Abstract
Extensive evidence indicates that serum response factor (SRF) regulates muscle-specific gene expression and that myocardin family SRF cofactors are critical for smooth muscle cell differentiation. In a yeast two hybrid screen for novel SRF binding partners expressed in aortic SMC, we identified four and a half LIM domain protein 2 (FHL2) and confirmed this interaction by GST pull-down and coimmunoprecipitation assays. FHL2 also interacted with all three myocardin factors and enhanced myocardin and myocardin-related transcription factor (MRTF)-A-dependent transactivation of smooth muscle α-actin, SM22, and cardiac atrial natriuretic factor promoters in 10T1/2 cells. The expression of FHL2 increased myocardin and MRTF-A protein levels, and, importantly, this effect was due to an increase in protein stability not due to an increase in myocardin factor mRNA expression. Treatment of cells with proteasome inhibitors MG-132 and lactacystin strongly upregulated endogenous MRTF-A protein levels and resulted in a substantial increase in ubiquitin immunoreactivity in MRTF-A immunoprecipitants. Interestingly, the expression of FHL2 attenuated the effects of RhoA and MRTF-B on promoter activity, perhaps through decreased MRTF-B nuclear localization or decreased SRF-CArG binding. Taken together, these data indicate that myocardin factors are regulated by proteasome-mediated degradation and that FHL2 regulates SRF-dependent transcription by multiple mechanisms, including stabilization of myocardin and MRTF-A.
Keywords: smooth muscle, ubiquitin, restenosis
the serum response transcription factor (SRF) regulates cell type-specific gene expression in cardiac, skeletal, and smooth muscle by binding to CArG elements present in most muscle differentiation marker gene promoters. Although SRF is highly expressed in all three muscle cell types, it is a ubiquitously expressed factor that also regulates several early response growth genes, including c-fos and early growth response-1 (1, 5, 30, 34, 35). Extensive evidence has indicated that SRF activity is regulated mainly by its physical interaction with additional general and cell type-specific transcription factors. The first SRF cofactors identified were the ternary complex factors (Elk-1, Sap-1, SAP-2/NET/ERP) that bind to SRF as well as to the Ets domain adjacent to the c-fos CArG following their phosphorylation by MAPK (for a review, see Ref. 2). SRF has also been shown to interact with cell type-specific factors such as MyoD and GATA-4 to regulate skeletal and cardiac muscle-specific gene expression, respectively (26, 29).
The discovery of myocardin was a major advance in our understanding of the mechanisms that regulate SMC differentiation. This SRF cofactor is specifically expressed in cardiac and smooth muscle, powerfully stimulates SRF-dependent transcription in a variety of cell-types, and is critical for smooth muscle cell (SMC) differentiation in vivo (6, 20, 36). Two myocardin-related transcription factors, MRTF-A and MRTF-B, have also been described that have similar activities to myocardin (37). Although MRTFs are expressed more widely, recent results in knockout mice have indicated that MRTF-B is required for SMC differentiation of cardiac neural crest cells, whereas MRTF-A is required for the SMC differentiation marker gene expression that normally occurs in the myoepithelial layer of the mammary gland during lactation (18, 19, 25, 31). Interestingly, the myocardin factors are differentially regulated by subcellular localization, and our laboratory and others have shown that RhoA-dependent nuclear translocalization of the MRTFs is an important mechanism by which some extrinsic factors stimulate SMC-specific gene expression (9, 13, 21, 22).
To identify additional factors involved in the regulation of SRF-dependent SMC-specific transcription, we conducted a yeast two hybrid screen of a human aortic library using an amino-terminal version of SRF as bait. Three of the clones identified coded for four and a half LIM domain-containing protein 2 (FHL2), a LIM-only protein that has been shown to be selectively expressed in the heart and SMCs during development (8, 16, 33) and that functions as a transcriptional coactivator or corepressor for a variety of transcription factors, including the androgen receptor, cAMP-responsive element-binding protein, activator protein-1, FOXO1, E4F1, and β-catenin (for a review, see Ref. 16). Since FHL2 does not bind DNA directly, these effects are thought to be mediated by the ability of FHL2 to facilitate protein-protein interactions through its multiple LIM domains.
In addition to its expression pattern during development, several features of FHL2 function led us to examine its role in regulating SMC- and cardiac-specific transcription. First, Müller et al. (24) demonstrated that, like the MRTFs, FHL2 nuclear localization and transactivation were dependent on RhoA signaling. Second, Philippar et al. (27) used a genetic screen in SRF−/− embryonic stem (ES) cells to identify FHL2 as an SRF target gene whose upregulation correlated with increased SMC-specific gene expression in an ES cell model of SMC differentiation. These authors demonstrated that FHL2 interacted physically with SRF and that overexpression of FHL2 inhibited RhoA-dependent activation of SM22. Finally, Chang et al. (3, 4) demonstrated that the SMC-specific LIM-only proteins cysteine- and glycine-rich protein (CRP)1 and CRP2 stimulated SMC-specific transcription by facilitating SRF's interaction with GATA factors.
Our results confirm that FHL2 interacts with SRF, but we also demonstrate that FHL2 binds directly to all three myocardin factors and has differential effects on their abilities to regulate cardiac and SMC-specific transcription. Importantly, FHL2 increased myocardin and MRTF-A transactivation, and this effect may be due to protection of these factors from proteasomal degradation.
MATERIALS AND METHODS
Yeast two hybrid screen and plasmid construction.
FHL2 was identified in a Matchmaker yeast two hybrid screen (Clontech) using the SRF NH2-terminus (amino acids 1-201) as bait. Full-length FHL2 was subcloned into flag-pcDNA3 and PGEX4T1 vectors. Expression constructs for SRF, L63RhoA, myocardin, MRTF-A, MRTF-B, and myocardin factor derivatives have been previously described (13, 21). GST fusions, FHL2 0-2 and FHL2 3/4, were generous gifts from Roland Schüle (University of Freiburg, Freiburg, Germany) and have been previously described (23).
Cell culture, transient transfections, and reporter assays.
The rat aortic SMC and 10T1/2 cultures and SMC-specific promoter assays used have been previously described (21). In brief, cells were maintained in 10% serum media and transfected 24 h after being plated at 70–80% confluency using LT-1 transfection reagent (Mirus) per protocol. Luciferase activity measurements were made 24 h posttransfection. Luciferase activity was measured in relative light units and expressed as fold activity over empty vector. For the harvesting of protein, cells were plated in 15-cm dishes and lysed in RIPA plus 0.5% triton.
GST fusion pull-downs and coimmunoprecipitations.
GST pull-down assays and coimmunoprecipitations were performed as previously described (32). In brief, GST fusion proteins were purified from bacterial lysates using glutathione Sepharose (Amersham Biosciences). Interacting proteins were in vitro translated and 35S labeled using the Promega TnT kit. Interacting complexes were pelleted by centrifugation and washed two times in NETN solution and one time in cold Tris-buffered saline. For coimmunoprecipitations, flag-FHL2 was expressed in 10T1/2 cells, and immunoprecipitations were performed using anti-flag or anti-SRF antibodies.
Semiquantitative PCR.
RNA was prepared from cell and tissue lysates using TRIzol reagent (Invitrogen) and was quantified by ribogreen assay (Molecular Probes). cDNA was generated from 1 μg RNA using in the iScript cDNA synthesis kit (Bio-Rad). Exon-spanning primers were used to amplify FHL2, myocardin, MRTF-A, MRTF-B, and GAPDH (sequences available upon request).
EMSAs.
FHL2, SRF, and myocardin factors were in vitro translated using the Promega TnT kit. Binding reactions contained 5 μl of total TnT lysate, a 32P-labeled smooth muscle α-actin Intronic CArG probe (20,000 counts/min), and 0.25 μg dI·dC in binding buffer [10 mM Tris (pH 7.5), 50 mM NaCl, 100 mM KCl, 1 mM DTT, 1 mM EDTA, and 5% glycerol]. Reactions were incubated for 30 min before samples were loaded on a nondenaturing 5% polyacrylamide gel.
Subcellular localization experiments.
10T1/2 cells were transfected with enhanced green fluorescent protein (EGFP)-MRTF-B with or without FHL2. After serum starvation, cells were fixed in 4% paraformaldehyde, and EGFP-MRTF-B localization was then scored into three categories: nuclear, cytoplasmic, and diffuse.
FHL2 knockdown.
The following short interfering (si)RNAs were ordered from Invitrogen: control (NTC) 5′-UGGUUUACAUGUCGACUAA-3′ and FHL2 5′-GCAAGGACUUGUCCUACAA-3′. siRNAs were transfected into primary rat aortic SMCs using dharmafect reagent 1 (Dharmacon) following the manufacturer's protocol. FHL2 protein expression in SMC RIPA lysates was measured by Western blot analysis using an anti-FHL2 antibody (Santa Cruz Biotechnology). For promoter-luciferase assays, transfections were performed 24 h after the oligonucleotide introduction.
Detection of ubiquitylated MRTF-A.
Myocardin factor-expressing or nonexpressing 10T1/2 cells were treated for 12 h with MG-132 (20 μM), lactacystin (10 μM), or DMSO vehicle. RIPA lysates were run on a SDS-PAGE gel, transferred to nitrocellulose membranes, and probed with anti-flag, anti-tubulin, or anti-MRTF-A antibodies. MRTF-A was immunoprecipitated from MG-132-treated cells using a monoclonal hamster anti-MRTF-A antibody coupled to protein G beads (Sigma). Western blots were performed on immunoprecipitants using the P4G7 anti-ubiquitin antibody (Covance).
Determination of the MRTF-A half-life.
10T1/2 cells with or without FHL2 were treated with cycloheximide (50 μg/ml) or vehicle. Endogenous MRTF-A expression was measured by Western blot analysis at 0, 0.5, 3, and 6 h. Similar experiments were done in COS cells expressing flag-MRTF-A and in 10T1/2 cells treated with MG-132.
RESULTS
FHL2 interacted with SRF.
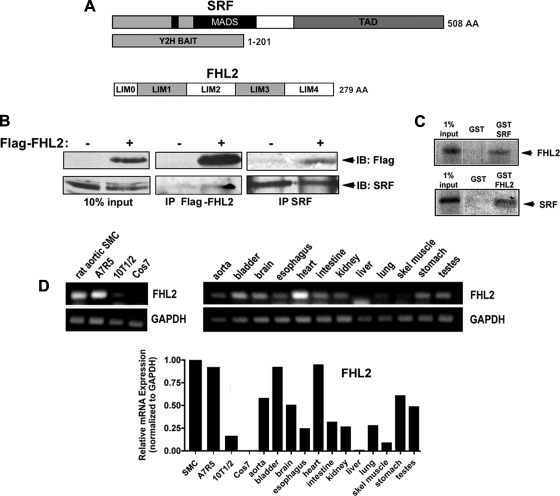
To identify novel members of the transcriptional complex required for the expression of SMC differentiation marker genes, we screened a human aortic yeast two hybrid library using the NH2-terminal third of SRF (amino acids 1-201) as bait. This SRF fragment included the inhibitory NH2-terminal region, nuclear localization signal, and MADS box motifs αI (amino acids 153-179), βI (amino acids 182-188), and βII (amino acids 194-198) (Fig. 1A). Three of the fifty interacting clones coded for the LIM-only protein FHL2, and a series of secondary yeast screens were performed to eliminate the possibility that the FHL2-Gal4 activation domain fusion construct activated yeast reporter genes on its own. Using coimmunoprecipitation assays and GST fusion pull-downs, we observed a consistent but relatively weak interaction between FHL2 and SRF (Fig. 1, B and C). These data confirm the observation by Philippar et al. (27) that FHL2 interacts with SRF.
Fig. 1.
Four and a half LIM domain protein 2 (FHL2) interacted with serum response factor (SRF) and was strongly expressed in cardiac cells and smooth muscle cells (SMCs). A: schematics of the SRF bait used in the yeast two hybrid (Y2H) screen and FHL2. MADS, MCM1, agamous, deficiens, SRF box; TAD, transcription activation domain; LIM0, half LIM domain; LIM1-4, LIM domains 1-4. B: SRF and flag-FHL2 were immunoprecipitated (IP) from 10T1/2 lysates. Immunoprecipitants were washed, subjected to SDS-PAGE, and immunoblotted (IB) with anti-flag and anti-SRF antibodies. C: pull-down assays were performed using the indicated GST fusions and in vitro translated, 35S-labeled proteins. D: semiquantitative RT-PCRs for FHL2 were performed on mRNA isolated from the indicated cultured cells and mouse tissues. Band intensities were quantified using ImageJ software and are expressed relative to GAPDH.
FHL2 was strongly expressed in SMCs and SMC-containing tissues.
FHL2 belongs to a subclass of LIM-only proteins that is characterized by four full LIM domains and one half LIM domain at the amino-termini separated by short linker peptides. To date, six members of this family (FHL1-5 and ACT) have been identified, which have varying expression patterns (8, 12). FHL2 was originally thought to be specifically expressed in the myocardium, but examination of mice containing LacZ knocked into the endogenous FHL2 locus revealed a high expression of FHL2 in the developing vasculature (8). To specifically examine FHL2 expression in adult smooth muscle, we used semiquantitative RT-PCR (Fig. 1D). As expected, the FHL2 message was highest in the heart, but significant levels were also detected in tissues that contain a large SMC component, including the aorta, bladder, esophagus, and stomach. In addition, FHL2 expression was very high in primary rat aortic and A7r5 SMCs, weak in multipotential 10T1/2 cells, and absent in COS-7 cells. FHL1, which has also been shown to be expressed in the developing heart and outflow tract, was expressed in a similar pattern (data not shown).
FHL2 interacted directly with all three myocardin family members.
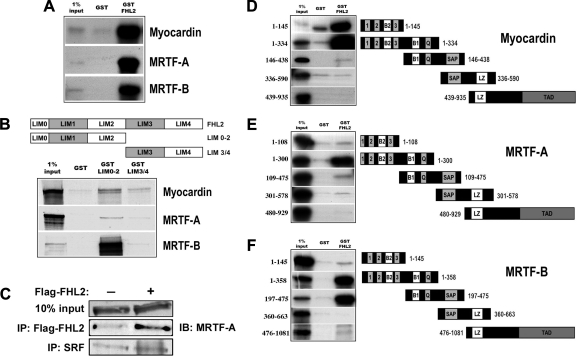
Based on previous studies demonstrating that CRP LIM-only proteins facilitated the formation of a SRF/GATA4 complex and that the transcriptional activity of FLH2, like that of MRTF-A and MRTF-B, was regulated by RhoA, we postulated that FHL2 may interact with the myocardin factors. As shown in Fig. 2A, GST-FHL2 strongly precipitated in vitro translated myocardin, MRTF-A, and MRTF-B. Further mapping experiments suggested that full-length FHL2 was required for strong interactions with myocardin and MRTF-A, whereas an FHL2 fragment containing only the NH2-terminal two and half LIM domains (LIM 0-2) could mediate binding with MRTF-B (Fig. 2B). In addition, endogenous MRTF-A coimmunoprecipitated with flag-FHL2 expressed in 10T1/2 cells, further suggesting that these proteins interact in vivo (Fig. 2C).
Fig. 2.
FHL2 interacted physically with myocardin factors. A: GST or GST-FHL2 were incubated with in vitro translated, 35S labeled myocardin, myocardin-related transcription factor (MRTF)-A or MRTF-B. Complexes were pelleted, washed three times, ran on a SDS-PAGE gel, and exposed to film. B: FHL2 truncations containing the first two and a half LIM domains (LIM 0-2) or the last two LIM domains (LIM 3/4) were fused to GST and used to precipitate myocardin factors in similar reactions. C: 10T1/2 cells were transfected with flag-FHL2 or empty expression vector. Forty-eight hours posttransfection, RIPA lystates were immunoprecipitated with anti-flag or anti-SRF antibodies. Immunoprecipitants were then probed using a monoclonal anti-MRTF-A antibody. Note that the expression of FHL2 had little effect on the interaction between SRF and MRTF-A. D–F: several myocardin factor deletions were generated and used in pull-down assays with GST and GST-FHL2. D: myocardin deletions; E: MRTF-A deletions; F: MRTF-B deletions.
We also mapped the domains of the myocardin factors that interacted with FHL2. As shown in Fig. 2, D–F, FHL2 bound most strongly to myocardin, MRTF-A, and MRTF-B through a NH2-terminal fragment that contained the RPEL motifs, the two basic domains, and the Q-rich region. Interestingly, FHL2 bound fairly strongly to a myocardin fragment that contained only the RPEL motifs and basic domain 2 (Fig. 2D) and to a MRTF-B fragment that contained only basic domain 1, the Q-rich region, and the SAP domain (Fig. 2F). Taken together, these results suggest that the region near basic domain 1 is probably the most important for FHL2 binding. The slight differences in FHL2 binding to the myocardin factors were somewhat surprising and suggested that FHL2 may have differential effects on the myocardin factors.
FHL2 enhanced myocardin and MRTF-A transactivation.
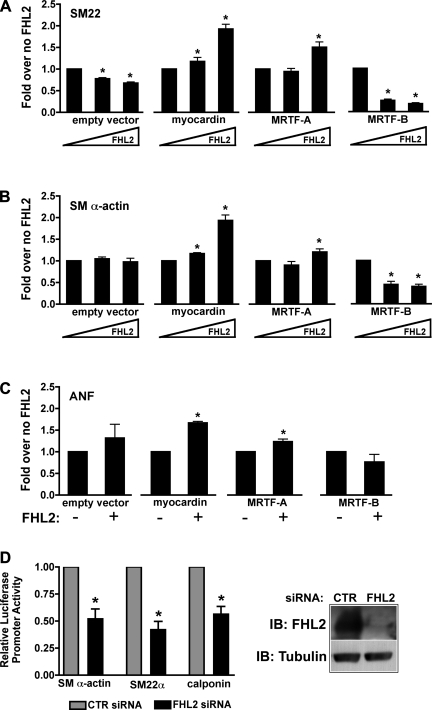
To test whether FHL2 had functional effects on myocardin factor activity, we coexpressed FHL2 with each of the myocardin factors along with the smooth muscle α-actin and SM22 promoters in multipotential 10T1/2 cells. In these experiments, we transfected submaximal levels of myocardin, MRTF-A, and MRTF-B, which activated these promoters by ∼60-, 45-, and 10-fold, respectively (data not shown). As shown in Fig. 3, coexpression of FHL2 dose dependently increased the ability of myocardin to stimulate SMC-specific promoter activity. In stark contrast, FHL2 inhibited transactivation by MRTF-B and slightly stimulated transactivation by MRTF-A, but only at high concentrations. Because FHL2 expression is the highest in the myocardium, we also examined the effects of FHL2 on the cardiac-specific atrial natriuretic factor (ANF) promoter. Similar to effects observed with SMC-specific promoters, FHL2 increased the transactivation of ANF by myocardin and MRTF-A. We initially hypothesized that FHL2 increased myocardin and MRTF-A activity by facilitating their interactions with SRF. However, expression of FHL2 had no effect on the association of SRF and MRTF-A, as measured by the coimmunoprecipitation of proteins from 10T1/2 lysates (Fig. 2C), and did not increase ternary complex formation between SRF and the myocardin factors in gel shift assays (see Fig. 6).
Fig. 3.
FHL2 differentially regulated myocardin factor transactivation. SM22 (A), smooth muscle (SM) α-actin (B), or atrial natriuretic factor (ANF; C) promoter-luciferase constructs were cotransfected into 10T1/2 cells along with one of the myocardin factors and increasing concentrations of FHL2. The total amount of expression plasmid in each transfection was equalized by the addition of empty expression vector. Cells were maintained in 10% serum media and assayed for luciferase activity at 24 h. Results are expressed relative to promoter activity in the presence of the indicated myocardin factor but without FHL coexpression. *P ≤ 0.05 vs. without (−) FHL2. D: after short interfering (si)RNA-mediated knockdown of FHL2 expression in SMCs (right), cells were transfected with the indicated luciferase reporter constructs, and luciferase activity was measured at 48 h (left). *P ≤ 0.05 vs. control (CTR) siRNA.
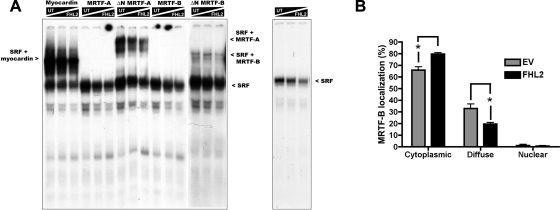
Fig. 6.
FHL2 modestly decreased SRF complex formation and nuclear translocation of MRTF-B. A: in vitro translated SRF and the indicated myocardin factors were incubated with a radiolabeled SM α-actin Intronic CArG probe. Increasing concentrations of in vitro translated FHL2 (0, 2, or 4 μl) were added to each binding reaction, and the total amount of TnT lysate in each reaction was maintained by the addition of unprogrammed TnT lysate (UT). After 30 min, binding reactions were run on a 5% nondenaturing gel, transferred to filter paper, and visualized by autoradiography. B: 10T1/2 cells were transfected with enhanced green fluorescent protein (EGFP)-MRTF-B with FHL2 or without FHL2 (EV) and maintained in serum-free media for 24 h. After fixation, EGFP-MRTF-B localization was scored as nuclear, cytoplasmic, or diffuse. Data represent means ± SE of 3 separate transfections. *P ≤ 0.05.
To further explore the role of FHL2 in SMC differentiation marker gene expression, we used siRNA to knock down FHL2 expression in primary rat aortic SMCs. As shown in Fig. 3D, knockdown of FHL2 expression by 90% led to an ∼50% decrease in the activities of smooth muscle α-actin, SM22, and calponin promoters.
FHL2 protected myocardin and MRTF-A from proteasome-mediated degradation.
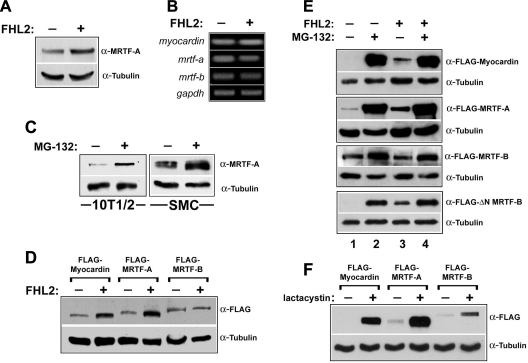
A recent report (10) has demonstrated that FHL2 is a target for the ubiquitin E3 ligase Murf3. Given that other LIM domain-containing proteins can inhibit proteasome-mediated degradation by interacting with E3 ligases or ubiquitin targets (15, 28), we tested whether myocardin factor protein levels were regulated by proteasomal degradation and whether the positive effects of FHL2 on myocardin factor activity were due to inhibition of this pathway. We have recently generated a highly specific monoclonal antibody for MRTF-A that we first used to determine whether endogenous MRTF-A protein levels were affected by FHL2 expression. Even with a transfection efficiency of only 40-50% (measured by parallel transfection of GFP), overexpression of FHL2 in 10T1/2 cells led to a significant increase (∼3-fold) in MRTF-A protein, as measured by Western blot (Fig. 4A). Importantly, overexpression of FHL2 did not affect myocardin factor mRNA levels (Fig. 4B). Treatment of cells with the proteasome inhibitor MG-132 resulted in a slightly larger increase in endogenous MRTF-A protein in both 10T1/2 cells and primary rat aortic SMCs (Fig. 4C).
Fig. 4.
FHL2 inhibited proteasome-mediated degradation of myocardin and MRTF-A. A: 10T1/2 cells were transfected with FHL2 or empty expression vector, lysed after 24 h, and subjected to Western blot analysis using a monoclonal antibody to MRTF-A. B: RT-PCR for each myocardin factor was performed on mRNA isolated from cells transfected with FHL2 or empty vector. C: MRTF-A expression was measured by Western blot in 10T1/2 cells and SMCs treated with the proteasome inhibitor MG-132 (20 μM) or DMSO for 12 h. D: exogenously expressed myocardin, MRTF-A, and MRTF-B were measured in 10T1/2 cells in the absence or presence of cotransfected FHL2. E: flag-tagged myocardin factors were cotransfected with FHL2 or empty vector and treated with MG-132 (20 μM) or DMSO for 12 h. F: exogenously expressed myocardin, MRTF-A, and MRTF-B were measured in 10T1/2 cells after treatment with 10 μM lactacystin or DMSO vehicle for 12 h.
We were also interested in the effects of FHL2 on myocardin and MRTF-B protein levels, but because we have had little success in measuring myocardin or MRTF-B protein levels with the available antibodies, we cotransfected FHL2 along with flag-tagged versions of all three myocardin factors into 10T1/2 cells. As shown in Fig. 4D, overexpression of FHL2 led to increased levels of exogenously expressed myocardin and MRTF-A but had no effect on levels of MRTF-B. MG-132 treatment led to a more dramatic increase in myocardin and MRTF-A protein and slightly increased MRTF-B levels (Fig. 4E, compare lanes 1 and 2). Interestingly, FHL2 expression had little, if any, effect on myocardin and MRTF-A levels in MG-132-treated cells (Fig. 4E, compare lanes 2 and 4). We observed very similar changes in myocardin factor protein levels upon treatment of cells with lactacystin, a more specific inhibitor of the proteasome (Fig. 4F).
Interestingly, the ability of FHL2 to protect myocardin factors from degradation correlated fairly well with myocardin factor nuclear localization. For example, myocardin, which is constitutively nuclear, was protected from degradation by FHL2. In contrast, MRTF-B, which is frequently cytoplasmic, was not upregulated by FHL2 expression. To test this more rigorously, we compared the effects of MG-132 and FHL2 on ΔN MRTF-B, which lacks the NH2-terminal RPEL motifs that are required for the maintenance of MRTF-B localization to the cytoplasm. ΔN MRTF-B levels were much lower than full-length MRFT-B levels under control conditions, and, unlike full-length MRTF-B, ΔN MRTF-B protein levels were upregulated by FHL2 coexpression and were strongly upregulated by MG-132 treatment (Fig. 4E, bottom).
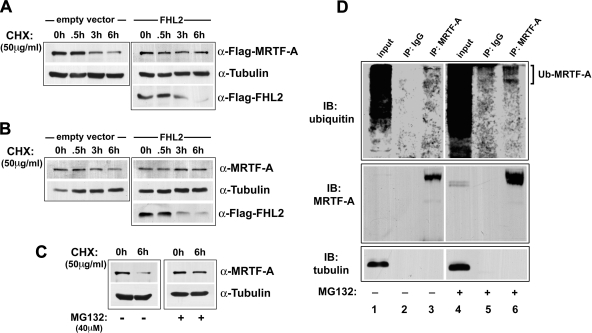
To directly measure the effects of FHL2 on MRTF-A protein stability, we treated cells with the protein synthesis inhibitor cycloheximide and measured MRTF-A protein by Western blot at 0.5, 3, and 6 h. As shown in Fig. 5, the half-life of overexpressed or endogenous MRTF-A was ∼3 h and was significantly extended by the expression of FHL2 (Fig. 5, A and B) or by proteasome inhibition (Fig. 5C). Note that the protein half-life of FHL2 was substantially shorter (∼1.5 h).
Fig. 5.
MRTF-A half-life was increased by the overexpression of FHL2 or treatment with MG-132. A: COS-7 cells were transfected with flag-MRTF-A with or without FHL2. Cells were treated with cycloheximide (CHX) and flag-MRTF-A, and flag-FHL2 levels were measured by Western blot at 0, 0.5, 3, or 6 h. B: 10T1/2 cells with or without flag-FHL2 were treated with CHX as described above, and endogenous MRTF-A expression was measured by Western blot at 0, 0.5, 3, or 6 h. C: MRTF-A expression was measured in 10T1/2 cells treated simultaneously with MG-132 and CHX. D: MRTF-A was immunoprecipitated from control and MG-132-treated SMC lysates. Immunoprecipitants were probed with an antibody to ubiquitin (Ub; top), MRTF-A (middle), or tubulin (bottom).
To determine whether myocardin factors were targeted to the proteasome by ubiquitylation, we immunoprecipitated MRTF-A from MG-132-treated and control SMCs and probed immunoprecipitates with an anti-ubiquitin antibody. As shown in Fig. 5D, ubiquitin immunoreactivity was only detected in MRTF-A (not control IgG) immunoprecipitants, and treatment of SMCs with MG-132 resulted in a substantial increase in this signal.
FHL2 did not increase myocardin factor association with SRF and may negatively regulate SRF-CArG binding.
Philippar et al. (20) has reported that FHL2 expression inhibited RhoA-dependent increases in SM22 and smooth muscle α-actin promoter activity in 293T cells and, based on gel shift analyses, suggested that this effect was due to competitive inhibition of myocardin factor binding to SRF. We observed similar effects of FHL2 on RhoA-dependent stimulation of SMC-specific transcription in our 10T1/2 model (data not shown) and also demonstrated that FHL2 had negative effects on MRTF-B transactivation (Fig. 3). However, our demonstration that FHL2 interacts directly with myocardin factors and has differential effects on the myocardin factor transactivation potentially complicates this model.
Based on our observation that FHL2 binds slightly differently to each myocardin factor, we used gel shift assays to test whether FHL2 had differential effects on myocardin factor binding to the SRF-CArG complex. Because the NH2-terminal RPEL domains of MRTF-A and MRTF-B inhibit their incorporation into the SRF-containing ternary complex, full-length and NH2-terminally truncated variants (ΔN) of MRTFs were used in these assays. The results shown in Fig. 6A demonstrate that only myocardin and the ΔN forms of MRTFs formed ternary complexes with SRF at the smooth muscle α-actin intronic CArG element. The addition of in vitro translated FHL2 to the binding reaction slightly decreased the intensity of all SRF-containing complexes, including those that did not contain a myocardin factor. This observation was particularly evident in the binding reactions that contained SRF only (Fig. 6A, far right). Taken together, although FHL2 did slightly decrease the levels of ternary complex, this was likely due to an overall reduction in SRF-CArG binding.
FHL2 modestly inhibited MRTF nuclear translocation.
FHL2, like the MRTFs, has been shown to translocate from the cytoplasm to the nucleus in response to external stimuli that activate RhoA (24). Given that the effects of RhoA are strongly dependent on nuclear translocation of the MRTFs, we hypothesized that the inhibitory effects of FHL2 could also be due to inhibition of MRTF-B nuclear translocation. To test this, we coexpressed flag-FHL2 along with EGFP-MRTF-B fusion proteins. MRTF-B localization under serum-free conditions was scored as nuclear, cytoplasmic, or diffuse (nuclear + cytoplasmic). As shown in Fig. 6B, expression of FHL2 slightly increased the percentage of cells exhibiting strictly cytoplasmic localization and decreased the percentage of cells exhibiting diffuse localization.
DISCUSSION
FHL2 has been shown to interact with a large number of transcription factors in a number of different model systems and has been shown to have both positive and negative effects on gene expression (for a review, see Ref. 16). The goal of the present study was to further characterize the effects of FHL2 on SRF-dependent transcription. While our results confirm a previous report showing that FHL2 interacts directly with SRF, they also suggest that the role of FLH2 in the regulation of SRF-dependent transcription is complicated by additional interactions with the myocardin family of SRF cofactors. We demonstrate, for the first time, that the expression of FHL2 stimulates myocardin-dependent transactivation of cardiac and SMC-specific promoter activity and that these effects are most likely due to protection of myocardin and MRTF-A from proteasomal degradation. Thus, based on the known expression pattern of FHL2 during development, we propose that FHL2 promotes cardiac and SMC differentiation by preserving myocardin factor protein levels. In addition, since the FHL2 and SRF promoters are both regulated by SRF (27) (and probably the myocardin factors), FHL2 could be a key component of a feedforward mechanism that maintains high levels of cardiac and/or SMC-specific gene expression.
This study demonstrates that the myocardin factors are tightly regulated by the proteasome and may provide a novel mechanism for the control of cardiac and SMC-specific transcription. Elberg et al. (10) reported that the addition of proteasome inhibitor MG-132 or Z-LLF prevented the degradation of MRTF-A in renal tubular epithelial cells, providing excellent support for the data presented here and suggesting that the myocardin factors are regulated by the proteasome in multiple cell types. Our finding that endogenous MRTF-A is ubiquitylated suggests that the myocardin factors may be direct targets for the proteasome, but it remains possible that myocardin factor stability is also regulated by another mechanism that is proteasome sensitive. In addition, although the effects of FHL2 on MRTF-A protein half-life strongly suggest that FHL2 stabilizes myocardin factor protein, we cannot completely rule out an additional effect of FHL2 on the translation of myocardin factor message.
Previous studies have shown that the effects of FHL2 on gene expression are determined by a number of parameters, including cellular localization and interactions with a variety of general and cell type-specific transcription factors. The positive and negative transcriptional effects of FHL2 observed in this study likely reflect a combination of these differences. For example, although FHL2 may attenuate cardiac and SMC-specific promoter activity by inhibiting ternary complex formation or MRTF-B nuclear translocation, these effects may be counteracted by increased myocardin and MRTF-A protein levels. The failure of FHL2 to protect MRTF-B from degradation may help explain the negative effects of FHL2 on MRTF-B transactivation. It is also possible that the negative effects of FHL2 may be due to sequestration of the myocardin factors or SRF into inactive complexes.
It is currently unclear why FHL2 does not affect MRTF-B stability. Our results, especially the comparison between full-length and ΔN MRTF-B, demonstrated that myocardin factor protein levels and the ability of FHL2 to protect individual myocardin factors from degradation were affected by nuclear localization. These results suggest the interesting possibility that myocardin factor ubiquitylation occurs mainly in the nucleus. It is also possible that small differences in FHL2 binding to each myocardin factor could affect interactions with the ubiquitylation or proteasome machinery.
Other LIM domain-containing proteins have also been shown to inhibit ubiquitylation. Sangadala et al. (28) demonstrated that LIM-mineralization protein-1 stimulated osteoblast differentiation in mesenchymal cells by an inhibitory interaction with the E3 ligase Smurf1 and that this interaction prevented SMAD degradation. In addition, Hiratani et al. (15) demonstrated that the Xenopus LIM homeodomain protein Xlim-1 prevented the degradation of its binding partner, Ldb1, by the E3 ligase XRnf12. Importantly, Fielitz et al. (11) identified FHL2 as a substrate for the E3 ligase Murf3, and a very important future goal is to determine whether myocardin factors are potential targets of MuRF proteins. Although we did not detect FHL2 in complex with the SRF myocardin factor complex by gel shift, flag-FHL2 was detected at the CArG-containing regions of smooth muscle α-actin and SM22 promoters in vivo (27). If FHL2 and the myocardin factors are targets of the same E3 ligase, FHL2 could shield the myocardin factors from degradation by competitively interfering with myocardin factor ubiquitylation. This would lead to the stabilization of an important component of the SMC-specific transcription initiation complex and increased SMC differentiation marker gene expression.
Deletion of FHL2 in the mouse did not result in an overt cardiac phenotype (7). However, it is unknown whether other FHL family members (most likely FHL1) can compensate for the loss of FHL2 in this model, and the fact that FHL2−/− mice are more susceptible to isoproterenol-induced cardiac hypertrophy (17) suggests that FHL2 does have a specific cardiac function. Although the effects of FHL2 deficiency on SMC function have not been directly examined, a recent study by Wixler et al. (38) has indicated that FHL2 regulates SMC differentiation marker gene expression in vivo. These authors observed that FHL2 was activated in skin myofibroblasts after wounding and that FHL2 expression correlated with the activation of smooth muscle α-actin and SM22 expression that is known to occur in this model (14). Furthermore, they demonstrated that FHL2−/− mice exhibited reduced smooth muscle α-actin expression and healing after wounding and that reexpression of FHL2 in FHL2−/− MEFs and mesenchymal stem cells upregulated smooth muscle α-actin promoter activity. These results support a closer examination of the role of FHL2 in the modulation of SMC phenotype after vessel injury.
In summary, FHL2 has multiple effects on SRF- and myocardin factor-dependent transcription. Our data suggest that FHL2 protects myocardin and MRTF-A from proteasome-mediated degradation, providing a novel mechanism for the control of myocardin factor activity. It will be very important to further characterize this regulatory mechanism in cardiac cells and SMCs and to test whether FHL2 (and perhaps other FHL family members) has similar effects on other transcription factors.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-070953 (to C. P. Mack) and HL-081844 and HL-071054 (to J. M. Taylor).
Acknowledgments
The authors thank Roland Schule (University of Freiburg, Freiburg, Germany) and Da-Zhi Wang (University of North Carolina, Chapel Hill, NC) for reagents.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Browning CL, Culberson DE, Aragon IV, Fillmore RA, Croissant JD, Schwartz RJ, Zimmer WE. The developmentally regulated expression of serum response factor plays a key role in the control of smooth muscle-specific genes. Dev Biol 194: 18–37, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Buchwalter G, Gross C, Wasylyk B. The ternary complex factor Net regulates cell migration through inhibition of PAI-1 expression. Mol Cell Biol 25: 10853–10862, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang DF, Belaguli NS, Chang J, Schwartz RJ. LIM-only protein, CRP2, switched on smooth muscle gene activity in adult cardiac myocytes. Proc Natl Acad Sci USA 104: 157–162, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang DF, Belaguli NS, Iyer D, Roberts WB, Wu SP, Dong XR, Marx JG, Moore MS, Beckerle MC, Majesky MW, Schwartz RJ. Cysteine-rich LIM-only proteins CRP1 and CRP2 are potent smooth muscle differentiation cofactors. Dev Cell 4: 107–118, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Chen CY, Croissant J, Majesky M, Topouzis S, McQuinn T, Frankovsky MJ, Schwartz RJ. Activation of the cardiac alpha-actin promoter depends upon serum response factor, Tinman homologue, Nkx-2.5, and intact serum response elements. Dev Genet 19: 119–130, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Kitchen CM, Streb JW, Miano JM. Myocardin: a component of a molecular switch for smooth muscle differentiation. J Mol Cell Cardiol 34: 1345–1356, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Chu PH, Bardwell WM, Gu Y, Ross J Jr, Chen J. FHL2 (SLIM3) is not essential for cardiac development and function. Mol Cell Biol 20: 7460–7462, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu PH, Ruiz-Lozano P, Zhou Q, Cai C, Chen J. Expression patterns of FHL/SLIM family members suggest important functional roles in skeletal muscle and cardiovascular system. Mech Dev 95: 259–265, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Du KL, Chen M, Li J, Lepore JJ, Mericko P, Parmacek MS. Megakaryoblastic leukemia factor-1 transduces cytoskeletal signals and induces smooth muscle cell differentiation from undifferentiated embryonic stem cells. J Biol Chem 279: 17578–17586, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Elberg G, Chen L, Elberg D, Chan MD, Logan CJ, Turman MA. MKL1 mediates TGF-β1-induced α-smooth muscle actin expression in human renal epithelial cells. Am J Physiol Renal Physiol 294: F1116–F1128, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Fielitz J, van Rooij E, Spencer JA, Shelton JM, Latif S, van der Nagel R, Bezprozvannaya S, de Windt L, Richardson JA, Bassel-Duby R, Olson EN. Loss of muscle-specific RING-finger 3 predisposes the heart to cardiac rupture after myocardial infarction. Proc Natl Acad Sci USA 104: 4377–4382, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fimia GM, De Cesare D, Sassone-Corsi P. A family of LIM-only transcriptional coactivators: tissue-specific expression and selective activation of CREB and CREM. Mol Cell Biol 20: 8613–8622, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinson JS, Medlin MD, Lockman K, Taylor JM, Mack CP. Smooth muscle cell-specific transcription is regulated by nuclear localization of the myocardin-related transcription factors. Am J Physiol Heart Circ Physiol 292: H1170–H1180, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol 170: 1807–1816, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiratani I, Yamamoto N, Mochizuki T, Ohmori SY, Taira M. Selective degradation of excess Ldb1 by Rnf12/RLIM confers proper Ldb1 expression levels and Xlim-1/Ldb1 stoichiometry in Xenopus organizer functions. Development 130: 4161–4175, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Johannessen M, Moller S, Hansen T, Moens U, Van Ghelue M. The multifunctional roles of the four-and-a-half-LIM only protein FHL2. Cell Mol Life Sci 63: 268–284, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kong Y, Shelton JM, Rothermel B, Li X, Richardson JA, Bassel-Duby R, Williams RS. Cardiac-specific LIM protein FHL2 modifies the hypertrophic response to beta-adrenergic stimulation. Circulation 103: 2731–2738, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Zhu X, Chen M, Cheng L, Zhou D, Lu MM, Du K, Epstein JA, Parmacek MS. Myocardin-related transcription factor B is required in cardiac neural crest for smooth muscle differentiation and cardiovascular development. Proc Natl Acad Sci USA 102: 8916–8921, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li S, Chang S, Qi X, Richardson JA, Olson EN. Requirement of a myocardin-related transcription factor for development of mammary myoepithelial cells. Mol Cell Biol 26: 5797–5808, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li S, Wang DZ, Wang Z, Richardson JA, Olson EN. The serum response factor coactivator myocardin is required for vascular smooth muscle development. Proc Natl Acad Sci USA 100: 9366–9370, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lockman K, Hinson JS, Medlin MD, Morris D, Taylor JM, Mack CP. Sphingosine 1-phosphate stimulates smooth muscle cell differentiation and proliferation by activating separate serum response factor co-factors. J Biol Chem 279: 42422–42430, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell 113: 329–342, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Muller JM, Isele U, Metzger E, Rempel A, Moser M, Pscherer A, Breyer T, Holubarsch C, Buettner R, Schule R. FHL2, a novel tissue-specific coactivator of the androgen receptor. EMBO J 19: 359–369, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller JM, Metzger E, Greschik H, Bosserhoff AK, Mercep L, Buettner R, Schule R. The transcriptional coactivator FHL2 transmits Rho signals from the cell membrane into the nucleus. EMBO J 21: 736–748, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh J, Richardson JA, Olson EN. Requirement of myocardin-related transcription factor-B for remodeling of branchial arch arteries and smooth muscle differentiation. Proc Natl Acad Sci USA 102: 15122–15127, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olson EN Regulation of muscle transcription by the MyoD family. The heart of the matter. Circ Res 72: 1–6, 1993. [DOI] [PubMed] [Google Scholar]
- 27.Philippar U, Schratt G, Dieterich C, Muller JM, Galgoczy P, Engel FB, Keating MT, Gertler F, Schule R, Vingron M, Nordheim A. The SRF target gene Fhl2 antagonizes RhoA/MAL-dependent activation of SRF. Mol Cell 16: 867–880, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Sangadala S, Boden SD, Viggeswarapu M, Liu Y, Titus L. LIM mineralization protein-1 potentiates bone morphogenetic protein responsiveness via a novel interaction with Smurf1 resulting in decreased ubiquitination of Smads. J Biol Chem 281: 17212–17219, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Sepulveda JL, Belaguli N, Nigam V, Chen CY, Nemer M, Schwartz RJ. GATA-4 and Nkx-2.5 coactivate Nkx-2 DNA binding targets: role for regulating early cardiac gene expression. Mol Cell Biol 18: 3405–3415, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sotiropoulos A, Gineitis D, Copeland J, Treisman R. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell 98: 159–169, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Sun Y, Boyd K, Xu W, Ma J, Jackson CW, Fu A, Shillingford JM, Robinson GW, Hennighausen L, Hitzler JK, Ma Z, Morris SW. Acute myeloid leukemia-associated Mkl1 (Mrtf-a) is a key regulator of mammary gland function. Mol Cell Biol 26: 5809–5826, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sundberg LJ, Galante LM, Bill HM, Mack CP, Taylor JM. An endogenous inhibitor of focal adhesion kinase blocks Rac1/JNK but not Ras/ERK-dependent signaling in vascular smooth muscle cells. J Biol Chem 278: 29783–29791, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Taira M, Evrard JL, Steinmetz A, Dawid IB. Classification of LIM proteins. Trends Genet 11: 431–432, 1995. [DOI] [PubMed] [Google Scholar]
- 34.Treisman R, Ammerer G. The SRF and MCM1 transcription factors. Curr Opin Genet Dev 2: 221–226, 1992. [DOI] [PubMed] [Google Scholar]
- 35.Walsh K Cross-binding of factors to functionally different promoter elements in c-fos and skeletal actin genes. Mol Cell Biol 9: 2191–2201, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang D, Chang PS, Wang Z, Sutherland L, Richardson JA, Small E, Krieg PA, Olson EN. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell 105: 851–862, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Wang DZ, Li S, Hockemeyer D, Sutherland L, Wang Z, Schratt G, Richardson JA, Nordheim A, Olson EN. Potentiation of serum response factor activity by a family of myocardin-related transcription factors. Proc Natl Acad Sci USA 99: 14855–14860, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wixler V, Hirner S, Muller JM, Gullotti L, Will C, Kirfel J, Gunther T, Schneider H, Bosserhoff A, Schorle H, Park J, Schule R, Buettner R. Deficiency in the LIM-only protein Fhl2 impairs skin wound healing. J Cell Biol 177: 163–172, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]