Abstract
Idiopathic dilated cardiomyopathy (IDC) is characterized by left ventricular (LV) enlargement with systolic dysfunction, other causes excluded. When inherited, it represents familial dilated cardiomyopathy (FDC). We hypothesized that IDC or FDC would show with cardiac magnetic resonance (CMR) increased myocardial accumulation of gadolinium contrast at steady state and decreased baseline myocardial blood flow (MBF) due to structural alterations of the extracellular matrix compared with normal myocardium. CMR was performed in nine persons affected with IDC/FDC. Healthy controls came from the general population (n = 6) or were unaffected family members of FDC patients (n = 3) without signs or symptoms of IDC/FDC or any structural cardiac abnormalities. The myocardial partition coefficient for gadolinium contrast (λGd) was determined by T1 measurements. LV shape and function and MBF were assessed by standard CMR methods. λGd was elevated in IDC/FDC patients vs. healthy controls (λGd = 0.56 ± 0.15 vs. 0.41 ± 0.06; P = 0.002), and correlated with LV enlargement (r = 0.61 for λGd vs. end-diastolic volume indexed by height; P < 0.01) and with ejection fraction (r = −0.80; P < 0.001). The extracellular volume fraction was higher in IDC patients than in healthy controls (0.31 ± 0.05 vs. 0.24 ± 0.03; P = 0.002). Resting MBF was lower in IDC patients (0.64 ± 0.13 vs. 0.91 ± 0.22; P = 0.01) than unaffected controls and correlated with both the partition coefficient (r = −0.57; P = 0.012) and the extracellular volume fraction (r = −0.56; P = 0.019). The expansion of the extracellular space correlated with reduced MBF and ventricular dilation. Expansion of the extracellular matrix may be a key contributor to contractile dysfunction in IDC patients.
Keywords: idiopathic dilated cardiomyopathy, partition coefficient, myocardial blood flow
idiopathic dilated cardiomyopathy (IDC) is characterized by left ventricular enlargement (LVE) and impaired contractility of unknown cause. In many cases, the disease is inherited and termed familial dilated cardiomyopathy (FDC; Ref. 4) and accounts for approximately 20 to 50% of IDC cases (2), with an autosomal dominant mode of inheritance predominating (4). Screening, in particular in first degree relatives of FDC-positive patients, and early detection are important because FDC is amenable to medical therapy (4, 6). The diagnosis of IDC is based on the presence of reduced LV global function by fractional shortening or ejection fraction, ventricular enlargement, and clinical exclusion of other etiology.
Pathological features of IDC include increased levels of interstitial and perivascular fibrosis(7) and decreased capillary density (17). The histological evidence from imaging and postmortem studies suggests that fibrosis in nonischemic cardiomyopathies affects the myocardium in a diffuse pattern (21, 29, 38). Currently, myocardial biopsy is primarily recommended to identify patients with new onset disease resulting from myocarditis or other rare conditions and is seldom indicated for those with an established diagnosis of IDC (5). Also, an endomyocardial biopsy is an invasive procedure and only yields information at the selected biopsy sites. For the development of novel therapies that could benefit IDC patients, it will be important to develop imaging-based markers to monitor structural remodeling in the myocardium. For example, treatment with an angiogenic factor in a hamster model of inherited dilated cardiomyopathy showed by histological analysis that an increase of capillary density was accompanied by a decrease of myocardial fibrosis compared with placebo-treated controls (23).
Cardiac magnetic resonance (CMR) imaging of myocardial contrast enhancement after intravenous administration of a gadolinium-based contrast agent has been useful for the detection of myocardial infarction (18) and fibrosis (33, 41). We hypothesized that patients diagnosed with IDC/FDC would demonstrate increased interstitial accumulation of gadolinium contrast at steady state due to structural alterations of the extracellular matrix compared with those without myocardial disease. As interstitial and perivascular fibrosis may be associated with a decrease of capillary density, we also hypothesized that expansion of the extracellular space would be associated with a reduction of baseline myocardial blood flow (MBF). To test these hypotheses, we measured MBF during the first pass of an injected gadolinium contrast bolus. Once the contrast attained an equilibrium distribution between blood and tissue, we measured the blood-tissue partition coefficient to determine the distribution volume for the extracellular contrast agent (9, 20).
METHODS
The study population comprised 18 participants (9 males and 9 females) that were recruited through the Oregon Health and Science University (OHSU) FDC research project (24) and the general population. FDC was diagnosed according to established criteria as previously published (6, 12, 32) and without the use of magnetic resonance imaging (MRI) findings. IDC was defined as LVE with systolic dysfunction (ejection fraction <0.50), with other causes of ventricular dilation excluded. The sample included 9 participants who had been diagnosed with IDC. Three of the nine patients were members of a family affected by FDC based on the previous diagnosis of IDC during clinical evaluation and their family history. Three further patients from a different family diagnosed with FDC were siblings. The remaining patients represented sporadic cases of IDC. Study participants classified as unaffected (n = 9) had no evidence of any cardiovascular abnormalities and had ventricular volume and function parameters within the normal ranges. They were recruited as volunteers from the general population (n = 6) or were unaffected relatives of FDC patients (n = 3) without the pathogenetic mutation found in their relatives and with normal LV function and no signs of LV dilatation. The study protocol was approved by the Human Subjects Protection Committee of OHSU. All study participants gave written, informed consent for study participation.
MRI protocol.
MRI was performed with a 3 Tesla Scanner (Intera 3T, Philips Medical Systems) using a dedicated phased-array cardiac coil. A respiratory bellows was placed on the patient's abdomen. Cine MRI images were obtained in the short axis view for 10–12 slices from base to apex to assess LV volumes and function. An ECG-gated gradient echo sequence with steady-state free precession was used for cine MRI to acquire images for 20 phases of the cardiac cycle during breath holding [repetition time per phase encoding (TR)/echo time (TE)/flip angle = 3.9/2.0 ms/45°; slice thickness = 6 mm; 192 × 170 matrix; field of view (FOV) = 380 × 320- to 380-mm; sensitivity encoding factor of 2]. T1 measurements were performed with a Look-Locker technique (27), which was based on a gradient-echo cine sequence with a temporal resolution of 40 ms, using a nonslice selective inversion pulse applied after the detection of an R-wave, followed by a segmented gradient-echo acquisition for 15–30 phases of the inversion recovery (IR) (TR/TE/flip angle = 3.4/1.7 ms/12°; slice thickness = 8 mm; 176 × 140 matrix; 40 ms per segment; FOV = 380 × 320- to 380-mm; SENSE factor of 2). ECG and respiratory gating were used for the Look-Locker acquisitions with a respiratory gating delay of 2 s or larger. T1 measurements were made in one slice at the midventricular level before contrast administration, and for two or more time points after contrast administration with measurements spaced at least 5–10 min apart and starting no earlier than 4–5 min after a bolus injection of contrast.
Myocardial perfusion imaging with the patient at rest was performed during the first contrast administration with a low-dosage bolus of gadolinium (0.03 mmol/kg body wt) injected intravenously in the antecubital fossa at a rate of 2 ml/s. Perfusion images were acquired for three slice levels during each heart beat with a single-shot gradient echo pulse sequence with nonslice selective saturation-recovery magnetization preparation (TR/TE/flip angle: 2.4/0.98 ms/20°; 160 × 140 matrix; FOV ∼380 mm and 80% rectangular FOV factor). The first postcontrast T1 measurements were made about 4–5 min after imaging the first pass of the contrast bolus based on model-based estimates of the time it would take to reach contrast equilibrium between blood and tissue after a contrast bolus injection. This was followed by injection of the remaining balance of contrast for a total of 0.1 mmol/kg body wt in each participant. Further one-to-four T1 measurements were made after this last contrast injection, depending on the remaining time available.
Image analysis.
Images of LV cines, T1 measurements, and resting perfusion were analyzed with the MASS CMR software (Laboratory for Clinical and Experimental Image Processing, Leiden University, The Netherlands). Global function parameters were determined by segmenting all cine images along the endo- and epicardial border and summing of the cavity and myocardial volumes in each slice. End-systole and end-diastole were identified by the minimum and maximum on the volume vs. cardiac phase curve. Images for T1 measurements and perfusion measurements were manually segmented along the endo- and epicardial borders. The gray-scale windowing was adjusted for each cardiac phase to achieve optimal conspicuity of the LV borders. The anterior LV-RV junction was used as landmark, and eight myocardial sectors were defined for each cardiac phase such that they had equal circumferential extent on a center-line between endo- and epicardial borders, with the first sector starting at said landmark.
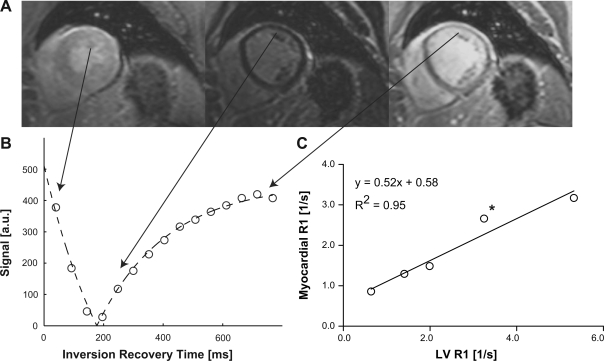
For the T1 measurements, the mean signal intensity (SI) in each sector was plotted against the delay after the inversion pulse. The SI data points were fit in MATLAB (The MathWorks, Natick, MA) with a nonlinear least-squares algorithm, using the analytical expression for the magnitude of a monoexponential IR, SI = |m0 − m1·exp(−delay/T1*)|, where |…| represents the magnitude operation and m0, m1, and T1* are adjustable model parameters. Examples of model fits to experimental inversion-recovery data for one myocardial sector and a region in the center of the LV are shown in Fig. 1. T1 values were derived from the IR fit parameters, using previously validated methods (3, 37). The gadolinium contrast partition coefficient (λGd) in myocardium was calculated for each patient from the change of relaxation rate (R1 = 1/T1) in myocardium and blood after contrast injection (9). In this study, multiple T1 measurements were used to calculate the partition coefficient from the slope of the linear regression line for the measured values of R1 = 1/T1 (myocardium) vs. R1 (blood) as shown in Fig. 2C.
Fig. 1.
A: a series of inversion recovery images was acquired in a female patient diagnosed with idiopathic dilated cardiomyopathy (IDC) [ejection fraction = 45%; end-diastolic volume (EDV) indexed by height = 96 ml/m] with a cardiac and respiratory-gated Look-Locker sequence with up to 30 phases, using a segmented gradient-echo readout with a train of low-angle (12°) excitation pulses. Only 3 out of 15 images are shown here and in order of increasing inversion times from left to right. B: the graph shows the measured signal intensity in 1 of 8 transmural wall segments (open circles) against the time delay after the nonslice-selective inversion pulse. The continuous lines represent least-squares fits to the data points, using a model equation for the inversion-recovery measured from magnitude images. C: the relaxation rate (R1 = 1/T1) in tissue was plotted against the corresponding R1 in the left ventricular (LV) blood pool. The partition coefficient was determined by linear regression and was 0.52 ± 0.07 (mean ± SE) in this patient. The asterisk denotes the measurement corresponding to the images in A and the graph in B. a.u., Arbitrary units.
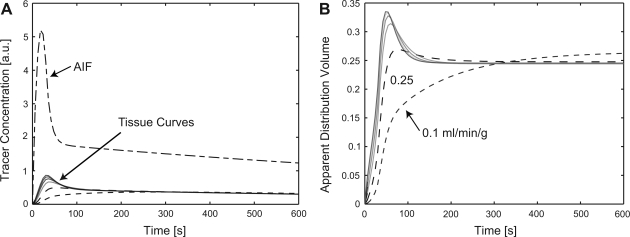
Fig. 2.
A: tracer concentration curves were simulated with a spatially distributed model for blood flows of 0.1, 0.25, 0.5, 0.75, 1.0, 1.25, and 1.5 ml·min−1·g−1 using an arterial input function (AIF) composed of an initial log-normal curve joined to an exponential that represents the slow clearance of contrast from blood with a time constant of ∼25 min. B: the apparent extracellular volume was calculated from the ratio of gadolinium concentrations in tissue and the arterial input. For flows above 0.5 ml·min−1·g−1, the apparent distribution volume agrees within 1% with the extracellular volume for times greater than 3 min from the beginning of the contrast bolus injection. In the patient studies, a time of at least 5 min was allowed to elapse after a contrast injection before acquiring T1 data for determination of the partition coefficient.
For quantification of MBF, SI curves were generated from the mean SI in each myocardial sector and plotted as a function of time. The initial SI, before the appearance of the contrast in the LV, was subtracted from all SI values for a baseline correction of the curves. MBF in milliliters per minute per gram was determined from the initial amplitude of the myocardial impulse response calculated by a customized software to perform a model-independent deconvolution for the SI curves with the arterial input function measured in the LV center. This method was previously validated in animal models using labeled microspheres (14, 15) and has been applied in patient studies (36, 39). To adjust for differences in cardiac workload, the resting MBF was divided by the participant's (heart-) rate-pressure product (RPP). The MBF normalized by the RPP is referred to here as RPP-normalized MBF.
Model simulations.
The myocardial partition coefficient for gadolinium contrast (λGd) is defined as the ratio of concentration of contrast in tissue (Ct), divided by the contrast concentration in blood (Cb):
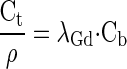 |
(1) |
where ρ represents the specific density of myocardial tissue (∼1.05 ml/g). It is assumed that the concentration of contrast reaches an equilibrium state where the concentration in the extravascular extracellular space (Ce) equals the plasma concentration (Cp):
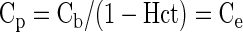 |
(2) |
and Hct represents the blood hematocrit. If Ve and Vp denote the volume fractions of the extravascular extracellular and the plasma spaces, respectively, one can express the tissue concentration as the weighted average of the concentrations in these spaces, with weight factors corresponding to the respective volume fractions, Vp and Ve:
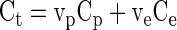 |
(3) |
By combining the above equations and assuming an equilibrium state (i.e., Ce = Cp), one can derive at the following expression for the partition coefficient (11), expressed in terms of the volume fractions and the blood hematocrit (Hct):
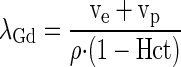 |
(4) |
A crucial aspect of any measurement protocol for determination of the partition coefficient is the time one has to allow after contrast injection to reach equilibrium of contrast agent between blood and tissue. The time to reach this equilibrium state depends on the tissue blood flow, the capillary permeability surface area product (PS), and to a lesser degree the extracellular distribution volume.
Simulations were performed with a spatially distributed two-space model to test the equilibrium assumption for a protocol with repeated bolus injections of contrast agent. A model of the blood-to-tissue exchange of contrast agent was implemented in JSIM, a Java-based simulation and modeling environment, available from the National Simulation Resource (http://physiome.org/jsim/). The following assumptions were applied in the definition of the model: 1) steady plasma flow (Fp), 2) uniform concentration gradients and diffusion in the radial direction, and 3) capillary length of 0.1 cm/unit volume. The following parameters were varied to determine the dependence of the partition coefficient on the parameters of Fp and PS permeability surface area product. Fp and PS do not vary independently, and it was assumed that vasodilation results in capillary recruitment, which increases the permeability-surface area product for gadolinium contrast in proportion to the increase of flow: PS = a + b·F (26).
After a contrast bolus injection, renal clearance of the gadolinium-based contrast agent results in a relatively slow decay of the blood concentration of the contrast agent. The clearance of contrast from the blood stream was measured in this study in six patients, and the mean of the first order blood clearance rates was used in the simulations to account for the decay of the gadolinium concentration in the arterial input. (The 6 patients were selected on the basis of having no less than 4 T1 measurements after the last contrast injection for fitting to an exponential function.) The arterial input function was modeled as combination of a lag-normal curve to which is joined an exponential decay. The lag-normal represents the peak from the initial bolus injection of contrast. The exponential tail of the arterial input continues from the point where the lag-normal has decayed to 60% of its peak value. The true distribution volume for gadolinium contrast corresponded in the simulations to the sum of the model parameters for the plasma and interstitial volumes. The plasma volume fraction for resting conditions was assumed to be 0.045, and the extracellular extravascular volume fraction was set equal to 0.2. The sum of plasma and extravascular extracellular volume fractions was close to the extracellular volume fraction measured previously (35). An apparent λGd and extracellular volume fraction (Ve + Vp) were calculated from the ratio of the gadolinium concentration in tissue to the gadolinium concentration in the blood pool at each time point in the simulation. They approach the true (i.e., model specified) λGd and extracellular volume fraction, respectively, as the gadolinium concentration in blood and tissue reach equilibrium.
Genetic analysis.
Genetic analysis was included in this study for further profiling of the affected and unaffected members of a family included in this study. Genomic DNA was extracted from whole blood according to a standard procedure. Each exon of MYH7 was PCR amplified by standard methods. Purified PCR products (individual exons and approximately 20–40 nucleotides of 5′ and 3′ intronic sequence) were sequenced in both directions using standard methods.
Statistical analysis.
Results for IDC/FDC patients and asymptomatic relatives are reported as means ± SD. Correlations between continuous variables are reported as Pearson's product-moment. Univariate and multivariable linear regression analysis was used to study associations between variables, and the coefficient estimates are given as means ± SE. Firth's method of bias-reduced logistic regression by maximum penalized likelihood (BRLR) was used to assess the value of λGd and the extracellular volume fraction for predicting who was affected by IDC (8). The BRLR method assures finite coefficient estimates and standard errors in situations of complete or quasi-complete separation of groups by the linear predictors (8). A P value <0.05 was chosen throughout to indicate statistical significance. All statistical analysis was performed using R [R release 2.6 (2008); ISBN 3-900051-07-0; R: a language and environment for statistical computing; R Development Core Team, R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org].
RESULTS
The general characteristics of the study sample are summarized in Table 1. A mutation (R1500W) in a highly conserved region of MYH7, the gene encoding β-myosin heavy chain, was identified in 3 family members included in this cohort who were affected with FDC. This mutation was not identified in the 3 family members classified as unaffected by FDC or in DNA specimens from 256 control subjects (512 chromosomes). A mutation in the same amino acid has been reported previously in a distal skeletal myopathy (31) where the wild-type arginine was substituted by proline rather than the tryptophan identified in the subjects affected with FDC in this family.
Table 1.
The P values for continuous variables were obtained from unpaired t-test and for categorical variables with a χ2 test
| IDC/FDC (n = 9) | Healthy Controls (n = 9) | P | |
|---|---|---|---|
| Age, yr | 59±15 | 45±11 | 0.03 |
| Sex (female/male) | 6/3 | 4/7 | 0.37 |
| Weight, kg | 81±17 | 79±21 | 0.83 |
| Height, cm | 168±12 | 168±10 | 1.00 |
| Body surface area, m2 | 1.9±0.23 | 1.88±0.25 | 0.84 |
| Heart rate, beats/min | 66±12 | 66±9 | 0.98 |
| Hematocrit, % | 0.44±0.02 | 0.41±0.02 | 0.04 |
| Systolic blood pressure, mmHg | 119±13 | 118±12 | 0.95 |
| Diastolic blood pressure, mmHg | 69±10 | 74±6 | 0.22 |
| EDV, ml | 276±156 | 125±27 | 0.02 |
| EDV/height, ml/cm | 1.66±1 | 0.74±0.14 | 0.03 |
| ESV, ml | 195±152 | 38±13 | 0.01 |
| ESV/height, ml/cm | 1.18±0.95 | 0.23±0.07 | 0.02 |
| Left ventricular mass, g | 146±64 | 82±26 | 0.02 |
| Ejection fraction, % | 38±17 | 69±9 | 0.00 |
| Cardiac output, ml/min | 5,257±920 | 5,507±1,771 | 0.71 |
| Cardiac output/body surface area, ml/min/m2 | 2,778±471 | 2,903±717 | 0.67 |
| Peak ejection rate/height, ml·min−1·cm−1 | 1.93±1.29 | 3.04±0.47 | 0.04 |
| Peak filling rate, ml·min−1·cm−1 | 1.42±1.09 | 2.93±1.02 | 0.02 |
EDV, end-diastolic volume; ESV, end-systolic volume; IDC, idiopathic dilated cardiomyopathy; FDC, familial dilated cardiomyopathy.
Figure 2A shows the simulated time course for the gadolinium concentration after the contrast injection. The tail of the arterial input in Fig. 2A was assumed to follow an exponential with the exponential blood clearance time constant for gadolinium contrast set to 26 min, the mean time constant measured in 6 patients (mean exponential time constant ±SD: 26 ± 5 min.). The apparent extracellular volume fraction represents the true extracellular volume fraction of the model (0.25) in the limit where the gadolinium concentrations in the plasma and interstitial spaces reach equilibrium. Figure 2B illustrates how the apparent extracellular volume fraction estimate approaches this true value of the extracellular volume fraction of 0.25 as time elapses after a contrast injection. For tissue blood flows above 0.5 ml·min−1·g−1, the calculated extracellular volume fraction deviated from the true extracellular volume fraction by a relative difference of less than 1% at 3 min and longer after the contrast injection. For the lowest value of tissue blood flows in the simulations of 0.1 ml·min−1·g−1, the apparent extracellular volume fraction is first underestimated and then overestimated, indicating that at such low tissue blood flows the concentration cannot reach equilibrium. For the experimental protocol, a minimum delay of 5 min after each contrast injection was chosen, as the blood clearance time constant and MBF range were not known a priori.
Study participants diagnosed with IDC were older than those unaffected (59.1 ± 14.8 vs. 44.0 ± 11.0; P = 0.007) but showed no differences in height, or weight. The end-diastolic volume (EDV) normalized by height was significantly higher in IDC patients compared with healthy controls (Table 1) and outside the published 95% confidence intervals for indexed EDV in normals (28). The stroke volume was positively associated with EDV (P < 0.001) in the normal controls as predicted by the Frank-Starling law. In the nine IDC/FDC patients, there was no significant association between stroke volume and EDV (P = 0.63), suggesting of a significant myopathic process leading to systolic dysfunction in IDC patients compared with healthy controls.
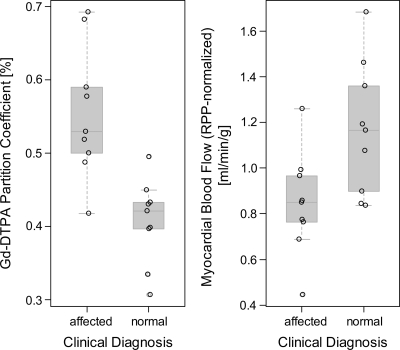
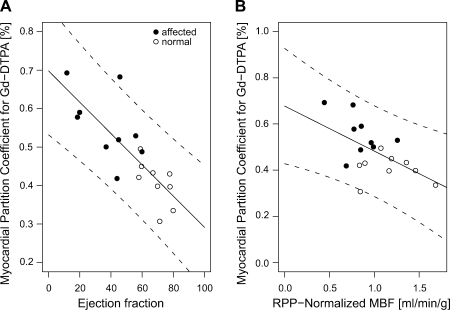
The average gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA) partition coefficient (λGd) (Fig. 3A) was significantly higher in patients diagnosed with IDC/FDC than in unaffected participants (0.56 ± 0.15 vs. 0.41 ± 0.06; P = 0.002). In a multivariable regression model for λGd that included clinical diagnosis, age, and sex as predictors, the differences of λGd between IDC patients and unaffected participants was highly significant (IDC diagnosis coefficient ±SE: +0.19 ± 0.05; P = 0.002) with no significant effect of age (P = 0.21) and sex (P = 0.75). The blood hematocrit in IDC patients averaged 0.44 ± 0.02 (n = 8, not available for 1 patient) and was within the normal range for males and females. For the healthy volunteers, the hematocrit was measured only in three participants and was otherwise assumed to average 0.44 in males and 0.40 in females corresponding to the means in healthy persons. The extracellular volume fraction, calculated from the measured or assumed hematocrit values, was significantly higher in IDC patients than in healthy controls (0.31 ± 0.05 vs. 0.24 ± 0.03; P = 0.002). In a multivariable linear regression model for the extracellular volume fraction with clinical diagnosis, age, and sex as predictors, the difference of extracellular volume fraction between IDC patients and unaffected participants was highly significant (IDC diagnosis coefficient ±SE: +0.10 ± 0.03; P = 0.002), whereas the effects of age (P = 0.17) and sex (P = 0.61) were not significant. The λGd values correlated with EDV normalized by height (r = 0.60; P < 0.01) and with the ejection fraction (r = −0.80; P < 0.001). The negative association between λGd and ejection fraction (P < 0.0001), shown in Fig. 4A with a linear regression line and confidence limits for the association, was not significantly modified by including age and sex as additional predictors in the multivariable regression model for λGd (P = 0.674 for variance test).
Fig. 3.
Left: gadolinium partition coefficient (λGd) in myocardium for healthy controls and patients affected by IDC and diagnosed independent from magnetic resonance imaging findings. Right: in IDC-affected patients, myocardial blood flow (MBF) at rest, normalized by the patients' rate pressure product (RPP; systolic blood pressure × heart rate/103) was significantly lower than in normal controls. Gd-DTPA, gadolinium-diethylenetriamine pentaacetic acid.
Fig. 4.
A: the myocardial partition coefficient for gadolinium contrast (λGd) correlated negatively with systolic function (r = 0.80; P < 0.001). The solid line represents the linear regression fit, and the dotted lines give the 95% confidence limits for prediction. B: there was also a significant association between λGd and MBF at rest and normalized by the RPP (P = 0.02).
MBF at rest was significantly lower in IDC patients (0.641 ± 0.131 vs. 0.905 ± 0.224; P = 0.01) compared with the MBF in normal controls. The results for RPP-normalized MBF are shown in Fig. 3B. The effect of clinical diagnosis remained significant (P = 0.038) when age (P = 0.45), sex (P = 0.46), and RPP (P = 0.60) were included with clinical diagnosis as predictors in a multivariable linear regression model for MBF. The RPP-normalized MBF showed a highly significant positive association with the cardiac output, indexed by LV mass (P < 0.001).
The MBF values correlated significantly with both the partition coefficient (r = −0.57; P = 0.012) and the extracellular volume fraction (r = −0.56; P = 0.019). The partition coefficient λGd was negatively associated with RPP-normalized MBF [coefficient: −0.39 ± 0.13 (ml·min−1·g−1); P < 0.01], as shown in Fig. 4B, and this association remained significant (P = 0.03) and was not modified if the univariate regression model was expanded by adding cardiac output, normalized by body-surface area, as additional predictor to the model. Both λGd and the extracellular volume fraction increased significantly with ventricular dilation. The association between λGd and EDV indexed by height (P < 0.02) was analyzed with a multivariable linear regression model for λGd, which also included sex as predictor to account for the significant difference of height-indexed EDV between males and females. The strong association between partition coefficient and EDV was significantly modified by patient sex (P = 0.02 for interaction between EDV and sex). As approximately the same increases of partition coefficient are observed in male and female patients with dilated cardiomyopathy, the known sex differences of EDV normalized by height lead to significantly different slopes of the respective regression lines. A similar pattern of association was observed between the extracellular volume fraction and height-indexed EDV, indicating that the elevated partition coefficient in IDC patients primarily reflects structural changes of the extracellular matrix that correlate with the degree of LV dilation independently of sex-related differences in LV size.
The values of λGd for each participant represent averages of λGd over eight myocardial sectors in a midlevel slice. In the group of healthy controls, the relative dispersion of λGd (defined as SD/mean) within each volunteer averaged 15% (range: 7–20%). In the patients diagnosed with IDC, the relative dispersion of λGd averaged 17% (range: 9–35%). For comparison, the MBF at rest is known to have a relative dispersion, corrected for methodological scatter and temporal variation, of ∼30% in the whole heart (19). The relative dispersion of λGd in our study could reflect both an underlying physiological heterogeneity of λGd and measurement error, but the total variation from all sources results in a relative dispersion that is low.
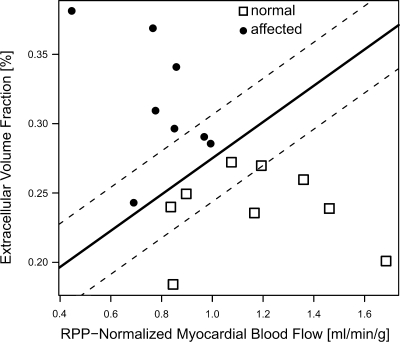
As summarized above, both extracellular volume fraction (or for that matter the partition coefficient) and MBF were found to be significantly different between the two groups in this study, which raised the question whether the combination of these two physiological parameters would enhance the ability to differentiate between IDC/FDC patients and unaffected study participants. Figure 5 shows a plot of extracellular volume fraction vs. the RPP-normalized MBF, with IDC diagnosis encoded as solid circles. With a logistic regression model for IDC diagnosis and with extracellular volume fraction and RPP-normalized MBF as the only independent predictors in the logistic regression model, one can draw in this plot the line corresponding to a probability of P = 0.5 for an IDC diagnosis. The solid line in Fig. 5, corresponding to P = 0.5, perfectly separated the two groups.
Fig. 5.
The extracellular volume fraction was plotted vs. the RPP-normalized MBF, with IDC diagnosis encoded as solid circles, to determine whether the IDC patients could be separated with these 2 parameters from unaffected participants. A logistic regression model including both extracellular volume fraction and RPP-normalized MBF as independent predictors was used to determine the line corresponding to a probability of P = 0.5 for an IDC diagnosis and drawn as solid line in the graph. It perfectly separated the 2 groups. The dashed lines correspond to probabilities of P = 0.05 and P = 0.95 for IDC diagnosis, respectively.
DISCUSSION
The present study demonstrates that the partition coefficient for gadolinium contrast (λGd) is higher among patients diagnosed with FDC or IDC than in their asymptomatic kindred and healthy volunteers. This suggests that λGd measured by CMR may be of use as a novel physiological marker for monitoring structural alterations of the extracellular matrix in FDC/IDC patients. An increase of λGd can result from an expansion of the extracellular space. The extracellular volume fraction estimated from λGd and the blood hematocrit, using Eq. 4, was significantly higher in IDC patients compared with healthy controls. From this we can conclude that in IDC patients an increase of the partition coefficient is primarily due to an expansion of the extracellular space, possibly caused by interstitial fibrosis. Varying degrees of interstitial fibrosis have been documented by endomyocardial biopsy in patients diagnosed with IDC and apparently healthy relatives (29). de Leeuw et al. (7) reported that distinct patterns of fibrosis were the sole significant histopathological difference between myocardial samples from patients with IDC and from those with heart diseases of known causes. For the IDC/FDC patient cohort in this study, the increase of λGd was generalized and not regionally confined. An increase of λGd in IDC patients correlated negatively with the degree of systolic dysfunction and positively with ventricular dilatation, and both of these latter parameters underpin the diagnostic criteria for IDC, after exclusion of other etiology. For three affected members of one of the two FDC families, the genetic analysis revealed a mutation in MYH7, the gene encoding the β-myosin heavy chain protein, one of the more common causes in patients with FDC (13). These mutations can impair contractile force generation by the sarcomere (16). Diffuse fibrosis may result from the volume overload of the dysfunctional ventricle, whereas reduced resting blood flow may, in part, reflect the impairment of systolic function and reduced cardiac output.
A further important finding is the observation of lower MBF at rest in IDC patients compared with healthy controls, particularly when put in the context of the expansion of the extracellular volume fraction in IDC patients. We observed a highly significant association between resting (RPP normalized) MBF and λGd, which could result from reduced capillary density (17). This finding supports the hypothesis that an expansion of the extracellular matrix, possibly due to fibrosis, is accompanied by a decline in both myocardial function and perfusion. A PET study (21) with the tracers H215O and C15O also indicated that the fraction of perfusable tissue was lower in IDC patients compared with healthy controls. In this study, the MBF at rest correlated closely with the measured cardiac output, normalized by LV mass. The latter can be considered a surrogate measure of the coronary flow if the fraction of cardiac output diverted to the coronary ostium is relatively constant. The strong correlation between MBF and cardiac output indexed by LV mass indicates that myocardial perfusion and global function are matched at rest. The hypothesis that MBF may be lower in IDC patients as a result of interstitial fibrosis has remained controversial (22), but there is at least one study supporting our observation of lower resting MBF (40). A possible reason for our observation of decreased MBF in IDC/FDC patients, contradicting one previous PET study (22), is the higher spatial resolution of MRI compared with PET, a distinction that may be particularly relevant for avoiding spillover effects in patients with dilated hearts, and relatively thinner walls, which would increase MBF estimates when the spatial resolution is reduced.
Both the extracellular volume fraction and the MBF are not used currently for the clinical diagnosis of IDC. This study shows that the extracellular volume fraction correlates with physiological parameters that characterize IDC, such as ventricular enlargement and systolic dysfunction. A classification of all study participants as healthy controls or affected by IDC was performed using only the extracellular volume fraction and RPP-adjusted MBF as predictors and resulting in a perfect separation of the groups, in agreement with their previous independent clinical classification, as shown in Fig. 5. These results seem to warrant further investigations of the question whether the extracellular volume fraction combined with MBF measurements will enhance current capabilities for early detection and diagnosis of IDC.
Imaging by CMR of contrast enhancement with gadolinium is widely applied for the detection of nonviable myocardium (18). Delayed contrast-enhancement has also been reported in the presence of interstitial fibrosis (33, 34, 41). The partition coefficient is an excellent quantitative measure to relate an elevated gadolinium distribution volume to a loss of myocardial viability (9, 20, 25, 42). We did not observe focal late gadolinium enhancement in the IDC/FDC patients, except for one equivocal case compared with 0% (22), 36% (30), and 65% (1) incidences in previous studies of patients with dilated cardiomyopathy, without evidence of ischemic heart disease. A pattern of reactive, diffuse fibrosis predominates in IDC (7), accompanied by a dramatic increase of collagen concentration (10). The absence of focal contrast enhancement does not preclude the presence of diffuse and generalized fibrosis. Therefore, the gadolinium partition coefficient was measured in this study to obtain an absolute measure of myocardial Gd-DTPA accumulation without relying on any differential contrast enhancement to detect abnormalities.
In the absence of a slow contrast infusion, it becomes important to ascertain whether the gadolinium concentration reaches a equilibrium between blood and tissue. This was investigated through simulations, which provided an estimate of the minimum blood flow required to reach equilibrium, and taking into account the rate of clearance of gadolinium from blood. Multiple T1 measurements in each person did not provide any evidence of a significant deviation of the R1 (tissue) vs. R1 (blood) relation from a linear relationship. Such a deviation would be expected if an equilibrium state would not be reached, and this condition can be simulated by decreasing the exponential time constant for gadolinium elimination from the arterial input or by reducing the MBF to very low levels. It is in principle possible to use a blood tissue-exchange model as employed here for the simulations to obtain flow-adjusted estimates of the extracellular volume fraction. It would render the analysis more complex, and the simulations showed that there was no benefit in doing so if the data were collected later than 4 min after contrast injection. Reduced ejection fraction and cardiac output are accounted for in the simulation model only through the effects they have on coronary blood flow. Further structural alterations in dilated cardiomyopathy such as changes in capillary density and permeability could also impact on the estimates of the time it takes to reach equilibrium between blood and tissue. Simple estimates of the changes in diffusion distance due to sparser capillaries and expansion of the extracellular space suggest that a 4-min waiting time still leaves sufficient time to attain an equilibrium state. Our estimates of the extracellular volume in normal myocardium (24 ± 3.4%) agree closely with published values (e.g., 23.6 ± 6.3%; Ref. 35). A recent study in an experimental animal model found that measurements with constant contrast infusion or a contrast bolus injection can give concordant results if a delay greater than 4 min is observed after a bolus injection for measurement of the contrast enhancement (43).
Limitations.
The generalization of the findings from this study is limited by the small sample size, and the patient cohort is dominated by cases of FDC from only two families. The sample of patients with dilated cardiomyopathy may not represent a good cross-section of the delayed enhancement patterns observed in larger studies (1, 22, 30). Nevertheless, the clinical appearance of the patients with familial clustering was similar to those without. Based on previous measurements of the partition coefficient in nonviable myocardium (42), one can expect that the partition coefficient is higher in areas with late gadolinium enhancement compared with areas without delayed contrast enhancement. Inclusion of patients with late gadolinium enhancement could have arguably magnified the differences of the partition coefficient between patients and healthy controls.
Quantification of fibrosis is only possible in explanted hearts or through endomyocardial biopsies. In this study, no histological samples by endomyocardial biopsy were obtained. Therefore, there is no direct evidence that the increased gadolinium coefficient in IDC family members is a result of myocardial fibrosis. The normal range of λGd in healthy persons has not been determined at 3 Tesla (T), and, at 1.5 T, the literature indicates significant variability (11). Nevertheless, our estimates of the extracellular volume fraction are in excellent agreement with previously reported results (35), and we used multiple T1 measurements to reduce the uncertainty of the λGd estimates.
The prognostic significance of λGd measurements with cardiac magnetic resonance imaging is not addressed in this study and will require long-term follow-up of patients and relatives.
Conclusions.
The myocardial partition coefficient for gadolinium contrast may in the future be of use as a novel marker for monitoring structural alterations of the extracellular matrix in IDC patients. This new marker could enhance phenotyping of patients with IDC or FDC and their at-risk kindred by noninvasive imaging. The current study has shown that the extracellular volume fraction is closely associated with ventricular dilation and systolic dysfunction, which are currently two pivotal predictors for the clinical diagnosis of IDC. The expansion of the extracellular space, possibly as a result of diffuse fibrosis and/or other forms of remodeling of the extracellular matrix may therefore constitute a key contributor to contractile dysfunction and reduced myocardial perfusion in IDC patients. It can also be foreseen that the measurement of the myocardial partition coefficient for an extracellular contrast agent may be useful for detecting and monitoring the progression of diffuse fibrosis in other cardiac diseases and may potentially reduce the need for myocardial biopsies. A prospective study would need to address the prognostic significance of λGd measurements by CMR in a clinical cohort affected with myocardial fibrosis.
GRANTS
This work was supported in part by National Institutes of Health Grants RO1-HL-58626 (R. E. Hershberger) and 5 M01 RR000334.
Acknowledgments
We thank the patients and families and their referring physicians for their participation in the OHSU Familial Dilated Cardiomyopathy Research Program, without whom this study would not have been possible. We thank Dr. Raymond Kwong for helpful comments.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, Sheppard MN, Poole-Wilson PA, Pennell DJ. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol 48: 1977–1985, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Baig MK, Goldman JH, Caforio AL, Coonar AS, Keeling PJ, McKenna WJ. Familial dilated cardiomyopathy: cardiac abnormalities are common in asymptomatic relatives and may represent early disease. J Am Coll Cardiol 31: 195–201, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Brix G, Schad LR, Deimling M, Lorenz WJ. Fast and precise T1 imaging using a TOMROP sequence. Magn Reson Imaging 8: 351–356, 1990. [DOI] [PubMed] [Google Scholar]
- 4.Burkett EL, Hershberger RE. Clinical and genetic issues in familial dilated cardiomyopathy. J Am Coll Cardiol 45: 969–981, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, Levine GN, Narula J, Starling RC, Towbin J, Virmani R. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation 116: 2216–2233, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Crispell KA, Wray A, Ni H, Nauman DJ, Hershberger RE. Clinical profiles of four large pedigrees with familial dilated cardiomyopathy: preliminary recommendations for clinical practice. J Am Coll Cardiol 34: 837–847, 1999. [DOI] [PubMed] [Google Scholar]
- 7.de Leeuw N, Ruiter DJ, Balk AH, de Jonge N, Melchers WJ, Galama JM. Histopathologic findings in explanted heart tissue from patients with end-stage idiopathic dilated cardiomyopathy. Transpl Int 14: 299–306, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Firth D Bias reduction of maximum likelihood estimates. Biometrika 80: 27–38, 1993.
- 9.Flacke SJ, Fischer SE, Lorenz CH. Measurement of the gadopentetate dimeglumine partition coefficient in human myocardium in vivo: normal distribution and elevation in acute and chronic infarction. Radiology 218: 703–710, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Gunja-Smith Z, Morales AR, Romanelli R, Woessner JF Jr. Remodeling of human myocardial collagen in idiopathic dilated cardiomyopathy. Role of metalloproteinases and pyridinoline cross-links. Am J Pathol 148: 1639–1648, 1996. [PMC free article] [PubMed] [Google Scholar]
- 11.Harris PA, Lorenz CH, Holburn GE, Overholser KA. Regional measurement of the Gd-DTPA tissue partition coefficient in canine myocardium. Magn Reson Med 38: 541–545, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Hershberger RE, Ni H, Crispell KA. Familial dilated cardiomyopathy: echocardiographic diagnostic criteria for classification of family members as affected. J Card Fail 5: 203–212, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Hershberger RE, Parks SB, Kushner JD, Li D, Ludwigsen S, Jakobs P, Nauman D, Burgess D, Partain J, Litt M. Coding sequence mutations identified in MYH7, TNNT2, SCN5A, CSRP3, LBD3, and TCAP from 313 patients with Familial or idiopathic dilated cardiomyopathy. Clinical and Translational Science 1: 21–26, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jerosch-Herold M, Hu X, Murthy NS, Rickers C, Stillman AE. MRI of myocardial contrast enhancement with MS-325 and its relation to myocardial blood flow and the perfusion reserve. J Magn Reson Imaging 18: 544–554, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Jerosch-Herold M, Swingen C, Seethamraju RT. Myocardial blood flow quantification with MRI by model-independent deconvolution. Med Phys 29: 886–897, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Kamisago M, Sharma SD, DePalma SR, Solomon S, Sharma P, McDonough B, Smoot L, Mullen MP, Woolf PK, Wigle ED, Seidman JG, Seidman CE. Mutations in sarcomere protein genes as a cause of dilated cardiomyopathy. N Engl J Med 343: 1688–1696, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Karch R, Neumann F, Ullrich R, Neumuller J, Podesser BK, Neumann M, Schreiner W. The spatial pattern of coronary capillaries in patients with dilated, ischemic, or inflammatory cardiomyopathy. Cardiovasc Pathol 14: 135–144, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 343: 1445–1453, 2000. [DOI] [PubMed] [Google Scholar]
- 19.King RB, Bassingthwaighte JB, Hales JR, Rowell LB. Stability of heterogeneity of myocardial blood flow in normal awake baboons. Circ Res 57: 285–295, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein C, Nekolla SG, Balbach T, Schnackenburg B, Nagel E, Fleck E, Schwaiger M. The influence of myocardial blood flow and volume of distribution on late Gd-DTPA kinetics in ischemic heart failure. J Magn Reson Imaging 20: 588–593, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Knaapen P, Boellaard R, Gotte MJ, Dijkmans PA, van Campen LM, de Cock CC, Luurtsema G, Visser CA, Lammertsma AA, Visser FC. Perfusable tissue index as a potential marker of fibrosis in patients with idiopathic dilated cardiomyopathy. J Nucl Med 45: 1299–1304, 2004. [PubMed] [Google Scholar]
- 22.Knaapen P, Gotte MJ, Paulus WJ, Zwanenburg JJ, Dijkmans PA, Boellaard R, Marcus JT, Twisk JW, Visser CA, van Rossum AC, Lammertsma AA, Visser FC. Does myocardial fibrosis hinder contractile function and perfusion in idiopathic dilated cardiomyopathy? PET and MR imaging study. Radiology 240: 380–388, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Komamura K, Tatsumi R, Miyazaki J, Matsumoto K, Yamato E, Nakamura T, Shimizu Y, Nakatani T, Kitamura S, Tomoike H, Kitakaze M, Kangawa K, Miyatake K. Treatment of dilated cardiomyopathy with electroporation of hepatocyte growth factor gene into skeletal muscle. Hypertension 44: 365–371, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Kushner JD, Nauman D, Burgess D, Ludwigsen S, Dutton D, Parks S, Pantely G, Burkett EL, Hershberger RE. Clinical characteristics of 304 kindreds evaluated for familial dilated cardiomyopathy. J Card Fail 12: 422–429, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Lekx KS, Prato FS, Sykes J, Wisenberg G. The partition coefficient of Gd-DTPA reflects maintained tissue viability in a canine model of chronic significant coronary stenosis. J Cardiovasc Magn Reson 6: 33–42, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Springer CS, Jerosch-Herold M. First-pass DCE-MRI with extravasating CR: evidence for human myocardial capillary recruitment in adenosine-induced hyperemia. NMR Biomed. In press. [DOI] [PubMed]
- 27.Look DC, Locker DR. Time saving in measurement of NMR and EPR relaxation times. Rev Sci Instrum 41: 250–251, 1976. [Google Scholar]
- 28.Lorenz CH, Walker ES, Morgan VL, Klein SS, Graham TP Jr. Normal human right and left ventricular mass, systolic function, and gender differences by cine magnetic resonance imaging. J Cardiovasc Magn Reson 1: 7–21, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Mahon NG, Madden BP, Caforio AL, Elliott PM, Haven AJ, Keogh BE, Davies MJ, McKenna WJ. Immunohistologic evidence of myocardial disease in apparently healthy relatives of patients with dilated cardiomyopathy. J Am Coll Cardiol 39: 455–462, 2002. [DOI] [PubMed] [Google Scholar]
- 30.McCrohon JA, Moon JC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJ, Pennell DJ. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation 108: 54–59, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Meredith C, Herrmann R, Parry C, Liyanage K, Dye DE, Durling HJ, Duff RM, Beckman K, de Visser M, van der Graaff MM, Hedera P, Fink JK, Petty EM, Lamont P, Fabian V, Bridges L, Voit T, Mastaglia FL, Laing NG. Mutations in the slow skeletal muscle fiber myosin heavy chain gene (MYH7) cause laing early-onset distal myopathy (MPD1). Am J Hum Genet 75: 703–708, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mestroni L, Maisch B, McKenna WJ, Schwartz K, Charron P, Rocco C, Tesson F, Richter A, Wilke A, Komajda M. Guidelines for the study of familial dilated cardiomyopathies. Collaborative Research Group of the European Human and Capital Mobility Project on Familial Dilated Cardiomyopathy. Eur Heart J 20: 93–102, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Moon JC, Sachdev B, Elkington AG, McKenna WJ, Mehta A, Pennell DJ, Leed PJ, Elliott PM. Gadolinium enhanced cardiovascular magnetic resonance in Anderson-Fabry disease. Evidence for a disease specific abnormality of the myocardial interstitium. Eur Heart J 24: 2151–2155, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Moon JC, Sievers B, Pennell DJ, Yacoub MH, Mohiaddin RH. Myocardial scarring caused by left ventricular assist device (LVAD) insertion demonstrated by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 5: 361–363, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Pack NA, Dibella EV, Wilson BD, McGann CJ. Quantitative myocardial distribution volume from dynamic contrast-enhanced MRI. Magn Reson Imaging 26: 532–542, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petersen SE, Jerosch-Herold M, Hudsmith LE, Robson MD, Francis JM, Doll HA, Selvanayagam JB, Neubauer S, Watkins H. Evidence for microvascular dysfunction in hypertrophic cardiomyopathy: new insights from multiparametric magnetic resonance imaging. Circulation 115: 2418–2425, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Pickup S, Wood AK, Kundel HL. A novel method for analysis of TOMROP data. J Magn Reson Imaging 19: 508–512, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Rossi MA Patterns of myocardial fibrosis in idiopathic cardiomyopathies and chronic Chagasic cardiopathy. Can J Cardiol 7: 287–294, 1991. [PubMed] [Google Scholar]
- 39.Selvanayagam JB, Jerosch-Herold M, Porto I, Sheridan D, Cheng AS, Petersen SE, Searle N, Channon KM, Banning AP, Neubauer S. Resting myocardial blood flow is impaired in hibernating myocardium: a magnetic resonance study of quantitative perfusion assessment. Circulation 112: 3289–3296, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Stolen KQ, Kemppainen J, Kalliokoski KK, Karanko H, Toikka J, Janatuinen T, Raitakari OT, Airaksinen KE, Nuutila P, Knuuti J. Myocardial perfusion reserve and peripheral endothelial function in patients with idiopathic dilated cardiomyopathy. Am J Cardiol 93: 64–68, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Tandri H, Saranathan M, Rodriguez ER, Martinez C, Bomma C, Nasir K, Rosen B, Lima JA, Calkins H, Bluemke DA. Noninvasive detection of myocardial fibrosis in arrhythmogenic right ventricular cardiomyopathy using delayed-enhancement magnetic resonance imaging. J Am Coll Cardiol 45: 98–103, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Thornhill RE, Prato FS, Wisenberg G, Moran GR, Sykes J. Determining the extent to which delayed-enhancement images reflect the partition-coefficient of Gd-DTPA in canine studies of reperfused and unreperfused myocardial infarction. Magn Reson Med 52: 1069–1079, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Thornhill RE, Prato FS, Wisenberg G, White JA, Nowell J, Sauer A. Feasibility of the single-bolus strategy for measuring the partition coefficient of Gd-DTPA in patients with myocardial infarction: independence of image delay time and maturity of scar. Magn Reson Med 55: 780–789, 2006. [DOI] [PubMed] [Google Scholar]