1-enhanced excitability in a reciprocal glutamate-dopamine feedback loop. By contrast, in striatopallidal MSNs, D2-receptor activation decreases excitability. A consequent decrease in H2O2 generation would lead to enhanced local dopamine release, resulting in further D2-receptor-mediated inhibition of MSNs excitability. The absence of activity-dependent H2O2 elevation in those MSNs, for example, would avoid an endless negative feedback process.
Last, glutamate-dopamine dysregulation has been implicated as a causal factor in Parkinson's disease (Bevan et al. 2006; Chase and Oh 2000), Huntington's disease (Di Filippo et al. 2007), schizophrenia (Sawa and Snyder 2002; Thompson et al. 2004), and addiction (Hyman and Malenka 2001; Kelley 2004). Oxidative stress is also linked causally to several of these disorders (Browne and Beal 2006; Orth and Schapira 2002; Klein and Schlossmacher 2006; Tosic et al. 2006). The finding that AMPAR activation in MSNs by endogenously release glutamate leads to enhanced H2O2 generation provides a new starting point for understanding glutamate-dopamine function and dysfunction.
GRANTS
The work was funded by National Institute of Neurological Disorders and Stroke Grant NS-36362 and the Attilio and Olympia Ricciardi Research Fund.
Acknowledgments
We are grateful to A. Quyyum for HPLC analysis, M. L. Chao for immunocytochemistry, C. Nicholson for assistance with photography, and C. R. Lee and Z. Sidló for insightful feedback during the preparation of this report.
Present address of M. V. Avshalumov: Dept. of Neurosurgery, Mount Sinai Medical Center, New York, USA.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Albin et al. 1989.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci 12: 366–375, 1989. [DOI] [PubMed] [Google Scholar]
- Avshalumov et al. 2007.Avshalumov MV, Bao L, Patel JC, Rice ME. H2O2 signaling in the nigrostriatal dopamine pathway via ATP-sensitive potassium channels: issues and answers. Antioxid Redox Signal 9: 219–231, 2007. [DOI] [PubMed] [Google Scholar]
- Avshalumov et al. 2005.Avshalumov MV, Chen BT, Koós T, Tepper JM, Rice ME. Endogenous hydrogen peroxide regulates the excitability of midbrain dopamine neurons via ATP-sensitive potassium channels. J Neurosci 25: 4222–4231, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avshalumov et al. 2003.Avshalumov MV, Chen BT, Marshall SP, Peña DM, Rice ME. Glutamate-dependent inhibition of dopamine release in striatum is mediated by a new diffusible messenger, H2O2. J Neurosci 23: 2744–2750, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avshalumov et al. 2004.Avshalumov MV, MacGregor DG, Sehgal LM, Rice ME. The glial antioxidant network and neuronal ascorbate: protective yet permissive for H2O2 signaling. Neuron Glia Biol 1: 365–376, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avshalumov and Rice 2003.Avshalumov MV, Rice ME. Activation of ATP-sensitive K+ (KATP) channels by H2O2 underlies glutamate-dependent inhibition of striatal dopamine release. Proc Natl Acad Sci USA 100: 11729–11734, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford et al. 2004a.Bamford NS, Robinson S, Palmiter RD, Joyce JA, Moore C, Meshul CK. Dopamine regulates release from corticostriatal terminals. J Neurosci 24: 9541–9552, 2004a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford et al. 2004b.Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron 42: 653–663, 2004b. [DOI] [PubMed] [Google Scholar]
- Bao et al. 2005.Bao L, Avshalumov MV, Rice ME. Partial mitochondrial inhibition causes striatal dopamine release suppression and medium spiny neuron depolarization via H2O2 elevation not ATP depletion. J Neurosci 25: 10029–10040, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour 2001.Barbour B An evaluation of synapse independence. J Neurosci 21: 7969–7984, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendse and Groenewegen 1990.Berendse HW, Groenewegen HJ. Organization of the thalamostriatal projections in the rat, with special emphasis on the ventral striatum. J Comp Neurol 299: 187–228, 1990. [DOI] [PubMed] [Google Scholar]
- Bergles et al. 1999.Bergles DE, Diamond JS, Jahr CE. Clearance of glutamate inside the synapse and beyond. Curr Opin Neurobiol 9: 293–298, 1999. [DOI] [PubMed] [Google Scholar]
- Berman and Hastings 1999.Berman SB, Hastings TG. Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: implications for Parkinson's disease. J Neurochem 73: 1127–1137, 1999. [DOI] [PubMed] [Google Scholar]
- Bernard and Bolam 1998.Bernard V, Bolam JP. Subcellular and subsynaptic distribution of the NR1 subunit of the NMDA receptor in the neostriatum and globus pallidus of the rat: colocalization at synapses with the GluR2/3 subunit of the AMPA receptor. Eur J Neurosci 10: 3721–3738, 1998. [DOI] [PubMed] [Google Scholar]
- Bernard et al. 1997.Bernard V, Somogyi P, Bolam JP. Cellular, subcellular, and subsynaptic distribution of AMPA-type glutamate receptor subunits in the neostriatum of the rat. J Neurosci 17: 819–833, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan et al. 2006.Bevan MD, Atherton JF, Baufreton J. Cellular principles underlying normal and pathological activity in the subthalamic nucleus. Curr Opin Neurobiol 16: 621–628, 2006. [DOI] [PubMed] [Google Scholar]
- Bienert et al. 2007.Bienert GP, Møller AL, Kristiansen KA, Schulz A, Møller IM, Schjoerring JK, Jahn TP. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem 282: 1183–1192, 2007. [DOI] [PubMed] [Google Scholar]
- Bienert et al. 2006.Bienert GP, Schjoerring JK, Jahn TP. Membrane transport of hydrogen peroxide. Biochim Biophys Acta 1758: 994–1003, 2006. [DOI] [PubMed] [Google Scholar]
- Bindokas et al. 1996.Bindokas VP, Jordan J, Lee CC, Miller RJ. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J Neurosci 16: 1324–1336, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris and Chance 1973.Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J 134: 707–716, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne and Beal 2006.Browne SE, Beal MF. Oxidative damage in Huntington's disease pathogenesis. Antioxid Redox Signal 8: 2061–2073, 2006. [DOI] [PubMed] [Google Scholar]
- Cagniard et al. 2006.Cagniard B, Beeler JA, Britt JP, McGehee DS, Marinelli M, Zhuang X. Dopamine scales performance in the absence of new learning. Neuron 51: 541–547, 2006. [DOI] [PubMed] [Google Scholar]
- Carriedo et al. 2000.Carriedo SG, Sensi SL, Yin HZ, Weiss JH. AMPA exposures induce mitochondrial Ca2+ overload and ROS generation in spinal motor neurons in vitro. J Neurosci 20: 240–250, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter and Sabatini 2004.Carter AG, Sabatini BL. State-dependent calcium signaling in dendritic spines of striatal medium spiny neurons. Neuron 44: 483–493, 2004. [DOI] [PubMed] [Google Scholar]
- Cepeda et al. 2001.Cepeda C, Hurst RS, Altemus KL, Flores-Hernandez JJ, Calvert CR, Jokel ES, Grandy DK, Low MJ, Rubinstein M, Ariano MA, Levine MS. Facilitated glutamatergic transmission in the striatum of D2 dopamine receptor-deficient mice. J Neurophysiol 85: 659–670, 2001. [DOI] [PubMed] [Google Scholar]
- Chase and Oh 2000.Chase TN, Oh JD. Striatal dopamine- and glutamate-mediated dysregulation in experimental parkinsonism. Trends Neurosci 23: S86–S91, 2000. [DOI] [PubMed] [Google Scholar]
- Chen et al. 2001.Chen BT, Avshalumov MV, Rice ME. H2O2 is a novel endogenous modulator of synaptic dopamine release. J Neurophysiol 85: 2468–2476, 2001. [DOI] [PubMed] [Google Scholar]
- Chen et al. 1998.Chen Q, Veenman L, Knopp K, Yan Z, Medina L, Song WJ, Surmeier DJ, Reiner A. Evidence for the preferential localization of glutamate receptor-1 subunits of AMPA receptors to the dendritic spines of medium spiny neurons in rat striatum. Neuroscience 83: 749–761, 1998. [DOI] [PubMed] [Google Scholar]
- Chuhma et al. 2004.Chuhma N, Zhang H, Masson J, Zhuang X, Sulzer D, Hen R, Rayport S. Dopamine neurons mediate a fast excitatory signal via their glutamatergic synapses. J Neurosci 24: 972–981, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen 1994.Cohen G Enzymatic/nonenzymatic sources of oxyradicals and regulation of antioxidant defenses. Ann NY Acad Sci 73: 8–14, 1994. [DOI] [PubMed] [Google Scholar]
- Costa et al. 2006.Costa RM, Lin SC, Sotnikova TD, Cyr M, Gainetdinov RR, Caron MG, Nicolelis MA. Rapid alterations in corticostriatal ensemble coordination during acute dopamine-dependent motor dysfunction. Neuron 52: 359–369, 2006. [DOI] [PubMed] [Google Scholar]
- Cragg and Rice 2004.Cragg SJ, Rice ME. DAncing past the DAT at a DA synapse. Trends Neurosci 27: 270–277, 2004. [DOI] [PubMed] [Google Scholar]
- Danbolt 2001.Danbolt NC Glutamate uptake. Prog Neurobiol 65: 1–105, 2001. [DOI] [PubMed] [Google Scholar]
- Deng et al. 2007.Deng YP, Xie JP, Wang HB, Lei WL, Chen Q, Reiner A. Differential localization of the GluR1 and GluR2 subunits of the AMPA-type glutamate receptor among striatal neuron types in rats. J Chem Neuroanat 33: 167–192, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Filippo et al. 2007.Di Filippo M, Tozzi A, Picconi B, Ghiglieri V, Calabresi P. Plastic abnormalities in experimental Huntington's disease. Curr Opin Pharmacol 7: 106–111, 2007. [DOI] [PubMed] [Google Scholar]
- Dugan et al. 1995.Dugan LL, Sensi SL, Canzoniero LM, Handran SD, Rothman SM, Lin TS, Goldberg MP, Choi DW. Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-d-aspartate. J Neurosci 15: 6377–6388, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan et al. 1999.Fan D, Grooms SY, Araneda RC, Johnson AB, Dobrenis K, Kessler JA, Zukin RS. AMPA receptor protein expression and function in astrocytes cultured from hippocampus. J Neurosci Res 57: 557–571, 1999. [PubMed] [Google Scholar]
- Finkel 2003.Finkel T Oxidant signals and oxidative stress. Curr Opin Cell Biol 15: 247–254, 2003. [DOI] [PubMed] [Google Scholar]
- Freund et al. 1984.Freund TF, Powell JF, Smith AD. Tyrosine hydroxylase-immunoreactive boutons in synaptic contact with identified striatonigral neurons, with particular reference to dendritic spines. Neuroscience 13: 1189–1215, 1984. [DOI] [PubMed] [Google Scholar]
- Fuxe and Agnati 1991.Fuxe K, Agnati LF. (editors). Volume Transmission in the Brain. New York: Raven, 1991.
- Galvan et al. 2006.Galvan A, Kuwajima M, Smith Y. Glutamate and GABA receptors and transporters in the basal ganglia: what does their subsynaptic localization reveal about their function? Neuroscience 143: 351–375, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris et al. 1994.Garris PA, Ciolkowski EL, Pastore P, Wightman RM. Efflux of dopamine from the synaptic cleft in the nucleus accumbens of the rat brain. J Neurosci 14: 6084–6093, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen and Wilson 1996.Gerfen CR, Wilson CJ.The basal ganglia. In: Handbook of Chemical Neuroanatomy. Integrated Systems of the CNS, edited by Swanson LW, Bjorklund A, Hokfelt T. Amsterdam: Elsevier Science, 1996, p. 371–468.
- Graybiel et al. 1994.Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptive motor control. Science 265: 1826–1831, 1994. [DOI] [PubMed] [Google Scholar]
- Hofmann et al. 2002.Hofmann B, Hecht HJ, Flohe L. Peroxiredoxins. Biol Chem 383: 347–364, 2002. [DOI] [PubMed] [Google Scholar]
- Hyman and Malenka 2001.Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci 2: 695–703, 2001. [DOI] [PubMed] [Google Scholar]
- Jiang and North 1991.Jiang ZG, North RA. Membrane properties and synaptic responses of rat striatal neurons in vitro. J Physiol 443: 533–553, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamsler and Segal 2004.Kamsler A, Segal M. Hydrogen peroxide as a diffusible signal molecule in synaptic plasticity. Mol Neurobiol 29: 167–178, 2004. [DOI] [PubMed] [Google Scholar]
- Kaneko et al. 1990.Kaneko T, Akiyama H, Nagatsu I, Mizuno N. Immunohistochemical demonstration of glutaminase in catecholaminergic and serotoninergic neurons of rat brain. Brain Res 507: 151–154, 1990. [DOI] [PubMed] [Google Scholar]
- Kelley 2004.Kelley AE Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron 44: 161–179, 2004. [DOI] [PubMed] [Google Scholar]
- Kemp and Powell 1971a.Kemp JM, Powell TPS. The structure of the caudate nucleus of the cat: light and electron microscopy. Philos Trans R Soc Lond B Biol Sci 262: 383–401, 1971a. [DOI] [PubMed] [Google Scholar]
- Kemp and Powell 1971b.Kemp JM, Powell TPS. The termination of fibres from the cerebral cortex and thalamus upon the dendritic spines in the caudate nucleus: a study with the Golgi method. Philos Trans R Soc Lond B Biol Sci 262: 429–439, 1971b. [DOI] [PubMed] [Google Scholar]
- Kennedy et al. 1992.Kennedy RT, Jones SR, Wightman RM. Simultaneous measurement of oxygen and dopamine: coupling of oxygen consumption and neurotransmission. Neuroscience 47: 603–612, 1992. [DOI] [PubMed] [Google Scholar]
- Kishida and Klann 2007.Kishida KT, Klann E. Sources and targets of reactive oxygen species in synaptic plasticity and memory. Antioxid Redox Signal 9: 233–244, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita 1996.Kita H Glutamatergic and GABAergic postsynaptic responses of striatal spiny neurons to intrastriatal and cortical stimulation recorded in slice preparations. Neuroscience 70: 925–940, 1996. [DOI] [PubMed] [Google Scholar]
- Kitai et al. 1979.Kitai ST, Preston RJ, Bishop GA, Koscis JD. Striatal projection neurons: morphology and electrophysiological studies. Adv Neurol 24: 45–51, 1979. [Google Scholar]
- Kulagina and Michael 2003.Kulagina NV, Michael AC. Monitoring hydrogen peroxide in the extracellular space of the brain with amperometric microsensors. Anal Chem 75: 4875–4881, 2003. [DOI] [PubMed] [Google Scholar]
- Klein and Schlossmacher 2006.Klein C, Schlossmacher MG. The genetics of Parkinson disease: Implications for neurological care. Nat Clin Pract Neurol 2: 136–146, 2006. [DOI] [PubMed] [Google Scholar]
- Koós and Tepper 1999.Koós T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci 2: 467–472, 1999. [DOI] [PubMed] [Google Scholar]
- Kreitzer and Malenka 2005.Kreitzer AC, Malenka RC. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J Neurosci 25: 10537–10545, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafon-Cazal et al. 1993.Lafon-Cazal M, Pietri S, Culcasi M, Bockaert J. NMDA-dependent superoxide production and neurotoxicity. Nature 364: 535–537, 1993. [DOI] [PubMed] [Google Scholar]
- Liss et al. 1999.Liss B, Bruns R, Roeper J. Alternative sulfonylurea receptor expression defines sensitivity to K-ATP channels in dopaminergic midbrain neurons. EMBO J 18: 833–846, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. 2002.Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem 80: 780–787, 2002. [DOI] [PubMed] [Google Scholar]
- MacGregor et al. 2001.MacGregor DG, Chesler M, Rice ME. HEPES prevents edema in rat brain slices. Neurosci Lett 303: 141–144, 2001. [DOI] [PubMed] [Google Scholar]
- Makino et al. 2004.Makino N, Sasaki K, Hashida K, Sakakura Y. A metabolic model describing the H2O2 elimination by mammalian cells including H2O2 permeation through cytoplasmic and peroxisomal membranes: comparison with experimental data. Biochim Biophys Acta 1673: 149–159, 2004. [DOI] [PubMed] [Google Scholar]
- McGeorge and Faull 1989.McGeorge AJ, Faull RL. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience 29: 503–537, 1989. [DOI] [PubMed] [Google Scholar]
- Millar and Pelling 2001.Millar J, Pelling CW. Improved methods for construction of carbon fibre electrodes for extracellular spike recording. J Neurosci Methods 110: 1–8, 2001. [DOI] [PubMed] [Google Scholar]
- Mourre et al. 1989.Mourre C, Ben Ari Y, Bernardi H, Fosset M, Lazdunski M. Antidiabetic sulfonylureas: localization of binding sites in the brain and effects on the hyperpolarization induced by anoxia in hippocampal slices. Brain Res 486: 159–164, 1989. [DOI] [PubMed] [Google Scholar]
- Nirenberg et al. 1997.Nirenberg MJ, Chan J, Liu Y, Edwards RH, Pickel VM. Vesicular monoamine transporter-2: immunogold localization in striatal axons and terminals. Synapse 26: 194–198, 1997. [DOI] [PubMed] [Google Scholar]
- Nirenberg et al. 1996.Nirenberg MJ, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons. J Neurosci 16: 436–447, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisenbaum and Wilson 1995.Nisenbaum ES, Wilson CJ. Potassium currents responsible for inward and outward rectification in rat neostriatal spiny projection neurons. J Neurosci 15: 4449–4463, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth and Schapira 2002.Orth M, Schapira AH. Mitochondrial involvement in Parkinson's disease. Neurochem Int 40: 533–541, 2002. [DOI] [PubMed] [Google Scholar]
- Pennartz et al. 1991.Pennartz CM, Boeijinga PH, Kitai ST, Lopes da Silva FH. Contribution of NMDA receptors to postsynaptic potentials and paired-pulse facilitation in identified neurons of the rat nucleus accumbens in vitro. Exp Brain Res 86: 190–198, 1991. [DOI] [PubMed] [Google Scholar]
- Ramasarma 1982.Ramasarma T Generation of H2O2 in biomembranes. Biochem Biophys Acta Rev Biomemb 694: 69–93, 1982. [DOI] [PubMed] [Google Scholar]
- Reynolds and Hastings 1995.Reynolds IJ, Hastings TG. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J Neurosci 15: 3318–3327, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee 2006.Rhee SG H2O2, a necessary evil for cell signaling. Science 312: 1882–1883, 2006. [DOI] [PubMed] [Google Scholar]
- Rhee et al. 2001.Rhee SG, Kang SW, Chang TS, Jeong W, Kim K. Peroxiredoxins: a novel family of peroxidases. IUBMB Life 52: 35–41, 2001. [DOI] [PubMed] [Google Scholar]
- Rhee et al. 2005.Rhee SG, Kang SW, Jeong W, Chang TS, Yang KS, Woo HA. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol 17: 183–189, 2005. [DOI] [PubMed] [Google Scholar]
- Rice 2000.Rice ME Distinct regional differences in dopamine-mediated volume transmission. Prog Brain Res 125: 277–290, 2000. [DOI] [PubMed] [Google Scholar]
- Rice and Cragg 2008.Rice ME, Cragg SJ. Dopamine spillover after quantal release: rethinking dopamine transmission in the nigrostriatal pathway. Brain Res Rev 58: 303–313, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice et al. 1997.Rice ME, Cragg SJ, Greenfield SA. Characteristics of electrically-evoked somatodendritic dopamine release in substantia nigra and ventral tegmental area in vitro.J Neurophysiol 77: 853–862, 1997. [DOI] [PubMed] [Google Scholar]
- Rusakov et al. 1999.Rusakov DA, Kullmann DM, Stewart MG. Hippocampal synapses: do they talk to their neighbours? Trends Neurosci 22: 382–388, 1999. [DOI] [PubMed] [Google Scholar]
- Sah and Schwartz-Bloom 1999.Sah R, Schwartz-Bloom RD. Optical imaging reveals elevated intracellular chloride in hippocampal pyramidal cells after oxidative stress. J Neurosci 19: 9209–9217, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa and Snyder 2002.Sawa A, Snyder SH. Schizophrenia: diverse approaches to a complex disease. Science 296: 692–695, 2002. [DOI] [PubMed] [Google Scholar]
- Sesack et al. 1994.Sesack SR, Aoki C, Pickel VM. Ultrastructural localization of D2-receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. J Neurosci 14: 88–106, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeal et al. 2007.Smeal RM, Gaspar RC, Keefe KA, Wilcox KS. A rat brain slice preparation for characterizing both thalamostriatal and corticostriatal afferents. J Neurosci Methods 159: 224–235, 2007. [DOI] [PubMed] [Google Scholar]
- Smith and Bolam 1990.Smith AD, Bolam JP. The neural artwork of the basal ganglia as revealed by the study of synaptic connections of identified neurons. Trends Neurosci 13: 259–265, 1990. [DOI] [PubMed] [Google Scholar]
- Stults et al. 1977.Stults FH, Forstrom JW, Chiu DTY, Tappel AL. Rat liver glutathione peroxidase: purification and study of multiple forms. Arch Biochem Biophys 183: 490–497, 1977. [DOI] [PubMed] [Google Scholar]
- Sulzer et al. 1998.Sulzer D, Joyce MP, Lin L, Geldwert D, Haber SN, Hattori T, Rayport S. Dopamine neurons make glutamatergic synapses in vitro. J Neurosci 18: 4588–4602, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier et al. 2007.Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci 30: 228–235, 2007. [DOI] [PubMed] [Google Scholar]
- Thompson et al. 2004.Thompson JL, Pogue-Geile MF, Grace AA. Developmental pathology, dopamine, and stress: a model for the age of onset of schizophrenia symptoms. Schizophr Bull 30: 875–900, 2004. [DOI] [PubMed] [Google Scholar]
- Tosic et al. 2006.Tosic M, Ott J, Barral S, Bovet P, Deppen P, Gheorghita F, Matthey ML, Parnas J, Preisig M, Saraga M, Solida A, Timm S, Wang AG, Werge T, Cuénod M, Do KQ. Schizophrenia and oxidative stress: glutamate cysteine ligase modifier as a susceptibility gene. Am J Hum Genet 79: 586–592, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau 2004.Trudeau LE Glutamate co-transmission as an emerging concept in monoamine neuron function. J Psychiatry Neurosci 29: 296–310, 2004. [PMC free article] [PubMed] [Google Scholar]
- Veal et al. 2007.Veal EA, Day AM, Morgan BA. Hydrogen peroxide sensing and signaling. Mol Cell 26: 1–14, 2007. [DOI] [PubMed] [Google Scholar]
- Wilson 1993.Wilson CJ The generation of natural firing patterns in neostriatal neurons. Prog Brain Res 99: 227–297, 1993. [DOI] [PubMed] [Google Scholar]
- Xia and Haddad 1991.Xia Y, Haddad GG. Major differences in CNS sulfonylurea receptor distribution between the rat (newborn, adult) and turtle. J Comp Neurol 314: 278–289, 1991. [DOI] [PubMed] [Google Scholar]
- Yung et al. 1995.Yung KKL, Bolam JP, Smith AD, Hersch SM, Ciliax BJ, Levey AI. Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: light and electron microscopy. Neuroscience 65: 709–730, 1995. [DOI] [PubMed] [Google Scholar]
- Zhang and Sulzer 2003.Zhang H, Sulzer D. Glutamate spillover in the striatum depresses dopaminergic transmission by activating group I metabotropic glutamate receptors. J Neurosci 19: 10585–10592, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zini et al. 1993.Zini S, Tremblay E, Pollard H, Moreau J, Ben-Ari Y. Regional distribution of sulphonylurea receptors in the brain of rodent and primate. Neuroscience 55: 1085–1091, 1993. [DOI] [PubMed] [Google Scholar]