Abstract
Evidence is accumulating to support the presence of P2X purinergic receptors in the heart. However, the biological role of this receptor remains to be defined. The objectives here were to determine the role of cardiac P2X receptors in modulating the progression of post-myocardial infarction ischemic heart failure and to investigate the underlying mechanism. The P2X4 receptor (P2X4R) is an important subunit of native cardiac P2X receptors, and the cardiac-specific transgenic overexpression of P2X4R (Tg) was developed as a model. Left anterior descending artery ligation resulted in similar infarct size between Tg and wild-type (WT) mice (P > 0.1). However, Tg mice showed an enhanced cardiac contractile performance at 7 days, 1 mo, and 2 mo after infarction and an increased survival at 1 and 2 mo after infarction (P < 0.01). The enhanced intact heart function was manifested by a greater global left ventricular developed pressure and rate of contraction of left ventricular pressure in vitro and by a significantly increased fractional shortening and systolic thickening in the noninfarcted region in vivo (P < 0.05). The salutary effects on the ischemic heart failure phenotype were seen in both sexes and were not the result of any difference in infarct size in Tg versus WT hearts. An enhanced contractile function of the noninfarcted area in the Tg heart was likely an important rescuing mechanism. The cardiac P2X receptor is a novel target to treat post-myocardial infarction ischemic heart failure.
Keywords: purines, contractility, infarction
receptors for purine nucleotides, known as P2 purinergic receptors, are activated by extracellular adenine nucleotides such as ATP and ADP (16, 17). The P2 receptor class includes the ligand-gated receptor channel P2X receptor and the G protein-coupled P2Y receptor (1, 9, 16). Evidence is accumulating to indicate that the cardiac myocyte P2X receptor mediates an important physiological role (9, 24). ATP or the P2X receptor agonist 2-methylthioATP causes an increase in myocyte contractility and in intact heart function (9, 24). Of the P2X receptor family members, the P2X4 receptor (P2X4R) is an important subunit of the native cardiac myocyte P2X receptor (21). Transgenic hearts with cardiac-specific overexpression of the P2X4R showed an enhanced basal cardiac contractile function (9). Although the cardiac P2X receptor can exert a rescuing effect in the calsequestrin (CSQ) model of heart failure (24), the role of this receptor in a pathophysiologically more relevant ischemic heart failure model is not known.
The objective of the present study was to investigate the role of cardiac P2X receptors in heart failure progression after left anterior descending coronary artery (LAD) ligation-induced infarction. Another goal was to determine and characterize the mechanism underlying a modulatory effect of this receptor. Transgenic mice with cardiac-restricted overexpression of the P2X4R (P2X4R Tg) were used as a model. Cardiac performance and survival were characterized in P2X4R Tg and wild-type (WT) animals after myocardial infarction (MI). The data showed that the P2X4R Tg animals displayed significantly enhanced cardiac contractile function compared with WT animals following LAD ligation-induced MI at 7 days, 1 mo, and 2 mo after infarction.
METHODS
LAD ligation and induction of MI.
Age-matched (14–16 wk old) WT and P2X4R Tg mice with similar body weights were used. The P2X4R construct was generated and bred as previously described (9). LAD ligation-mediated ischemic cardiomyopathy was induced in both WT and P2X4R Tg male and female mice. All animals that recovered from anesthesia and lived for 24 h were analyzed for survival. There was no difference between WT and Tg animals in the death rate within the first 24 h after LAD ligation (P > 0.05, Fisher exact test). All data were presented as means ± SE. Ligation of LAD in anesthetized mice was carried out using a procedure similar to those previously described (4, 23). Adult WT (BL6) or P2X4R Tg mice 14–16 wk old of either sex were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) intraperitoneally. After endotracheal intubation under a dissecting microscope at 37°C, the cannula was connected to a small rodent ventilator (Hugo Sachs-Harvard Apparatus, Minivent Type 845, Holliston, MA) on room air with a stroke volume of 0.3 ml at a rate of 150 per min. After left intercostal thoracotomy, MI was produced by ligating the LAD with an 8-0 nylon suture within 2 mm below the edge of left atrium near the origin of the artery. All animal procedures were performed according to guidelines of and approved by the University of Connecticut School of Medicine Review Board.
Measurements of intact heart function and infarct size.
Various parameters of intact heart function, such as left ventricular (LV) developed pressure (LVDP) and rates of contraction and relaxation of LV pressure (±dP/dt), were quantitatively determined using the working heart model as previously described (9, 12). In brief, LVDP and ±dP/dt were quantitatively determined using the working heart model (12). Following injection of heparin via tail vein (500 U/kg iv) and anesthetization with Nembutal (150 mg/kg ip), the aorta was then cannulated with a 20-gauge catheter and was positioned about 2 mm above the coronary ostia. A column of Krebs-Henseleit solution buffer produced a constant hydrostatic pressure of 55 mmHg. The opening of the pulmonary vein was connected to a reservoir of Krebs-Henseleit solution buffer that maintained an adequate flow into the left atrium. The LVDP was the difference between LV systolic and diastolic pressures and was measured directly using a fluid-filled system of pressure transducer. The system was channeled from a precalibrated amplifier (Kent Scientific, Litchfield, CT), and the signals were digitized via a PCM-DAS 16S/330 interface board (Computer Boards, Mansfield, MA). The basal heart rate was determined in the absence of pacing. Data were analyzed by computer software (WorkBench for Windows+, Kent Scientific). The signals from the transducers were constantly displayed and analyzed.
The infarct size was quantified as previously described (3, 4). After being fixed in 10% formalin, the LV was cut into five 1.0 mm-thick transverse sections from the apex toward the base. Sections were embedded in paraffin, cut into 4-μm slices, and stained with Masson's trichrome to measure area of fibrosis (infarcted myocardium). The lengths of the infarcted and noninfarcted endocardial and epicardial surfaces were traced with a planimeter image analyzer (ImageProPlus). Infarct size was calculated as the ratio of infarct length to the circumference of both the endocardium and the epicardium.
Echocardiography.
Transthoracic echocardiography was performed using a linear 30-MHz transducer according to the manufacturer's instructions (Vevo 660 High Resolution Imaging System from VisualSonics, Toronto, Canada) similar to previously described methods (4, 9). Two-dimensional-targeted M-mode echocardiographic measurements were carried out at mid-papillary muscle level. Mice were anesthetized with 1% isoflurane using a vaporizer as previously described (23). LV end-diastolic (LVEDD) and end-systolic (LVESD) diameters, LV posterior wall (LVPW) systolic thickening (defined as the increase in wall thickness between systole and diastole), and fractional shortening (FS) (defined as LVEDD − LVESD/LVEDD) were measured. LVPW was measured digitally on the M-mode tracings, and all echocardiogram parameters were averaged from more than three cardiac cycles. There were no significant differences in heart rate between WT and Tg mice at all time points after the infarction. The various echocardiographic parameter values in postinfarction hearts were similar to those obtained in WT mice from other studies (2, 6, 13, 15). To compare WT versus P2X4R Tg differences or to compare WT versus P2X4R Tg mice treated under a specific condition or at a specific time point after infarction, an unpaired t-test was used.
RESULTS
Cardiac-specific overexpression of the P2X4R resulted in an improved cardiac contractile performance in the postinfarct heart failure mice.
The baseline echocardiographic parameters, such as LVEDD and LVESD (LV internal dimension at diastole and systole, respectively), systolic thickening of the LVPW (LV posterior wall) and interventricular septum, heart rate, FS, as well as heart weight-to-body weight ratios, were similar between P2X4R Tg and WT animals (P > 0.1). These data suggest a normal cardiac phenotype of the P2X4R Tg animals under basal physiological conditions, consistent with previous findings (9). To characterize its phenotype under the pathological condition of ischemic heart failure, cardiac function was examined in LAD-ligated P2X4R Tg mice. LAD ligation resulted in significant decreases in the various cardiac function indexes in both WT and P2X4R Tg animals. When compared with sham-operated animals, both WT and Tg mice showed significantly impaired FS by echocardiography as well as LVDP and +dP/dt by the working heart preparation at 1 mo after MI (Table 1).
Table 1.
Effects of LAD ligation on cardiac contractile performance in WT and P2X4R Tg mice
| FS, % | LVDP, mmHg | +dP/dt, mmHg/s | |
|---|---|---|---|
| WT | |||
| Sham | 32.7±1.7 (8) | 130±2.6 (13) | 4,727±191 (13) |
| Ligated | 18.5±0.5 (33) | 106±2.3 (26) | 3,504±110 (26) |
| P2X4R Tg | |||
| Sham | 31.1±0.5 (9) | 141±2.5 (16) | 5,403±124 (16) |
| Ligated | 23.6±0.66 (24) | 117±1.9 (25) | 4,210±128 (25) |
Values are means ± SE (number of mice shown in parentheses). At 1 mo after left anterior descending coronary (LAD) ligation-induced myocardial infarction (MI), fractional shortening (FS) derived from echocardiography as well as left ventricular (LV) developed pressure (LVDP) and rate of contraction of LV pressure (+dP/dt) derived from isolated working heart preparation were obtained in wild-type (WT) and P2X4 receptor transgenic (P2X4R Tg) mice as described in methods. For all parameters, P < 0.05 in sham-operated vs. ligated animals in both WT and P2X4R Tg mice.
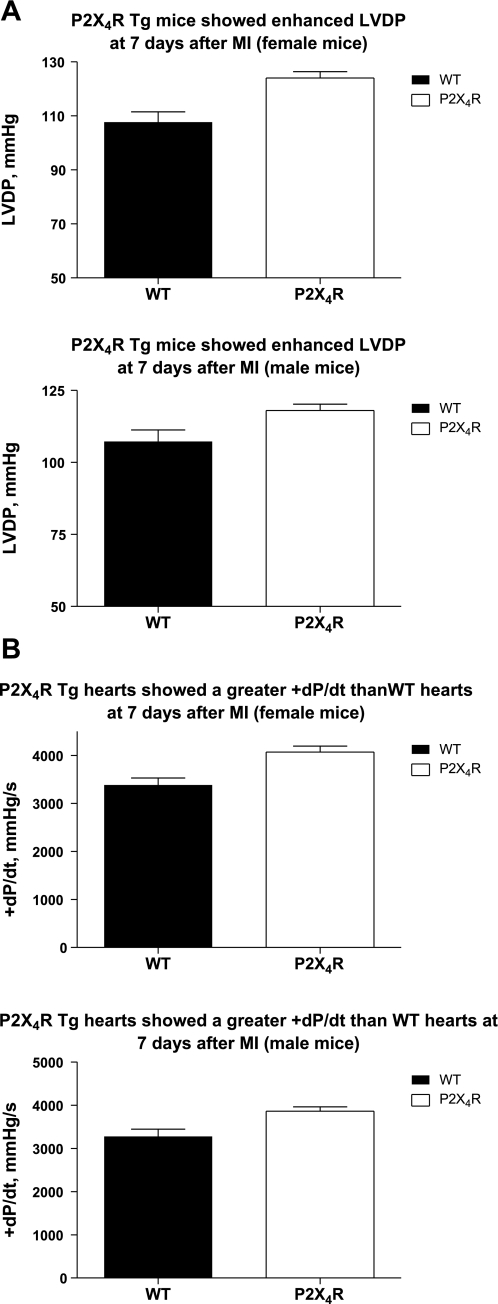
As early as 7 days after LAD ligation, however, the P2X4R Tg mice displayed enhanced LVDP (Fig. 1A), +dP/dt (Fig. 1B), and −dP/dt (not shown), compared with the WT mice, in an isolated working heart preparation in both sexes. The in vivo cardiac function was determined by echocardiographically measured FS and LVPW thickening. FS as well as thickening of the LVPW (Tables 2 and 3), a noninfarcted region, was also significantly greater in Tg than in WT hearts for both sexes. After permanent LAD ligation, there was no difference in the infarct size between WT and Tg animals in either sex. There was no difference in the infarct size between male and female hearts in either WT or Tg animals (P > 0.1) after permanent LAD ligation. At 7 days after infarction, the LVEDD and LVESD were similar between female WT and Tg mice, whereas male Tg animals had significantly smaller LVEDD and LVESD than male WT mice (Tables 2 and 3). Thus, during the acute healing or necrotic phase after LAD ligation (up to 5–7 days after infarction), cardiac-specific overexpression of the P2X4R resulted in an improved cardiac contractile performance by both in vitro and in vivo heart function indexes.
Fig. 1.
Cardiac-specific overexpression of P2X4 receptors (P2X4Rs) reversed the depressed left ventricular (LV) developed pressure (LVDP) and the rate of contraction of LV pressure (+dP/dt) at 7 days after myocardial infarction (MI) in both sexes. Isolated working heart preparations of wild-type (WT) and P2X4R transgenic (P2X4R Tg) animals were carried out as described in methods. The basal values for LVDP (A, top, for 8 WT and 10 Tg female; and A, bottom, for 9 WT and 15 Tg male) and +dP/dt (B, top, for 8 WT and 10 Tg female; and B, bottom, for 9 WT and 15 Tg male) were summarized as means ± SE. P2X4R Tg mice showed significantly higher LVDP and +dP/dt than did the WT mice (P < 0.05, unpaired t-test).
Table 2.
Echocardiographic parameters in female WT and P2X4R Tg animals
| Time Points After MI | n | FS, % | LVPW Thickening, mm | LVEDD, mm | LVESD, mm | MI Size, % |
|---|---|---|---|---|---|---|
| 7 days | ||||||
| WT | 28 | 20.50±0.59† | 0.059±0.0047† | 3.87±0.0840† | 3.08±0.0770† | 40.4±4.02† |
| P2X4R Tg | 21 | 22.60±0.84* | 0.079±0.0100* | 3.91±0.0970† | 3.05±0.0850† | 38.3±3.37 |
| 1 mo | ||||||
| WT | 19 | 18.80±0.59 | 0.053±0.0055 | 3.91±0.1100 | 3.18±0.0950 | 43.2±2.90 |
| P2X4R Tg | 14 | 24.20±0.77* | 0.080±0.0100* | 4.19±0.1350 | 3.19±0.1220 | 40.5±3.14 |
| 2 mo | ||||||
| WT | 22 | 18.49±0.56 | 0.040±0.0052 | 4.27±0.1600 | 3.51±0.1430 | 27.0±2.03 |
| P2X4R Tg | 17 | 23.14±0.76* | 0.102±0.0140* | 4.39±0.1590 | 3.39±0.1370 | 30.5±3.20 |
Values are means ± SE. At 7 days, 1 mo, and 2 mo after LAD ligation-induced myocardial infarction, WT and P2X4R Tg mice of either sex were subjected to echocardiography as described in methods. The various echocardiographic measurements were obtained as shown.
P < 0.05 vs. WT mice at the same time point after MI.
P < 0.05 vs. the 2-mo time point.
Table 3.
Echocardiographic parameters in male WT and P2X4R Tg animals
| Time Points After MI | n | FS, % | LVPW Thickening, mm | LVEDD, mm | LVESD, mm | MI Size, % |
|---|---|---|---|---|---|---|
| 7 days | ||||||
| WT | 28 | 17.65±0.49 | 0.051±0.0047 | 4.34±0.0870 | 3.53±0.10 | 44.8±3.67† |
| P2X4R Tg | 21 | 24.50±0.95*† | 0.090±0.0083* | 3.98±0.1600* | 3.14±0.13* | 38.1±4.22 |
| 1 mo | ||||||
| WT | 14 | 18.23±0.85 | 0.035±0.0056 | 4.33±0.1500 | 3.57±0.14 | 44.0±1.92 |
| P2X4R Tg | 10 | 22.72±1.14* | 0.090±0.0160* | 4.57±0.2190 | 3.55±0.21 | 42.3±1.83 |
| 2 mo | ||||||
| WT | 23 | 17.15±0.88 | 0.053±0.0055 | 4.29±0.1600 | 3.53±0.15 | 31.9±1.73 |
| P2X4R Tg | 18 | 20.42±0.98* | 0.087±0.0096* | 4.62±0.2900 | 3.68±0.26 | 33.6±4.90 |
Values are means ± SE. At 7 days, 1 mo, and 2 mo after LAD ligation-induced myocardial infarction, WT and P2X4R Tg mice of either sex were subjected to echocardiography as described in methods. The various echocardiographic measurements were obtained as shown.
P < 0.05 vs. WT mice at the same time point after MI.
P < 0.05 vs. the 2-mo time point.
Improvement in cardiac contractile performance of the Tg mice was sustained during the progression of heart failure after infarction.
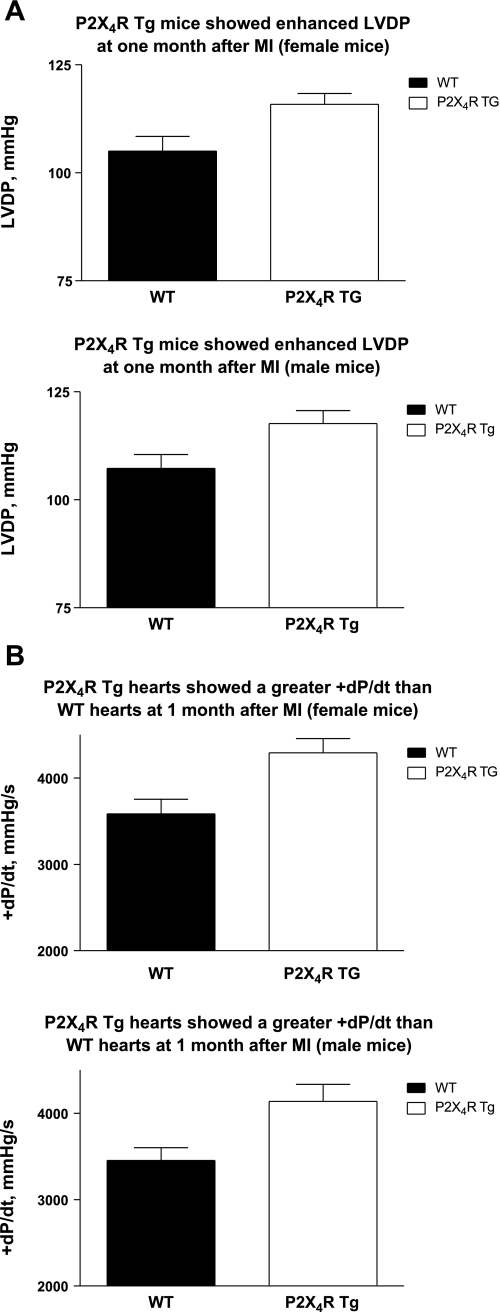
The improvement in cardiac contractile performance in Tg animals was present at 1 mo after infarction. At this time point, P2X4R Tg mice also displayed enhanced LVDP (Fig. 2A), +dP/dt (Fig. 2B), and −dP/dt (not shown) in an isolated working heart preparation of both sexes. At 1 mo after infarction, the Tg hearts showed greater overall FS and systolic thickening of LVPW (Tables 2 and 3) than did the WT animals for either sex (P < 0.05). Thus, similar to data obtained at 7 days after infarction, Tg animals exhibited greater cardiac contractile function based on in vitro isolated working heart and in vivo echocardiographic measurements. There was no difference in the infarct size between WT and Tg animals in either sex at 1 mo after infarction (Tables 2 and 3). The LVEDD and LVESD were similar between WT and Tg mice of either sex at this time point.
Fig. 2.
Cardiac-specific overexpression of P2X4Rs reversed the depressed LVDP and +dP/dt and showed enhanced LV fractional shortening (FS) at 1 mo after MI in both sexes. Isolated working heart preparations of WT and P2X4R Tg animals and in vivo echocardiography were carried out as described in methods. The basal values for LVDP (A, top, for 10 WT and 13 Tg female; and A, bottom, for 16 WT and 12 Tg male) and +dP/dt (B, top, for 10 WT and 12 Tg female; and B, bottom, for 16 WT and 13 Tg male) were summarized as means ± SE. P2X4R Tg mice showed significantly higher LVDP and +dP/dt than did the WT mice (P < 0.05, unpaired t-test).
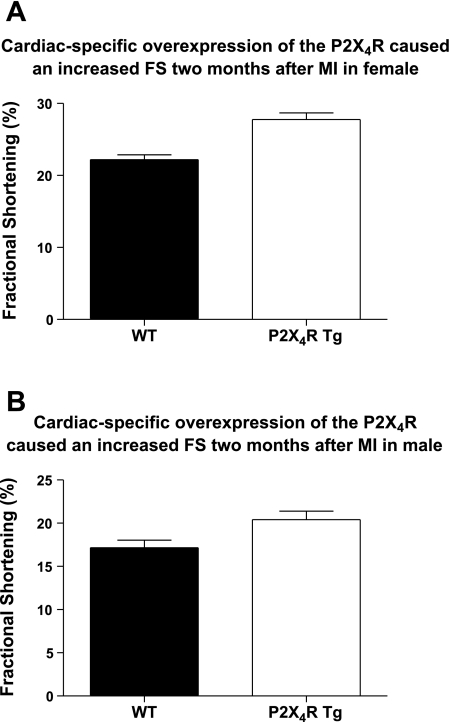
The improvement in cardiac contractile performance was also evident at 2 mo after infarction. Echocardiographically measured FS and LVPW thickening showed that at 2 mo after infarction, the Tg hearts exhibited greater FS (Fig. 3) and systolic thickening of LVPW (Tables 2 and 3) than did the WT animals in either sex (P < 0.05). Again, there was no difference in the infarct size between WT and Tg hearts (P > 0.1). Similar to data on the MI size at 7 days after infarct, there was no difference in the MI size between female and male hearts in either WT or Tg animals 2 mo after permanent LAD ligation (P > 0.1). At 2 mo after infarction, the LVEDD and LVESD were similar between WT and Tg mice of either sex.
Fig. 3.
The increase in LV basal FS in P2X4R Tg mice was sustained at 2 mo after infarction in both sexes. Two-dimensionally directed M-mode echocardiography was carried out as described in methods. The basal values for LV FS of 22 WT and 17 Tg female (A) and of 23 WT and 15 Tg male (B) were summarized as means ± SE. P2X4R Tg mice showed significantly greater FS than did the WT mice (P < 0.05, unpaired t-test).
During the progression of heart failure after LAD ligation, the LVPW systolic thickening and FS were significantly depressed at 2 mo versus 7 days after infarction in WT female mice (Table 2, P < 0.05). The depressed LVPW thickening and FS occurred even though mice at the 2-mo time point had a significantly smaller infarct size than mice studied at 7 days (P < 0.05). On the other hand, female P2X4R Tg mice showed preserved LVPW thickening and FS at 2 mo compared with 7 days after infarction, even though the infarct size obtained at these two time points were similar (Table 2). Thus, with the use of both LVPW thickening and FS as indexes of cardiac function, Tg female hearts maintained better contractile performance than WT female hearts 2 mo after infarction.
In examining cardiac chamber dilatation after infarction, LVEDD and LVESD increased significantly at 2 mo after infarction compared with those obtained at 7 days in female WT and Tg hearts (Table 2, P < 0.05). In Tg mice, the LV chamber size became more dilated with time in the presence of similar infarct sizes at the two time points. However, WT female mice showed significant LV chamber dilatation despite a significantly smaller infarct size at 2 mo versus 7 days after infarction (Table 2). In the 2-mo vs. 7-day comparison in male mice, there was no decrease in LVPW systolic thickening or an enlargement in LVEDD or LVESD in both WT and Tg mice (Table 3). In male WT mice, the preservation of LVPW thickening and LV chamber size was associated with, or perhaps, was due to a smaller infarct size of the hearts at 2 mo. In male Tg mice, the preservation of LVPW thickening or LV chamber size could not be the result of a smaller infarct size of the 2-mo hearts, since the infarct size at 2 mo was similar to that at 7 days (Table 3).
Cardiac-specific overexpression of the P2X4R was associated with an enhanced survival after LAD ligation.
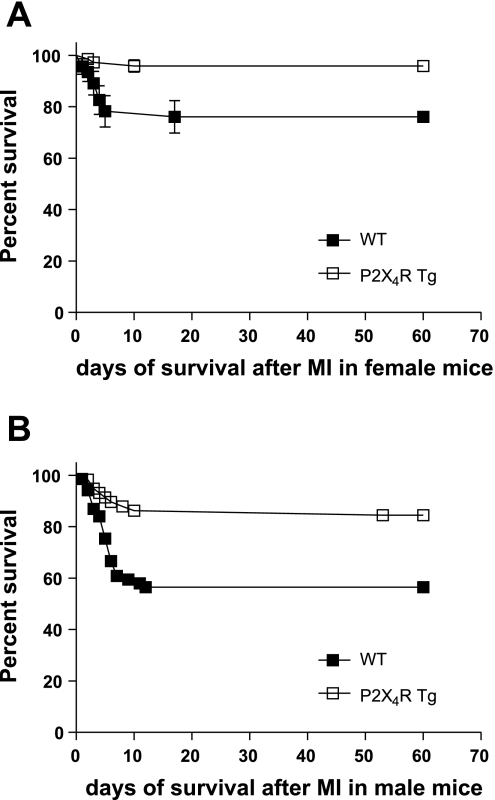
To test whether the enhanced cardiac contractile function of the Tg animals is associated with a survival benefit, the mortality rate of WT and Tg mice after LAD ligation were compared. Survival data were compared using Kaplan-Meier survival curve with a log-rank method of statistical analysis. The overall postinfarct survival was significantly greater in P2X4R Tg than WT mice at 2 mo after the infarction in both sexes (Fig. 4). The infarct sizes were similar in male WT (38 ± 2% infarction, n = 69 mice) and male P2X4R Tg (36 ± 1.7%, n = 58, P > 0.05) animals. Female WT and P2X4R Tg mice also showed similar infarct size at 2 mo after infarction (not shown, P > 0.1). A survival benefit of the P2X4R Tg animals was also observed at 1 mo after infarction (log-rank test, P < 0.01). The improved survival after infarction was due to a lower incidence of death during the first 7–10 days after LAD ligation.
Fig. 4.
P2X4R Tg animals showed improved survival after MI. Kaplan-Meier analysis was used to determine the survival probability in female P2X4R Tg (n = 72) and female WT (n = 46) mice (A) or in male P2X4R Tg (n = 58) and male WT (n = 69) animals (B) after infarction. Log-rank test method was used to analyze the survival curves. P2X4R Tg animals showed significantly enhanced survival at 2 mo after MI (P = 0.0011 for female comparison; P = 0.0007 for male comparison).
DISCUSSION
The activation of cardiac P2X receptors is capable of enhancing the contractile state of the myocyte and intact heart (9, 24, 12). Although a growing body of evidence supports a biological role of cardiac myocyte P2X receptors (9, 20, 21, 24), its function in mediating or modulating ischemic heart failure progression is not known (1, 16, 17). With the use of transgenic mice with cardiac-specific overexpression of P2X4R as a model, the present data showed that the overexpression of this receptor channel was capable of enhancing cardiac contractile performance and survival after MI.
Of the P2X receptor subfamily, the P2X4R is an important subunit of the cardiac myocyte P2X receptor (21). Previous studies suggested a beneficial, protective function of the cardiac P2X receptor in the calsequestrin (CSQ) model of cardiomyopathy and heart failure (24). The CSQ mice are a model of heart failure with abnormal calcium handling and metabolism (10, 19). Since the cardiac P2X receptor can induce an increase in the sarcoplasmic reticulum (SR) calcium content and cause an enhanced cardiac performance, it was not surprising that the receptor played a salutary role in the CSQ model of cardiomyopathy and heart failure. The present data showed that cardiac-specific overexpression of P2X4R could also in fact rescue the ischemic heart failure phenotype following MI, a clinically more relevant model of heart failure.
The salutary effect of cardiac transgenic expression of the P2X4R was supported by a number of lines of evidence. First, although both WT and Tg hearts showed significant reductions in LVDP and ±dP/dt in vitro as well as in FS in vivo after infarction, as early as 7 days after infarction when the injured myocardium was undergoing acute healing, the P2X4R Tg animal maintained a greater cardiac contractile function by both in vitro and in vivo indexes. The Tg hearts showed a higher LVDP and ±dP/dt in an isolated working heart preparation as well as a greater FS and systolic thickening of the noninfarcted LVPW. The enhanced contractile performance of the postinfarction Tg hearts was sustained at 1 and 2 mo after infarction, time points that correspond to the fibrotic and remodeling phases, respectively (11, 18). When compared with LVPW thickening before LAD ligation, Tg hearts maintained a similar level of thickening in this noninfarcted region at all time points after infarction in both sexes (P > 0.1), whereas WT hearts showed a significant decrease in the LVPW systolic thickening in the pre- versus post-LAD ligation comparison (P < 0.05). The data further supported an enhanced contractile function of the Tg hearts in the postinfarction ischemic heart failure model. Second, P2X4R Tg animals showed an improved survival after the infarction. All deaths occurred within 7 to 8 days after LAD ligation. Excluding deaths associated with acute injury from surgery, trauma, or anesthesia during the immediate 24-h postoperative period, significantly fewer deaths occurred in the P2X4R Tg than the WT mice. The survival effect was not due to a decreased infarct size in the P2X4R Tg animals since both Tg and WT hearts had similar infarct sizes. An altered postinfarction survival could be seen early (5, 13, 14, 23) or late (18) after LAD ligation. The enhanced survival of P2X4R Tg animals occurred early after the infarction. Finally, Tg hearts showed some difference in LV chamber dilatation during the postinfarction course compared with the WT hearts. In female mice, Tg hearts showed LV dilatation at 2 mo versus 7 days after infarction in the presence of similar infarct sizes at these two time points. WT female mice studied at 2 mo after infarction had a significantly smaller infarct size than those studied at 7 days. Yet, these WT animals still exhibited a significant LV dilatation at 2 mo. It is possible that if the infarct size were similar at these two time points, the WT female hearts would show more LV dilatation than the Tg female hearts. In male mice, WT hearts did not dilate with time, possibly as a result of significantly smaller infarct size of the mice studied at 2 mo versus that at 7 days after infarction. Since infarct size correlates directly with the extent of heart failure (11, 8), differences in the infarct size between mice studied at the two time points may have influenced the extent of LV dilatation with time. If the infarct size were similar at 2 mo versus 7 days, WT male animals may have displayed cardiac dilatation. On the other hand, despite similar infarct sizes at the 2-mo versus 7-day time points, the Tg male hearts did not dilate with time. Although there is no established mathematical method to correct the LVEDD or LVESD for infarct size, Tg hearts appeared to exhibit a modest difference from WT hearts in cardiac chamber dilatation after infarction in either sex. Possible differences between WT versus Tg mice in the various components of remodeling, such as myocyte area, length, width, and extracellular fibrosis, deserve further investigation.
Overall, although phenotypically normal under physiological conditions, Tg mice with cardiac overexpression of the P2X4R showed a salutary phenotype during the pathological progression of heart failure after infarction. Since the P2X4R Tg animals did not show any decrease in the infarct size after LAD ligation, the beneficial effect on survival and contractile function could not be due to a smaller infarct size in the Tg versus WT mice. Instead, an enhanced contractile function of the noninfarcted region, mediated via its overexpressed P2X4R, was responsible for the greater global contraction performance of the Tg hearts. We could not exclude an antiarrhythmic effect of P2X4R overexpression as a reason for the increased survival of Tg animals after infarction. However, the better preserved intact heart function was likely an important salutary mechanism. Although the cellular mechanism is not known, an enhanced SR calcium store and an improved excitation-contraction coupling may be the myocyte signaling mechanism by which the cardiac P2X4R, stimulated by its endogenous agonist ATP, achieves its salutary effect. This hypothesis, deserving of further investigation, is compatible with the observations that the failing human cardiac myocytes exhibited decreased SR calcium stores and that the restoration of this content was correlated with recovery of cardiac function during ventricular assist device implantation in patients (22). In summary, the cardiac myocyte P2X receptor represents a novel pathway by which a ligand-gated cell surface ion channel can rescue postinfarction ischemic heart failure in both sexes and is a potential new target in treating this form of heart failure. That ATP can cause an increased contractile performance in human cardiac atrium, possibly mediated via P2X4-like receptors (7), further implicates an important function for this receptor in the heart.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grant RO1-HL-48225 and Ray Neag Distinguished Professorship (to B. T. Liang). K. A. Jacobson acknowledges support from the National Institute of Diabetes and Digestive and Kidney Diseases Intramural Research Program.
Acknowledgments
We thank Carol McGuiness for capable technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International union of pharmacology. Update of the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 59: 281–341, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bridgman P, Aronovitz MA, Kakkar R, Oliverio MI, Coffman TM, Rand WM, Konstam MA, Mendelsohn ME, Patten RD. Gender-specific patterns of left ventricular and myocyte remodeling following myocardial infarction in mice deficient in the angiotension II type 1a receptor. Am J Physiol Heart Circ Physiol 289: H586–H592, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Chen CX, Gan XT, Beier N, Scholz W, Karmazyn M. Inhibition and reversal of myocardial infarction-induced hypertrophy and heart failure by NHE-1 inhibition. Am J Physiol Heart Circ Physiol 286: H381–H387, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Curcio A, Noma T, Prasad SVN, Wolf MJ, Lemaire A, Perrino C, Mao L, Rockman HA. Competitive displacement of phosphoinoside 3-kinase from β-adrenergic receptor kinase-1 improves postinfarction adverse myocardial remodeling. Am J Physiol Heart Circ Physiol 291: H1754–H1760, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Du XJ, Gao XM, Kiriazis H, Moore XL, Ming Z, Su Y, Finch AM, Hannan RA, Dart AM, Graham RM. Transgenic α1A-adrenergic activation limits post-infarct ventricular remodeling and dysfunction and improves survival. Cardiovasc Res 71: 735–743, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Franz S, Brandes RP, Hu K, Rammelt K, Wolf J, Scheuermann H, Ertl G, Bauersachs J. Left ventricular remodeling after myocardial infarction in mice with targeted deletion of the NADPH oxidase subunit gp91PHOX. Basic Res Cardiol 101: 127–132, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Gergs U, Boknik P, Schmitz W, Simm A, Silber RF, Neumann J. A positive inotropic effect of ATP in the human cardiac atrium. Am J Physiol Heart Circ Physiol 294: H1716–H1723, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Holmes JW, Borg TK, Covell JW. Structure and mechanics of healing myocardial infarcts. Annu Rev Biomed Eng 7: 223–253, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Hu B, Mei Q, Smith E, Barry WH, Liang BT. A novel cardiac inotropic phenotype with cardiac transgenic expression of human P2X4 Receptor transgenic mouse. FASEB J 15: 2739–2741, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Knollman BC, Knollmann-Ritschel BE, Weissman NJ, Jones LR, Morad M. Remodeling of ionic currents in hypertrophied and failing hearts of transgenic mice overexpressing calsequestrin. J Physiol 525: 483–498, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol 23: 845–856, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Mei Q, Liang BT. P2 purinergic receptor activation enhances cardiac contractility in isolated rat and mouse hearts. Am J Physiol Heart Circ Physiol 281: H334–H341, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Miyamoto S, Kawamura T, Morimoto T, Ono K, Wada H, Kawase Y, Matsumori A, Nishio R, Kita T, Hasegawa K. Histone acetyltransferase activity of p300 is required for the promotion of left ventricular remodeling after myocardial infarction in adult mice in vivo. Circulation 113: 679–690, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Nakanishi M, Saito Y, Kishimoto I, Harado M, Kuwahara K, Takahashi N, Kawakami R, Nakagawa Y, Tanimoto K, Yasuni S, Usami S, Li Y, Adachi Y, Kukamizu A, Garbers DL, Nakao K. Role of natriuretic peptide receptor guanylyl cyclase-A in myocardial infarction evaluated using genetically engineered mice. Hypertension 46: 441–447, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Niizeki T, Takeishi Y, Arimoto T, Takahashi H, Shishido T, Koyama Y, Goto K, Walsh RA, Kubota I. Cardiac-specific overexpression of diacylglycerol kinase ζ attenuates left ventricular remodeling and improves survival after myocardial infarction. Am J Physiol Heart Circ Physiol 292: H1105–H1112, 2007. [DOI] [PubMed] [Google Scholar]
- 16.North RA Molecular physiology of P2X receptors. Physiol Rev 82: 1013–1067, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 50: 413–492, 1998. [PubMed] [Google Scholar]
- 18.Sam F, Sawyer DB, Xie Z, Change DLF, Ngoy S, Brenner DA, Siwik DA, Singh K, Apstein CS, Colucci WS. Mice lacking inducible nitric oxide synthase have improved left ventricular contractile function and reduced apoptotic cell death late after myocardial infarction. Circ Res 89: 351–356, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Sato Y, Kiriazis H, Yatani A, Schmidt AG, Hahn H, Ferguson DG, Sako H, Mitarai S, Honda R, Mesnard-Rouiller L, Frank KF, Beyermann B, Wu G, Fujimori K, Dorn GW 2nd, Kranias EG. Rescue of contractile parameters and myocyte hypertrophy in calsequestrin overexpressing myocardium by phospholamban ablation. J Biol Chem 276: 9392–9399, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Shen JB, Cronin C, Sonin D, Joshi BV, Nieto M, Harrison D, Jacobson KA, Liang BT. P2X purinergic receptor-mediated ionic current in cardiac myocytes of calsequestrin model of cardiomyopathy: implications for the treatment of heart failure. Am J Physiol Heart Circ Physiol 292: H1077–H1084, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen JB, Pappano A, Liang BT. Extracellular ATP-stimulated current in wild-type and P2X4 receptor transgenic mouse ventricular myocytes: implications for a cardiac physiologic role of P2X4 receptors. FASEB J 20: 277–284, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Terracciano CMN, Hardy J, Birks EJ, Khaghani A, Banner NR, Yacoub MH. Clinical recovery from end-stage heart failure using left-ventricular assist device and pharmacological therapy correlates with increased sarcoplasmic reticulum calcium content but not with regression of cellular hypertrophy. Circulation 109: 2263–2265, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Tsoporis JN, Marks A, Haddad A, Dawood F, Liu PP, Parker TG. S100B expression modulates left ventricular remodeling after myocardial infarction in mice. Circulation 111: 598–606, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Yang A, Sonin D, Jones L, Liang BT. A beneficial role of cardiac P2X4 receptors in heart failure: rescuing the calsequestrin-overexpression model of cardiomyopathy. Am J Physiol Heart Circ Physiol 287: H1096–H1103, 2004. [DOI] [PubMed] [Google Scholar]