vascular oxygen sensing has intrigued and puzzled physiologists for more than 50 years. Despite some controversies, significant progress has been made in identifying candidate sensor, mediator, and effector systems in different vascular beds (17). There is a general agreement that O2 sensors typically reside within the vascular smooth muscle cells (VSMCs) and respond to changes in O2 tension by inducing redox signals/mediators that, in turn, regulate critical cellular effector systems (13). Under hypoxia, vascular tone is also modulated by vasoactive substances released from the endothelium. However, the basis for the diversity of O2-sensing systems within the vascular system remains unknown. Specifically, it is not known why vessels in the lung [pulmonary arteries (PA)] and placenta, the “O2-supplier” organs, constrict in hypoxia, while vessels in the “O2-consuming” organs [systemic arteries (SA)] dilate, thus achieving optimal blood and O2 distribution in the body at any given level of O2 supply (13). When discovered, the basis for this critical difference will shed more light on the molecular basis for vascular O2 sensing.
Differences in the physiology of the PAs vs. SAs might provide some clues. First, the PAs have to respond to essentially one input signal, i.e., decreased Po2. The lungs are much less metabolically active than systemic organs like the heart, the brain, the kidneys, or muscle. In contrast, the SAs in such organs have to respond to metabolic signals that reflect ischemia, in addition to pure hypoxia. For example, ischemia may also result because of anemia, decreased blood flow, increased O2 demand, etc., conditions that generate additional metabolic signals like acidosis, lactate, or increased ADP/ATP. SAs must integrate many of these signals, which are usually sensed through membrane and cytoplasmic systems, whereas O2 is best sensed at the site of its primary destination, i.e., the mitochondrial electron transport chain (ETC). Second, being adjacent to alveoli, resistance PAs are normally exposed to much higher O2 levels (Po2 ∼80 Torr) compared with resistance SAs (∼Po2 <50 Torr), which are hypoxic, compared with the PAs.
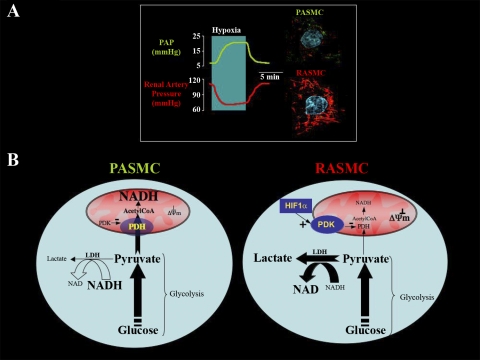
In 1977, Liang (8) showed that, in normoxic anesthetized dogs, intravenous fluoroacetate (an inhibitor of the mitochondrial Krebs' cycle) simultaneously caused a decrease in the systemic and an increase in the pulmonary vascular resistance, mimicking hypoxia. Many years later, in rat lungs perfused in series with rat kidneys, mitochondrial ETC inhibitors decreased renal, while increasing pulmonary, vascular resistance, again mimicking hypoxia (12). This suggested that metabolism and, more specifically, mitochondrial function differences might be the basis for the opposing response of the PA vs. SA to hypoxia. Indeed, renal artery smooth muscle cell (SMC) mitochondria were shown to be more hyperpolarized compared with PA SMC (PASMC) when studied under identical conditions (12) (Fig. 1A).
Fig. 1.
A: in rat lungs and kidneys perfused in series, hypoxia causes simultaneous pulmonary vasoconstriction and renal vasodilatation; this might be due to different O2 sensors, i.e., smooth muscle cell (SMC) mitochondria. Pulmonary artery SMC (PASMC) have decreased mitochondrial membrane potential (ΔΨm) compared with the renal artery SMC (RASMC). Note the more red fluorescence of the mitochondrial voltage-sensitive dye tetramethylrhodamine methyl ester in the confocal images to the right (nuclei stained with 4,6-diamidino-2-phenylindole) (12). B: a potentially different compartmentalization of metabolism and redox signaling in PASMC compared with RASMC (which are more hypoxic during normal conditions) might be due to a hypoxia-inducible factor-1α-induced inhibition of pyruvate dehydrogenase (PDH) (see text). PDK, pyruvate dehydrogenase kinase; PAP, pulmonary arterial pressure; LDH, lactate dehydrogenase.
Metabolism and mitochondrial function are much less studied in the VSMC compared with other organs like the heart (3). There is also evidence that VSMC have a unique metabolism, since they are known to be glycolytic and produce high levels of lactate in normal conditions (aerobic glycolysis) (1, 9). Initially, this was thought to be either an artifact or represent a metabolic deficiency. It is difficult to explain why the energy-hungry contracting VSMC (particularly in SAs that always have tone, i.e., the myogenic tone) rely on the cytoplasmic glycolysis (generating 2 mol of ATP per molecule of glucose) and not the mitochondrial glucose oxidation, which would generate 36 mol of ATP per molecule of glucose. This “lactate paradox” is impressively similar to the “Warburg paradox” in cancer. In 1930, Otto Warburg (16) said that the energy-hungry cancer cells are also using aerobic glycolysis and hypothesized and that this was due to cancer mitochondrial abnormalities.
There is now emerging evidence that aerobic glycolysis might, in fact, offer cancer a survival benefit (2, 14). When the entry of pyruvate (the final product of gycolysis) into the mitochondria is limited by inhibiting the gate-keeping enzyme in the inner mitochondrial membrane [i.e., pyruvate dehydrogenase (PDH)], the Krebs' cycle cannot generate the electron donors required to feed the ETC and sustain respiration. Eventually, mitochondria hyperpolarize, inhibiting the voltage- and redox-sensitive mitochondrial transition pore, through which pro-apoptotic mediators efflux during apoptosis, thus suppressing mitochondrial apoptosis. While by upregulating glucose uptake and glycolysis, cancer can “catch up” in terms of ATP production. Activation of PDH with dichloroacetate (DCA) reverses this, reactivating apoptosis and decreasing tumor growth (2).
Similarly, it has been proposed that, in VSMC, aerobic glycolysis is teleologically advantageous, and several mechanisms have been proposed. For example, VSMC aerobic glycolysis and lactate production were shown to be linked to Na+-K+ transport in the plasma membrane (10, 15). It was speculated that the ATP generation from the glycolytic enzymes at the plasma membrane is spatially advantageous to the ATPase required for Na+-K+ transport, compared with mitochondria-derived ATP (9), and a similar link between ion transport and glycolysis was shown in cancer as well (5). One can speculate further that such a compartmentalization in ATP production might also be relevant to the function of ion channels, like the ATP-sensitive potassium channels that are more important for the excitability of the SA SMC (SASMC) than of the PASMC.
A compartmentalization of glucose metabolism in VSMC was clearly described in 1983 (9). It was shown that exogenous glucose was the sole source of lactate in VSMC and that >90% of the glucose taken up was consumed in glycolysis and produced lactate, never making it to mitochondria to be oxidized. Rather, on VSMC contraction, intrinsic glucose was released by glycogenolysis, and it was that glucose that was oxidized in mitochondria, meeting the ATP demand during contraction (9). By promoting pyruvate entry into the mitochondria, DCA decreases glycogen breakdown in KCl-contracted SAs (1). These data suggest that the VSMC lactate paradox can be (at least in part) explained by a tonically inhibited PDH, comparable to cancer.
Are these observations relevant to vascular O2 sensing? In this issue of the American Journal of Physiology: Heart and Circulatory Physiology, Gao and Wolin (6) provide evidence for compartmentalization of both metabolism and redox signaling between the cytoplasm and mitochondria in bovine coronary artery VSMC in response to hypoxia. In an attempt to resolve some controversies in the field of O2 sensing [do reactive O2 species (ROS) increase or decrease in acute hypoxia, and are mitochondrial ETC or cytoplasmic sources of ROS more relevant?], they carefully measured mitochondrial and cytoplasmic ROS generation using mitosox vs. dehydroethidium fluorescence probes. Using confocal microscopy and taking advantage of NAD(P)H's autofluorescence, they studied its levels in mitochondria vs. cytoplasm/nucleus in response to hypoxia. These, along with the measurement of lactate-to-pyruvate ratios, allowed them to estimate the redox potential in different cellular compartments in response to hypoxia, hypoxia + 30 mM KCl, or exogenous lactate and pyruvate.
Hypoxia increased mitochondrial and cytoplasmic NAD(P)H and increased mitochondrial ROS, while decreasing cytoplasmic ROS. However, KCl + hypoxia prevented the NAD(P)H increase in the mitochondria, but not its increase in the cytoplasm, and decreased ROS in both mitochondria and cytoplasm. Hypoxia increased the lactate-to-pyruvate ratio compared with normoxia, and KCl + hypoxia increased the ratio more than hypoxia alone. Exogenous pyruvate failed to produce a sustained increase in mitochondrial NAD(P)H, while it caused a sustained increase in the cytoplasm.
Although there are many shuttles between the cytoplasm and the mitochondria that eventually will redistribute and equilibrate redox signaling, there is clear evidence that acute hypoxia differentially regulates redox signaling between the two compartments. There is also indirect evidence that these effects might be relevant to the compartmentalization of metabolism in VSMC, as described above. For example, the fact that exogenous pyruvate failed to alter mitochondrial redox might be due to the fact that PDH is inhibited. Although pyruvate can enter the Kreb's cycle at other levels (for example, through carboxylation to oxalacetate), conversion to acetyl-CoA by PDH is the main and rate-limiting step (Fig. 1B).
The provocative work by Gao and Wolin (6) does not give us immediate or clear answers to the real basis of vascular O2 sensing but offers several lessons that need to be seriously considered in the field, help address some of the controversies, and allow for the generation of new hypotheses. First, it is clear that the simultaneous measurements of ROS and redox signaling among different cellular compartments is a critical technique that may clarify some of the controversies in the field. For example, the opposite effects of hypoxia in mitochondria vs. cytoplasmic redox suggest that one or the other is more relevant to VSMC response to hypoxia and that, while one compartment might be more relevant to SAs, the other might be more relevant to PAs. As discussed earlier, the “simplicity” of the PA O2-sensing system suggests that it might be more directly responsive to mitochondrial ROS, whereas the more complex and more integrative SA O2-sensing system might be more responsive to cytoplasmic redox signals.
Second, such a dependence on different compartment ROS might be relevant to the fact that the compartmentalization and degree of aerobic glycolysis could be different between the PA and SA. For example, we hypothesize that PDH might be more inhibited in the SASMC compared with the PASMC, limiting pyruvate entry into the Krebs' cycle and promoting more cytoplasmic aerobic glycolysis in the former. Why would that be? It was recently shown in cancer that hypoxia-inducible factor-1α induces the expression of PDH kinase (7), a mitochondrial enzyme that tonically phosphorylates and inhibits PDH. Therefore, the more hypoxic SAs could have more inhibited PDH than the normoxic PAs (Fig. 1B). The inhibition of PDH in cancer is associated with more hyperpolarized mitochondria in cancer vs. noncancer cells (2). It is thus intriguing that SASMCs, as in cancer, were shown to have more hyperpolarized mitochondria compared with PASMCs (12).
Third, the opposite effects of hypoxia vs. hypoxia + KCl in mitochondria redox and ROS signaling remind us of the importance of studying cells and vessels in as close to physiological conditions as possible. Particularly, SASMCs have myogenic tone and, therefore, need to be studied in conditions that mimic this, to achieve physiological relevance. The mechanism by which KCl does this is unknown, but it is intriguing that, since 1976, aerobic glycolysis in both cancer and VSMC has been linked to K+ transport (5, 10, 15). It is also interesting that KCl induces glycogenolysis and promotes glucose oxidation in SAs, a phenomenon reversible by DCA (1, 9). The KCl-induced increase in intracellular Ca2+ will also activate a number of cytoplasmic enzymes, including ROS-producing systems. On the other hand, whether some of this increase in cytoplasmic calcium will enter the mitochondria and also activate Krebs' cycle enzymes depends on how negative the mitochondria are (4); thus the more hyperpolarized SASMC mitochondria will tend to attract more Ca2+ than the more depolarized PASMC mitochondria.
In summary, Gao and Wolin (6) describe redox compartmentalization in addition to the already described metabolic compartmentalization in VSMC. Since metabolic and redox signals are closely related and recognized to play a critical role in O2 sensing, this compartmentalization needs to be considered in future studies. Direct comparison of this compartmentalization between PASMCs and SASMCs should be studied. In addition, it appears that PDH might be critically involved in VSMC energetics and regulate diverse O2-sensing systems, acting as the gatekeeper between the two compartments of glucose metabolism. These concepts may also play a role in disease states. It is indeed intriguing that DCA has been shown to reverse both cancer growth (2) and pulmonary hypertension (11).
GRANTS
E. D. Michelakis is supported by funds from the Canadian Institutes for Health Research, the Alberta Herritage Foundation for Medical Research, and the Canadian Heart and Stroke Foundation. E. K. Weir is supported by National Heart, Lung, and Blood Institute Grant R01-HL65322.
REFERENCES
- 1.Barron JT, Parrillo JE. Production of lactic acid and energy metabolism in vascular smooth muscle: effect of dichloroacetate. Am J Physiol Heart Circ Physiol 268: H713–H719, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Bonnet S, Harry G, Hashimoto K, Porter CJ, Andrade MA, Thebaud B, Michelakis ED. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell 11: 37–51, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Chace KV, Odessey R. The utilization by rabbit aorta of carbohydrates, fatty acids, ketone bodies, and amino acids as substrates for energy production. Circ Res 48: 850–858, 1981. [DOI] [PubMed] [Google Scholar]
- 4.Duchen MR Contributions of mitochondria to animal physiology: from homeostatic sensor to calcium signalling and cell death. J Physiol 516: 1–17, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galeotti T, van Rossum GD, Russo MA, Palombini G. Interaction of Na+ and K+ transport with aerobic energy metabolism in slices of Morris hepatoma 3924A. Cancer Res 36: 4175–4184, 1976. [PubMed] [Google Scholar]
- 6.Gao Q, Wolin MS. Effects of hypoxia on relationships between cytosolic and mitochondrial NAD(P)H redox and superoxide generation in coronary arterial smooth muscle. Am J Physiol Heart Circ Physiol (Jun 2008); doi: 10.1152/ajpheart.00316.2008. [DOI] [PMC free article] [PubMed]
- 7.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 3: 177–185, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Liang CS Metabolic control of circulation. Effects of iodoacetate and fluoroacetate. J Clin Invest 60: 61–69, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lynch RM, Paul RJ. Compartmentation of glycolytic and glycogenolytic metabolism in vascular smooth muscle. Science 222: 1344–1346, 1983. [DOI] [PubMed] [Google Scholar]
- 10.Lynch RM, Paul RJ. Glucose uptake in porcine carotid artery: relation to alterations in active Na+-K+ transport. Am J Physiol Cell Physiol 247: C433–C440, 1984. [DOI] [PubMed] [Google Scholar]
- 11.McMurtry MS, Bonnet S, Wu X, Dyck JR, Haromy A, Hashimoto K, Michelakis ED. Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis. Circ Res 95: 830–840, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Michelakis ED, Hampl V, Nsair A, Wu X, Harry G, Haromy A, Gurtu R, Archer SL. Diversity in mitochondrial function explains differences in vascular oxygen sensing. Circ Res 90: 1307–1315, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Michelakis ED, Thebaud B, Weir EK, Archer SL. Hypoxic pulmonary vasoconstriction: redox regulation of O2-sensitive K+ channels by a mitochondrial O2-sensor in resistance artery smooth muscle cells. J Mol Cell Cardiol 37: 1119–1136, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Pan JG, Mak TW. Metabolic targeting as an anticancer strategy: dawn of a new era? Sci STKE 2007: pe14, 2007. [DOI] [PubMed]
- 15.Paul RJ, Bauer M, Pease W. Vascular smooth muscle: aerobic glycolysis linked to sodium and potassium transport processes. Science 206: 1414–1416, 1979. [DOI] [PubMed] [Google Scholar]
- 16.Warburg O Ueber den Stoffwechsel der Tumoren. London: Constable, 1930.
- 17.Weir EK, Lopez-Barneo J, Buckler KJ, Archer SL. Acute oxygen-sensing mechanisms. N Engl J Med 353: 2042–2055, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]