ischemic preconditioning (PC) was originally described by Murry et al. (6) in 1986. In the ensuing years, much has been learned about the signaling pathways that are activated by PC, but we still know little about how these signals reduce cell death (5). Brief intermittent periods of ischemia and reperfusion are thought to lead to the release of agonists such as adenosine, bradykinin (BK), and opioids from the heart. These agonists are thought to bind to G protein-coupled receptors (GPCRs), where they initiate a signaling cascade that leads to cardioprotection. Gi appears to be involved as the Gi inhibitor pertussis toxin blocks the protection afforded by PC. A number of kinases have been shown to be activated by PC and inhibitors of these kinases attenuate protection, suggesting a role for these kinases in PC. Among the kinases that are thought to be involved downstream of Gi protein-coupled receptors is phosphatidylinositol 3-kinase (PI3K), which phosphorylates and activates Akt. Downstream signaling from Akt activates nitric oxide (NO) synthase (NOS) isoforms and inactivates glycogen synthase kinase (GSK) through phosphorylation. The NO generated by NOS is reported to activate PKG, which, in turn, leads to the stimulation of mitochondrial PKC-ɛ and activation of the mitochondrial ATP-sensitive K+ (mitoKATP) channel, which has been reported to be an important component of the protection afforded by PC. As discussed previously, activation of these signaling pathways is thought to converge on the mitochondria, resulting in inhibition of the mitochondrial permeability transition pore (MPT). Although previous studies have identified many signaling pathways involved in PC, the challenges for the future are to understand how these signals generated by GPCRs are targeted to the mitochondria, establish the identity of the mitochondrial targets, and determine how these mitochondrial targets result in reduced cell death. The study by Quinlan et al. (8) provides some novel insights into these issues.
Quinlan et al. (8), studying pharmacological PC mediated by BK, showed that after BK stimulation, the BK receptor localizes with caveolin, endothelial NOS (eNOS), and PKG in a light layer of mitochondria that they have termed the signalosome. PKG was present in this light mitochondrial layer in the absence of BK stimulation, but the BK receptor, caveolin, and eNOS were all recruited to the signalosome after stimulation of the receptor. The addition of this signalosome to mitochondria from a non-PC heart resulted in the activation of the mitoKATP channel in the non-PC mitochondria. BK-mediated activation of the mitoKATP channel was blocked by bafilomycin or methyl-β-cyclodextrin, suggesting a role for endosomal and caveolin signaling, respectively.
These finding fit with recent data suggesting that GPCR localization in endosomal vesicles can result in unique signaling (4, 10, 11). Endosomal localization of GPCRs was originally described as part of receptor desensitization. For example, after agonist stimulation of the β-adrenergic receptor, the receptor is phosphorylated by G protein receptor kinase 2 (GRK2). Phosphorylation by GRK2 recruits β-arrestin, which decouples the receptor from G protein signaling. PI3K is also recruited to this complex, and the receptor complex is internalized in endosomes. This mechanism was originally thought to function solely for receptor desensitization. However, a recent study (4) has shown that the receptor-β-arrestin complex recruits a number of signaling kinases, such as ERK and GSK, which can result in novel signaling. For example, activation of the angiotensin receptor results in an increase in ERK in the nuclear compartment; however, overexpression of β-arrestin results in increased endosomal localization of the angiotensin receptor and increased cytosolic ERK (10). Interestingly, inhibition of GRK2 signaling, by overexpression of a peptide that blocks GRK2 binding to the receptor and receptor phosphorylation, inhibits the protective effects of PC and the PC-mediated increase in ERK (11). It is thus tempting to speculate that perhaps PC (or pharmacological PC) results in spatial localization of kinase signaling through the assembly of endosomal vesicles, which traffic to mitochondria and coordinate the movement of kinases to the mitochondria (see Fig. 1). These endosomal vesicles may be the source of the light mitochondrial fraction. This mechanism would allow the selective transfer of signals from the GPCR to the mitochondria. The presence of the BK receptor, caveolin, and eNOS, along with kinases such as PKG, in the light mitochondrial fraction would be consistent with BK-mediated assembly and transfer of vesicles containing these components from the plasma membrane to the mitochondria. Regarding a role for caveolin, it is also interesting to note that for the β-adrenergic receptor, phosphorylation with GRK2 resulted in internalization of the receptor via clathrin-coated vesicles, whereas phosphorylation with PKA resulted in internalization of the receptor into caveolae (9).
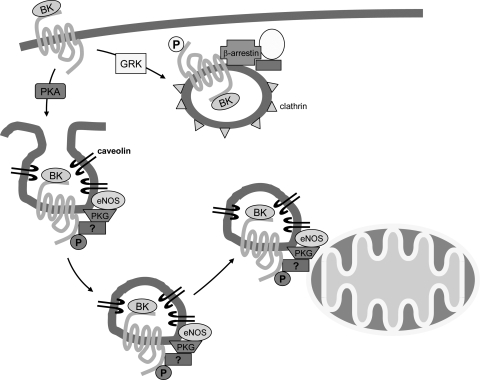
Fig. 1.
Ischemic preconditioning (PC) results in the release of agonists, such as bradykinin (BK), that bind to their receptors. It is hypothesized that BK binding to its receptor initiates the internalization of receptors into caveolin-containing vesicles and that these endosomal vesicles recruit additional signaling molecules, such as endothelial nitric oxide synthase (eNOS) and PKG. It will be interesting to determine whether additional signaling molecules such as β-arrestin, Akt, or glycogen synthase kinase are contained in this “signalosome.” It will also be interesting to determine whether PKA is involved in targeting the receptor to caveolin-containing vesicles, as shown previously. Phosphorylation (P) of the β-adrenergic receptor has been shown to target the receptor to clathrin-coated vesicles. It will interesting to determine whether BK and its receptor can also be targeted to clathrin-coated vesicles and, if so, whether this leads to different signaling. GRK, G protein receptor kinase.
There is mounting evidence that signaling in cells does not occur by free diffusion in the cytosol. The cytosolic or whole cell levels of signals such as cAMP (1) or ERK (10) tell us little about signaling in the different regions of the cell. Rather, there is considerable evidence that adapter proteins such as β-arrestin, 14-3-3, A-kinase anchoring proteins, and others assemble signaling complexes that are recruited to the appropriate compartments, likely by mechanisms that involve movement by microtubules (3) or by similar mechanisms. It is noteworthy that PC has been reported to be blocked by inhibitors of microtubules (7). Thus, it is not surprising that cardioprotection involves the assemblage of signalosomes by agonist binding to GPCRs and that these signaling complexes are directed to the mitochondria. It will be important for future studies to provide insight as to how these signalosomes are directed to the mitochondria and whether there are multiple signalosomes derived from different GPCRs. If multiple signalosomes are generated, do they work in concert or in competition with one another? Does phosphorylation by different kinases, which is reported to direct receptors to caveolae or clathrin-coated vesicles, direct the signalosome to different locations? Or does the composition of the complex differ depending on the phosphorylation site? It will also be of interest to determine whether other kinases suggested to be involved in PC are contained in this signalosome. A number of other kinases, such as ERK and GSK, have been reported to localize to mitochondria with PC. It will be interesting to see if any of these kinases are present in this caveolin signalosome.
In addition to providing some insight into how GPCR signaling complexes might be transferred to the mitochondria, the study by Quinlan et al. also provided new insights regarding mitochondrial targets of the signalosome. Inhibition of PKC-ɛ or PKG or addition of the protein phosphatase 2A (PP2A) were shown by Quinlan et al. to block the BK-activated signalosome stimulation of the mitoKATP channel, suggesting that signalosome activation of mitoKATP channels requires active PKC-ɛ and PKG and is inhibited by PP2A activity. These data are consistent with the hypothesis that the signalosome containing eNOS leads to the activation of PKG, resulting in the activation of mitoKATP channels by a mechanism involving PKC and requiring phosphorylation.
There are still a number of questions regarding how PC reduces myocyte death. It has been proposed that opening of the mitoKATP channel results in the inhibition of the MPT. However, the molecular identity of both the mitoKATP channel and MPT is unknown. The mechanistic link between opening of the mitoKATP channel and inhibition of the MPT remains to be elucidated (2). There are also questions regarding the time frame in which PC inhibits the MPT. It has been suggested that PC initiates a trigger that then results in the inhibition of the MPT at the end of ischemia and beginning of reperfusion. The signalosomes appear to be formed during the trigger phase as the signalosome can be isolated after BK addition but before sustained ischemia. Does the signalosome mediate protection during ischemia or only at the start of reperfusion? Postconditioning has recently been reported to mediate protection when administered immediately at the start of reperfusion. Postconditioning has also been reported to inhibit the MPT. Preliminary data (not included) have indicated that postconditioning also generates a mitochondrial light layer that opens the mitoKATP channel in untreated mitochondria.
In summary, this elegant study by Quinlan et al. has extended our understanding of the mechanistic link between GPCR activation and mitochondrial responses that characterize PC. As with most important scientific advances, this study also raises questions for further investigation.
GRANTS
This research was supported in part by the Intramural Research Program of the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health. C. Steenbergen was supported by National Heart, Lung, and Blood Institute (NHLBI) Grant RO1-HL-39752.
REFERENCES
- 1.Bers DM, Ziolo MT. When is cAMP not cAMP? Effects of compartmentalization. Circ Res 89: 373–375, 2001. [PubMed] [Google Scholar]
- 2.Gross GJ, Peart JN. KATP channels and myocardial preconditioning: an update. Am J Physiol Heart Circ Physiol 285: H921–H930, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Hanson SM, Cleghorn WM, Francis DJ, Vishnivetskiy SA, Raman D, Song X, Nair KS, Slepak VZ, Klug CS, Gurevich VV. Arrestin mobilizes signaling proteins to the cytoskeleton, and redirects their activity. J Mol Biol 368: 375–387, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lefkowitz RJ, Rajagopal K, Whalen EJ. New roles for beta-arrestins in cell signaling: not just for seven-transmembrane receptors. Mol Cell 24: 643–652, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev 88: 581–609, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74: 1124–1136, 1986. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura Y, Miura T, Nakano A, Ichikawa Y, Yano T, Kobayashi H, Ikeda Y, Miki T, Shimamoto K. Role of microtubules in ischemic preconditioning against myocardial infarction. Cardiovasc Res 64: 322–330, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Quinlin C, Costa A, Costa C, Pierre S, Dos Santos P, Garlid K. Preconditioning the heart induces the formation of signalosomes that interact with mitochondria to open mitoKATP channels. Am J Physiol Heart Circ Physiol. First published July 11, 2008; doi: 10.1152/ajpheart.00520.2008 [DOI] [PMC free article] [PubMed]
- 9.Rapacciuolo A, Suvarna S, Barki-Harrington L, Luttrell LM, Cong M, Lefkowitz RJ, Rockman HA. Protein kinase A and G protein-coupled receptor kinase phosphorylation mediates beta-1 adrenergic receptor endocytosis through different pathways. J Biol Chem 278: 35403–35411, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Tohgo A, Pierce KL, Choy EW, Lefkowitz RJ, Luttrell LM. β-Arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation. J Biol Chem 277: 9429–9436, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Tong H, Rockman HA, Koch WJ, Steenbergen C, Murphy E. G protein-coupled receptor internalization signaling is required for cardioprotection in ischemic preconditioning. Circ Res 94: 1133–1141, 2004. [DOI] [PubMed] [Google Scholar]