Abstract
Cellular hypertrophy is regulated by coordinated pro- and antigrowth machineries. Foxo transcription factors initiate an atrophy-related gene program to counter hypertrophic growth. This study was designed to evaluate the role of Akt, the forkhead transcription factor Foxo3a, and atrophy genes muscle-specific RING finger (MuRF)-1 and atrogin-1 in cardiac hypertrophy and contractile dysfunction associated with high-fat diet-induced obesity. Mice were fed a low- or high-fat diet for 6 mo along with a food-restricted high-fat weight control group. Echocardiography revealed decreased fractional shortening and increased end-systolic diameter and cardiac hypertrophy in high-fat obese but not in weight control mice. Cardiomyocytes from high-fat obese but not from weight control mice displayed contractile and intracellular Ca2+ defects including depressed maximal velocity of shortening/relengthening, prolonged duration of shortening/relengthening, and reduced intracellular Ca2+ rise and clearance. Caspase activities were greater in high-fat obese but not in weight control mouse hearts. Western blot analysis revealed enhanced basal Akt and Foxo3a phosphorylation and reduced insulin-stimulated phosphorylation of Akt and Foxo3a without changes in total protein expression of Akt and Foxo3a in high-fat obese hearts. RT-PCR and immunoblotting results displayed reduced levels of the atrogens atrogin-1 and MuRF-1, the upregulated hypertrophic markers GATA4 and ciliary neurotrophic factor receptor-α, as well as the unchanged calcineurin and proteasome ubiquitin in high-fat obese mouse hearts. Transfection of H9C2 myoblast cells with dominant-negative Foxo3a adenovirus mimicked palmitic acid (0.8 mM for 24 h)-induced GATA4 upregulation without an additive effect. Dominant-negative Foxo3a-induced upregulation of pAkt and repression of phosphatase and tensin homologue were abrogated by palmitic acid. These results suggest a cardiac hypertrophic response in high-fat diet-associated obesity at least in part through inactivation of Foxo3a by the Akt pathway.
Keywords: myocardial function, cardiomyocytes, contractile function
obesity, if uncorrected, leads to cardiac hypertrophy and compromised myocardial function and energy metabolism, contributing to enhanced cardiac morbidity and mortality (11, 24, 43). Cardiac hypertrophy characterized by increased cell size and protein synthesis is associated with an overtly increased risk of ventricular dysfunction, heart failure, and malignant arrhythmias in obese individuals (21). Weight loss in obese patients has been shown to reduce heart size and improve cardiac performance in the absence of any systemic hemodynamic alteration (1), suggesting an independent role for hypertrophy in cardiac dysfunction in sustained obesity. To date, a plethora of cellular signaling pathways have been identified as participating in the hypertrophic response including tonic activation of the serine-threonine kinase Akt in response to growth factors, angiotensin II, mechanical stress, oxidative stress, and calcineurin and reduced degradation of terminally misfolded proteins by the ubiquitin-proteasome system (13, 27, 32, 39). Akt may regulate a wide variety of signaling molecules involved in hypertrophic response such as the mammalian target of rapamycin (38), eukaryotic initiation factor 4E-binding proteins (48), p70S6k (15, 39), GSK-3β (15, 16), forkhead transcriptional factors (20), and GATA4 (25). Moreover, the activity of Akt is also under the negative regulation of phosphatase and tensin homologue (PTEN; Ref. 47). However, little information is available regarding the precise regulatory machinery of Akt in the cardiac hypertrophic response that results from diet-induced obesity.
The Foxo subfamily of forkhead transcription factors, including Foxo1 (FKHR), Foxo3a (FKHRL-1), and Foxo4 (AFX), is a downstream target of Akt (20). Akt phosphorylation results in nuclear exclusion (inhibition) of Foxo. In addition to the well-established cellular responses elicited by Foxo, including differentiation, metabolism, proliferation, survival, and skeletal muscle atrophy (20, 37), this transcription factor was also indicated in cardiomyocyte atrophy involving upregulation of a cascade of atrogenes (36, 37, 46). In skeletal muscle, atrogenes are controlled by the growth factor-Akt-mediated transcriptional regulation of Foxo factors (35, 37). Recently, it was demonstrated that the Foxo transcription factors are expressed in cardiomyocytes under the regulation of growth factors/Akt signaling. Foxo may control an atrogene transcriptional program to regulate myocyte size downstream of multiple regulators of cardiac hypertrophy (40).
To better understand the mechanism behind obesity-associated cardiac hypertrophy and the resultant myopathic changes, fat-enriched diets are used to foster diet-induced obesity (33). Recent evidence from our laboratory as well as others has shown that diet-induced obesity is associated with insulin resistance, cardiac hypertrophy, and myocardial dysfunction (33, 44). However, disparate findings in myocardial function have been seen for diet-induced obesity. Wilson et al. (50) found moderate cardiac dysfunction in rats consuming a “Western diet” (45% calories from fat, identical to our high-fat diet used in the present study). Evidence (9, 33) from our group revealed myocardial and cardiomyocyte contractile dysfunction associated with cardiac hypertrophy in both rat and mouse models of the “Western diet” (45% calories from fat)-induced obesity. To the contrary, Morgan and et al. (28) failed to identify cardiac remodeling and contractile dysfunction after a 12-wk “Western” high-fat diet (45% fat) feeding in rats with established heart failure. Interestingly, no adverse cardiac effects were detected in rats fed a diet with a higher fat component (60% of the energy from fat; Ref. 50). Consistently, similar high-fat diet (60% fat) feeding after coronary artery ligation triggers increased oxidative phosphorylation and electron transport chain complex activities without adversely affecting left ventricular contractile function or remodeling (34). Similarly, Stanley and colleagues (4, 29) also reported absence of overt cardiac remodeling and contractile dysfunction in mice and rats fed a high-fat diet (60% fat) for 12–16 wk albeit before the onset of obesity. To elucidate the interplay behind cardiac hypertrophy and contractile dysfunction after high-fat diet intake, this study was designed to examine the role of Akt, Foxo transcription factor, and atrophy-specific gene transcription in high-fat diet-induced cardiac geometric and functional alterations. Given the prominent roles of ubiquitin-related protein clearance and calcineurin in the regulation of cardiac remodeling and contractile function (13, 22, 31, 31), myocardial expression of ubiquitin and calcineurin along with other hypertrophic markers was also evaluated after high-fat diet feeding.
MATERIALS AND METHODS
High-fat diet feeding and serum parameters.
The experimental procedure described here was approved by the Institutional Animal Use and Care Committee at the University of Wyoming (Laramie, WY). In brief, 4 mo-old male FVB mice weighing ∼20 g were randomly assigned to low-fat (10 and 70% of total calorie from fat and carbohydrate, respectively, catalogue #D12450B) or high-fat [45 and 35% of total calorie from fat and carbohydrate, respectively (37% saturated and 46% mono- and 19% polyunsaturated fatty acids), catalogue #D12451, which is often referred to as the “Western diet” (50)] diets (Research Diets, New Brunswick, NJ) for 6 mo (33, 49). Cholesterol content was 18 and 196.5 mg/kg for low- and high-fat diets, respectively. High-fat diet was calorically rich (4.83 vs. 3.91 kcal/g in low-fat diet) due to higher fat composition. To discern the effect of high-fat diet from high-fat diet-induced obesity, a third group of mice received food-restricted high-fat diet (∼80% of the ad libitum high-fat diet intake) to match the body weight gain of low-fat diet group. Mice were housed individually in a climate-controlled environment with a 12:12-h light-dark cycle and free access to diets (unless food restricted) as well as water. Serum glucose levels (after 12 h of fasting) were determined using an Accu-Chek III glucose analyzer (12). Systolic and diastolic blood pressures were examined with a semi-automated, amplified tail cuff device (IITC, Woodland Hills, CA). Blood insulin levels were measured using a mouse insulin ELISA kit. Plasma leptin concentrations were determined using a RIA kit (Linco Research, St. Charles, MO).
Echocardiographic assessment.
Cardiac geometry and function were evaluated in anesthetized (Avertin 2.5%, 10 μl/g body wt ip) mice using two-dimensional guided M-mode echocardiography (Phillips Sonos 5500) equipped with a 15-6 MHz linear transducer (Phillips Medical Systems, Andover, MD). Anterior and posterior wall thicknesses and diastolic and systolic left ventricular dimensions were recorded from M-mode images using method adopted by the American Society of Echocardiography. Fractional shortening was calculated from end-diastolic diameter (EDD) and end-systolic diameter (ESD) using the equation of (EDD-ESD)/EDD. Estimated echocardiographic left ventricular (LV) mass was calculated as [(LVEDD + septal wall thickness + posterior wall thickness)3 − LVEDD3] × 1.055, where 1.055 (mg/mm3) is the density of myocardium. Heart rates were averaged over 10 cardiac cycles (14).
Isolation of cardiomyocytes.
After ketamine/xylazine sedation, hearts were removed and perfused with Krebs-Henseleit bicarbonate buffer containing (in mM) the following: 118 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, 10 HEPES, and 11.1 glucose. Hearts were digested with collagenase D for 20 min. Left ventricles were removed and minced before being filtered. The myocyte yield was ∼75%, which was not affected by high-fat diet feeding. Only rod-shaped myocytes with clear edges were selected for mechanical and intracellular Ca2+ study (12).
Cell shortening and relengthening.
Mechanical properties of cardiomyocytes were assessed using an IonOptix soft-edge system (IonOptix, Milton, MA). Myocytes were placed in a chamber mounted on the stage of an Olympus IX-70 microscope and superfused (∼2 ml/min at 25°C) with a Krebs-Henseleit bicarbonate buffer containing 1 mM CaCl2. Myocytes were field stimulated at 0.5 Hz unless otherwise stated. Cell shortening and relengthening were assessed including peak shortening (PS) − peak contractility; time-to-PS (TPS) − contraction duration; time-to-90% relengthening (TR90) − relaxation duration; and maximal velocities of shortening/relengthening (±dL/dt) − and maximal pressure development and decline (12).
Intracellular Ca2+ transients.
A cohort of myocytes was loaded with fura 2-AM (0.5 μM) for 10 min, and fluorescence intensity was recorded with a dual-excitation fluorescence photomultiplier system (Ionoptix). Myocytes were placed onto an Olympus IX-70 inverted microscope and imaged through a Fluor ×40 oil objective. Cells were exposed to light emitted by a 75-W lamp and passed through either a 360- or a 380-nm filter, while being stimulated to contract at 0.5 Hz. Fluorescence emissions were detected between 480–520 nm, and the qualitative change in fura 2 fluorescence intensity (FFI) was inferred from FFI ratio at the two wavelengths (360/380). Fluorescence decay time (single or biexponential decay) was calculated as an indicator of intracellular Ca2+ clearing (12).
Caspase-3 assay.
Caspase-3 activity was determined according to the published method (23). Briefly, 1 ml PBS was added to a flask containing left ventricular tissue homogenates before centrifugation at 10,000 g at 4°C for 10 min. The supernatant was discarded, and the homogenates were lysed in 100 μl of ice-cold cell lysis buffer (50 mM HEPES pH 7.4, 0.1% CHAPS, 1 mM DTT, 0.1 mM EDTA, and 0.1% NP-40). The assay was carried out in a 96-well plate with each well containing 30 μl cell lysate, 70 μl of assay buffer (50 mM HEPES, 0.1% CHAPS, 100 mM NaCl, 10 mM DTT, and 1 mM EDTA), and 20 μl of caspase-3 colorimetric substrate Ac-DEVD-pNA (Sigma). The 96-well plate was incubated at 37°C for 1 h, during which time the caspase in the sample was allowed to cleave the chromophore p-NA from the substrate molecule. Absorbency was detected at 405 nm with caspase-3 activity being proportional to color reaction. Protein content was determined using the Bradford method. The caspase-3 activity was expressed as picomoles of pNA released per micrograms of protein per minute.
Caspase-3/7 assay.
Caspase-3 and caspase-7 activity was determined using an Apo-ONE homogeneous caspase-3/7 assay kit (Promega, Madison, WI). Caspase-3 and caspase-7 are members of the cysteine aspartic acid-specific protease (caspase) family that play key roles in apoptosis in mammalian cells. In brief, caspase-3 and caspase-7 activities were detected in cells undergoing apoptosis via cleavage of a rhodamine 110, bis-N-CBZ-l-aspartyl-l-glutamyl-l-valyl-l-aspartic acid amide (Z-DEVD-R110) substrate, which exists as a profluorescent substrate before the assay. To perform the Apo-ONE caspase-3/7 assay, we mixed and added a caspase-3/7 buffer and the Z-DEVD-R110 substrate to the left ventricular tissue homogenates. Upon sequential cleavage and removal of the DEVD peptides by caspase-3 and caspase-7 activity, the R110 leaving group will become intensely fluorescent at an excitation wavelength of 499 nm and an emission wavelength of 521 nm. The caspase-3 and caspase-7 activity was directly proportional to R110 fluorescence and was expressed as the net fluorescence (2).
Ex vivo dominant-negative Foxo3a transfection and Western blot analysis.
For the ex vivo dominant-negative (DN) Foxo3a transfection study, H9C2 myoblast cells (ATCC, Manassas, VA) were first incubated at 37°C in the DMEM medium (5.5 mM glucose and 10% FBS) with or without 2 μl of DN Foxo3a virus (1:1,000) for 6 h before exposure to free fatty acid palmitic acid (0.8 mM) for 24 h. The dosage and treatment duration were largely based on previous experience from our laboratory as well as others (7, 9). The DN Foxo3a (purchased from Vector Biolabs, PA) is a truncated version of Foxo3a, which is devoid of the transactivation domain from the C terminus (D256) of the full-length Foxo3a. It has previously been shown that truncated Foxo functions as DN inhibitor of transcription induced by Foxo3a (41). Proteins were then extracted from H9C2 cells treated with or without DN Foxo3a as described previously (10). For insulin stimulation, mice were injected intraperitoneally with insulin (1.5 U/100 g body wt) for 10 min before the death of the animal and tissue collection. Samples containing equal amount of proteins were separated on 10% SDS-polyacrylamide gels in a minigel apparatus (Mini-PROTEAN II, Bio-Rad) and transferred to nitrocellulose membranes. The membranes were blocked with 5% milk in TBS-Tween, and were incubated overnight at 4°C with anti-Akt, anti-pAkt, anti-Foxo3a, and anti-pFoxo3a (Thr32) (all from Cell Signaling Technology, Beverly, MA); anti-GATA4 (Santa Cruz Biotenology, Santa Cruz, CA); anti-ciliary neurotrophic factor receptor (CNTFR)-α (Cell Signaling); anti-calcineurin A (Abcam, Cambridge, MA); anti-ubiquitin (Sigma-Aldrich, St. Louis, MO) at a dilution ratio of 1:1,000; and anti-GAPDH (as loading control, 1:2,000; Cell Signaling). After immunoblotting, the film was scanned and the intensity of immunoblot bands was detected with a Bio-Rad calibrated densitometer (12).
Total RNA extraction, cDNA synthesis, reverse transcription, and real-time PCR.
Total RNA was isolated from left ventricles using the TRIzol reagent (Invitrogen), followed by DNase digestion to eliminate genomic DNA contamination. RNAs were quantified with spectrophotometer A260 readings. Synthesis of cDNA was performed at 37°C for 60 min using 1 μg of total RNA in a 20 μl system by Superscript III (http://www.invitrogen.com/content.cfm?pageid=10281; Invitrogen). Primers were designed using Beacon Designer 5.0 software. The primers for mouse were as follows: atrogin-1: sense, 5′-GCAGAGAGTCGGCAAGTC-3′ and antisense, 5′-CAGGTCGGTGATCGTGAG-3′; and muscle-specific RING finger (MuRF)-1: sense, 5′-TGGAAACGCTATGGAGAACC-3′; and antisense, 5′-ATTCGCAGCCTGGAAGATG-3′. The primers for the housekeeping gene GAPDH (mouse) were as follows: sense, 5′-AATGGTGAAGGTCGGTGTGAAC-3′; and antisense, 5′-CGTGAGTGGAGTCATACTGGAAC-3′. All primers were obtained from Integrated DNA Technologies (Coralville, IA). Real-time PCR was performed by using an iCycler iQ real-time PCR detection system (Bio-Rad) with a SYBR green qPCR SuperMixes kit (Invitrogen). The thermocycling program was 40 cycles of 95°C for 15 s and 55°C for 45 s with an initial cycle of 95°C for 10 min. Melting curve analysis was performed over the range 55–95°C by monitoring SYBR green fluorescence with increasing temperature (0.5°C increment with a 10-s interval). PCR-specific products were determined as a clear single peak at the melting curves >80°C. Real-time PCR was duplicated for each cDNA sample. Each gene mRNA level was acquired from the value of the threshold cycle (Ct) of the real-time PCR as related to that of GAPDH using the comparative Ct method through the formula 2ΔCt (ΔCt = GAPDH Ct − gene of interest Ct; Ref. 19).
Data analysis.
Data are means ± SE. Statistical comparison was performed by ANOVA followed by Newman-Keuls post hoc tests. Significance was set as P < 0.05.
RESULTS
General feature, echocardiographic property, and apoptosis of low- and high-fat fed mice.
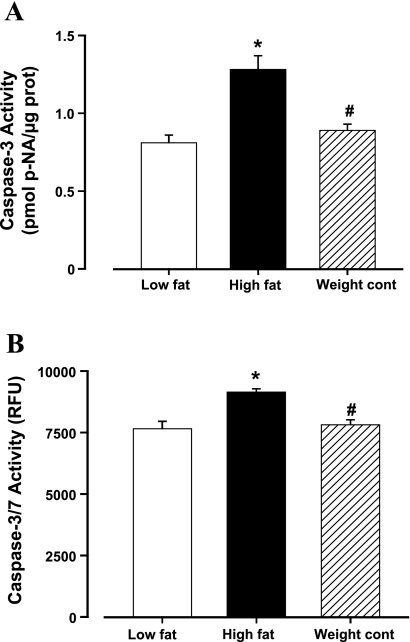
High-fat diet feeding ad libitum significantly increased body wt as well as heart, liver, and kidney organ weights. Food-restricted high-fat diet feeding (weight control) did not affect body and organ weights. Fasting serum glucose level, blood pressure (both diastolic and systolic), heart rate, wall thickness, left ventricular EDD, and normalized left ventricular mass were comparable among all three groups. High-fat diet-induced obesity was accompanied with significantly increased serum insulin and leptin levels, left ventricular mass, ESD, and reduced fractional shortening. High-fat diet food restriction significantly attenuated hyperinsulinemia, hyperleptinemia, and myocardial aberration associated with high-fat diet-induced obesity, although levels of plasma insulin and leptin were moderately elevated in the weight control group (Table 1). High-fat diet feeding ad libitum triggered elevated myocardial apoptosis, as assessed by the caspase-3 and caspase-3/7 activity assays, which was alleviated by the high-fat diet food restriction (Fig. 1).
Table 1.
Biometric and echocardiographic parameters of mice fed low- or high-fat diet for 6 mo
| Parameter | Low-Fat Diet (n = 19) | High-Fat Diet Ad Libitum (n = 20) | Weigh-Matched Control (n = 13) |
|---|---|---|---|
| Body weight, g | 25.86±0.66 | 32.58±0.84* | 26.85±0.57† |
| Heart weight, mg | 121±7 | 150±7* | 132±6† |
| Liver weight, g | 1.37±0.08 | 1.64±0.08* | 1.40±0.05† |
| Kidney weight, g | 0.32±0.02 | 0.43±0.03* | 0.34±0.02† |
| Fasting serum glucose, mM | 5.17±0.31 | 5.98±0.31 | 5.69±0.36 |
| Fasting serum insulin, ng/ml | 0.17±0.02 | 7.32±0.35* | 0.57±0.09*† |
| Fasting serum leptin, ng/ml | 3.39±0.38 | 8.36±1.29* | 4.65±0.41*† |
| Diastolic blood pressure, mmHg | 74.0±4.1 | 80.5±4.2 | 76.6±3.1 |
| Systolic blood pressure, mmHg | 104±6 | 113±5 | 111±6 |
| Sedated heart rate, beats/min | 413±9 | 422±15 | 419±19 |
| Wall thickness, mm | 0.83±0.04 | 0.89±0.04 | 0.89±0.05 |
| EDD, mm | 2.70±0.09 | 2.80±0.15 | 2.75±0.14 |
| ESD, mm | 1.39±0.11 | 1.83±0.14* | 1.48±0.08† |
| LV mass, mg | 66.9±6.2 | 87.4±7.5* | 68.4±6.8† |
| Normalized LV mass, mg/g | 2.38±0.18 | 2.71±0.20 | 2.47±0.24 |
| Fraction shortening, % | 49.1±3.0 | 37.4±1.9* | 49.5±3.9† |
Weight-matched control is high-fat diet food restriction. EDD, end-diastolic diameter; ESD, end-systolic diameter; LV, left ventricular. Data are means ± SE; n = no. of animals.
P < 0.05 vs. low-fat diet group;
P < 0.05 vs. high-fat diet group.
Fig. 1.
Effect of fat diet feeding on apoptosis in mouse left ventricles from low-fat, high-fat, and high-fat diet food restriction (weight control) groups. A: caspase-3 activity. B: capsase-3/7 activity. Data are means ± SE; n = 5–6 mice per group. *P < 0.05 vs. low-fat group; #P < 0.05 vs. high-fat group. RFU, relative fluorescent units.
Cardiomyocyte contractile and intracellular Ca2+ properties.
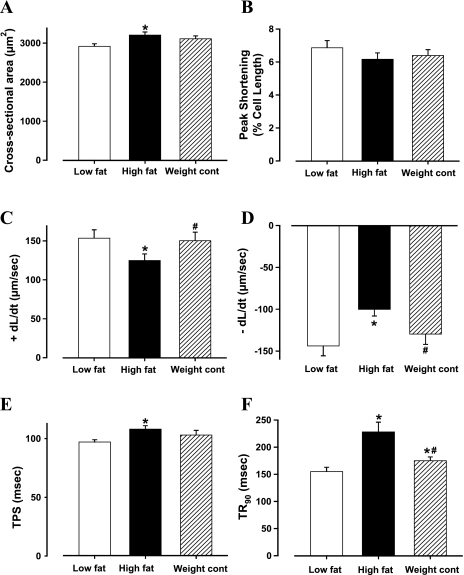
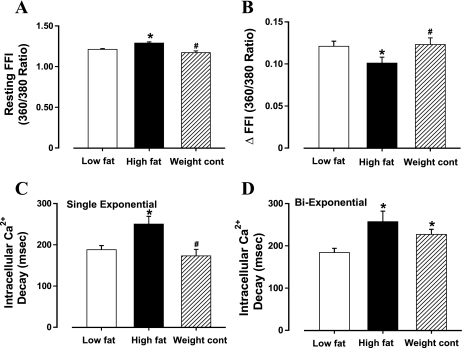
High-fat diet-induced obesity was accompanied with significantly increased cardiomyocyte cross-sectional area, reduced ±dL/dt, and prolonged TPS and TR90 with normal PS (Fig. 2), somewhat reminiscent of our earlier finding (33). In addition, cardiomyocytes from high-fat-fed obese mice displayed a significantly elevated baseline intracellular Ca2+, depressed intracellular Ca2+ rise in response to electrical stimulus (ΔFFI), and reduced intracellular Ca2+ decay rate (either single or biexponential curve fit; Fig. 3). These cardiomyocyte mechanical and intracellular Ca2+ defects associated with high-fat diet-induced obesity were significantly attenuated by the weight control maneuver. Nonetheless, food restriction of high-fat diet slightly but significantly prolonged TR90 and biexponential intracellular Ca2+ decay without affecting any other indices (Fig. 2F and 3D).
Fig. 2.
Contractile properties of cardiomyocytes from low-fat, high-fat, and high-fat diet food restriction (weight control) groups. A: cross-section area. B: peak shortening (normalized to cell length); C: maximal velocity of shortening (+dL/dt). D: maximal velocity of relengthening (−dL/dt). E: time-to-peak shortening (TPS). F: time-to-90% relengthening (TR90). Data are means ± SE; n = 75 cells from 6 mice per group. *P < 0.05 vs. low-fat group; #P < 0.05 vs. high-fat group.
Fig. 3.
Intracellular Ca2+ transients in cardiomyocytes from low-fat, high-fat, and high-fat diet food restriction (weight control) groups. A: resting fura 2 fluorescence intensity (FFI). B: electrically stimulated rise in FFI (ΔFFI). C: single exponential intracellular Ca2+ decay. D: biexponential intracellular Ca2+ decay. Data are means ± SE; n = 60 cells from 6 mice per group. *P < 0.05 vs. low-fat group; #P < 0.05 vs. high-fat group.
Expression of Akt, pAkt, Foxo3a, pFoxo3a, hypertrophic factors, MuRF-1, and atrogin-1.
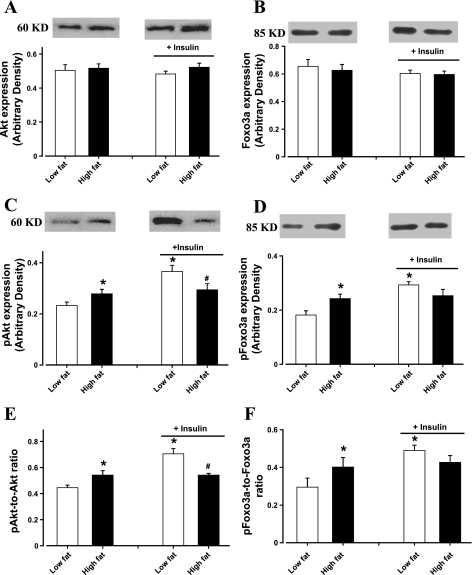
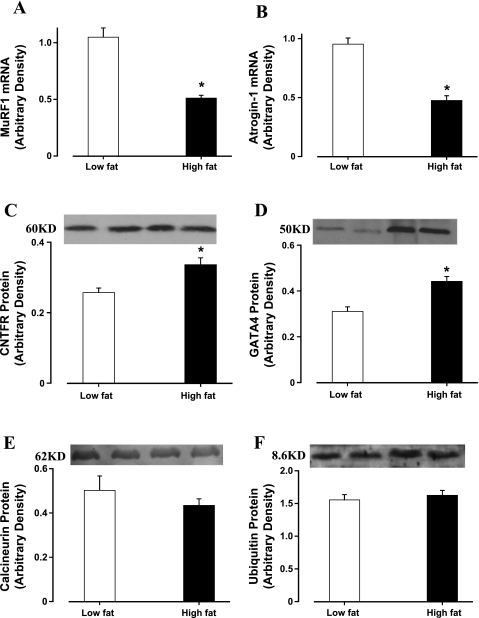
Immunoblotting data revealed that high-fat diet-induced obesity significantly enhanced basal phosphorylation of Akt and Foxo3a (shown as absolute or normalized phosphorylation) without affecting the total protein expression of Akt and Foxo3a. Moreover, high-fat diet-associated obesity abrogated the insulin-stimulated increase in phosphorylation of Akt and Foxo3a (shown as absolute or normalized phosphorylation). Insulin exposure did not affect the levels of nonphosphorylated Akt and Foxo3a (Fig. 4). Our data further revealed that high-fat diet-induced obesity significantly reduced the transcriptional expression of atrogens including MuRF-1 and atrogin-1. Both atrogens exhibited a similar decline in mRNA expression of ∼50%. In addition, markers for hypertrophy GATA4 and CNTFR-α were significantly upregulated in murine hearts after 6 mo of high-fat diet feeding. However, myocardial levels of calcineurin A and ubiquitin were unaffected in response to 6 mo of high-fat diet feeding (Fig. 5).
Fig. 4.
Western blot analysis of total and phosphorylated Akt and Foxo3a in cardiomyocytes from low-fat and high-fat-fed mice in the absence or presence of insulin stimulation (1.5 U/100 g body wt for 10 min). A: total Akt. B: total Foxo3a. C: phosphorylated Akt (pAkt). D: phosphorylated Foxo3a (pFoxo3a). E: pAkt-toAkt ratio. F: pFoxo3a-to-Foxo3a ratio. Insets: representative gels of Akt, pAkt, Foxo3a, and pFoxo3a using specific antibodies. Data are means ± SE; n = 5–8 per group. *P < 0.05 vs. low-fat group; #P < 0.05 vs. insulin-stimulated low-fat group.
Fig. 5.
A and B: RT-PCR measurement of atrogin-1 (A) and muscle-specific RING finger (MuRF) (B) in left ventricles from low-fat and high-fat fed mice. C and D: Western blot analysis of hypertrophic proteins ciliary neurotrophic factor receptor (CNTFR)-α (C) and GATA4 (D) in left ventricles from low-fat and high-fat fed mice. E and F: Western blot analysis of hypertrophic proteins calcineurin A (E) and ubiquitin (F) in left ventricles from low-fat and high-fat fed mice. Data are means ± SE; n = 4–8. *P < 0.05 vs. low-fat group.
Effect of DN foxo3a adenovirus on palmitic acid-induced response on Akt, PTEN, and GATA4.
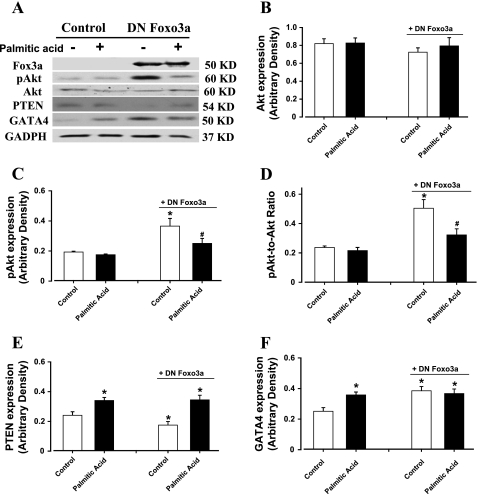
To examine the causal relationship between high-fat diet-induced change in Akt-Foxo3a and cardiac hypertrophy, ex vivo adenoviral transfection study was performed to transfect DN Foxo3a virus into the H9C2 myoblasts for 6 h before the cells were exposed to palmitic acid (0.8 mM) for 24 h. Our immunoblotting analysis revealed that palmitic acid significantly upregulated hypertrophic markers including PTEN and GATA4 without an overt change in Akt and pAkt. Interestingly, DN Foxo3a adenovirus mimicked palmitic acid-induced upregulation of GATA4 without eliciting any additive effect with palmitic acid. DN Foxo3a itself induced upregulation of pAkt and repression of PTEN, the effect of which was abrogated by palmitic acid (Fig. 6). These data suggested that DN Foxo3a may trigger Akt phosphorylation and cardiac hypertrophic marker expression, reminiscent of high-fat diet and palmitic acid, respectively.
Fig. 6.
Western blot analysis of total and phosphorylated Akt, phosphatase and tensin homologue (PTEN), and GATA4 in H9C2 myoblast cells treated with 0.8 mM palmitic acid for 24 h. A cohort of cell had been transfected with dominant-negative (DN) Foxo3a virus (1:1,000) for 6 h before exposure of palmitic acid. A: representative gel blots using specific antibodies. B: total Akt. C: phosphorylated Akt (pAkt). D: pAkt-toAkt ratio. E: PTEN. F: GATA4. Data are means ± SE; n = 6–8 per group. *P < 0.05 vs. control group; #P < 0.05 vs. DN Foxo3a/control group.
DISCUSSION
The major findings of our study revealed that high-fat diet-associated obesity elicits cardiac hypertrophy, myocardial contractile dysfunction, and impaired intracellular Ca2+ handling in mice, the effect of which may be reconciled by food restriction of high-fat diet feeding. The myocardial geometric and functional aberrations seen in high-fat diet-induced obesity were associated with myocardial apoptosis, elevated basal phosphorylation of Akt and Foxo3a, dampened insulin-stimulated increase in phosphorylation of Akt and Foxo3a, and reduced atrogene expression including atrogin-1 and MuRF-1, as well as upregulated the hypertrophic markers GATA4 and CNTFR-α. The levels of calcineurin and ubiquitin were not affected after the 6-mo high-fat diet feeding in our current experimental setting. These data suggest a predominant role of elevated basal Akt-Foxo3a drive and repressed atrogene transcriptional expression in cardiac hypertrophy associated with high-fat diet-induced obesity. Our ex vivo study using the DN Foxo3a virus revealed that palmitic acid, a main free fatty acid component in high-fat diet, upregulated hypertrophic protein expression in H9C2 myoblasts, reminiscent of mutant Foxo3a adenovirus. Collectively, our data suggest that the high-fat diet-associated obesity rather than the high-fat diet itself may be essential to the development of cardiac remodeling and cardiac dysfunction.
High-fat diet intake triggers dyslipidemia, insulin resistance, obesity, and type 2 diabetes (3, 44). This is supported by our current findings of elevated plasma insulin and leptin levels despite weight control. Although lifestyle modification and pharmacological intervention have shown some promise against hypertrophied and dysfunctional hearts in obesity (26, 43), no unique target has been identified to radically reconcile cardiac geometric and functional defects in obese individuals. Our data of cardiac hypertrophy and compromised myocardial and cardiomyocyte contractile function (reduced fraction shortening, enlarged ESD, depressed ±dL/dt, and prolonged duration of contraction and relaxation) after ad libitum high-fat diet intake are consistent with previous clinical and experimental observations (4, 6, 8, 29, 33, 43). The presence of overt cardiac contractile dysfunction after the 6-mo ad libitum high-fat diet feeding favors the notion that cardiac hypertrophy has prompted distinct pathological conditions progressing from a compensated into a decompensated stage in obesity. The 6-mo high-fat feeding regimen (regardless food restriction) elicited little change in blood pressure and blood glucose, excluding the possible existence of concomitant hypertension and full-blown diabetes mellitus. The impaired intracellular Ca2+ handling shown as elevated resting intracellular Ca2+ levels, reduced intracellular Ca2+ clearance rate, and reduced intracellular Ca2+ rise (ΔFFI) in high-fat diet-fed obese mouse cardiomyocytes is in line with data from a rat model of high-fat-induced obesity (33) and is likely responsible for prolonged relaxation, reduced ±dL/dt, and fraction shortening in high-fat diet-fed mouse hearts. The fact that weight control alleviated high-fat diet-induced cardiac remodeling and contractile dysfunction suggests a key role of obesity in the high-fat diet-induced cardiac abnormalities. The presence of obesity in addition to the duration of fat feeding seems to explain the discrepant findings between our study and those by Stanley's group (4, 29) regarding the high-fat diet-induced cardiac remodeling and contractile response. It is worth mentioning that our data revealed prolonged relaxation duration and intracellular Ca2+ clearance in the weight-controlled high-fat diet group, indicating the likelihood of the presence of a high-fat-elicited myocardial effect independent of body weight gain.
Cardiac hypertrophy occurs during normal physiological growth as an adaptive response to pressure or volume stress, mutations in cardiac proteins, or metabolic perturbations (40). This adaptive response may become maladaptive and contribute to cardiac dysfunction (13). In our present study, high-fat diet-induced obesity promoted basal phosphorylation of Akt and Foxo3a without changes in Akt and Foxo3a expression. Insulin-stimulated phosphorylation of Akt and Foxo3a was blunted, supporting reduced insulin sensitivity. These data favor a role for Akt and its downstream signal Foxo3a in cardiac hypertrophy in high-fat diet-triggered obesity. While increased basal phosphorylation of Akt and Foxo3a after ad libitum high-fat diet feeding promotes cardiac hypertrophy and suppresses atrophy-specific gene transcription involving atrogin-1 and MuRF-1, reduced insulin-stimulated phosphorylation of Foxo3a favors apoptosis. This is supported by elevated caspase activities in cardiomyocytes after high-fat diet intake. Akt signaling is an important regulator of cardiac growth, and its overexpression leads to enhanced contractility, cell survival, and pathological cardiac hypertrophy (5, 38). Our observation of enhanced basal Foxo3a phosphorylation and suppressed atrophy-specific gene suppression coincides with cardiac hypertrophy under high-fat intake-induced obesity.
One rather interesting finding from our study depicted that DN Foxo3a virus mimicked increased basal Akt phosphorylation and hypertrophic protein GATA4 in high-fat diet-associated obesity. Upregulation of GATA4 in high-fat diet-induced obesity is synchronized with downregulation of atrophy-specific gene transcription to promote cardiac hypertrophy and likely hypertrophic cardiomyopathy. This notion is reinforced by our findings that palmitic acid directly promoted GATA4 expression in H9C2 myoblasts. The levels of palmitic acid, the predominant saturated free fatty acid released from adipose tissue, are elevated in obesity and contribute to obesity-associated cardiovascular complications (51). The cellular mechanism responsible for repressed atrophy gene transcription in high-fat diet-induced obesity is not fully understood, although interplay between the transcriptional coactivator PGC-1α (peroxisome proliferator-activated receptor-γ coactivator) and Foxo transcriptional factor may play a role (36). Further study is warranted to examine atrophy gene transcription regulation after high-fat diet intake with or without development of obesity.
Our ex vivo data also suggested a possible feed-forward mechanism between Akt and its downstream signaling molecule Foxo3a, as transfection of the mutant Foxo3a stimulated Akt phosphorylation. This feed-forward scenario is supported by the notion that the atrophy gene atrogin-1 inhibits Akt-dependent cardiac hypertrophy via ubiquitin-dependent coactivation of forkhead proteins (22). Nonetheless, our present study failed to detect any change in ubiquitin expression in response to high-fat diet-induced obesity, not favoring a role of ubiquitin-associated protein degradation in cardiac hypertrophy and cardiac dysfunction associated with high-fat diet-induced obesity. The ubiquitin-proteasome is a barrel-shaped protease capable of recognizing and destroying proteins decorated with at least four ubiquitin residues (31). Likewise, our data also indicated an unlikely role of calcineurin in cardiac hypertrophy and contractile dysfunction in high-fat diet-induced obesity.
Atrogin-1 is an F-box protein that inhibits cardiac hypertrophy by participating in an Akt- and ubiquitin ligase-dependent pathway. As a result, the hypertrophic promoter calcineurin may be degraded. It was suggested that atrogin-1 does not affect Akt activity itself but rather serves as a coactivator for members of the forkhead transcription factors downstream of Akt (22). Mice with cardiac overexpression of atrogin-1 displayed upregulated forkhead transcriptional factors concomitant with suppression of cardiac hypertrophy, while mice lacking atrogin-1 demonstrated the opposite physiological phenotype, suggesting that atrogin-1 may disrupt cardiac hypertrophy through its effects on forkhead transcription factors (22). This notion is supported by our experimental data of suppressed atrogin-1 mRNA expression and elevated basal Foxo3a phosphorylation (less expression of the active transcriptional factor), although this process may be independent of the ubiquitin-proteasome system and calcineurin.
In our study, palmitic acid failed to recapitulate the effect of high-fat diet-induced obesity on Akt activation, despite the comparable finding of GATA4 in response to palmitic acid and high-fat diet feeding. This apparent discrepancy may be attributed to the possible contribution from other long-chain fatty acids in the high-fat diet and the difference in treatment duration. Our observation of elevated PTEN levels in response to palmitic acid indicates a possible contribution of PTEN to the palmitic acid-elicited hypertrophic response in the absence of high basal Akt phosphorylation. PTEN signaling is known to be positively correlated with cardiac hypertrophy, thus representing a novel target to retard progression of heart failure (30). In addition, PTEN is known to negatively regulate Akt activity, although it is unknown if PTEN contributes to the lack of responsiveness in pAkt after palmitic acid treatment (17). CNTF is essential to tissue growth and metabolism (42). Usually, a reciprocal relationship exists between the CNTFR and cardiac hypertrophy (42). Our finding of enhanced CNTFR-α in light of cardiac hypertrophy may represent a compensatory response in obesity-associated organ hypertrophy. Last but not the least, our data also revealed elevated cardiac hyprotrophic signaling molecule leptin (18) in response to high-fat diet feeding, the effect of which may be alleviated by weight control. These observations suggest a potential role of overt hyperleptinamia in cardiac hypertrophy and cardiac dysfunction in high-fat diet-induced obesity.
Experimental Limitations
In a study of this scale, there are many limitations. Foremost, the diet used in our study was also relatively high in sugar; therefore, the high-fat diet-induced effect may have been due to the surplus of both fat and sugar. Also, recent evidence (45) has suggested that a soy-based diet worsens cardiac dysfunction, although the soybean oil component was identical between our two diets. Second, we evaluated cardiac systolic and diastolic function using isolated cardiomyocytes in an effort to minimize the potential influence from neurohumoral factors and connective tissues, which both could affect inherent alterations in cardiac performance. However, cardiomyocyte contractile function was recorded in a similar extracellular milieu, thus discounting possible differences in circulating levels of fatty acids in vivo. Third, a relatively high concentration of palmitic acid was used in our ex vivo DN Foxo3a transfection study instead of oleate, the main component of lard (responsible for high-fat component in diet). Our preliminary evidence failed to show any significant response from oleate in Akt phosphorylation and GATA4 expression (data not shown). Such an apparent discrepancy between the effects of oleate and palmitate suggests that the precise composition of fatty acid may directly affect the phenotype of cardiomyocytes and how these cells respond to hypertrophic stimuli. It is possible that the duration of fatty acid exposure may play a role in the onset of hypertrophic signaling, although our transfection study did not allow any longer exposure duration due to cytotoxicity reasons. Finally, our data between cardiomyocyte function and the phosphorylation status of Akt/Foxo3a are essentially correlative. Direct assessment of cardiomyocyte contractile function using a bioengineering technique targeted to Akt and Foxo3a (such as using transgenic mice) should provide more direct evidence regarding the role of Akt/Foxo3a phosphorylation in cardiomyocyte function.
In conclusion, our study offered evidence that geometric, myocardial contractile and intracellular Ca2+ abnormalities in high-fat diet-induced obesity may be associated with suppressed forkhead transcription factor (elevated basal Foxo3a phosphorylation) and atrophy-specific gene transcription. In light of the DN Foxo3a adenovirus-elicited effect on Akt phosphorylation and upregulation of hypertrophic proteins reminiscent of high-fat diet or palmitic acid, our data support the novel hypothesis that high-fat diet-induced obesity (possibly insulin resistance and type 2 diabetes) suppresses forkhead transcription factor via chronic activation of Akt. Chronic Akt activation is capable of overriding the antigrowth program induced by Foxo. Likewise, other hypertrophic agonists such as angiotensin II may trigger inactivation of Foxo proteins in cardiomyocytes via a phosphatidylinositol 3-kinase/Akt-dependent mechanism. It is imperative to scrutinize the role of Akt-forkhead transcription factors in obesity and diabetes-induced cardiac hypertrophy and hypertrophic cardiomyopathy so that optimal therapeutic strategies may be achieved targeting on this signaling cascade.
GRANTS
This work was supported in part by the American Heart Association Pacific Mountain Affiliate (#0355521Z) and the National Institutes of Health University of Wyoming Northern Rockies Regional Institutional Development Award Network of Biomedical Research Excellence (#5P20RR016474).
Acknowledgments
We thank Dr. Ji Li for generous support as well as Drs. Qun Li and Nair Sreejayan for assistance on the Foxo3a transfection study.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev 88: 389–419, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J. Human ICE/CED-3 protease nomenclature. Cell 87: 171, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Axen KV, Dikeakos A, Sclafani A. High dietary fat promotes syndrome X in nonobese rats. J Nutr 133: 2244–2249, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Chess DJ, Lei B, Hoit BD, Azimzadeh AM, Stanley WC. Effects of a high saturated fat diet on cardiac hypertrophy and dysfunction in response to pressure overload. J Card Fail 14: 82–88, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Condorelli G, Drusco A, Stassi G, Bellacosa A, Roncarati R, Iaccarino G, Russo MA, Gu Y, Dalton N, Chung C, Latronico MV, Napoli C, Sadoshima J, Croce CM, Ross J Jr. Akt induces enhanced myocardial contractility and cell size in vivo in transgenic mice. Proc Natl Acad Sci USA 99: 12333–12338, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Divitiis O, Fazio S, Petitto M, Maddalena G, Contaldo F, Mancini M. Obesity and cardiac function. Circulation 64: 477–482, 1981. [DOI] [PubMed] [Google Scholar]
- 7.Dimopoulos N, Watson M, Sakamoto K, Hundal HS. Differential effects of palmitate and palmitoleate on insulin action and glucose utilization in rat L6 skeletal muscle cells. Biochem J 399: 473–481, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobrian AD, Davies MJ, Schriver SD, Lauterio TJ, Prewitt RL. Oxidative stress in a rat model of obesity-induced hypertension. Hypertension 37: 554–560, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Dong F, Li Q, Sreejayan N, Nunn JM, Ren J. Metallothionein prevents high-fat diet induced cardiac contractile dysfunction: role of peroxisome proliferator activated receptor gamma coactivator 1alpha and mitochondrial biogenesis. Diabetes 56: 2201–2212, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Dong F, Ren J. Fidarestat improves cardiomyocyte contractile function in db/db diabetic obese mice through a histone deacetylase Sir2-dependent mechanism. J Hypertens 25: 2138–2147, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Eckel RH, Barouch WW, Ershow AG. Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases Working Group on the pathophysiology of obesity-associated cardiovascular disease. Circulation 105: 2923–2928, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Fang CX, Dong F, Ren BH, Epstein PN, Ren J. Metallothionein alleviates cardiac contractile dysfunction induced by insulin resistance: role of Akt phosphorylation, PTB1B, PPARgamma and c-Jun. Diabetologia 48: 2412–2421, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Fiedler B, Wollert KC. Targeting calcineurin and associated pathways in cardiac hypertrophy and failure. Expert Opin Ther Targets 9: 963–973, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Gardin JM, Siri FM, Kitsis RN, Edwards JG, Leinwand LA. Echocardiographic assessment of left ventricular mass and systolic function in mice. Circ Res 76: 907–914, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Hannigan GE, Coles JG, Dedhar S. Integrin-linked kinase at the heart of cardiac contractility, repair, and disease. Circ Res 100: 1408–1414, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Hardt SE, Sadoshima J. Glycogen synthase kinase-3beta: a novel regulator of cardiac hypertrophy and development. Circ Res 90: 1055–1063, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Hay N The Akt-mTOR tango and its relevance to cancer. Cancer Cell 8: 179–183, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Karmazyn M, Purdham DM, Rajapurohitam V, Zeidan A. Leptin as a cardiac hypertrophic factor: a potential target for therapeutics. Trends Cardiovasc Med 17: 206–211, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Kinkel MD, Horton WE Jr. Coordinate down-regulation of cartilage matrix gene expression in Bcl-2 deficient chondrocytes is associated with decreased SOX9 expression and decreased mRNA stability. J Cell Biochem 88: 941–953, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Lam EW, Francis RE, Petkovic M. FOXO transcription factors: key regulators of cell fate. Biochem Soc Trans 34: 722–726, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 322: 1561–1566, 1990. [DOI] [PubMed] [Google Scholar]
- 22.Li HH, Willis MS, Lockyer P, Miller N, McDonough H, Glass DJ, Patterson C. Atrogin-1 inhibits Akt-dependent cardiac hypertrophy in mice via ubiquitin-dependent coactivation of forkhead proteins. J Clin Invest 117: 3211–3223, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li SY, Gomelsky M, Duan J, Zhang Z, Gomelsky L, Zhang X, Epstein PN, Ren J. Overexpression of aldehyde dehydrogenase-2 (ALDH2) transgene prevents acetaldehyde-induced cell injury in human umbilical vein endothelial cells: role of ERK and p38 mitogen-activated protein kinase. J Biol Chem 279: 11244–11252, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Li SY, Yang X, Ceylan-Isik AF, Du M, Sreejayan N, Ren J. Cardiac contractile dysfunction in Lep/Lep obesity is accompanied by NADPH oxidase activation, oxidative modification of sarco(endo)plasmic reticulum Ca2+-ATPase and myosin heavy chain isozyme switch. Diabetologia 49: 1434–1446, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Liang Q, Molkentin JD. Divergent signaling pathways converge on GATA4 to regulate cardiac hypertrophic gene expression. J Mol Cell Cardiol 34: 611–616, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Matthaei S, Stumvoll M, Kellerer M, Haring HU. Pathophysiology and pharmacological treatment of insulin resistance. Endocr Rev 21: 585–618, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Meiners S, Dreger H, Fechner M, Bieler S, Rother W, Gunther C, Baumann G, Stangl V, Stangl K. Suppression of cardiomyocyte hypertrophy by inhibition of the ubiquitin-proteasome system. Hypertension 51: 302–308, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Morgan EE, Rennison JH, Young ME, McElfresh TA, Kung TA, Tserng KY, Hoit BD, Stanley WC, Chandler MP. Effects of chronic activation of peroxisome proliferator-activated receptor-α or high-fat feeding in a rat infarct model of heart failure. Am J Physiol Heart Circ Physiol 290: H1899–H1904, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Okere IC, Young ME, McElfresh TA, Chess DJ, Sharov VG, Sabbah HN, Hoit BD, Ernsberger P, Chandler MP, Stanley WC. Low carbohydrate/high-fat diet attenuates cardiac hypertrophy, remodeling, and altered gene expression in hypertension. Hypertension 48: 1116–1123, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Oudit GY, Kassiri Z, Zhou J, Liu QC, Liu PP, Backx PH, Dawood F, Crackower MA, Scholey JW, Penninger JM. Loss of PTEN attenuates the development of pathological hypertrophy and heart failure in response to biomechanical stress. Cardiovasc Res 78: 505–514, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Patterson C, Ike C, Willis PW, Stouffer GA, Willis MS. The bitter end: the ubiquitin-proteasome system and cardiac dysfunction. Circulation 115: 1456–1463, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Pham FH, Cole SM, Clerk A. Regulation of cardiac myocyte protein synthesis through phosphatidylinositol 3′ kinase and protein kinase B. Adv Enzyme Regul 41: 73–86, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Relling DP, Esberg LB, Fang CX, Johnson WT, Murphy EJ, Carlson EC, Saari JT, Ren J. High-fat diet-induced juvenile obesity leads to cardiomyocyte dysfunction and upregulation of Foxo3a transcription factor independent of lipotoxicity and apoptosis. J Hypertens 24: 549–561, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Rennison JH, McElfresh TA, Okere IC, Vazquez EJ, Patel HV, Foster AB, Patel KK, Chen Q, Hoit BD, Tserng KY, Hassan MO, Hoppel CL, Chandler MP. High-fat diet postinfarction enhances mitochondrial function and does not exacerbate left ventricular dysfunction. Am J Physiol Heart Circ Physiol 292: H1498–H1506, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Sacheck JM, Ohtsuka A, McLary SC, Goldberg AL. IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF-1. Am J Physiol Endocrinol Metab 287: E591–E601, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, Goldberg AL, Spiegelman BM. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci USA 103: 16260–16265, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117: 399–412, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shioi T, McMullen JR, Kang PM, Douglas PS, Obata T, Franke TF, Cantley LC, Izumo S. Akt/protein kinase B promotes organ growth in transgenic mice. Mol Cell Biol 22: 2799–2809, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shiojima I, Yefremashvili M, Luo Z, Kureishi Y, Takahashi A, Tao J, Rosenzweig A, Kahn CR, Abel ED, Walsh K. Akt signaling mediates postnatal heart growth in response to insulin and nutritional status. J Biol Chem 277: 37670–37677, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Skurk C, Izumiya Y, Maatz H, Razeghi P, Shiojima I, Sandri M, Sato K, Zeng L, Schiekofer S, Pimentel D, Lecker S, Taegtmeyer H, Goldberg AL, Walsh K. The FOXO3a transcription factor regulates cardiac myocyte size downstream of AKT signaling. J Biol Chem 280: 20814–20823, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skurk C, Maatz H, Kim HS, Yang J, Abid MR, Aird WC, Walsh K. The Akt-regulated forkhead transcription factor FOXO3a controls endothelial cell viability through modulation of the caspase-8 inhibitor FLIP. J Biol Chem 279: 1513–1525, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Sleeman MW, Garcia K, Liu R, Murray JD, Malinova L, Moncrieffe M, Yancopoulos GD, Wiegand SJ. Ciliary neurotrophic factor improves diabetic parameters and hepatic steatosis and increases basal metabolic rate in db/db mice. Proc Natl Acad Sci USA 100: 14297–14302, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sowers JR Obesity as a cardiovascular risk factor. Am J Med 115 Suppl 8A: 37S–41S, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Sparks LM, Xie H, Koza RA, Mynatt R, Hulver MW, Bray GA, Smith SR. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes 54: 1926–1933, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Stauffer BL, Konhilas JP, Luczak ED, Leinwand LA. Soy diet worsens heart disease in mice. J Clin Invest 116: 209–216, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14: 395–403, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Sun H, Kerfant BG, Zhao D, Trivieri MG, Oudit GY, Penninger JM, Backx PH. Insulin-like growth factor-1 and PTEN deletion enhance cardiac L-type Ca2+ currents via increased PI3Kalpha/PKB signaling. Circ Res 98: 1390–1397, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Thomas G, Hall MN. TOR signalling and control of cell growth. Curr Opin Cell Biol 9: 782–787, 1997. [DOI] [PubMed] [Google Scholar]
- 49.van HM, Compton DS, France CF, Tedesco RP, Fawzi AB, Graziano MP, Sybertz EJ, Strader CD, Davis HR Jr. Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest 99: 385–390, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson CR, Tran MK, Salazar KL, Young ME, Taegtmeyer H. Western diet, but not high fat diet, causes derangements of fatty acid metabolism and contractile dysfunction in the heart of Wistar rats. Biochem J 406: 457–467, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson-Fritch L, Nicoloro S, Chouinard M, Lazar MA, Chui PC, Leszyk J, Straubhaar J, Czech MP, Corvera S. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J Clin Invest 114: 1281–1289, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]